Introduction
Ovarian cancer is a lethal gynecological malignancy
and the fifth leading cause of cancer-associated mortality
worldwide. In 2013, there were >2 million novel cases of ovarian
cancer and 1 million mortalities worldwide (1–3).
Chemotherapy following surgical resection is the current primary
treatment for localized ovarian cancer. Progress has been made in
early diagnosis of patients; however, treatment options for the
majority of patients with advanced stage ovarian cancer remain
limited. Particularly, resistance to chemotherapeutical drugs often
leads to poor prognosis (4–6).
Therefore, it is important to investigate the potential molecular
mechanisms underlying the pathogenesis of ovarian cancer, and to
identify novel therapeutic agents and combination regimens in order
to improve the treatment and prognosis of patients with ovarian
cancer.
Nitidine chloride (NC) (Fig. 1A) is isolated from the root of
Zanthoxylum nitidum (Roxb). Numerous pharmacological
properties of NC have been previously reported, including
anti-oxidant, anti-inflammatory, anti-fungal, analgesic and
anti-human immunodeficiency virus functions (7,8).
Previous studies have determined that NC exhibits anti-tumor
activity in several types of cancer. Additionally, NC inhibits cell
proliferation and induces apoptosis in renal cancer, gastric
cancer, breast cancer and hepatocellular carcinoma (9–12).
NC was also capable of suppressing the invasion and metastasis of
renal cancer via the Akt serine/threonine kinase 1 (Akt) pathway,
and breast cancer by inhibiting the SRC proto-oncogene,
non-receptor tyrosine kinase/focal adhesion kinase-associated
signaling pathway (13,14). However, to the best of our
knowledge, no evidence has previously reported whether NC exerts
any effect on ovarian cancer proliferation, apoptosis and its
underlying mechanisms. NC may be successful in treating ovarian
cancer; however, the synergistic effect of NC and other therapeutic
agents requires further investigation.
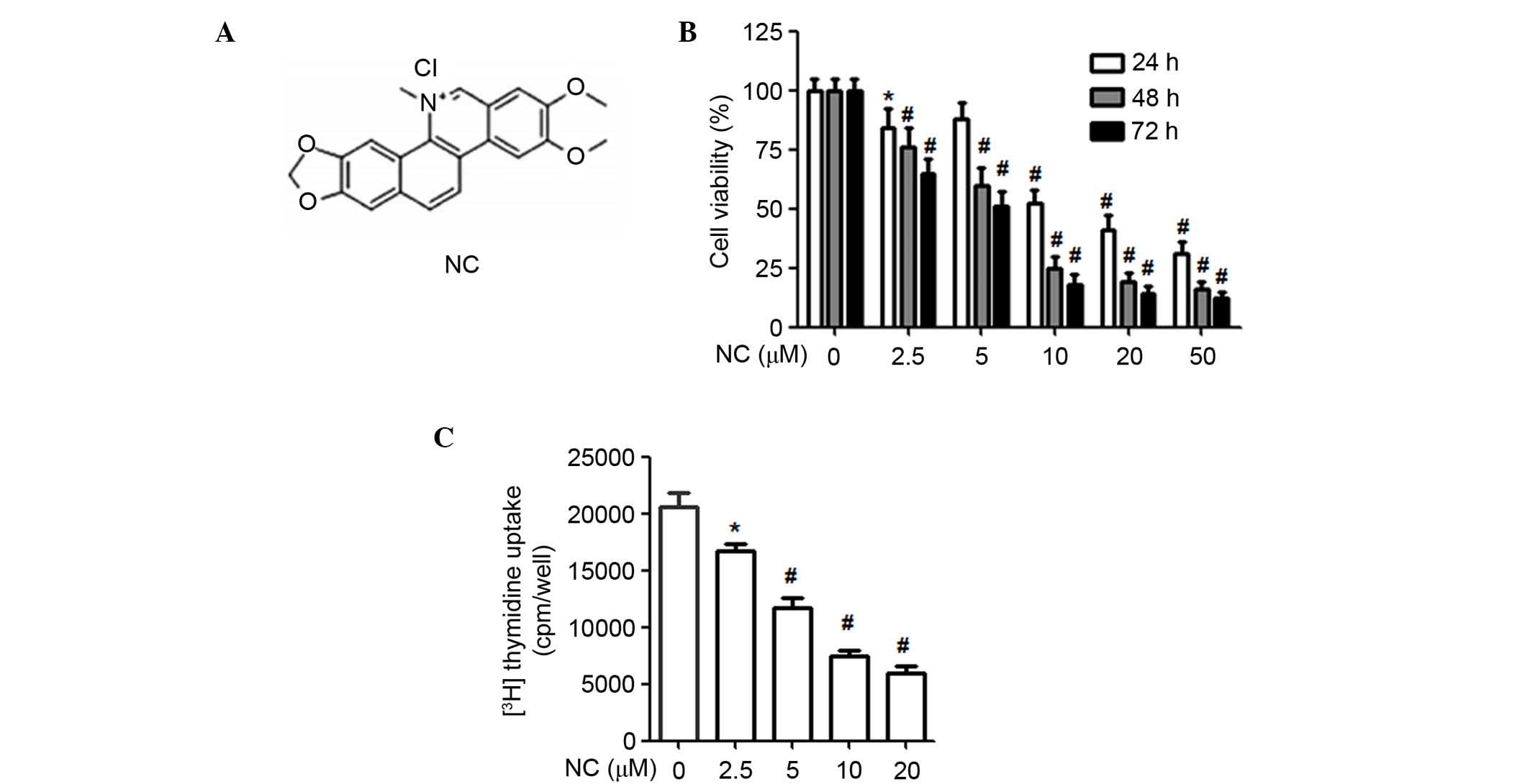 | Figure 1NC inhibited the proliferation of
A2780 ovarian cancer cells. (A) Chemical structure of NC. (B) NC
treatment for different time periods (24, 48 and 72 h) and
different concentration gradients (0, 2.5, 5, 10, 20 and 50
µM), methyl thiazolyl tetrazolium assay was used to
determine the viability of A2780 cells. (C) Following 40 h
treatment with NC at different concentrations (0, 2.5, 5, 10 and 20
µM), 3H-thymidine incorporation assay was used to
detect the proliferation of A2780 cells. *P<0.05,
#P<0.01 vs. control group. Data are presented as the
mean ± standard deviation of three independent experiments. NC,
nitidine chloride. |
The present study investigated the effects of NC on
ovarian cancer cell proliferation and apoptosis. It was also
determined that the Akt pathway is important for NC-induced
apoptosis. Additionally, the synergistic effect of NC with
doxorubicin (DOX) on ovarian cancer cells was evaluated.
