Introduction
Multiple myeloma (MM) is a hematological malignancy
derived from clonal B cells. Different stages of differentiation of
the neoplastic clone may cause heterogeneity in MM (1). MM is relatively uncommon but can be
fatal. Patients with MM initially respond to treatment, but
eventually relapse and succumb to disease after several months or
years (2,3). Thus, the development of novel
treatment strategies to effectively control MM are required.
Cancerous inhibitor of protein phosphatase 2A
(CIP2A) is a novel human oncoprotein that can promote tumor
transformation and maintain the malignant phenotype in various
types of cancer (4). The oncogenic
effects of CIP2A may occur in part through stabilization of c-Myc
(5). CIP2A levels are
significantly elevated in several malignancies, compared with
normal tissue (6–8). To the best of our knowledge, CIP2A
expression has not been reported in MM. Thus, the present study
aimed to determine the levels of CIP2A in patients with MM and in
MM cell lines. In addition to the effect of CIP2A on MM cell growth
and apoptosis following knockdown using shRNA, phosphorylation of
key signaling molecules was analyzed in vitro.
Materials and methods
Serum samples
In total, 40 serum samples were obtained from
patients with MM who underwent treatment at the Second Hospital of
Shanxi Medical University (Taiyuan, Shanxi) between October 2010
and August 2014 (20 cases with κ-type, 16 cases with λ-type and 4
cases with non-secretory type). There were 20 serum samples from
the normal control without any detectable bone marrow abnormalities
observed during physical examination. The controls had a median age
of 42 years (range, 31–60 years), controls were excluded if they
were deceased or had been previously diagnosed with a hematological
malignancy at the corresponding MM patient diagnosis. The
diagnostic criteria of all MM patients was determined using the
International Myeloma Working Group 2012 system (9). This study was approved by the Ethics
Committee of the Second Hospital of Shanxi Medical University. All
patients provided informed consent.
Cell lines and cell culture
U266, KM3 and RPMI-8226 human myeloma cell lines
were obtained from American Type Culture Collection (Manassas, VA,
USA). All cells were maintained in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., St. Louis, MO, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C, in a humidified atmosphere containing 5% CO2. The
cells at the logarithmic growth phase were harvested for the
subsequent experiments when the cells reached 80% confluence.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction
Total RNA was extracted from samples and cell lines
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Subsequently,
complementary DNA (cDNA) was synthesized from 2 µg total RNA
using the GoScript™ Reverse Transcription System kit (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
instructions. Briefly, the samples were pre-incubated at 70°C for
10 min, cooled on ice and then added to a reaction mixture
consisting of 10 mmol/l deoxynucleotide triphosphate, 25 mmol/l
MgCl2, 15 units avian myoblastosis virus reverse
transcriptase, 10X reverse transcription buffer, 0.5 units
RNasin® and 0.5 µg oligo-(dT) 15 primer (all
provided in the GoScript™ Reverse Transcription System kit). The
reaction mixture (final volume, 20 ml) was incubated at 44°C for 15
min, 99°C for 5 min and 4°C for 5 min. The cDNA was maintained at
20°C prior to use. The PCR reaction used 5 µg cDNA. RT-qPCR
was performed using SYBR Master mix [Takara Biotechnology (Dalian)
Co. Ltd., Dalian, China] on a LightCycler 480 system (Roche Applied
Science, Penzberg, Germany). The thermocycling conditions were as
follows: 40 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for
30 sec. A human glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
gene served as an endogenous control for sample normalization.
Results were presented as the fold expression relative to that of
GAPDH. PCR primers were as follows: Forward
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse 5′-GACAAGCTTCCCGTTCTCAG-3′
for human GAPDH; and forward 5′-GGGAATTCCCTGATTCCTCTTCA-3′ and
reverse 5′-CCCTCGAGCTAGAAGCTTACTTCCAT-3′ for CIP2A. PCR products
were separated by electrophoresis on 1.5% agarose gels. The gel
images were captured with a camera and analyzed using NIH Image 2.0
(rsb.info.nih.gov/nih-image).
