Introduction
With the wide application of intravenous
thrombolysis, percutaneous coronary intervention and coronary
artery bypass grafting, physicians and researchers are beginning to
realize the importance of myocardial ischemia/reperfusion injury
(MIRI) (1). The most important
therapeutic principle regarding ischemic heart disease and acute
myocardial infarction is to open the related blood vessels as early
as possible, in order to recover perfusion of ischemic myocardium
and rescue myocardial tissue from necrosis (2). However, reperfusion therapy is a
double-edged sword, which may aggravate tissue damage at the same
time as recovering coronary blood flow (3). Issues including cardiac
insufficiency, arrhythmia and extended myocardial infarction may
occur; therefore, it is important to identify effective measures
for the alleviation of MIRI.
MIRI is a complex pathological process. Well-defined
pathogenic mechanisms associated with MIRI include oxidative stress
injury, intracellular calcium overload, cell apoptosis, loss of
cellular energy, and activation of the inflammatory response via
neutrophils (4). Furthermore, MIRI
is associated with the activation and inactivation of various
regulatory mechanisms and signal pathways.
High mobility group box 1 (HMGB1) is a highly
conserved DNA-binding nonhistone protein, which exists in the
karyon of eukaryotic cells (5). It
has previously been demonstrated that HMGB1 participates in
inflammation, where it acts as a proinflammatory cytokine (6). Under inflammatory conditions, HMGB1
is passively released or actively secreted from affected
monocytes/macrophages into the extracellular environment (6). Consequently, it can combine with
receptor for advanced glycation end products or Toll-like receptors
(TLRs). Finally, nuclear factor (NF)-κB can be activated and the
inflammatory response mediated (7).
Ghrelin is found in the stomachs of mice, and was
the first endogenous ligand of growth hormone secretagogue receptor
to be reported to possess biological activity. Ghrelin is
synthesized in the stomach and can promote the secretion of gastric
acid and growth hormones via paracrine, autocrine and internal
secretion (8). In addition, the
functions of ghrelin include improving ingestion, gastric acid
secretion and gastrointestinal motility, and protecting the mucous
membrane of the digestive system (9). Previous studies have reported that
ghrelin exerts anti-inflammatory, antioxidative and neuroprotective
effects (10–12). Therefore, the present study aimed
to determine the protective effects of ghrelin against oxidative
stress, inducible nitric oxide synthase (iNOS) and inflammation in
MIRI mice, and to investigate the mechanisms underlying the
cardioprotective effects of ghrelin.
Materials and methods
Experimental animals, grouping and MIRI
model
A total of 30 male C57BL/6 mice (weight, 20–25 g;
age, 6–8 weeks) were obtained from the Shanghai SLAC Laboratory
Animal Center Co., Ltd. (Shanghai, China) and were maintained in a
standard animal room (temperature, 25±1°C; humidity, 70–80%; 12-h
light/dark cycle). All mice received humane care in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the National Institutes of Health (Bethesda, MD, USA) and the
present study was approved by the ethics committee of Tianjin
Medical University General Hospital. All mice were randomly
distributed into three groups of 10 mice: (i) Control group, mice
were anesthetized and a suture was passed under the left anterior
descending artery without occlusion; (ii) MIRI group, mice were
anesthetized and used to generate a MIRI model, the mice were
pretreated with 0.5 ml normal saline administered by gavage; (iii)
MIRI + ghrelin (Sigma-Aldrich, St. Louis, MO, USA) group, MIRI mice
were pretreated with 0.8 mg/kg ghrelin twice daily by gavage for 3
days.
Briefly, mice were intraperitoneally injected with 7
mg/kg ketamine and 45 mg/kg pentobarbital sodium. The left anterior
descending coronary artery was ligated and sutured using 7–0 silk.
Visual observation of pale color development in the myocardium was
considered to indicate regional ischemia. Mice in the control group
were also anesthetized and a suture was passed under the left
anterior descending artery without occlusion. Mice were sacrificed
by overdose of pentobarbital sodium (100 mg/kg). Blood samples were
collected from the cardiopuncture of each mouse, and the heart
tissues were harvested and washed with ice-cold normal saline.
Serum creatine kinase (CK) and lactate
dehydrogenase (LDH) levels
The CK and LDH serum levels were measured in each
mouse to determine the degree of myocardial injury using commercial
kits (Wuhan Elabscience Biotechnology Co., Ltd., Wuhan, China),
according to the manufacturers' protocols.
Assessment of myocardial infarct
size
Following 2 h reperfusion, the left anterior
descending artery was re-occluded and 0.3 ml Evan's
Blue-triphenyltetrazolium chloride (TTC, 2%) was injected into the
right jugular vein to determine myocardial infarct size and
identify the area prone to ischemic damage. Subsequently, the heart
was rapidly excised and rinsed in normal saline, and the left
ventricle was isolated and frozen at −80°C for 10–15 min. The
frozen left ventricle was cut into 1-mm slices, and was incubated
in 1% TTC for 15 min at 37°C. The infarct areas were measured using
an image analyzer.
Assessment of caspase activity
Left ventricular samples were homogenized in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Nanjing, China) followed by centrifugation at 12,000
× g for 30 min at 4°C. The supernatant was collected and used to
measure protein concentration with the bicinchoninic acid method.
Lysate proteins (100 µg) were treated with 50 µl
Ac-DEVD-pNA and Ac-LEHD-pNA (Beyotime Institute of Biotechnology)
for 1 h at 37°C. Caspase-3 and caspase-9 activities were monitored
using a microplate reader at 405 nm.
