Introduction
Hepatocellular carcinoma (HCC), which accounts for
80–90% of primary liver cancer, is the fifth most common type of
cancer worldwide and the third most common cause of cancer-related
mortality (1). Liver resection is
currently the first line of treatment in non-cirrhotic patients,
yet the reported overall 5-year survival after HCC liver resection
is 30–60%, with a high incidence of recurrence (50–80%) (1–5).
Currently, there are no widely accepted chemopreventive strategies
to limit the progression of HCC once liver cirrhosis is established
(6). Liver transplantation
provides a good outcome for patients with HCC meeting the Milan
criteria (single nodule ≤5 cm or 2 or three nodules ≤3 cm), and
leads to 5-year survival rates of 70% and low recurrence rates
(7). The application of liver
transplantation is rare, however, due to a limited source of liver
donors, high cost and organ rejection following
transplantation.
Adult and embryonic hepatocytes, hepatic
stem/progenitor cells and extrahepatic stem cells have been used as
transplantable cell sources for liver regeneration (8). Such cellular therapy may provide a
novel approach for the treatment of advanced stages of HCC.
Umbilical cord mesenchymal stem cells (UC-MSCs) are a subgroup of
MSCs that possess the potential to differentiate into several
mesodermal tissues (bone, cartilage, tendon, muscle and adipose
tissue) (9), endodermal tissue
(hepatocytes) (10), and
ectodermal tissue (neurons) (11,12).
However, the effects of MSCs on tumor cells remain controversial.
Li et al (13) demonstrated
that MSCs enhanced tumor growth but significantly inhibited the
invasiveness and metastasis of HCC. However, another group
demonstrated that MSCs possess intrinsic antineoplastic properties
in a Kaposi's sarcoma model (14).
A recent study indicated that human UC-MSCs significantly inhibited
the growth of breast cancer stem cells (CSCs) in vitro and
in vivo (15).
Based on these data, it was hypothesize that UC-MSCs
may have antitumor effects on cancer cells, such as HepG2 HCC
cells. The present study investigated the effects of UC-MSCs on
HepG2 cells using a Transwell co-culture approach. The results
provide valuable insights into the biological characteristics of
UC-MSCs and provide evidence for their potential therapeutic use
for the treatment of HCC.
Materials and methods
Reagents
Fetal bovine serum (FBS), Dulbecco's modified
Eagle's medium (DMEM)/F12, 0.25% trypsin and phosphate-buffered
saline (PBS) were purchased from GE Healthcare (Logan, UT, USA).
Fluorescein isothiocyanate-conjugated (FITC) mice monoclonal
antibodies, including anti-CD29-FITC (cat no. 6604105; 1:50),
anti-CD34-FITC (cat. no. IM1870; 1:50), anti-CD44-FITC (cat. no.
IM1219U; 1:50), anti-CD45-FITC (cat. no. IM0782U; 1:50),
anti-CD90-FITC (cat. no. IM1839U; 1:50), anti-CD105-PE (cat. no.
B76299; 1:50), as well as intra-1 (cat. no. A07803; 1:50) and
intra-2 (cat. no. A07803; 1:50) were obtained from Beckman Coulter,
Inc. (Brea, CA, USA). Monoclonal phycoerythrin (PE)-conjugated
antibodies, anti-Bcl-2-PE (cat. no. A15796; 1:50; Invitrogen,
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
anti-Survivin-PE (cat. no. 129176; 1:50; eBio-science, Inc., San
Diego, CA, USA). Anti-α fetoprotein (AFP) primary antibody and
FITC-conjugated IgG (H+L) antibody were provided by LsBio (Seattle,
WA, USA) and Invitrogen, Thermo Fisher Scientific Inc.,
respectively. An RNA extraction kit was purchased from TianGen
BioTech Co., Ltd. (Beijing, China). An RNA reverse transcription
reaction kit and RNA quantification kit were purchased from Bio-Rad
(Hercules, CA, USA). Primers were synthesized by Sangon Biotech
Co., Ltd. (Shanghai, China).
Cell culture
Third passage human UC-MSCs were obtained from Beike
Biotechnology (Shenzhen, China). These cells were obtained from
informed, healthy donors after normal spontaneous vaginal
deliveries. The procedure for cell collection and mononuclear cell
extraction, cultivation and harvest, has been reported in a
previous publication (16). Human
HCC HepG2 cells were kindly provided by Professor Lin Wang
(17) from the Department of
Hepatobiliary Surgery, The 2nd Affiliated Hospital of Kunming
Medical University (Kunming, China). UC-MSCs and HepG2 cells were
maintained in DMEM/F12 containing 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin (GE Healthcare Life Sciences, Logan,
UT, USA). Cells were incubated in a 5% CO2-humidified
incubator at 37°C. The culture medium was changed every two or
three days. When cells reached 80–90% confluency, cell passage was
conducted using 0.25% trypsin for digestion. Passage seven to eight
UC-MSCs were used for the following experiments.
Co-culture procedure
For co-culture of UC-MSCs with HepG2 cells, a
Transwell co-culture system (pore size, 0.4 µm; Corning,
Corning, NY, USA) was utilized. UC-MSCs were collected by trypsin
digestion, resuspended in fresh culture medium, adjusted to the
appropriate cell density (2.5×105–4×106
cells/well) and seeded onto a six-well plate (2 ml cell suspension
for each well). After the initial seeding of UC-MSCs (24 h),
1×106 HepG2 cells suspended in 2 ml culture medium were
seeded into the upper compartment of the Transwell. Cell morphology
was observed under a phase contrast microscopy (BX53, Olympus,
Tokyo, Japan).
Flow cytometric analysis of UC-MSC
surface biomarkers
Passage three and passage seven UC-MSCs were used
for cell identification. Cells were washed with PBS and digested
with 0.25% trypsin. After centrifuging at 300 x g for 5 min, the
supernatant was removed and cells were washed twice with PBS. Cells
were then incubated with FITC-conjugated primary antibodies against
CD29, CD34, CD44, CD45, CD90 or CD105 for 30 min at room
temperature in the dark. The expression of cell surface biomarkers
was determined using flow cytometric analysis. Fluorescence
acquisition was performed in a Coulter-EPICS XL flow cytometer
(Beckman-Coulter, Inc.) with a 488 nm argon-ion laser.