Materials and methods
Cell lines and reagents
The A2780 human ovarian cancer cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured in RPMI-1640 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin, and 100 µg/ml streptomycin in 5% CO2
at 37°C. Rabbit anti-Akt (cat. no. 4685), phosphorylated-Akt (cat.
no. 4058), B-cell CLL/lymphoma 2 (Bcl-2; cat. no. 2870),
Bcl-2-associated X protein (Bax; cat. no. 502), p53 (cat. no.
2527), caspase-3 (cat. no. 9665) and -9 (cat. no. 9502) antibodies
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). NC was purchased from Tauto Biotech Co., Ltd. (Shanghai,
China) and dissolved in dimethyl sulfoxide (DMSO). LY294002, a
selective phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) Akt
inhibitor, was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
apoptosis detection kit was purchased from BD Biosciences (Franklin
Lakes, NJ, USA).
Methyl thiazolyl tetrazolium (MTT) assay
of cell viability and proliferation
Cell viability and proliferation were detected by
using MTT assay. A2780 cells in 100 µl RPMI-1640 were seeded
at a density of 5.0×103 cells/well in 96-well plates.
Following treatment with NC (0, 2.5, 5, 10, 20 or 50 µM)
and/or DOX (0, 0.25, 0.5, 1, 2, 5 µM; Aladdin Reagents Co.,
Ltd., Shanghai, China) for different time-points (24, 48 or 72 h),
20 µl MTT (5 mg/ml) was added to each well. Subsequently,
the cells were incubated for 4 h, then 100 µl DMSO was added
to each well for another 15 min. Finally, the absorbance values
were detected using a microplate luminometer (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) at 490 nm.
3H-thymidine incorporation
assay
A2780 cells were cultured in RPMI-1640 with 10% FBS
at 50% confluence. Next, the A2780 cells were cultured in
serum-free RPMI-1640 for 24 h and treated with NC at different
concentrations (0, 2.5, 5, 10, 20 µM) for 40 h.
3H-thymidine (final concentration 1 uCi/ml) was added to
the media during the last 24 h of culturing. The cells were then
washed with ice-cold phosphate-buffered saline (PBS), and
precipitated with ice-cold 5% trichloroacetic acid (TCA;
Sigma-Aldrich) for 4 h. The cells were washed with ice-cold 5% TCA
twice, followed by washed with ice-cold PBS. Subsequently, the
cells were lysed using 200 µl 0.5 M NaOH for 30 min at 37°C.
DNA synthesis was determined by 3H-thymidine uptake
using a liquid scintillation counter (LS-6500; Beckman Coulter,
Inc., Brea, CA, USA).
Western blot analysis
Total cell protein concentrations were detected by
using the Pierce bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). Equal quantity of protein from cell
lysates (10 µg) were loaded on 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gels. Following
electrophoresis, proteins were transferred to polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA), blocked
with 5% fat-free milk at room temperature for 1 h, and incubated
with the aforementioned primary antibodies overnight at 4°C. The
membranes were subsequently washed with Tris-buffered saline
Tween-20 and incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibodies (cat. no. ZDR-5306; OriGene
Technologies, Inc., Shanghai, China) for 1 h at room temperature.
Immune complexes were detected with enhanced chemiluminescence
reagents (EMD Millipore), and the blots were quantified by
densitometric analysis using the AlphaImager IS 2200.
Apoptosis analysis by Annexin V/PI
assay
Apoptosis of A2780 cells was detected by using the
Annexin V-FITC/PI assay. A2780 cells were seeded into 6-well plates
at a density of 1.0×106 cells/well. Following NC
treatment (0, 10, 20 µM) for 24 h the A2780 cells were
harvested, washed and resuspended in PBS. Apoptotic cells were
determined using an Annexin V-FITC apoptosis detection kit
according to the manufacturer's protocol. Briefly, the A2780 cells
were washed and then incubated for 15 min at room temperature in
the dark in 200 µl 1X binding buffer containing 5 µl
Annexin V-FITC and 10 µl PI. The apoptotic rate was detected
by the BD Accuri C6 flow cytometer (BD Biosciences) and processed
using FlowJo software (version 7.0; Tree Star, Inc., Ashland, OR,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
All of the experiments were repeated at least three times.