Assessment of tumor necrosis factor
(TNF)-α, interleukin (IL)-6, superoxide dismutase (SOD),
glutathione (GSH), GSH-peroxidase (GSH-PX) and malondialdehyde
(MDA) activities
Whole blood samples were homogenized by
centrifugation at 12,000 × g for 10 min at 4°C. The samples were
used to measured TNF-α, IL-6, SOD, GSH, GSH-PX and MDA activities
in the mice according to the manufacturer's protocols (Beyotime
Institute of Biotechnology).
Western blotting
Protein extracts from the heart samples were
subjected to western blotting. Briefly, the heart samples were
homogenized by centrifugation at 12,000 × g for 10 min at 4°C in
Nuclear and Cytoplasmic Protein Extraction kit (Beyotime Institute
of Biotechnology). Protein concentrations were measured using a
Bicinchoninic Acid Protein Assay kit (PerkinElmer, Waltham, MA,
USA). Equal amounts of protein (50–80 µg) were separated by
8–10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(Beyotime Institute of Biotechnology) and were transferred to
polyvinylidene fluoride membranes.
The membranes were then blocked with 5% skim milk
solution, followed by an overnight incubation at 4°C with rabbit
anti-HMGB1 (1:4,000; Cell Signaling Technology, Inc., Danvers, MA,
USA; cat. no. 6893), rabbit anti-TLR4 (1:3,000; Cell Signaling
Technology, Inc.; cat. no. 14358), rabbit anti-NF-κB (1:3,000;
Beyotime Institute of Biotechnology; cat. no. AN365) and mouse
anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5,000;
Beyotime Institute of Biotechnology; cat. no. AF0006). The
membranes were then incubated with goat anti-mouse immunoglobulin
(Ig)G (1:5,000; Beyotime Institute of Biotechnology; cat. no.
A0216) and goat anti-rabbit IgG (1:5,000; Beyotime Institute of
Biotechnology; cat. no. A0208) secondary antibodies for 1 h at room
temperature and washed with Tris-buffered saline/0.1% Tween-20. The
relative intensity of the bands was quantified using ImageJ 3.0
software (imagej.nih.gov).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical comparisons for continuous variables were performed
using analysis of variance and Tukey's test with SPSS 12.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Protective effects of ghrelin against CK
and LDH levels in MIRI mice
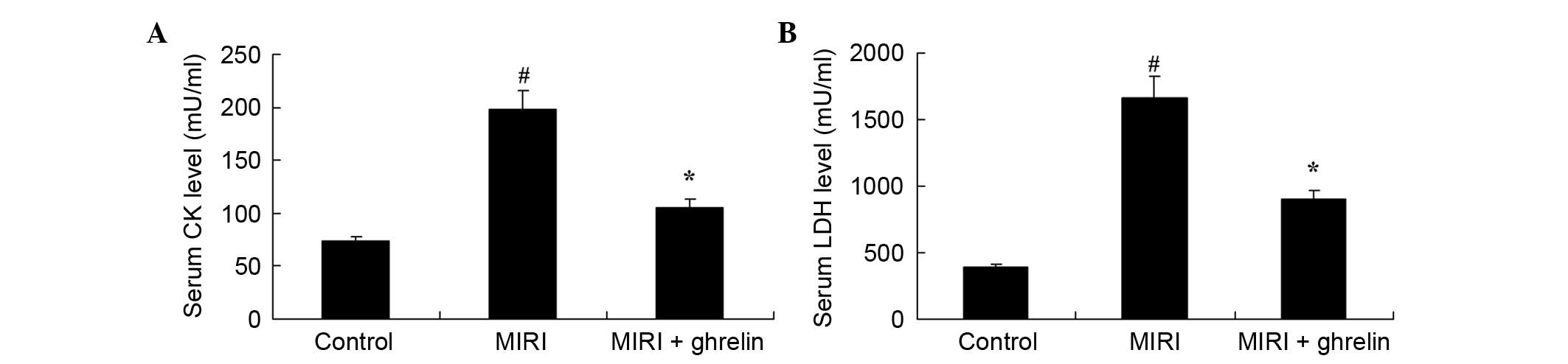
As shown in Fig. 1,
ghrelin exerted protective effects against CK and LDH levels in
MIRI mice. Compared with the control group, MIRI induced a
significant increase in CK levels (Fig. 1A). Furthermore, the LDH levels were
markedly increased in the MIRI group compared with in the control
mice (Fig. 1B). Conversely, CK and
LDH levels were markedly reduced following ghrelin treatment
compared with in the MIRI group (Fig.
1A and B).