Western blot analysis
Total proteins from RPMI-8226 cells were extracted
in lysis buffer (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) and quantified using the Bradford method
(Pierce Biotechnology, Inc., Rockford, IL, USA). Protein (20
µg) was separated on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gels (Bio-Rad
Labratories, Inc., Hercules, CA, USA), and transferred to
nitrocellulose membrane (Bio-Rad Labratories, Inc.), and blocked by
incubation with 5% non-fat milk in Tris-buffered saline with
Tween-20 at room temperature for 1 h. The membrane was then
incubated overnight at 4°C with the following primary antibodies:
Anti-CIP2A mouse monoclonal IgG1 (1:500; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA; cat. no. sc-80662),
rabbit monoclonal (PE Conjugate) c-Myc (D84C12) XP®
[1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA (cat.
no. 12189)], rabbit monoclonal Akt (1:1,000; Cell Signaling
Technology, Inc.; cat. no. 4691), rabbit monoclonal p-Akt (Ser473)
(D9E) XP® (1:2,000; Cell Signaling Technology, Inc.;
cat. no. 4060) and rabbit polyclonal IgG anti-actin (H-196)
(1:1,000; Santa Cruz Biotechnology, Inc.; cat. no. sc-7210). The
membranes were washed with phosphate-buffered saline three times
for 5 min each time. This was followed by incubation with
horseradish peroxidase-conjugated goat anti-mouse (1:2,000; Santa
Cruz Biotechnology, Inc.; cat. no. sc-2972) and goat anti-rabbit
(1:2,000; Santa Cruz Biotechnology, Inc.; cat. no. sc-2030)
secondary antibodies. Membranes were then washed again three times
for 10 min each with TBST. Target protein bands were visualized
using enhanced chemiluminescence (GE Healthcare Life Sciences,
Chalfont, UK). All western immunoblot analyses were performed three
times.
CIP2A short hairpin (sh)RNA
transfection
The MM cells were seeded onto the 6-well plates at a
density of 2×105 cells per well prior to transfection.
Sequences of CIP2A shRNA and shRNA control (Santa Cruz
Biotechnology, Inc.) were as follows: 5′-AAACTTCTCTCAACATACTAGC-3′
and 5′-CCTAAGGTTAAGTCGCCCTCG-3′, respectively. Cells were plated in
6-well plates and infected with lentivirus constructs using 8 ng/ml
polybrene (Santa Cruz Biotechnology, Inc.) as described in a
previous study (10). The stable
cell lines were selected in the presence of 1 µg/ml
puromycin (Sigma-Aldrich, St. Louis, MO, USA) for 2 weeks.
Cell proliferation assay
An MTT assay was used to measure relative cell
growth. Briefly, cells transduced shRNA for CIP2A and control shRNA
were seeded into 96-well micro-plates (3×103 cells/well)
for 48 h in RPMI-1640 medium with 10 % FBS. After culture, cells
were incubated with 20 µl/well MTT (Sigma-Aldrich) at 37°C
for 4 h, and then 200 µl dimethyl sulfoxide was added to
each well. Cells were subjected to absorbance reading at 570 nm
using a 96-well microplate reader (Synergy NEO; Bio-Tek
Instruments, Inc., Winooski, VT, USA).
Colony formation assay
Cells were plated into three 6-cm cell culture
dishes after transfection with CIP2A shRNA or control shRNA. Cells
were incubated for two weeks in complete growth media. Cell
colonies were fixed with cold methanol stained with 0.1% crystal
violet (Beyotime Institute of Biotechnology, Haimen, China) for 30
min. The colonies were manually counted using an inverted
microscope (CKX41SF; Olympus Corporation, Tokyo, Japan).
Apoptosis analysis
At transfection, cells were harvested and washed
with ice-cold PBS twice. Then, cells were re-suspended in Annexin
V-binding buffer, and 5 µl propidium iodide and 5 µl
Annexin V-fluorescein isothiocyanate (FITC; BD Pharmingen, San
Diego, CA, USA) was added. Cells were analyzed by FACSCalibur flow
cytometry (BD Biosciences, Franklin Lakes, NJ, USA). The proportion
of apoptotic cells (Annexin V-positive cells) was presented as the
mean ± standard deviation.