Evaluation of cell proliferation
After 24, 48 or 72 h of co-culture, HepG2 cells were
collected and seeded onto a 96-well plate. Six wells were used for
each treatment. Cells were maintained with the culture medium
collected from the co-culture system. After 24 h, the co-culture
medium was removed and replaced with 180 µl fresh culture
medium. Then, 20 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
5 mg/ml; Beckman-Coulter) was added to each well. After an
additional 4 h of incubation at 37°C, the medium was removed and
150 µl dimethyl sulfoxide was added to each well to
resuspend the MTT metabolic product. The absorbance of the
dissolved formazan was measured at 490 nm (A490) using a
scanning microplate spectrophotometer (Bio-Rad). The proliferation
inhibition rate was calculated using the following formula:
Proliferation inhibition rate = (A490,
Control-A490, Sample) / A490, Control ×
100.
Measurement of cell apoptosis
Cell apoptosis was measured by flow cytometry and
confirmed by terminal deoxynucleotidyl transferase (TdT)-mediated
nick end labeling (TUNEL). Using the Transwell system, UC-MSCs were
co-cultured with HepG2 cells. A range of initial seeding densities
for UC-MSCs was tested (0.25×106, 0.5×106,
1×106, 2×106 or 4×106 cells/well).
After co-culture, HepG2 cells were collected and cell density was
adjusted to 1×106 cells/ml. After washing with PBS,
cells were fixed with 4% paraformaldehyde (Sigma-Aldrich, St.
Louis, MO, USA) for 30 min at 4°C. Then, cells were washed with
0.2% bovine serum albumin (BSA; GE Healthcare Life Sciences) in PBS
and incubated with 70% ethanol at 20°C for an additional 30 min.
After washing twice with 0.2% BSA in PBS, cells were treated with
the TdT solution (1.5 µl TdT, 27 µl TdT buffer and
1.5 µl FITC-dUTP; GE Healthcare Life Sciences) or a negative
control solution (28.5 µl TdT buffer and 1.5 µl
FITC-dUTP) for 1 h at 37°C. After washing, samples were incubated
with 20 µl of 10 mg/ml RNase (Sigma-Aldrich) and 20
µl of 0.1% Triton X-100 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 15 min at 37°C followed by 100 µg/ml
propidium iodide (PI; Sigma-Aldrich) staining for 15–30 min.
Fluorescence acquisition and analysis were performed using a flow
cytometer (Beckman-Coulter, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
UC-MSCs were seeded and co-cultured with HepG2 cells
for 72 h with a range of initial seeding densities
(0.5×106, 0.75×106, 1×106,
1.25×106 or 1.5×106 cells/well). Total RNA
was extracted from HepG2 cells using an RNA extraction kit
according to the manufacturer's instructions. RNA concentration and
purity were assayed by gel electrophoresis. Total RNA (1 µg)
was reverse transcribed using a reverse transcription kit according
to the manufacturer's instructions. PCR amplification was conducted
with specific primers designed by Primer 5.0 software (PREMIER
Biosoft, Palo Alto, CA, USA) as follows: Forward: 5′-TGC GTT TCT
CGT TGC TTACA-3′ and reverse: 5′-GCT GCC ATT TTT CTG GTGAT-3′ for
AFP; forward: 5′-GTG GAT GAC TGA GTA CCT GAA CC-3′ and reverse:
5′-AGA CAG CCA GGA GAA ATC AAAC-3′ for BCL2 gene; forward: 5′-GAC
CAC CGC ATC TCT ACA TTC-3′ and reverse: 5′-AAG TCT GGC TCG TTC TCA
GTG-3′ for baculoviral IAP repeat containing fifth (BIRC5) gene
encoding survivin; and RPS13 was used as a housekeeping gene
forward: 5′-GTT GCT GTT CGA AAG CAT CTTG-3′ and reverse: 5′-AAT ATC
GAG CCA AAC GGT GAA-3′. The PCR reactions were heated to 94°C for 5
min, and subjected to 30 cycles of 94°C for 30 sec, 60°C for 30 sec
and 72°C for 45 sec. Each experimental condition was repeated in
triplicate. The amplified product lengths were 81, 124 and 116 bp
for AFP, Bcl-2 and Survivin, respectively. The relative expression
from amplified RNA samples was calculated using the
2−ΔΔCq method (18)
using Applied Biosystems 7500 thermo-cycler 2.3 (Thermo Fisher
Scientific, Inc.).
Evaluation of protein expression
UC-MSCs were seeded and co-cultured with HepG2 cells
for 72 h with a range of initial seeding densities
(0.25×106, 0.5×106, 1×106,
2×106 or 4×106 cells/well). HepG2 cells were
collected and incubated with 0.5% Tween-20, intra-1 and intra-2 for
membrane permeability. Cells were treated with 20 µl
AFP-FITC, Bcl-2-PE or Survivin-PE antibodies. The primary antibody
(20 µl) was added to tube one, washed with PBS after 30 min,
then FITC (H+L) IgG was added. Concurrently, 20 µl IgG-FITC
was added into tube two as negative control. After washing three
times with a 0.5% Tween-20 solution, fluorescence intensities were
analyzed with a flow cytometer (Beckman-Coulter).