Protective effects of ghrelin against
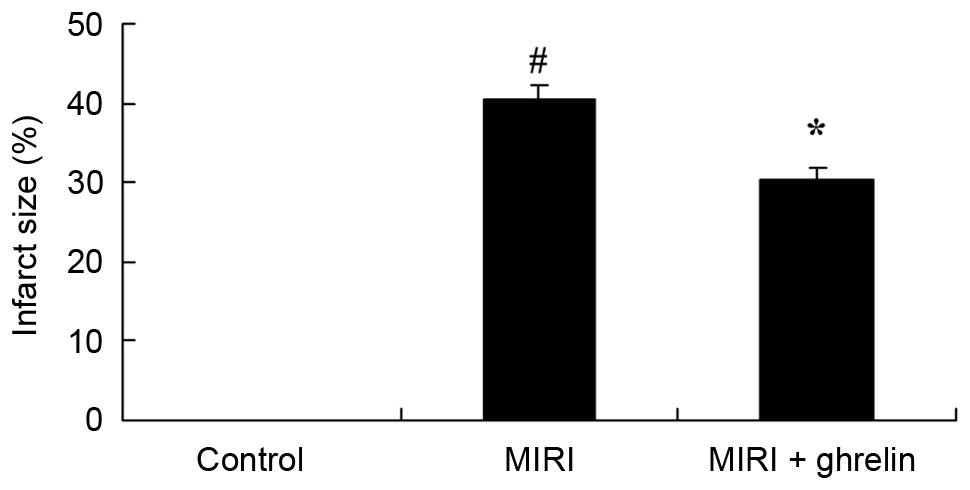
infarct size in MIRI mice
The effects of ghrelin on representative myocardial
infarct size in MIRI mice are presented in Fig. 2. Compared with the control group,
MIRI increased myocardial infarct size (Fig. 2). Conversely, ghrelin treatment
effectively suppressed myocardial infarct size compared with in the
MIRI group (Fig. 2).
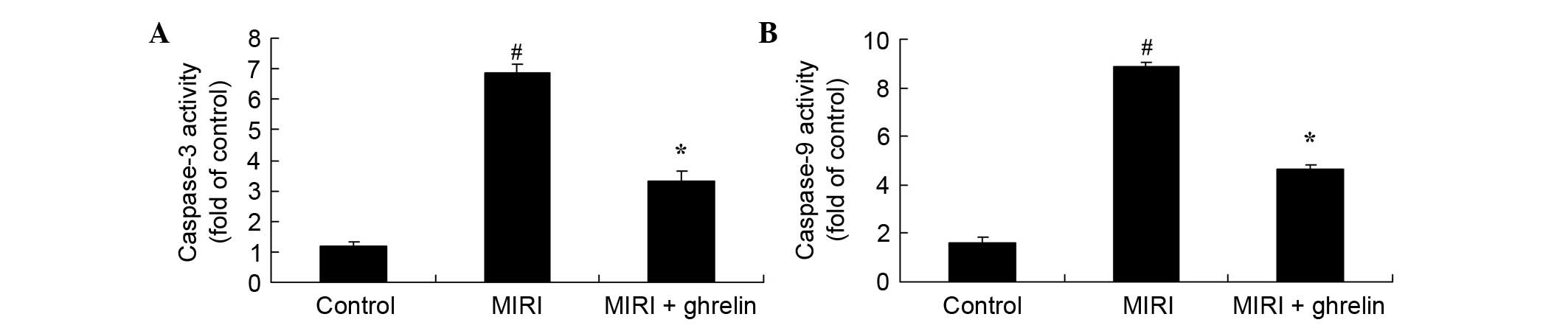
Protective effects of ghrelin against
caspase-3/caspase-9 activities in MIRI mice
Apoptosis is the major mechanism of cell death
following MIRI; therefore, in the present study,
caspase-3/caspase-9 activities were measured using a microplate
reader. The effects of ghrelin treatment on caspase-3/caspase-9
activities in MIRI mice are presented in Fig. 3. Compared with the control group,
MIRI increased caspase-3/caspase-9 activities. Conversely,
pretreatment with ghrelin effectively attenuated the increase in
caspase-3/caspase-9 activities in MIRI mice.
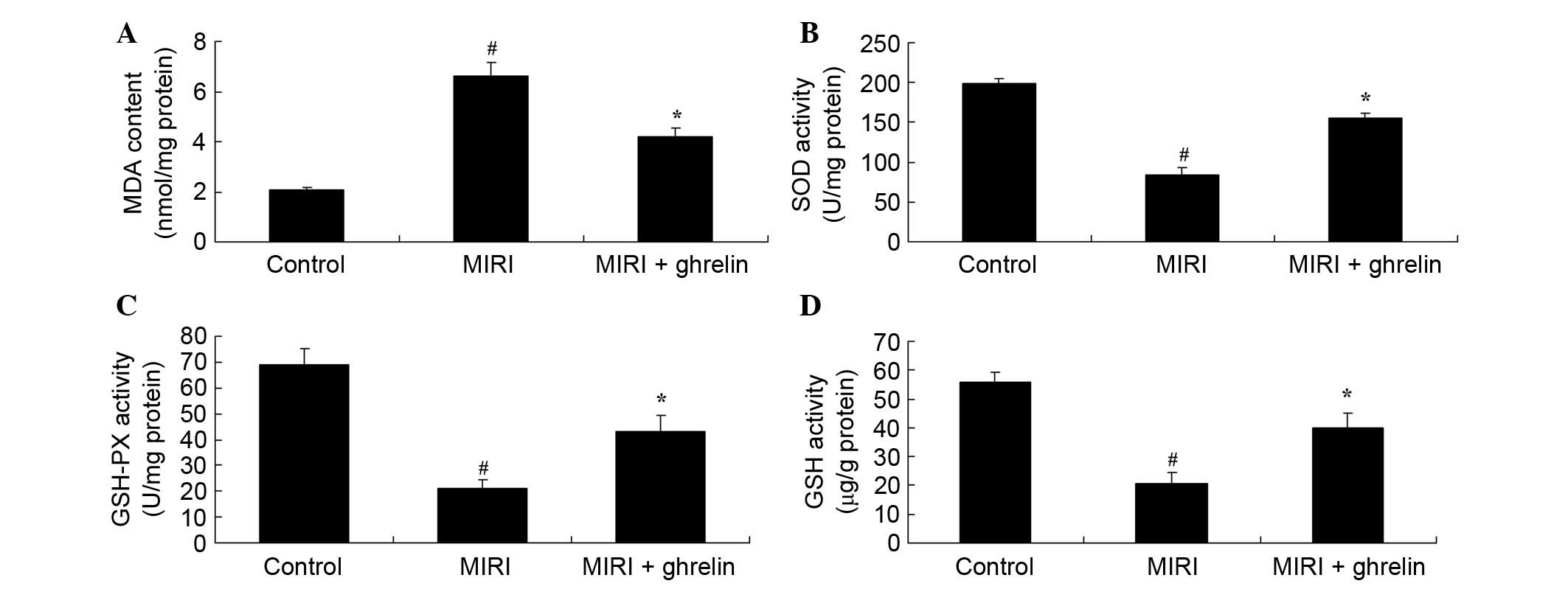
Protective effects of ghrelin against
oxidative stress in MIRI mice
A microplate reader was used after 3 days to
determine the effects of ghrelin on oxidative stress following
MIRI. MDA activity levels were significantly higher in the MIRI
group compared with in the control mice (Fig. 4). However, SOD, GSH and GSH-PX
activities were significantly lower in the MIRI group compared with
in the control mice (Fig. 4).