Comparisons among values for all groups were performed using a
one-way analysis of variance with Dunnett's test for comparison of
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
NC inhibits the proliferation of ovarian
cancer cells
An MTT assay was performed to determine the effect
of NC on ovarian cancer cell proliferation. As presented in
Fig. 1B, NC significantly reduced
the proliferation of ovarian cancer cells in a time- and
dose-dependent manner compared with the control group (P<0.01).
To verify the aforementioned findings, a 3H-thymidine
incorporation assay was used to determine the importance of NC on
the proliferation of ovarian cancer cells. As presented in Fig. 1C, NC (2.5, 5, 10 and 20 µM)
significantly suppressed the proliferation of ovarian cancer cells
compared with the control (P=0.038, P=0.0071, P=0.0062, P=0.0050,
respectively). The 10 and 20 µM NC concentrations were
selected for the subsequent experiments. These data suggested that
NC effectively suppressed the proliferation of ovarian cancer
cells.
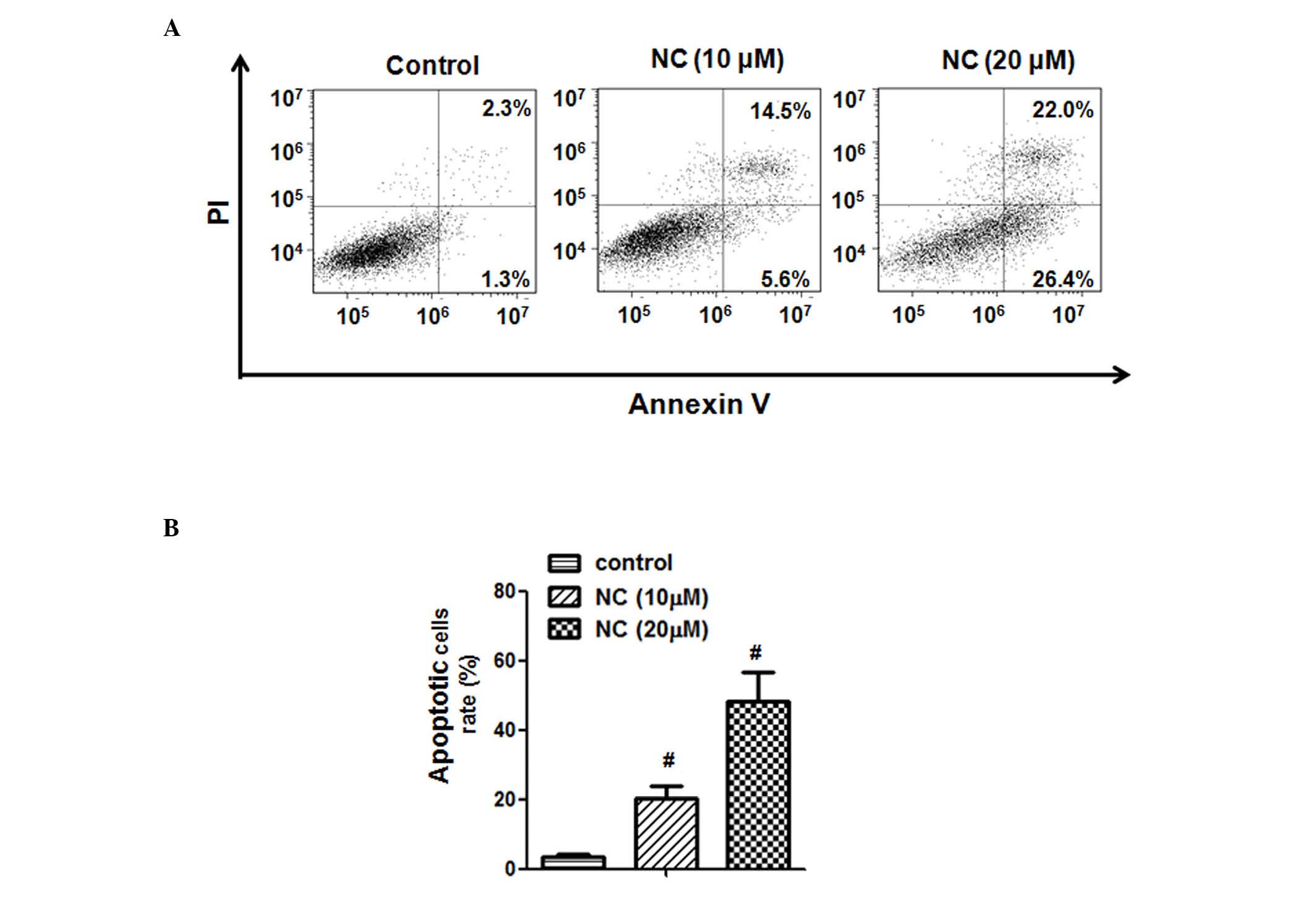
NC induces apoptosis in ovarian cancer
cells
To confirm that the inhibitory effect of NC on the
proliferation of A2780 ovarian cancer cells was due to the direct
effect on apoptosis, the A2780 cells were treated with NC (0, 10
and 20 µM) for 24 h. As presented in Fig. 2 it was determined that 10 and 20
µM NC significantly promoted the apoptosis of ovarian cancer
cells compared with the control group (P=0.0035 and P=0.0010,
respectively). The apoptotic rate of ovarian cancer cells increased
from 2.3±1.3 (control) to 14.5±5.6 and 22.0±26.4% following
treatment with 10 and 20 µM, respectively, for 24 h. These
findings demonstrated the anti-tumor effect of NC on ovarian cancer
cells.