Statistical analysis
Data were analyzed using SPSS 16.0 software (SPSS
Inc., Chicago, IL, USA). Statistical significance was determined
using repeated measures analysis of variance, a Bonferroni
correction was then performed as a post-hoc test. Data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
In vitro culture and identification of
UC-MSCs
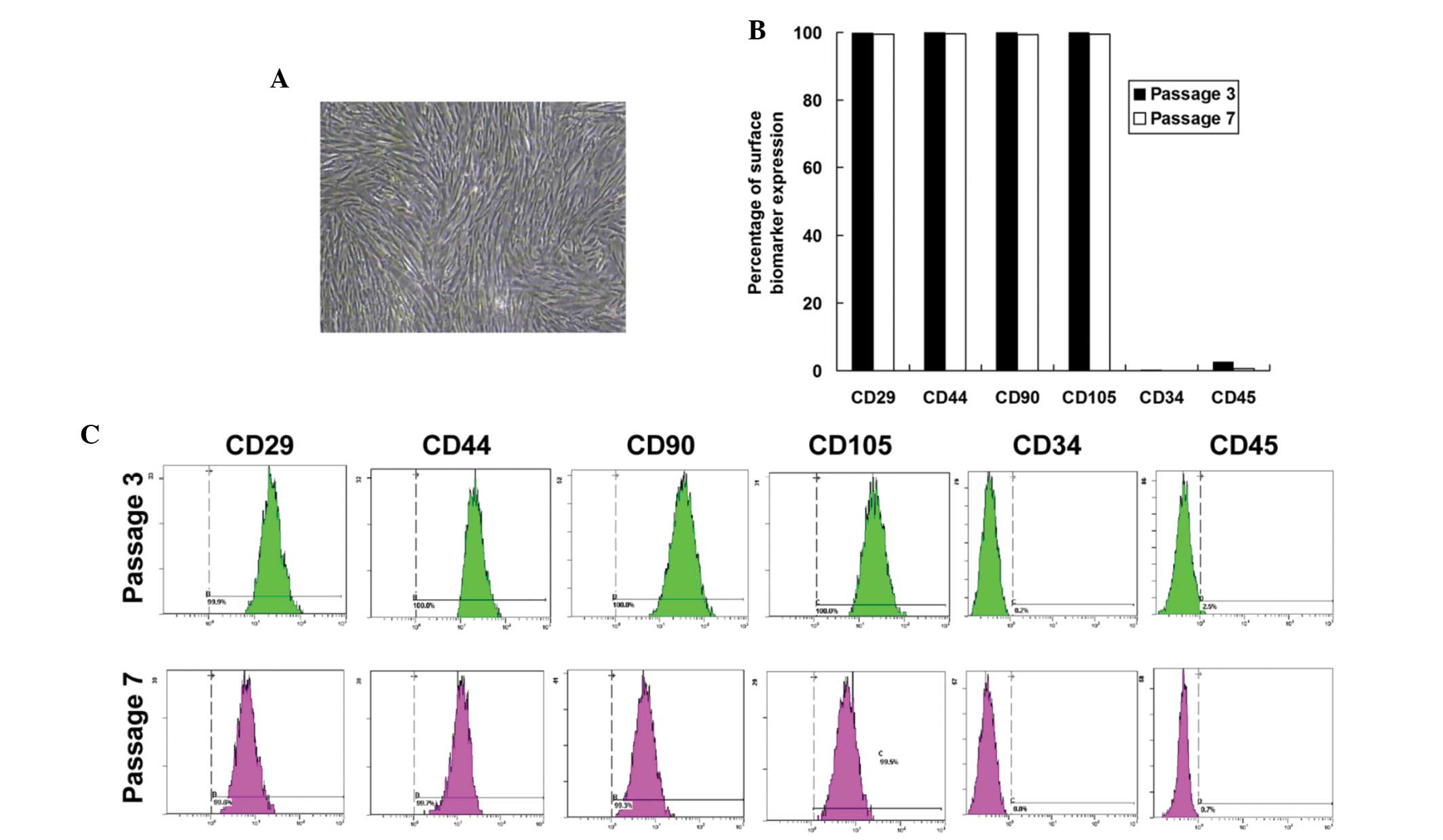
In vitro cultured UC-MSCs exhibited a
spindle-like structure and were arranged in parallel or in swirls
(Fig. 1A). These morphological
characteristics could be maintained until passage 20 (data not
shown), which is consistent with the results of previous studies
(19,20). Using flow cytometric analysis, the
cell surface expression of stem cell biomarkers, CD29, CD44, CD90,
CD105, CD34 and CD45 were determined. As shown in (Fig. 1B and C), UC-MSCs expressed CD29,
CD44, CD90 and CD105 but lacked CD34 and CD45, confirming the
typical phenotype of MSCs derived from the umbilical cord. No
significant difference was observed in the expression of these
biomarkers between passage three and passage seven UC-MSCs. These
data identified the MSCs derived from the umbilical cord and
passage seven to eight UC-MSCs were used for the remaining
experiments.
Co-culture of UC-MSCs suppresses the
proliferation of HepG2 cells
In order to investigate the effects of UC-MSCs on
cultured HepG2 HCC cells, the two cell types were co-cultured using
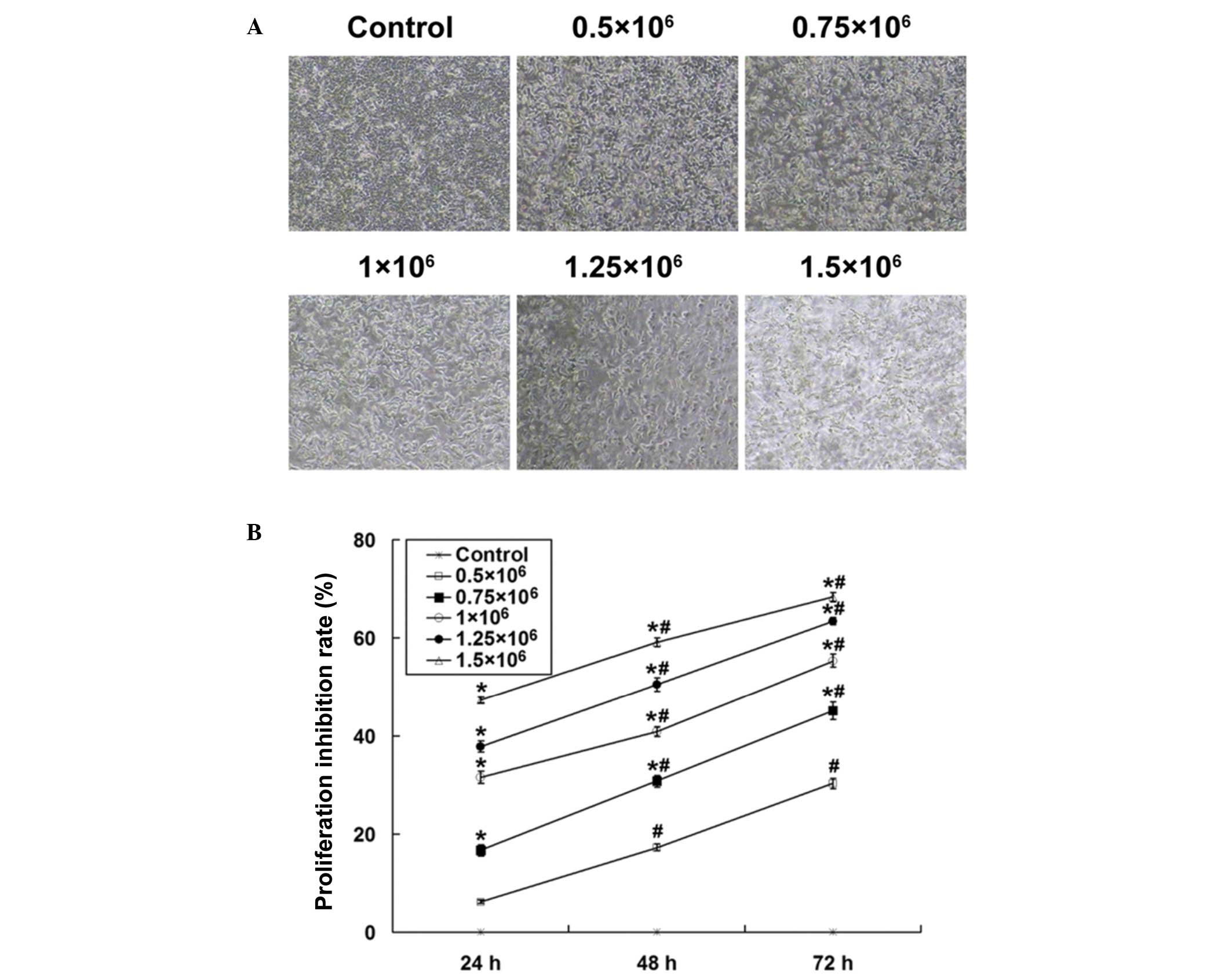
a Transwell system. Under normal conditions, cultured HepG2 cells
were multi-polar and had a cobblestone-like appearance when they