Compared with the MIRI group, MDA levels were markedly decreased,
whereas SOD, GSH and GSH-PX activities were markedly increased
following treatment with ghrelin (Fig.
4).
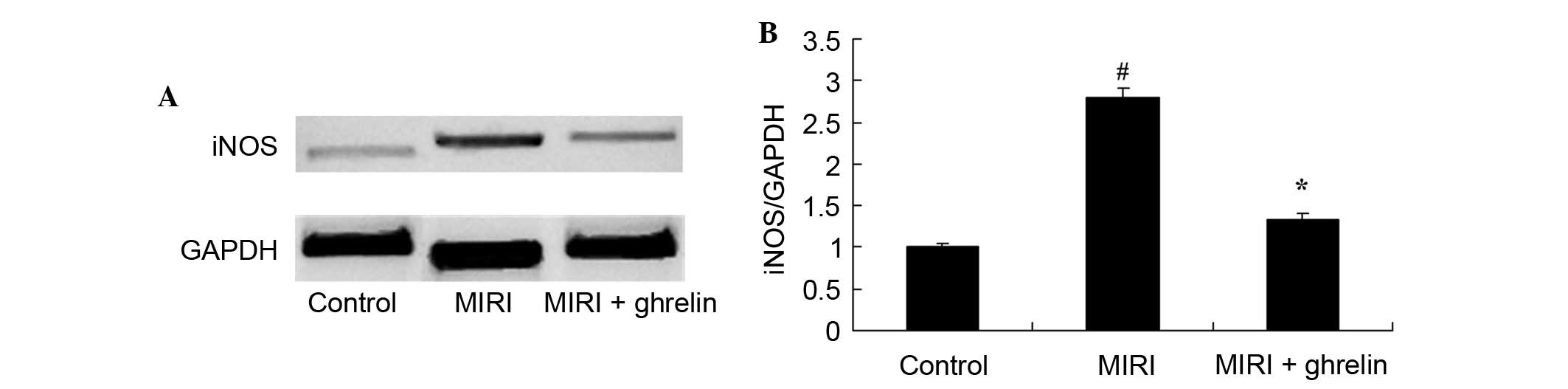
Protective effects of ghrelin against
iNOS in MIRI mice
The protein expression levels of iNOS following MIRI
were detected by western blotting (Fig. 5). Compared with the control group,
the protein expression levels of iNOS were significantly increased
in response to MIRI. Pretreatment with ghrelin significantly
alleviated MIRI-induced iNOS protein expression.
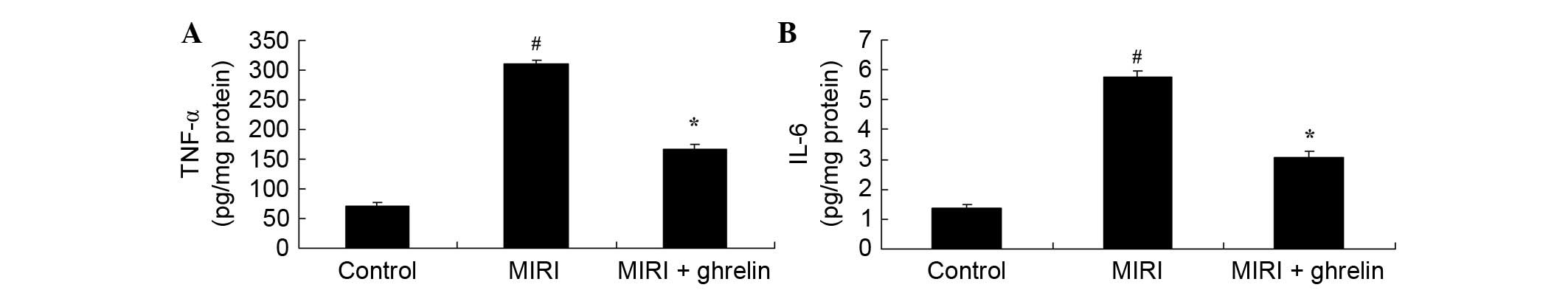
Protective effects of ghrelin against
inflammation in MIRI mice
The extent of inflammation was determined to assess
the protective effects of ghrelin against MIRI. TNF-α and IL-6
activities were markedly increased in the MIRI group compared with
in the control group (Fig. 6).