Statistical analysis
All experiments were repeated at least three times.
The data are presented as the mean ± standard deviation and
analyzed using SPSS software 15.0 (SPSS Inc., Chicago, IL, USA).
Student's t-test was used to compare differences, and data were
analyzed using the Pearson's χ2 test and Fisher's exact
test. P<0.05 and P<0.01 were considered to indicate a
statistically significant difference.
Results
CIP2A is overexpressed in MM plasma and
cell lines
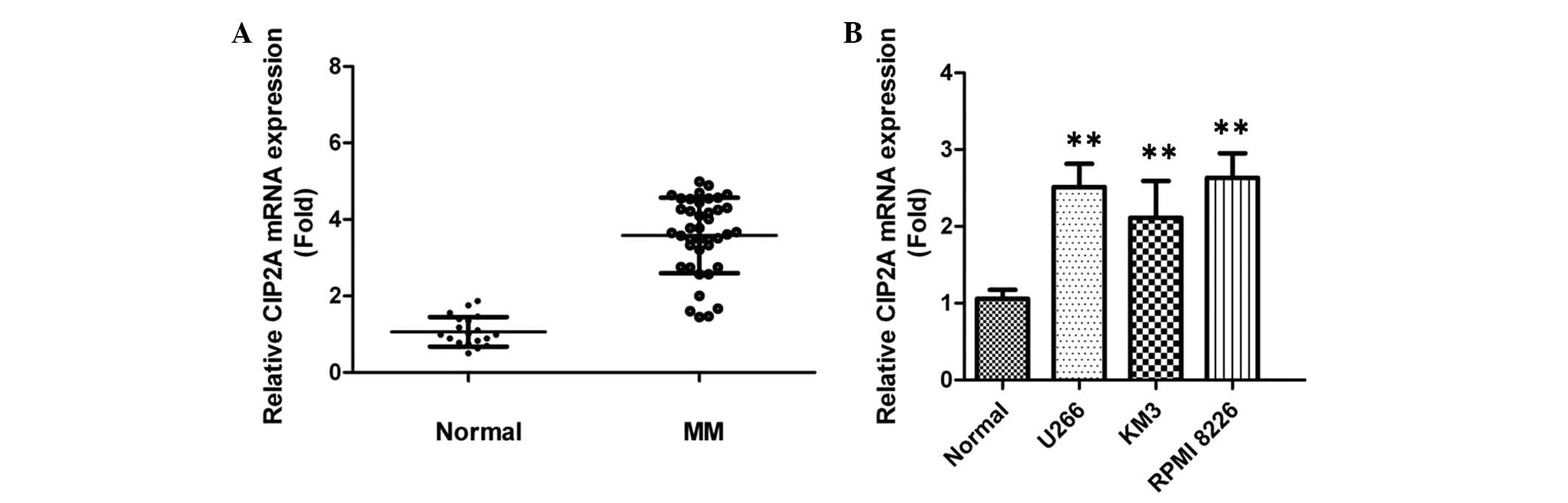
The expression of CIP2A in the plasma of 40 patients
with MM and 20 normal donors was examined by RT-qPCR. It was found
that the level of CIP2A in plasma samples was upregulated in
patients with MM compared with healthy control subjects (Fig. 1A), indicating that CIP2A may
contribute to the development of MM. In addition, the expression
level of CIP2A in MM cell lines was evaluated. Endogenous CIP2A was
significantly increased with different levels in all of these MM
cell lines, compared with normal plasma cells) from healthy donors
(Fig. 1B). The RPMI-8226 cell line
was used as the research model in the rest of the experiments.