reached confluence (Fig. 2A).
However, upon co-culture, the number of the HepG2 cells was reduced
and cells displayed an irregular morphology. Additionally, HepG2
cell loss was accelerated when the initial seeding number of
UC-MSCs was increased (Fig. 2A).
The growth inhibition capability of UC-MSCs on HepG2 cells was
further confirmed using an MTT assay. It was demonstrated that
UC-MSCs time-dependently inhibited the proliferation of HepG2 cells
(P<0.01 for different incubation times; Fig. 2B). Enhanced proliferation
inhibition was observed by increasing the initial seeding number of
UC-MSCs into the Transwell compartment (P<0.01 compared with
different seeding densities). These results suggest that UC-MSCs
can inhibit HepG2 cell proliferation.
Co-culture of UC-MSCs induces the
apoptosis of HepG2 cells
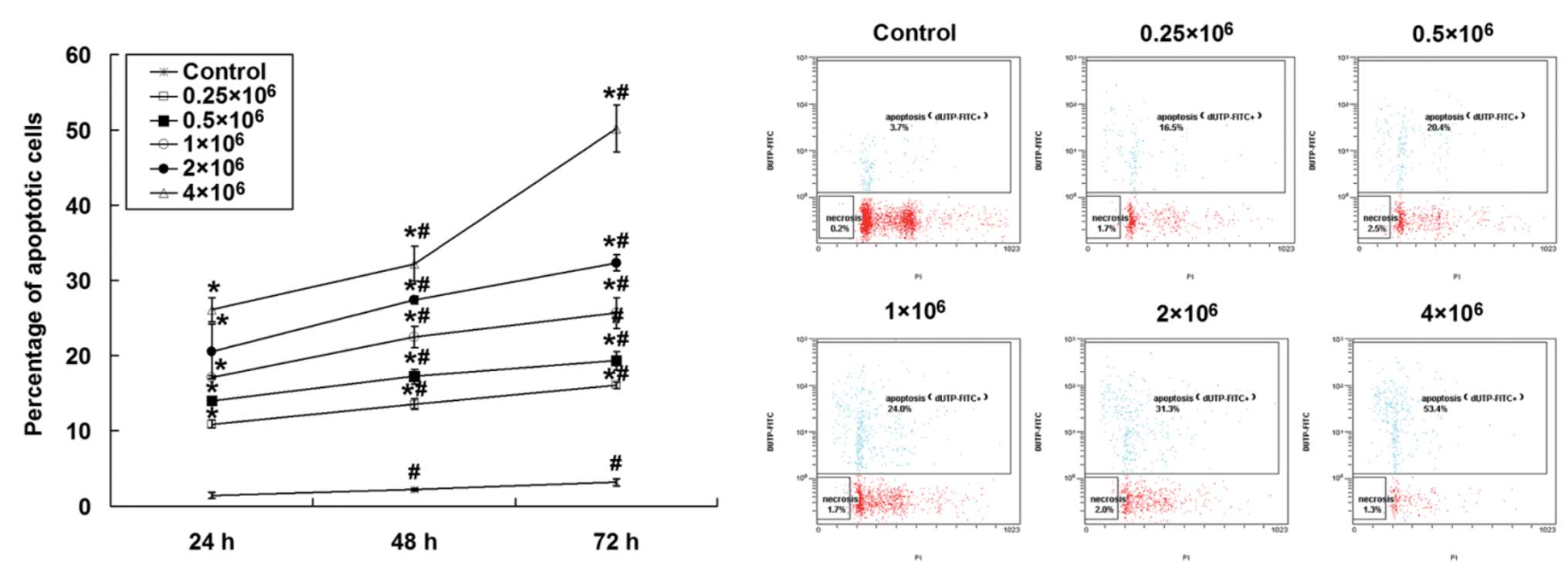
The UC-MSC-induced apoptosis of HepG2 cells was
investigated using a TUNEL assay. As shown in Fig. 3, co-culture of UC-MSCs induced the
apoptosis of HepG2 cells in a time-dependent manner (P<0.01
between different time points). Moreover, the initial seeding
density of UC-MSCs also influenced the percentage of apoptotic
HepG2 cells, as elevated apoptosis was detected with an increased
number of UC-MSCs (P<0.01 compared with different initial
seeding cell number). It is important to note that over 50% of the
HepG2 cells underwent apoptosis following a 72 h incubation with
UC-MSCs when 4×106 UC-MSCs were seeded into the
co-culture system. These data imply that UC-MSCs promote apoptosis
in HepG2 cells.