However, ghrelin treatment significantly inhibited the increase in
TNF-α and IL-6 activities in MIRI mice (Fig. 6).
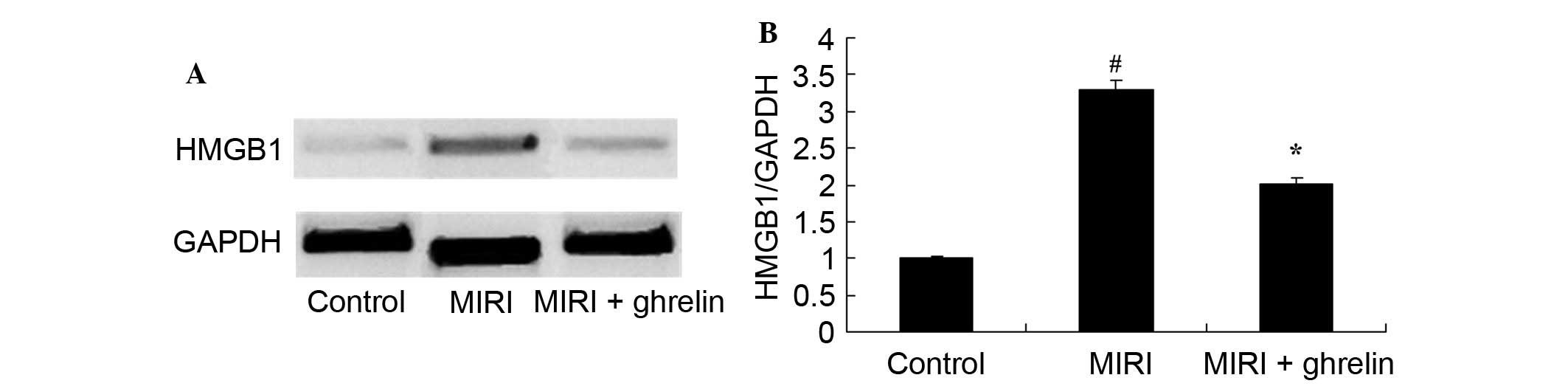
Protective effects of ghrelin on HMGB1 in
MIRI mice
MIRI induced HMGB1 expression (Fig. 7). Compared with in the control
group, HMGB1 protein expression levels were significantly increased
in the MIRI group. However, ghrelin pretreatment significantly
inhibited the MIRI-induced increase in HMGB1 protein expression
(Fig. 7).
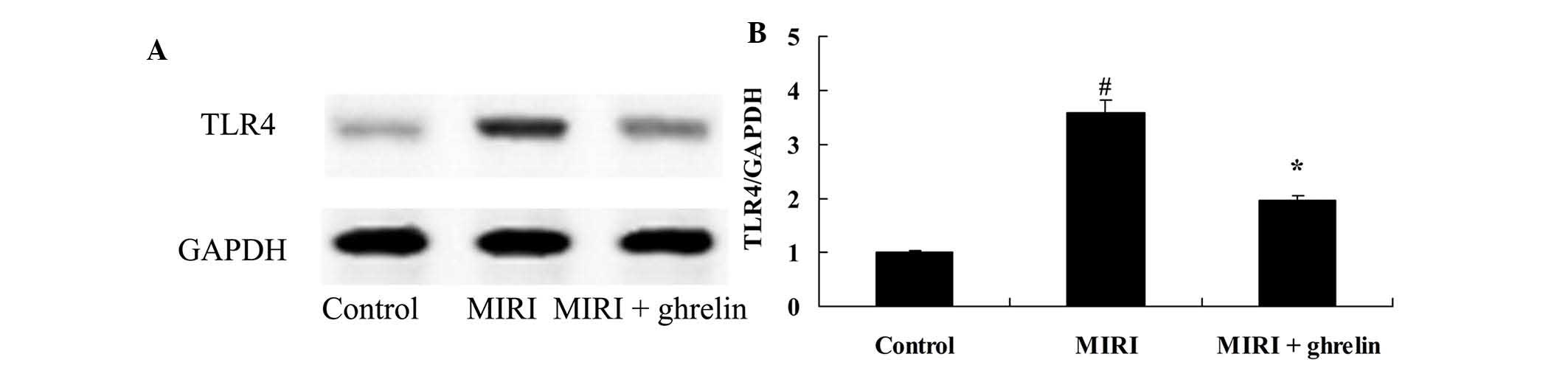
Protective effects of ghrelin on TLR4 in
MIRI mice
The present study examined the protective effects of
ghrelin on TLR4 in MIRI mice. MIRI induced an increase in TLR4
protein expression levels compared with in the control group
(Fig. 8). Conversely, ghrelin
pretreatment inhibited TLR4 protein expression in MIRI mice
(Fig. 8).