NC induces the apoptosis of ovarian
cancer cells via altered expression levels of apoptosis-associated
proteins
In order to determine the underlying mechanism of
ovarian cancer cell apoptosis induced by NC, the protein expression
levels of Bax, Bcl-2, p53, caspase-3 and -9 were determined.
Western blot analysis demonstrated that, following treatment with
NC (10 and 20 µM), the expression levels of pro-apoptotic
protein Bax were significantly upregulated (P=0.0074 and P=0.0058,
respectively; Fig. 3) and the
expression levels of the anti-apoptotic protein, Bcl-2 were
significantly downregulated compared with the control group
(P=0.0062 and P=0.0044, respectively; Fig. 3). Simultaneously, the protein
expression levels of p53 (10 µM NC, P=0.031; 20 µM
NC, P=0.0040), caspase-3 (10 µM NC, P=0.0054; 20 µM
NC, P=0.0057) and −9 (10 µM NC, P=0.0055; 20 µM NC,
P=0.0054) were also significantly upregulated compared with the
control group (Fig. 3).
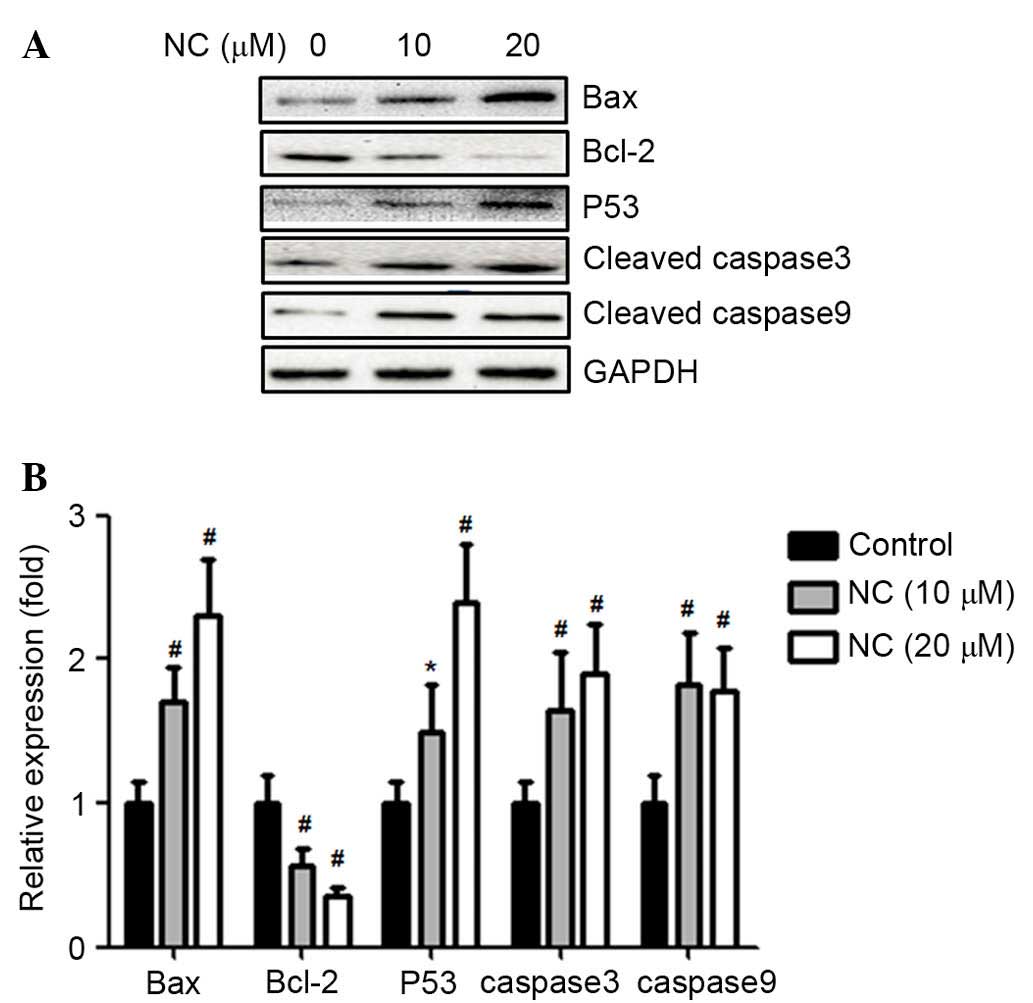 | Figure 3NC reduced the expression of Bcl-2 and
increased the expression of Bax, Bcl-2, p53, caspase-3 and -9 in
A2780 ovarian cancer cells. (A) A2780 cells were treated with NC
(0, 10 and 20 µM) for 24 h. The expression levels of Bax,
Bcl-2, p53, cleaved caspase-3 and -9 in cell lysates were detected
using western blot analysis. (B) Statistical analysis of the
western blot results. *P<0.05, #P<0.01
vs. control group. Data are presented as the mean ± standard
deviation from three independent experiments. NC, nitidine
chloride; Bax, Bcl-2-associated X protein; Bcl-2, B-cell
CLL/lymphoma 2. |
NC inhibits Akt phosphorylation in
ovarian cancer cells
The Akt signaling pathway is important for tumor
progression. To determine the molecular mechanism involved in the
effect of NC on the proliferation and apoptosis of ovarian cancer
cells, the Akt signaling pathway was examined. As presented in
Fig. 4A and B, Akt phosphorylation
was significantly downregulated by treatment with NC (10 and 20
µM; P=0.0041 and P=0.0045, respectively) for 24 h, compared
with the control group (P<0.01). This indicated that the Akt
signaling pathway may be involved in the viability and apoptosis of
ovarian cancer cells.