Co-culture of UC-MSCs downregulates mRNA
and protein expression of AFP, Bcl-2 and Survivin mRNA in HepG2
cells
In order to understand the underlying molecular
mechanism of UC-MSC-induced proliferation inhibition and apoptosis
induction of HepG2 cells, mRNA and protein expression of AFP, Bcl-2
and Survivin in HepG2 cells were examined by RT-qPCR and flow
cytometry, respectively. As shown in Fig. 4, AFP, Bcl-2 and Survivin mRNA
expression were observed in control HepG2 cells. After 72 h of
co-culture with UC-MSCs, the expression of these three genes in
HepG2 cells was gradually reduced and the level of reduction was
dependent on initial UC-MSC seeding density. At a low density of
UC-MSCs (0.5×106 or 0.75×106 cells/well),
mRNA levels of AFP, Bcl-2 and Survivin were reduced when compared
with the control (P<0.01), whereas a high initial seeding
density of UC-MSCs (1×106, 1.25×106 or
1.5×106 cells/well) essentially eliminated detection of
AFP, Bcl-2 and Survivin expression.
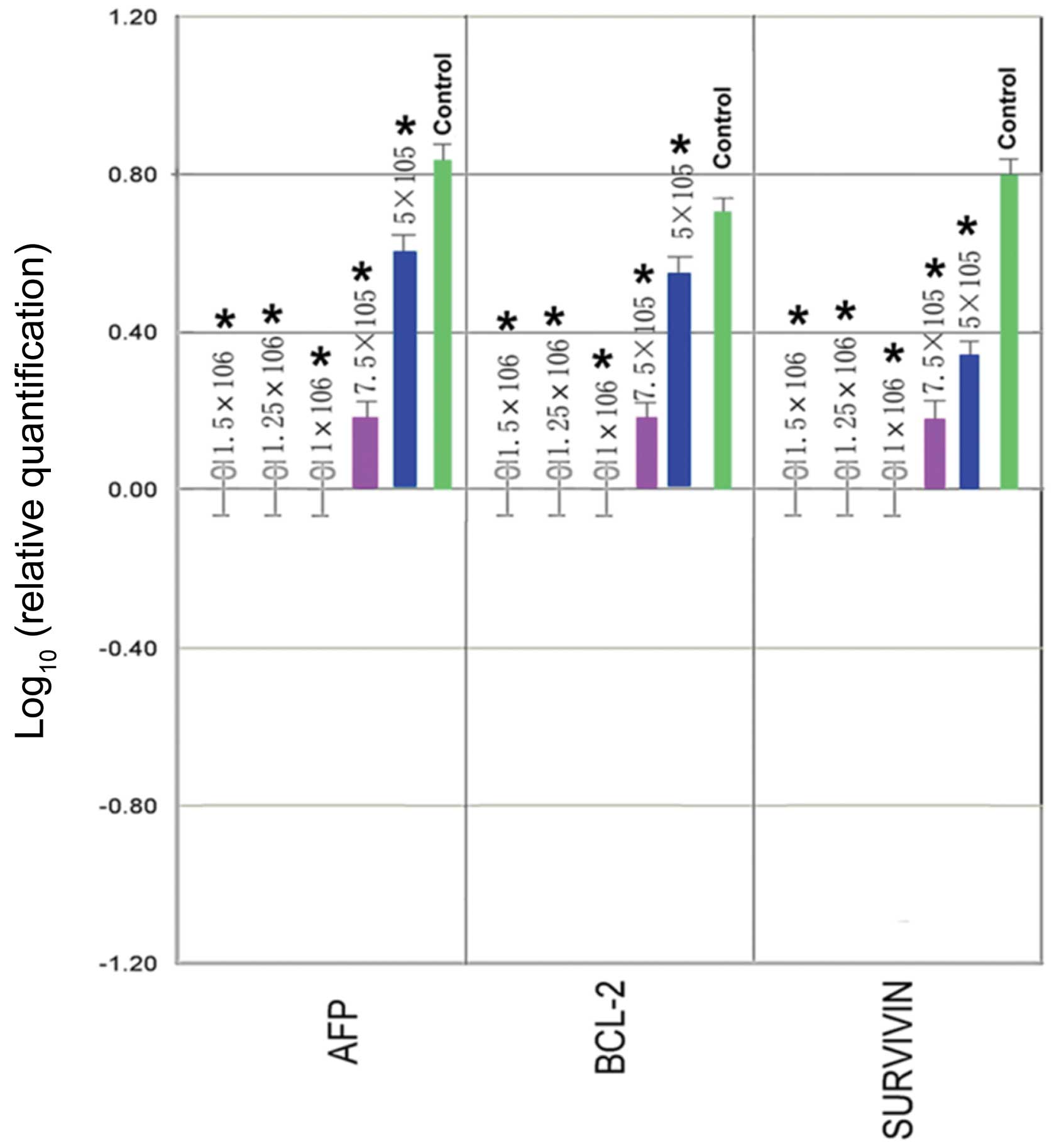
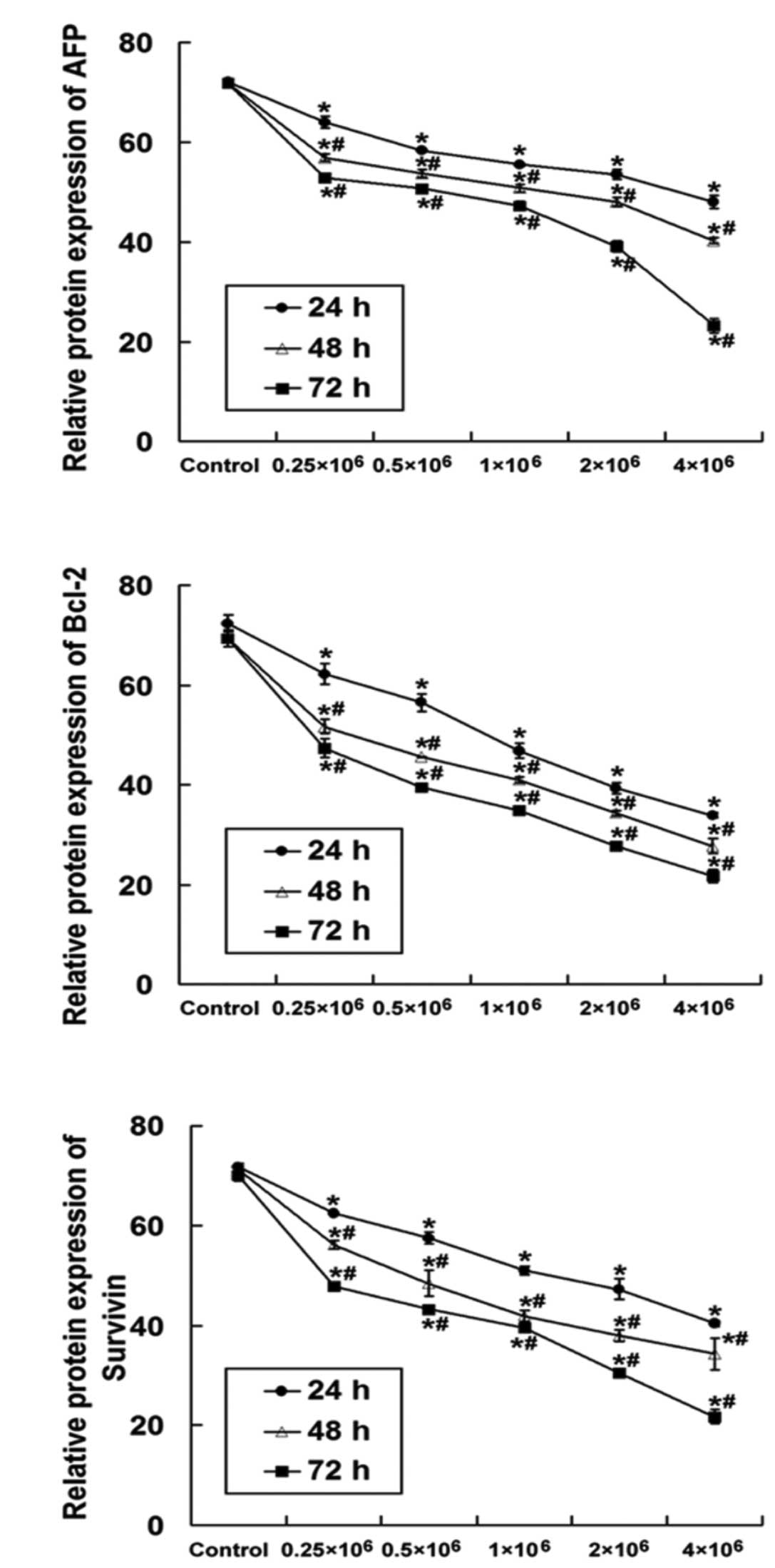
To confirm the effects of UC-MSCs on AFP, Bcl-2 and
Survivin expression in HepG2 cells, protein levels of these genes
were analyzed by flow cytometric analysis after the indicated time
period of co-culture. As shown in Fig.
5, co-culture of UC-MSCs significantly downregulated the
protein levels of AFP, Bcl-2 and Survivin in HepG2 cells (P<0.01
compared with control). Moreover, downregulation of protein levels
appeared to be dependent on the initial seeding density of UC-MSCs,
as a higher initial seeding density of UC-MSCs more efficiently
decreased HepG2 AFP, Bcl-2 and Survivin protein expression
(P<0.01). In addition, co-culture with UC-MSCs time-dependently
reduced the expression of these proteins in HepG2 cells within 72 h
of co-culture (24 h vs. 48 h, 24 h vs. 72 h, 48 h vs. 72 h;
P<0.01). Therefore, it is possible that UC-MSCs may inhibit cell
proliferation and promote apoptosis of HepG2 cells via regulating
the expression of these proteins.
Discussion
Human MSCs, multipotent cells that can self-renew,
proliferate and differentiate into a variety of cell types
(21,22), are emerging as novel cell-based
delivery agents for cancer therapy (23). Nevertheless, the effect of MSCs on
the growth, progression and metastasis of human malignancies,
including HCC, remains controversial and the underlying molecular