CIP2A depletion in MM cell lines inhibits
cell proliferation
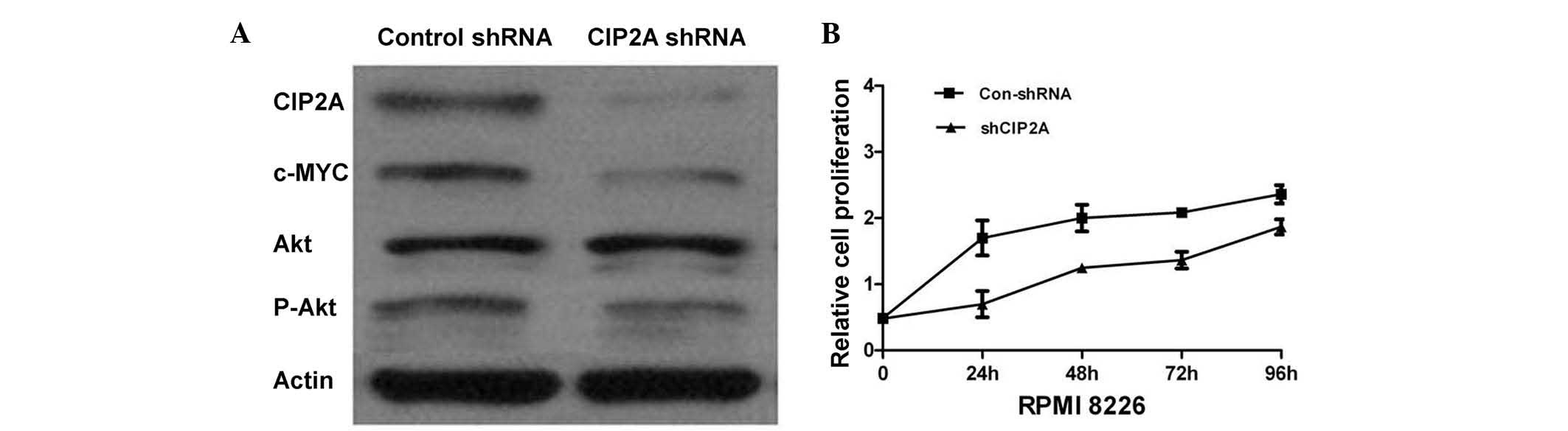
The RPMI-8226 cell line was used to generate cell
lines stably expressing CIP2A shRNA, in order to investigate the
role of CIP2A in the pathogenesis of MM. The knockdown of CIP2A was
confirmed by western blotting. CIP2A shRNA effectively decreased
CIP2A expression in the RPMI-8226 cell line (Fig. 2A). The proliferation of RPMI-8226
cells was measured by an MTT assay. Targeting CIP2A with specific
shRNA inhibited RPMI-8226 cell proliferation (Fig. 2B), suggesting the function of CIP2A
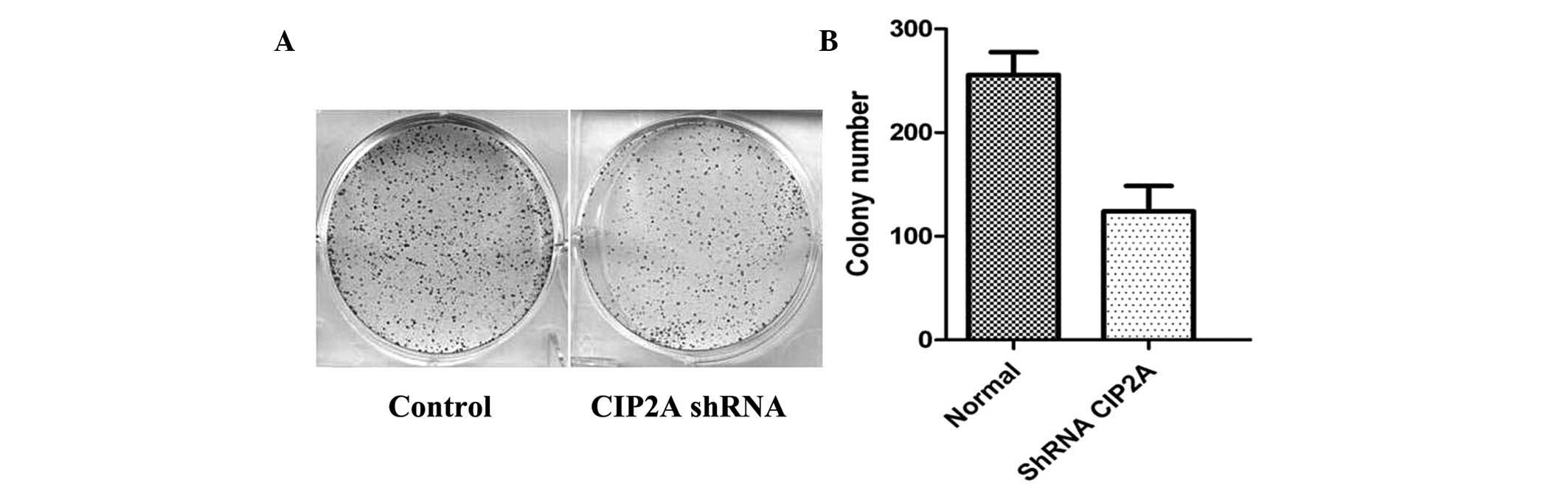
in promoting MM cell proliferation. Furthermore, the role of CIP2A
on proliferation was determined by colony formation assays. As
shown in Fig. 3A and B, the total
number of colonies and average colony diameter was markedly