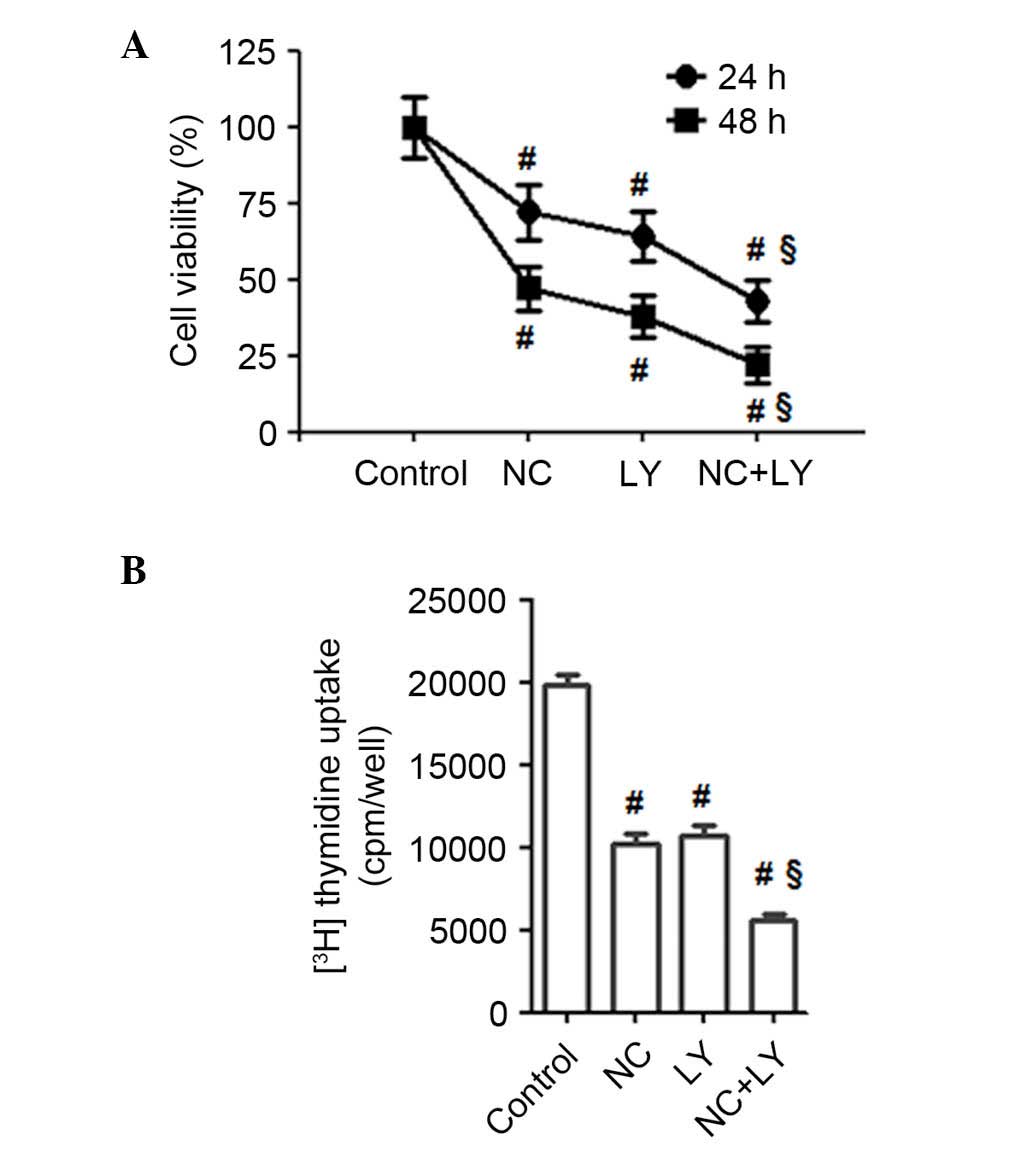
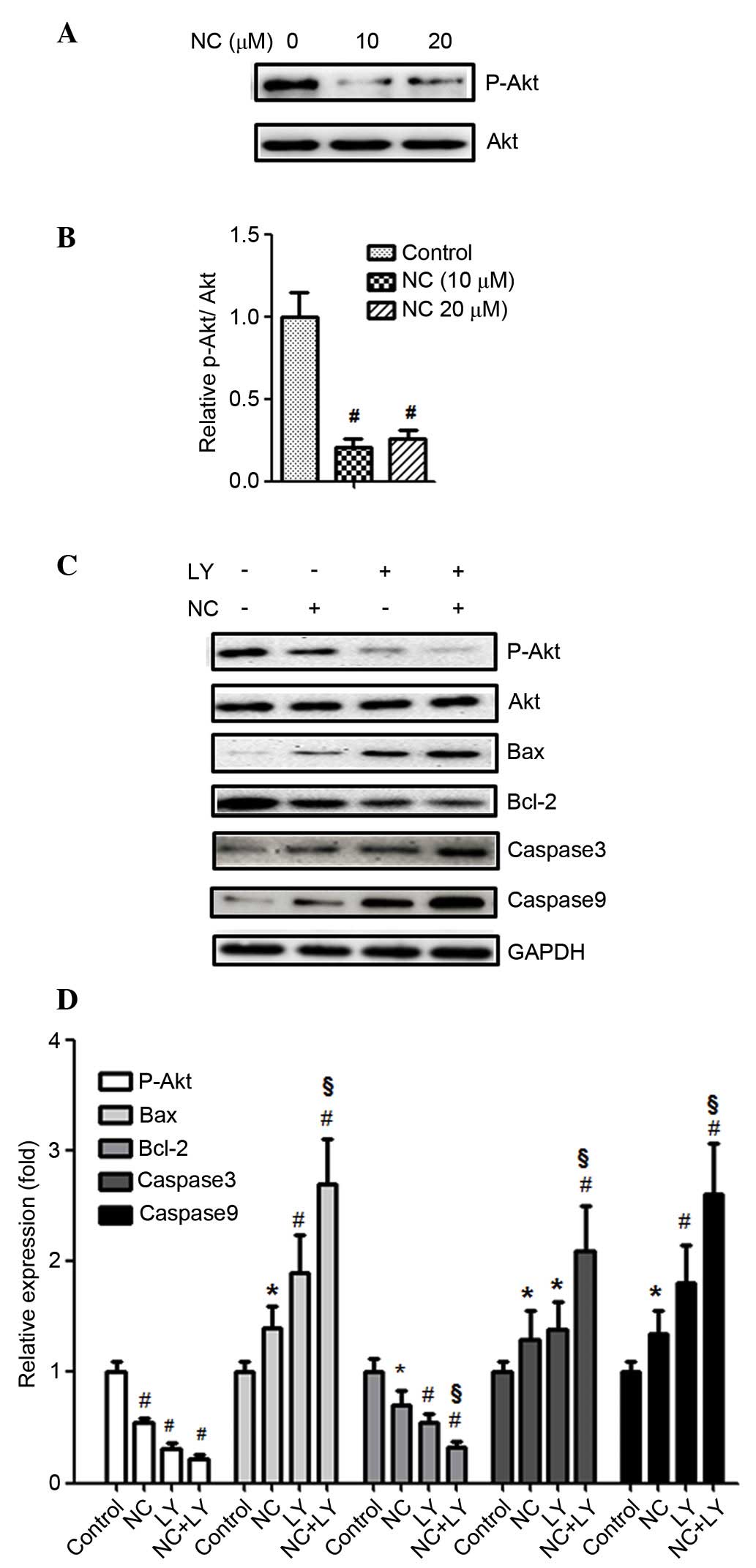 | Figure 4NC inhibited Akt phosphorylation and
the pro-apoptotic effect on A2780 cells was Akt-dependent. (A)
A2780 cells were treated with NC (0, 10 and 20 µM) for 24 h
and the levels of p-Akt, Akt were analyzed by western blotting. (B)
Statistical analysis of the western blotting results.
#P<0.01 vs. control group. (C) A2780 cells were
pretreated with the Akt inhibitor LY (50 µM) for 30 min
followed by incubation with or without NC (20 µM) for 24 h.
The expression levels of p-Akt, Akt, Bax, Bcl-2, caspase-3 and -9
were detected by western blotting with GAPDH as an internal
control. (D) Statistical analysis of the western blotting results.
*P<0.05, #P<0.01 vs. control group,
§P<0.01 vs. NC-treated group. Data are presented as
the mean ± standard deviation from three independent experiments.
p-Akt, phosphorylated-AKT serine/threonine kinase 1; NC, nitidine
chloride; Bax, Bcl-2-associated X protein; Bcl-2, B-cell
CLL/lymphoma 2; LY, LY294002. |
Suppression of the Akt signaling pathway
enhanced the pro-apoptotic and anti-proliferative effect of NC on
ovarian cancer cells
LY294002, a PI3K inhibitor, was used to stop Akt
phosphorylation. As presented in Fig.
4C and D, the inhibition of Akt phosphorylation with LY294002
significantly enhanced the NC-induced downregulation of the Bcl-2
expression levels compared with NC treatment only (P=0.0061),
whereas the expression levels of Bax, caspase-3 and -9 were
significantly upregulated by LY294002 + NC treatment compared with
20 µM NC treatment alone (P=0.0033, P=0.0041 and P=0.0052).
These findings indicated that NC-induced apoptosis may be
PI3K/Akt-dependent. Furthermore, the effect of NC on the
proliferation of ovarian cancer cells and the PI3K/Akt signaling
pathway was determined using an MTT assay (Fig. 5A) and 3H-thymidine
incorporation assay (Fig. 5B). As
presented in Fig. 5A, the PI3K/Akt
inhibitor LY294002, significantly enhanced the NC-induced
suppression of the proliferation of ovarian cancer cells compared
with the control and the NC-only treated group (P=0.0057, 24 h;
P=0.0051, 48 h). As demonstrated in Fig. 5B, LY294002 enhanced
3H-thymidine incorporation compared with NC alone
(P=0.0066). Therefore, blocking Akt activation may significantly
enhance the NC-induced suppression of the proliferation of ovarian
cancer cells.