and cellular mechanisms are not yet fully elucidated.
Emerging lines of evidence suggest that MSCs are the
potential precursor for tumor stroma (24). Other studies have shown that MSCs
mediate the inhibition of tumor growth in vivo and in
vitro (25-27). Application of genetically modified
human MSCs, which express human pigment epithelium-derived factor,
have been shown to inhibit HCC in nude mice, and thus it is has
been suggested to be a promising approach for the treatment of HCC
(28). Moreover, MSCs derived from
the umbilical cord are hypothesized to be potent candidates for the
clinical application of allogenic MSC-based therapies as they can
easily be isolated from umbilical cord blood, cultured or modified
in vitro, and autologously transplanted into patients, thus
overcoming the difficulties associated with immune rejection of
transplanted cells. Compared with the 'gold standard' of bone
marrow MSCs, UC-MSCs showed a higher proliferative potential and
were capable of osteogenic, chondrogenic and adipogenic
differentiation (29,30). Moreover, these cells have been
shown to home to tumor tissues but not to healthy tissues and they
do not form teratomas when injected into severe-combined
immunodeficiency mice (31).
In the present study, MSCs derived from the
umbilical cord were identified and were characterized by expression
of genetic and surface markers (positive for CD29, CD44, CD90 and
CD105 and negative for CD34 and CD45). They appeared to be stable
in terms of their surface marker expression in early passage
(passages three to seven), which is in accordance with a previous
study (32). Additionally, to the
best of our knowledge, this study demonstrated for the first time,
that UC-MSCs have the ability to inhibit the proliferation of HCC
HepG2 cells in vitro, which suggests that MSCs from the
umbilical cord exhibit antitumor properties. These data are
consistent with previous studies, which showed antitumor effects of
UC-MSCs on breast cancer (15,33,34)
and bronchioloalveolar carcinoma (35). It is possible that UC-MSCs may act
in a paracrine manner through secretion of cytokines, interleukins
and growth factors. A recent study identified several factors,
including interleukins, fibroblast growth factor and insulin-like
growth factor binding protein family members, that were secreted by
UC-MSCs (36). However, the
underlying mechanisms of these cytokines and factors remain
unknown.