decreased with CIP2A knockdown. These findings suggested that CIP2A
may regulate cell proliferation and potential tumorigenicity of
MM.
CIP2A depletion induces apoptosis and
inhibits c-Myc expression in MM cells
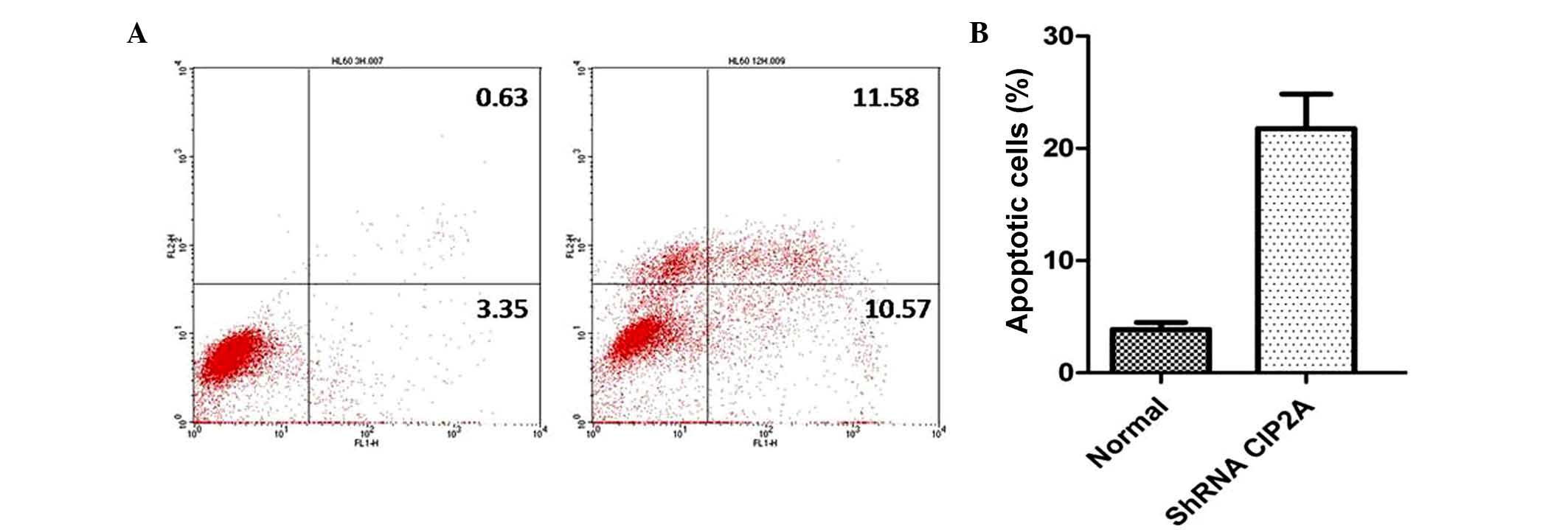
Annexin V-FITC analysis was used to detect the rate
of apoptosis in RPMI-8226 cells. The results showed that CIP2A
depletion could induce a notable population of early and late
apoptotic RPMI-8226 cells compared with controls (Fig. 4). To investigate the mechanism
underlying the effects of CIP2A, the c-Myc expression level was
analyzed following knockdown of CIP2A. Western blot analysis
revealed that CIP2A depletion downregulated the c-Myc protein
expression in RPMI-8226 cells, indicating that CIP2A could regulate
cell apoptosis and growth through the c-Myc signaling pathway
(Fig. 2A).