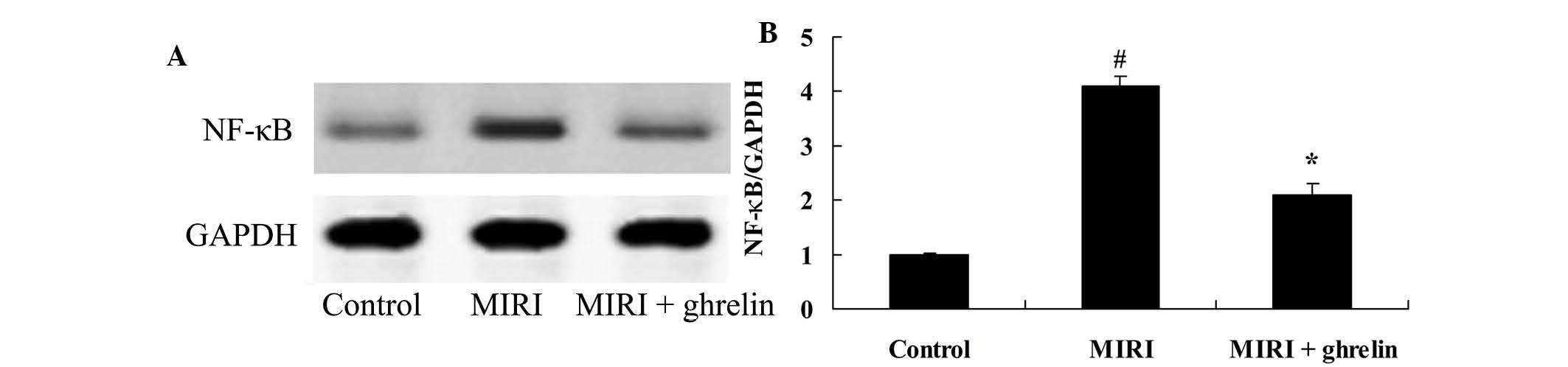
Protective effects of ghrelin on NF-κB in
MIRI mice
In order to investigate whether ghrelin pretreatment
protected against MIRI-induced NF-κB, the expression levels of
NF-κB were detected. MIRI markedly increased NF-κB protein
expression compared with in the control group (Fig. 9). Following ghrelin treatment,
MIRI-induced NF-κB protein expression was downregulated (Fig. 9).
Discussion
MIRI refers to serious damage to previously ischemic
myocardial tissues after a short period of myocardial blood flow
failure (13). The clinical
manifestations of MIRI after recanalization of an obstructed
coronary artery include reperfusion arrhythmias, extension of the
area of infarcted cardiac muscle, cardiac insufficiency or sudden
mortality (14). Post-reperfusion
of ischemic myocardium, metabolic disturbances to myocardial cells
and morphological structural damage are also serious complications
(15). In addition, the number of
necrotic myocardial cells is increased by ~25% (16). Therefore, methods of coronary
artery recanalization, including thrombolysis, are a double-edged
sword (17), since MIRI may lead
to novel complications, which greatly decrease the efficacy of
therapies attempting to improve myocardial blood supply and rescue
infarcted myocardium. The present study demonstrated that the
protective effects of ghrelin inhibited MIRI-induce CK and LDH
levels, and reduced infarct size in a mouse model of MIRI.
HMGBl shares features with cytokines, and can be
actively secreted by activated immune cells and act on the surface
receptors of immune cells and endothelial cells in order to induce
expression of inflammatory factors and further HMGB1 release,
resulting in promotion of the inflammatory cascade (18). Furthermore, HMGB1 has been reported
to stimulate the release of cytokines and adhesion molecules;
promote inflammatory cells; destroy epithelial barriers and extend
the inflammatory response (19).
The results of the present study suggested that ghrelin
pretreatment inhibited caspase-3 and caspase-9 activities;
alleviated oxidative stress, iNOS protein expression and
inflammation; and suppressed HMGB1 protein expression in MIRI mice.
Wang et al (20)
demonstrated that ghrelin protects mice against endotoxemia-induced
acute kidney injury via the suppression of nitric oxide, and
inhibition of TNF-α, IL-1β and IL-6. Koyuturk et al
(10) indicated that ghrelin
inhibited apoptosis, cell proliferation and the oxidant-antioxidant
system via caspase-8 and caspase-3 in the liver of neonatal
diabetic rats. Furthermore, Ercan et al (21) suggested that ghrelin treatment
suppressed sodium metabisulfite-induced oxidative stress and
caspase-3 expression in rat gastric mucosa.
TLRs are receptors of HMGB1. TLRs are signal
transduction transmembrane receptors that are found on the cell
surface. Among the TLR family, TLR2/4 are able to recognize the
majority of pathogenic microorganisms (22). Distributed in mononuclear cells,
macrophages, neutrophils, dendritic cells and cancer cells, TLR2/4
exerts innate immune functions against bacteria, viruses, fungi and
other pathogenic infections (23).
It acts as a bridge between congenital immunity and acquired
immunity (24). TLR2 and TLR4 can
integrate with HMGB1, which is secreted by macrophages and
neutrophils, and induce the occurrence of inflammation (23). In addition, it has been reported
that TLR2 and TLR4 are potential receptors of HMGB1. The present
study indicated that ghrelin treatment inhibited TLR4 protein
expression in MIRI mice. Liu et al (25) reported that ghrelin reduced high
glucose-induced PC12 cell apoptosis via the TLR4/NF-κB pathway.
Following integration of cytokines and receptor
proteins, a series of signal transduction molecules are required to
transmit signals into cells. The signal transduction pathway
mediated by TLR2/4 is as follows. Specific ligands that are
recognized by the TLR2/4 transmembrane domain induce the release of
signals into the cells (26).