The potential role of UC-MSCs in promoting the
apoptosis of HepG2 cells upon co-culture was also investigated. The
results demonstrated that UC-MSCs significantly enhanced HepG2
apoptosis. AFP functions as a regulatory factor in tumor cell
growth, as it acts as a protein-binding partner for caspase-3 and
blocks apoptotic signaling in human AFP-producing hepatoma cells
(37). Moreover, the level of AFP
is positively correlated with another well-defined oncop-rotein,
Bcl-2, in patients with HCC (38).
AFP may inhibit the translocation of the retinoic acid receptor
(RAR)-β into the nucleus through competition with all
trans-retinoic acid for binding to RAR-β and diminish the negative
regulatory effect of RAR-β on Survivin (39). These data indicate an association
of AFP, Bcl-2 and Survivin with apoptosis inhibition. The present
study also demonstrated that co-culture of UC-MSCs downregulated
the expression of these three genes in HepG2 cells, suggesting
UC-MSCs may promote apoptosis through regulating the expression of
genes participating in apoptosis signaling.
Future studies will focus on investigating the
underlying molecular mechanism involved in UC-MSC-mediated tumor
growth inhibition and apoptosis. Accumulating evidence has also
highlighted the tumor-promoting properties of MSCs (40-43).
It has been shown that MSCs enhanced tumor growth but significantly
inhibited the invasiveness and metastasis of HCC (13). This discrepancy, in relation to the
present results, may be due to the different characteristics of
bone marrow-derived MSCs used previously and the UC-MSCs used in
this study. The invasiveness and metastatic activities of tumor
cells following co-culture with UC-MSCs warrants further
investigation.
In conclusion, the present study shows that UC-MSCs
efficiently suppress the proliferation of HepG2 cells. In addition,
UC-MSCs promote apoptosis through downregulation of AFP, Bcl-2 and
Survivin gene expression, which are associated with apoptotic
signaling pathways. These data provide valuable insights into the
biological properties of UC-MSCs and suggest that UC-MSCs may be a
promising cell source for MSC-based therapy for HCC.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81360072), the Natural
Science Foundation of Yunnan Province (grant no. 2013FB050) and the
Health Science and Technology Project of Yunnan Province (grant
nos. 2014NS108 and 2014NS109).
References
|
1
|
Lau WY and Lai EC: Hepatocellular
carcinoma: Current management and recent advances. Hepatobiliary
Pancreat Dis Int. 7:237–257. 2008.PubMed/NCBI
|
|
2
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai EC and Lau WY: The continuing
challenge of hepatic cancer in Asia. Surgeon. 3:210–215. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lau WY: Primary liver tumors. Semin Surg
Oncol. 19:135–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lau WY: Management of hepatocellular
carcinoma. J R Coll Surg Edinb. 47:389–399. 2002.PubMed/NCBI
|
|
6
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM, Schwartz M and Mazzaferro V:
Resection and liver transplantation for hepatocellular carcinoma.
Semin Liver Dis. 25:181–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Russo FP and Parola M: Stem and progenitor
cells in liver regeneration and repair. Cytotherapy. 13:135–144.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karahuseyinoglu S, Kocaefe C, Balci D,
Erdemli E and Can A: Functional structure of adipocytes
differentiated from human umbilical cord stroma-derived stem cells.
Stem Cells. 26:682–691. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Campard D, Lysy PA, Najimi M and Sokal EM:
Native umbilical cord matrix stem cells express hepatic markers and
differentiate into hepatocyte-like cells. Gastroenterology.
134:833–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu YS, Shih YT, Cheng YC and Min MY:
Transformation of human umbilical mesenchymal cells into neurons in
vitro. J Biomed Sci. 11:652–660. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma L, Feng Xy, Cui BL, Law F, Jiang XW,
Yang LY, Xie QD and Huang TH: Human umbilical cord Wharton's
Jelly-derived mesenchymal stem cells differentiation into
nerve-like cells. Chin Med J (Engl). 118:1987–1993. 2005.
|
|
13
|
Li GC, Ye QH, Xue YH, Sun HJ, Zhou HJ, Ren
N, Jia HL, Shi J, Wu JC, Dai C, et al: Human mesenchymal stem cells
inhibit metastasis of a hepatocellular carcinoma model using the
MHCC97-H cell line. Cancer Sci. 101:2546–2553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khakoo AY, Pati S, Anderson SA, Reid W,
Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, et al:
Human mesenchymal stem cells exert potent antitumorigenic effects
in a model of Kaposi's sarcoma. J Exp Med. 203:1235–1247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma Y, Hao X, Zhang S and Zhang J: The in
vitro and in vivo effects of human umbilical cord mesenchymal stem
cells on the growth of breast cancer cells. Breast Cancer Res
Treat. 133:473–485. 2012. View Article : Google Scholar
|
|
16
|
Yang WZ, Zhang Y, Wu F, Min WP, Minev B,
Zhang M, Luo XL, Ramos F, Ichim TE, Riordan NH and Hu X: Safety
evaluation of allogeneic umbilical cord blood mononuclear cell
therapy for degenerative conditions. J Transl Med. 8:752010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Li H, Zhang Y, Santella RM and
Weinstein IB: HINT1 inhibits beta-catenin/TCF4, USF2 and NFkappaB
activity in human hepatoma cells. Int J Cancer. 124:1526–1534.
2009. View Article : Google Scholar :
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-timequantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Kulterer B, Friedl G, Jandrositz A,
Sanchez-Cabo F, Prokesch A, Paar C, Scheideler M, Windhager R,
Preisegger KH and Trajanoski Z: Gene expression profiling of human
mesenchymal stem cells derived from bone marrow during expansion
and osteoblast differentiation. BMC Genomics. 8:702007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Troyer DL and Weiss ML: Wharton's
jelly-derived cells are a primitive stromal cell population. Stem
Cells. 26:591–599. 2008. View Article : Google Scholar
|
|
21
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mareschi K, Ferrero I, Rustichelli D,
Aschero S, Gammaitoni L, Aglietta M, Madon E and Fagioli F:
Expansion of mesenchymal stem cells isolated from pediatric and
adult donor bone marrow. J Cell Biochem. 97:744–754. 2006.