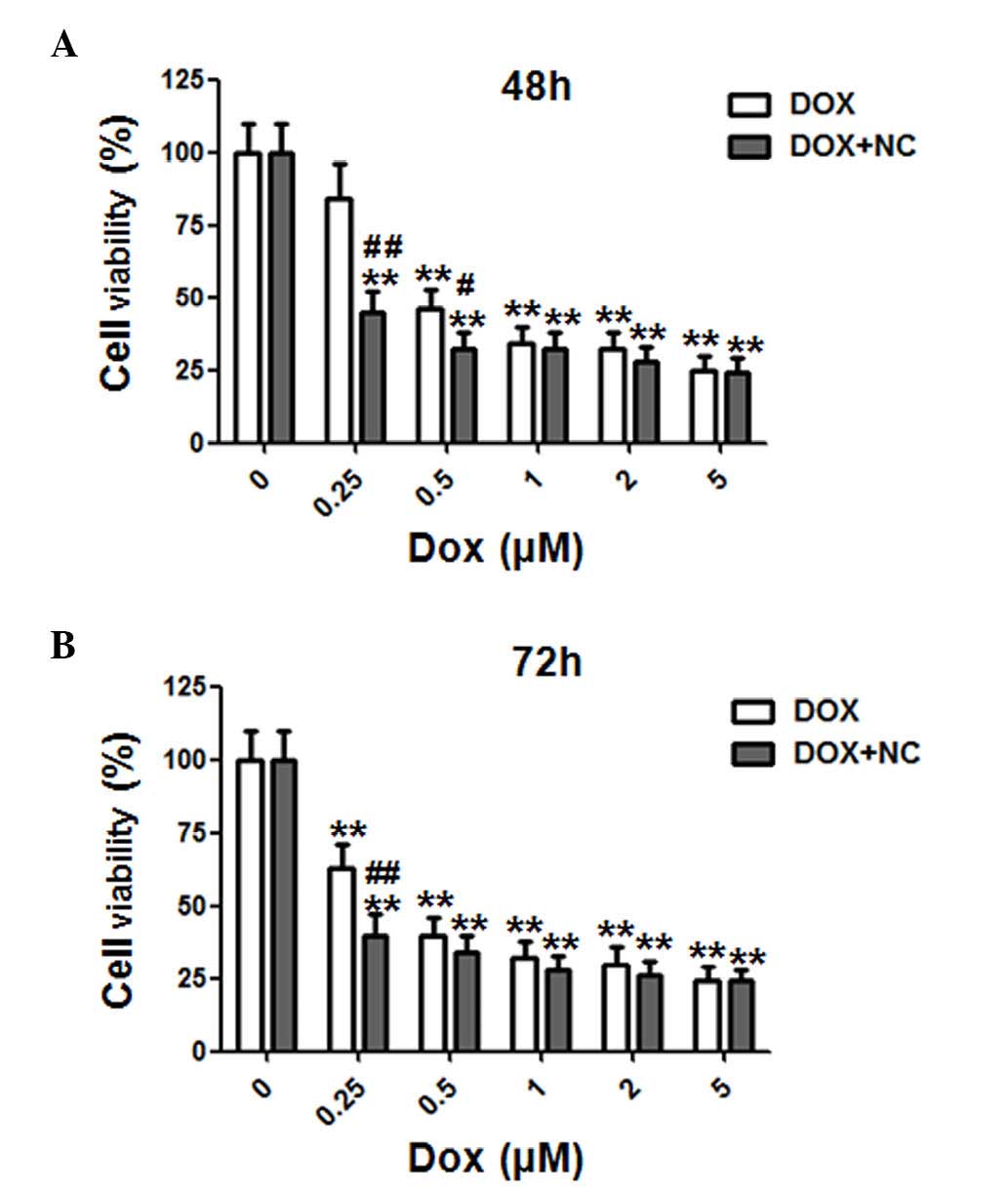
NC exhibits a synergistic effect with DOX
on limiting cell viability of ovarian cancer cells
A previous study on anti-tumor agents focused on
limiting drug resistance of traditional chemotherapeutic agents by
combining with novel anti-tumor drugs to achieve optimal curative
effects (14). In order to
investigate the synergistic effect of NC and DOX on A2780 ovarian
cancer cells, the cells were treated with various doses of DOX (0,
0.25, 0.5, 1, 2, 5 µM) and NC (2 µM) for 48 and 72 h
(Fig. 6A and B). The cell
viability was examined by MTT assays. DOX alone or in combination
with NC (2 µM) inhibited the viability of ovarian cancer
cells in a time- and dose-dependent manner. Notably, the viability
of A2780 cells was decreased from 84.2±11.2 to 43.5±7.1 (P=0.0063),
and 45.7±6.8 to 31.8±5.3% (P=0.034), respectively, when treated
with 0.25 and 0.5 µM DOX, in combination with NC (2
µM) at 48 h. For the 72 h treatment, the viability of A2780
cells decreased from 55.9±9.2 to 44.6±6.1% (P=0.0083) when treated
with 0.25 µM DOX in combination with NC (2 µM). These
findings indicated that the combination of DOX and NC exerted a
synergistic inhibitory effect on the viability of the ovarian
cancer cells.
Discussion
Uncontrolled proliferation and resistance to
apoptosis of tumor cells is considered to contribute to mortality
in patients with ovarian cancer (15,16).
Conventional chemotherapeutic agents have limited long-term
application due to their side effects. Therefore, understanding the
molecular mechanisms of proliferation and apoptosis, and developing
effective approaches to inhibit proliferation and induce apoptosis
of cancer cells are the crucial for cancer research. Therefore,
investigations of the therapeutic features of natural products in
tumor therapy are frequently performed (17,18).