Subsequently, the toll-IL-1 receptor cytoplasmic domain of TLR2/4
combines with various downstream molecular regulators, thus
activating downstream signal transduction cascades by
MyD88-dependent or -independent pathways (27). Finally, transcription factors, such
as NF-κB and interferon regulatory factor 3, are activated;
inflammatory cytokines, such as IFN-β, and thus inflammation are
induced; inflammatory cell infiltration is induced by chemotaxis,
and innate immunity is activated (28). The present study revealed the
protective effects of ghrelin, which was shown to inhibit NF-κB
expression in MIRI mice. Liu et al (25) reported that ghrelin reduced high
glucose-induced PC12 cell apoptosis via the TLR4/NF-κB pathway.
Furthermore, Rezaeian et al (29) demonstrated that ghrelin protects
musculocutaneous tissue via NF-κB in ischemic necrosis.
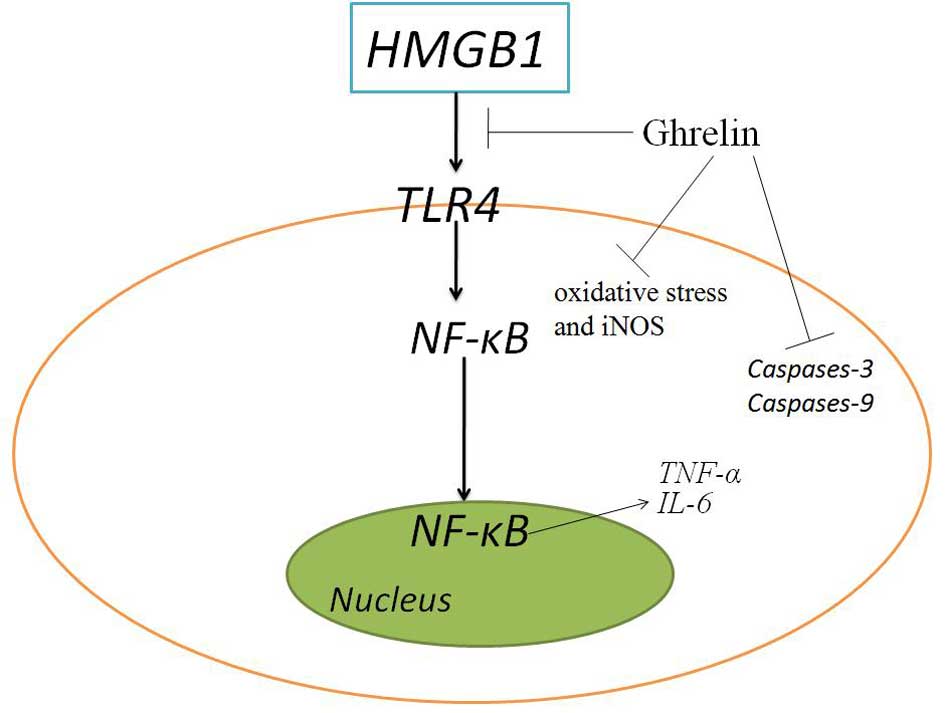
In conclusion, as shown in Fig. 10, ghrelin exerts protective
effects against oxidative stress, iNOS and inflammation in MIRI
mice via the HMGB1 andTLR4/NF-κB pathway. Therefore, ghrelin may be
a clinically useful agent in the treatment of MIRI.
Acknowledgments
The present study was supported the by Tianjin
Municipal Health Bureau Science Foundation (grant no. 2011KE113)
and the Tianjin Municipal Natural Science Foundation (grant no.
13JCYBJC36400).
References
|
1
|
Garcia Gómez-Heras S, Alvarez-Ayuso L,
Torralba Arranz A and Fernandez-Garcia H: Purkinje fibers after
myocardial ischemia-reperfusion. Histol Histopathol. 30:841–853.
2015.PubMed/NCBI
|
|
2
|
Smiley D, Smith MA, Carreira V, Jiang M,
Koch SE, Kelley M, Rubinstein J, Jones WK and Tranter M: Increased
fibrosis and progression to heart failure in MRL mice following
ischemia/reperfusion injury. Cardiovasc Pathol. 23:327–334. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao J, Wang F, Zhang Y, Jiao L, Lau WB,
Wang L, Liu B, Gao E, Koch WJ, Ma XL and Wang Y: Sevoflurane
preconditioning attenuates myocardial ischemia/reperfusion injury
via caveolin-3-dependent cyclooxygenase-2 inhibition. Circulation.
128(Suppl 1): S121–S129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xue L, Wu Z, Ji XP, Gao XQ and Guo YH:
Effect and mechanism of salvianolic acid B on the myocardial
ischemia-reperfusion injury in rats. Asian Pac J Trop Med.
7:280–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park SY, Lee SW, Kim HY, Lee WS, Hong KW
and Kim CD: HMGB1 induces angiogenesis in rheumatoid arthritis via
HIF-1α activation. Eur J Immunol. 45:1216–1227. 2015. View Article : Google Scholar
|
|
6
|
Kornblit B, Munthe-Fog L, Madsen HO, Strom
J, Vindelov L and Garred P: Association of HMGB1 polymorphisms with
outcome in patients with systemic inflammatory response syndrome.
Crit Care. 12:R832008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ibrahim ZA, Armour CL, Phipps S and Sukkar
MB: RAGE and TLRs: Relatives, friends or neighbours? Mol Immunol.