Discussion
MM is an incurable disease characterized by plasma
cell malignancy with heterogeneity (11). CIP2A is a tumor-associated antigen
that has been identified in patients with different types of
cancer. It has been reported that CIP2A is amplified and
overexpressed in various solid and hematological tumors (12,13),
however, the role of CIP2A in patients with MM requires further
investigation. Previous studies demonstrated cytoplasmic CIP2A
overexpression in various types of cancer (14–16).
In addition, Böckelman et al (17) noted that strong CIP2A expression
could be associated with poor survival in ovarian cancer. The
present study investigated the expression of CIP2A in plasma
samples from patients with MM and normal donors, and found a
significant upregulation of CIP2A in patients with MM, indicating
that CIP2A may be important in MM. This result in patients was also
in line with results in MM cell lines, which showed that endogenous
CIP2A was significantly higher in all three MM cell lines compared
with normal plasma cells from healthy donors. This suggests that
CIP2A has a functional role in MM carcinogenesis.
CIP2A is a novel human oncoprotein that can protect
c-Myc S62 from dephosphorylation and inhibit c-Myc associated
protein phosphatase 2 activity (4). In the present study, our clinical
investigation was extended and function studies in RPMI-8226 cells
were conducted due to their high abundance of CIP2A. The results
showed that the knockdown of CIP2A significantly decreased
clonogenic formation and cell growth ability. The results
demonstrated that CIP2A contributed to the colony formation and
proliferation abilities of MM in vitro and may therefore
represent a novel therapeutic target in patients with MM.
Apoptosis is regulated through two major pathways,
the extrinsic and the intrinsic pathways, and these pathways can
induce the activation of downstream effector caspases pathway.
Recently, studies reported that the downregulation of CIP2A
expression is associated with apoptosis in human cancer cells. In
this study, the results showed that compared with control groups,
CIP2A knockdown led to a significant increase in the apoptosis rate
of RPMI-8226 cells, which suggests that inhibition of CIP2A may be
a potential anticancer agent for patients with MM.
Previous studies revealed that CIP2A could regulate
cancerous transformation and growth of cancer cells via c-Myc
signaling pathway regulation (15,18).
Furthermore, CIP2A promotes c-Myc expression, which is required for
the proliferation of cancer cells. This study demonstrated that
CIP2A silencing strongly suppresses cell proliferation by means of
decreasing the expression of c-Myc and the level of AKT
phosphorylation in vitro to a certain degree.
In conclusion, CIP2A is upregulated in MM, and CIP2A
expression promotes cell proliferation and clonogenic formation
ability by regulating the expression of AKT phosphorylation and
c-Myc. This study indicated that CIP2A may be important in the
carcinogenesis and progression of MM.
Acknowledgments
This study was supported by the grants from the
Science and Technology Research Projects of Shanxi Province (grant
no. 20120313020-6).
References
|
1
|
Hatzimichael E, Dasoula A, Benetatos L,
Syed N, Dranitsaris G, Crook T and Bourantas K: Study of specific
genetic and epigenetic variables in multiple myeloma. Leuk
Lymphoma. 51:2270–2274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richards T and Weber D: Advances in
treatment for relapses and refractory multiple myeloma. Med Oncol.
27(Suppl 1): S25–S42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laubach JP, Richardson PG and Anderson KC:
The evolution and impact of therapy in multiple myeloma. Med Oncol.