View Article : Google Scholar
|
|
23
|
Sasportas LS, Kasmieh R, Wakimoto H,
Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza
RL, Weissleder R and Shah K: Assessment of therapeutic efficacy and
fate of engineered human mesenchymal stem cells for cancer therapy.
Proc Natl Acad Sci USA. 106:4822–4827. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Studeny M, Marini FC, Dembinski JL,
Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE and Andreeff
M: Mesenchymal stem cells: Potential precursors for tumor stroma
and targeted-delivery vehicles for anticancer agents. J Natl Cancer
Inst. 96:1593–1603. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuzuka T, Rachakatla RS, Doi C, Maurya
DK, Ohta N, Kawabata A, Pyle MM, Pickel L, Reischman J, Marini F,
et al: Human umbilical cord matrix-derived stem cells expressing
interferon-beta gene significantly attenuate bronchioloalveolar
carcinoma xenografts in SCID mice. Lung Cancer. 70:28–36. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohlsson LB, Varas L, Kjellman C, Edvardsen
K and Lindvall M: Mesenchymal progenitor cell-mediated inhibition
of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol
Pathol. 75:248–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian
C, Li J, Yan X, Liu Y, Shao C and Zhao RC: Human mesenchymal stem
cells inhibit cancer cell proliferation by secreting DKK-1.
Leukemia. 23:925–933. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao Y, Yao A, Zhang W, Lu S, Yu Y, Deng L,
Yin A, Xia Y, Sun B and Wang X: Human mesenchymal stem cells
overex-pressing pigment epithelium-derived factor inhibit
hepatocellular carcinoma in nude mice. Oncogene. 29:2784–2794.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baksh D, Yao R and Tuan RS: Comparison of
proliferative and multilineage differentiation potential of human
mesenchymal stem cells derived from umbilical cord and bone marrow.
Stem Cells. 25:1384–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeong JA, Hong SH, Gang EJ, Ahn C, Hwang
SH, Yang IH, Han H and Kim H: Differential gene expression
profiling of human umbilical cord blood-derived mesenchymal stem
cells by DNA microarray. Stem Cells. 23:584–593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rachakatla RS, Marini F, Weiss ML, Tamura
M and Troyer D: Development of human umbilical cord matrix stem
cell-based gene therapy for experimental lung tumors. Cancer Gene
Ther. 14:828–835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weiss ML, Medicetty S, Bledsoe AR,
Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G and
Troyer D: Human umbilical cord matrix stem cells: Preliminary
characterization and effect of transplantation in a rodent model of
Parkinson's disease. Stem Cells. 24:781–792. 2006. View Article : Google Scholar
|
|
33
|
Ayuzawa R, Doi C, Rachakatla RS, Pyle MM,
Maurya DK, Troyer D and Tamura M: Naive human umbilical cord matrix
derived stem cells significantly attenuate growth of human breast
cancer cells in vitro and in vivo. Cancer Lett. 280:31–37. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ganta C, Chiyo D, Ayuzawa R, Rachakatla R,
Pyle M, Andrews G, Weiss M, Tamura M and Troyer D: Rat umbilical
cord stem cells completely abolish rat mammary carcinomas with no
evidence of metastasis or recurrence 100 days post-tumor cell
inoculation. Cancer Res. 69:1815–1820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsuzuka T, Rachakatla RS, Doi C, Maurya
DK, Ohta N, Kawabata A, Pyle MM, Pickel L, Reischman J and Marini
F: Human umbilical cord matrix-derived stem cells expressing
interferon-beta gene significantly attenuate bronchioloalveolar
carcinoma xenografts in SCID mice. Lung Cancer. 70:28–36. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu CH and Hwang SM: Cytokine interactions
in mesenchymal stem cells from cord blood. Cytokine. 32:270–279.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li M, Li H, Li C, Zhou S, Guo L, Liu H,
Jiang W, Liu X, Li P, McNutt MA and Li G: Alpha fetoprotein is a
novel protein-binding partner for caspase-3 and blocks the
apoptotic signaling pathway in human hepatoma cells. Int J Cancer.
124:2845–2854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ali MA, Koura BA, El-Mashad N and Zaghloul
MH: The Bcl-2 and TGF-beta1 levels in patients with chronic
hepatitis C, liver cirrhosis and hepatocellular carcinoma. Egypt J
Immunol. 11:83–90. 2004.
|
|
39
|
Li M, Li H, Li C, Guo L, Liu H, Zhou S,
Liu X, Chen Z, Shi S, Wei J, et al: Cytoplasmic alpha-fetoprotein
functions as a co-repressor in RA-RAR signaling to promote the
growth of human hepatoma Bel 7402 cells. Cancer Lett. 285:190–199.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ke CC, Liu RS, Suetsugu A, Kimura H, Ho
JH, Lee OK and Hoffman RM: In vivo fluorescence imaging reveals the
promotion of mammary tumorigenesis by mesenchymal stromal cells.
PLoS One. 8:e696582013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ljujic B, Milovanovic M, Volarevic V,
Murray B, Bugarski D, Przyborski S, Arsenijevic N, Lukic ML and
Stojkovic M: Human mesenchymal stem cells creating an
immunosuppressive environment and promote breast cancer in mice.
Sci Rep. 3:22982013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiao W, Mohseny AB, Hogendoorn PC and
Cleton-Jansen AM: Mesenchymal stem cell transformation and sarcoma
genesis. Clin Sarcoma Res. 3:102013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang P, Dong L, Yan K, Long H, Yang TT,
Dong MQ, Zhou Y, Fan QY and Ma BA: CXCR4-mediated osteosarcoma
growth and pulmonary metastasis is promoted by mesenchymal stem
cells through VEGF. Oncol Rep. 30:1753–1761. 2013.PubMed/NCBI
|