56:739–744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muller TD, Nogueiras R, Andermann ML,
Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers
CY, Broglio F, et al: Ghrelin. Mol Metab. 4:437–460. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jonsson E: The role of ghrelin in energy
balance regulation in fish. Gen Comp Endocrinol. 187:79–85. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koyuturk M, Sacan O, Karabulut S, Turk N
and Bolkent S, Yanardag R and Bolkent S: The role of ghrelin on
apoptosis, cell proliferation and oxidant-antioxidant system in the
liver of neonatal diabetic rats. Cell Biol Int. 39:834–841. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang CX, Yuan MJ, Huang H, Wu G, Liu Y,
Yu SB, Li HT and Wang T: Ghrelin inhibits post-infarct myocardial
remodeling and improves cardiac function through anti-inflammation
effect. Peptides. 30:2286–2291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto A, Yamafuji M, Tachibana T,
Nakabeppu Y, Noda M and Nakaya H: Oral 'hydrogen water' induces
neuroprotective ghrelin secretion in mice. Sci Rep. 3:32732013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Yang H, Hu X, Fu W, Xie J, Zhou X,
Xu W and Jiang H: Dobutamine-mediated heme oxygenase-1 induction
via PI3K and p38 MAPK inhibits high mobility group box 1 protein
release and attenuates rat myocardial ischemia/reperfusion injury
in vivo. J Surg Res. 183:509–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
King AL and Lefer DJ: Cytoprotective
actions of hydrogen sulfide in ischaemia-reperfusion injury. Exp
Physiol. 96:840–846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ekeløf S, Rosenberg J, Jensen JS and
Gögenur I: Pharmacological attenuation of myocardial reperfusion
injury in a closed-chest porcine model: A systematic review. J
Cardiovasc Transl Res. 7:570–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruiz-Meana M and Garcia-Dorado D:
Translational cardiovascular medicine (II). Pathophysiology of
ischemia-reperfusion injury: New therapeutic options for acute
myocardial infarction. Rev Esp Cardiol. 62:199–209. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quintana M, Kahan T and Hjemdahl P:
Pharmacological prevention of reperfusion injury in acute
myocardial infarction. A potential role for adenosine as a
therapeutic agent. Am J Cardiovasc Drugs. 4:159–167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ramasamy R, Yan SF and Schmidt AM:
Stopping the primal RAGE reaction in myocardial infarction:
Capturing adaptive responses to heal the heart? Circulation.
117:3165–3167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Cheng Y, Xue FS, Yuan YJ, Xiong J,
Li RP, Liao X and Liu JH: Postconditioning with vagal stimulation
attenuates local and systemic inflammatory responses to myocardial
ischemia reperfusion injury in rats. Inflamm Res. 61:1273–1282.
2012. View Article : Google Scholar
|
|
20
|
Wang W, Bansal S, Falk S, Ljubanovic D and
Schrier R: Ghrelin protects mice against endotoxemia-induced acute
kidney injury. Am J Physiol Renal Physiol. 297:F1032–F1037. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ercan S, Basaranlar G, Gungor NE, Kencebay
C, Sahin P, Celik-Ozenci C and Derin N: Ghrelin inhibits sodium
metabisulfite induced oxidative stress and apoptosis in rat gastric
mucosa. Food Chem Toxicol. 56:154–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herzog C, Lorenz A, Gillmann HJ, Chowdhury
A, Larmann J, Harendza T, Echtermeyer F, Müller M, Schmitz M,
Stypmann J, et al: Thrombomodulin's lectin-like domain reduces
myocardial damage by interfering with HMGB1-mediated TLR2
signalling. Cardiovasc Res. 101:400–410. 2014. View Article : Google Scholar
|
|
23
|
Kwak MS, Lim M, Lee YJ, Lee HS, Kim YH,
Youn JH, Choi JE and Shin JS: HMGB1 binds to lipoteichoic acid and
enhances TNF-α and IL-6 Production through HMGB1-Mediated Transfer
of Lipoteichoic Acid to CD14 and TLR2. J Innate Immun. 7:405–416.
2015. View Article : Google Scholar
|
|
24
|
Liu Q, Wang J, Liang Q, Wang D, Luo Y, Li
J, Janicki JS and Fan D: Sparstolonin B attenuates
hypoxia-reoxygenation-induced cardiomyocyte inflammation. Exp Biol
Med (Maywood). 239:376–384. 2014. View Article : Google Scholar
|
|
25
|
Liu X, Xiao Q, Zhao K and Gao Y: Ghrelin
inhibits high glucose-induced PC12 cell apoptosis by regulating
TLR4/NF-kappaB pathway. Inflammation. 36:1286–1294. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song R, Ao L, Zhao KS, Zheng D, Venardos
N, Fullerton DA and Meng X: Soluble biglycan induces the production
of ICAM-1 and MCP-1 in human aortic valve interstitial cells
through TLR2/4 and the ERK1/2 pathway. Inflamm Res. 63:703–710.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hao H, Gufu H, Lei F, Dang L and
Zhongliang Y: Baicalin suppresses expression of TLR2/4 and NF-κB in
chlamydia trachomatis-infected mice. Immunopharmacol Immunotoxicol.
34:89–94. 2012. View Article : Google Scholar
|
|
28
|
Tu XK, Yang WZ, Chen JP, Chen Y, Ouyang
LQ, Xu YC and Shi SS: Curcumin inhibits TLR2/4-NF-κB signaling
pathway and attenuates brain damage in permanent focal cerebral
ischemia in rats. Inflammation. 37:1544–1551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rezaeian F, Wettstein R, Scheuer C,
Bäumker K, Bächle A, Vollmar B, Menger MD and Harder Y: Ghrelin
protects musculocutaneous tissue from ischemic necrosis by
improving microvascular perfusion. Am J Physiol Heart Circ Physiol.
302:H603–H610. 2012. View Article : Google Scholar
|