27:S1–S6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khanna A, Böckelman C, Hemmes A, Junttila
MR, Wiksten JP, Lundin M, Junnila S, Murphy DJ, Evan GI, Haglund C,
et al: MYC-dependent regulation and prognostic role of CIP2A in
gastric cancer. J Natl Cancer Inst. 101:793–805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson MD, Reeder JE, O'Connell M,
Woodford M and Walter K: CIP2A and PP2A in human leptomeninges,
arachnoid granulations and meningiomas. J Clin Neurosci.
21:2228–2232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Ge Z, Liu C, Liu Z, Björkholm M, Jia
J and Xu D: CIP2A is overexpressed in gastric cancer and its
depletion leads to impaired clonogenicity, senescence, or
differentiation of tumor cells. Clin Cancer Res. 14:3722–3728.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Junttila MR, Puustinen P, Niemelä M, Ahola
R, Arnold H, Böttzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, et
al: CIP2A inhibits PP2A in human malignancies. Cell. 130:51–62.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi YA, Park JS, Park MY, Oh KS, Lee MS,
Lim JS, Kim KI, Kim KY, Kwon J, Yoon do Y, et al: Increase in CIP2A
expression is associated with doxorubicin resistance. FEBS Lett.
585:755–760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Terpos E, Morgan G, Dimopoulos MA, Drake
MT, Lentzsch S, Raje N, Sezer O, García-Sanz R, Shimizu K, Turesson
I, et al: International myeloma working group recommendations for
the treatment of multiple myeloma-related bone disease. J Clin
Oncol. 31:2347–2357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nasri M, Karimi A and Allahbakhshian
Farsani M: Production, purification and titration of a
lentivirus-based vector for gene delivery purposes. Cytotechnology.
66:1031–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhan F, Huang Y, Colla S, Stewart JP,
Hanamura I, Gupta S, Epstein J, Yaccoby S, Sawyer J, Burington B,
et al: The molecular classification of multiple myeloma. Blood.
108:2020–2028. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pang X, Fu X, Chen S, Zhu X, Qi H, Li Y,
Li F and Tan W: Overexpression of CIP2A promotes bladder cancer
progression by regulating EMT. Clin Transl Oncol. 18:289–295. 2016.
View Article : Google Scholar
|
|
13
|
Khanna A, Rane JK, Kivinummi KK, Urbanucci
A, Helenius MA, Tolonen TT, Saramäki OR, Latonen L, Manni V,
Pimanda JE, et al: CIP2A is a candidate therapeutic target in
clinically challenging prostate cancer cell populations.
Oncotarget. 6:19661–19670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ventelä S, Sittig E, Mannermaa L, Mäkelä
JA, Kulmala J, Löyttyniemi E, Strauss L, Cárpen O, Toppari J,
Grénman R and Westermarck J: CIP2A is an Oct4 target gene involved
in head and neck squamous cell cancer oncogenicity and
radioresistance. Oncotarget. 6:144–158. 2015.
|
|
15
|
Junttila MR, Puustinen P, Niemelä M, Ahola
R, Arnold H, Böttzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, et
al: CIP2A inhibits PP2A in human malignancies. Cell. 130:51–62.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De P, Carlson J, Leyland-Jones B and Dey
N: Oncogenic nexus of cancerous inhibitor of protein phosphatase 2A
(CIP2A): An oncoprotein with many hands. Oncotarget. 5:4581–4602.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Böckelman C, Lassus H, Hemmes A, Leminen
A, Westermarck J, Haglund C, Bützow R and Ristimäki A: Prognostic
role of CIP2A expression in serous ovarian cancer. Br J Cancer.
105:989–995. 2011. View Article : Google Scholar :
|
|
18
|
Yeh E, Cunningham M, Arnold H, Chasse D,
Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida
T, et al: A signaling pathway controlling c-Myc degradation that
impacts oncogenic transformation of human cells. Nat Cell Biol.
6:308–318. 2004. View
Article : Google Scholar : PubMed/NCBI
|