As a bioactive phytochemical alkaloid extracted from Zanthoxylum
nitidum (Roxb), NC exhibits multiple pharmacological effects on
oxidation and inflammation (7,8).
Previous studies have demonstrated the capacity of NC to induce
apoptosis and inhibit of proliferation, migration and invasion in
breast, renal, hepatocellular carcinoma and gastric cancer cells
(9–14). However, the effect of NC on ovarian
cancer apoptosis and proliferation had not been fully elucidated
and the molecular mechanisms were unclear. To the best of our
knowledge, the current study was the first to demonstrate that NC
may effectively suppress the viability of ovarian cancer cells and
induce their apoptosis. The mechanism of proliferation inhibition
by NC was due to downregulation of the Akt signaling pathway.
Therefore, a novel molecular mechanism that allows NC to exhibit
the anti-tumor activity in ovarian cancer cells was identified.
Cell apoptosis is regulated by various factors,
including Bcl-2 protein family members and caspases (19). The Bcl-2 protein family members,
including the anti-apoptotic proteins (Bcl-2, Bcl-2-like 1,
Bcl-2-like 2 and myeloid cell leukemia 1) and pro-apoptotic
proteins [Bax and Bcl-2-associated agonist of cell death (Bad)] are
important for apoptosis and act via caspases (19,20).
Previous studies have determined that in the process of apoptosis,
the release of cytochrome C was regulated by Bax mitochondria
translocation and oligomerization, which in turn activated
caspase-3 and -9 (21,22). Subsequently, cell apoptosis is
triggered by the inhibition of Bcl-2 and Bax oligomerization. Thus,
Bcl-2 was understood to stabilize the mitochondrial membrane by
blocking internal calcium release into the cytoplasm (23,24).
A previous study demonstrated that the tumor suppressor, p53, was
associated with several Bcl-2 protein family members involved in
cell apoptosis (25). Previous
studies have determined that NC inhibited the proliferation of
renal and breast cancer cells by inducing apoptosis, and regulating
the expression levels of Bcl-2 and Bax (9,12).
The present study demonstrated that NC inhibited the proliferation
of ovarian cancer cells and induced their apoptosis in a time- and
dose-dependent manner. The expression levels of
apoptosis-associated proteins (Bax and Bcl-2) in ovarian cancer
cells, treated with NC were also investigated. It was determined
that NC altered the expression level ratio of Bax to Bcl-2.
Additionally, caspase-3 and -9 were activated by NC treatment,
which resulted in the apoptosis of ovarian cancer cells. The
current study also examined the effect of NC on the expression
levels of p53 in ovarian cancer cells and demonstrated the
upregulation of p53 in a dose-dependent manner. These results
indicated that NC-induced apoptosis of ovarian cancer cells may be
a result of the imbalance between Bcl-2 and Bax expression levels,
and the activation of caspase-3 and -9. Additionally, p53 was also
demonstrated to be involved in NC-induced apoptosis of ovarian
cancer cells.
Akt has been previously established as an important
regulator of cell proliferation and survival (26). A previous study demonstrated that
Akt mediated cell survival and apoptosis via its downstream
targets, including Bad and caspase-9 (27). Another previous study indicated
that Akt affected the regulation of Bax activity (28). The present study determined that
the expression of p-Akt was decreased when cells were treated with
NC. To determine whether the Akt signaling pathway was involved in
NC-induced apoptosis of ovarian cancer cells and inhibited their
proliferation, Akt phosphorylation was blocked by using the
PI3K/Akt inhibitor, LY294002. It was demonstrated that the
inhibition of Akt activity by LY294002 enhanced the apoptotic rate
induced by NC, which was determined by western blot analysis and
MTT assays. These results suggested that NC-induced apoptosis of
ovarian cancer cells by inhibition of Akt phosphorylation and
altering the expression levels of the Bcl-2 protein family members.
The PI3K/Akt inhibitor (LY294002) enhancing the apoptotic rate
induced by NC also suggested that Akt was located upstream of Bcl-2
and Bax.
To reduce the resistance to therapeutic agents,
combination therapy is frequently used in cancer treatment
(29). The anticancer agent DOX is
highly effective for the treatment multiple types of cancer.
However, there are problems with side effects, including acute and
chronic cardiotoxicity, and neutropenia, which significantly limit
its chemotherapeutic usage (30).
Thus, it is important to develop combination therapies in order to
decrease the side effects of chemotherapy agents. The present study
determined the synergistic effect of NC and DOX in ovarian cancer
cells. NC exerted a synergistic inhibitory effect on the viability
of ovarian cancer cells and enhanced the anti-tumor effect when
administered in conjunction with a low dose of DOX. This indicates
that a combination of NC and DOX at certain concentrations may
reduce its side effects. The precise mechanisms by which NC
improves chemotherapeutic efficacy require further
investigation.
In conclusion, to the best of our knowledge, the
present study was the first to demonstrate that NC suppressed the
proliferation of ovarian cancer cells and induced their apoptosis.
Additionally, the effect of NC was demonstrated to be mediated by
the Akt signaling pathway. NC exhibited a synergistic effect with
DOX in ovarian cancer cells. Therefore, it was determined that NC
is a promising agent for ovarian cancer therapy and additional
in vivo studies are required to confirm its efficacy.
Acknowledgments
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no.
ZR2014HP005).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen M, Pierredon S, Wuillemin C, Delie F
and Petignat P: Acellular fraction of ovarian cancer ascites induce
apoptosis by activating JNK and inducing BRCA1, Fas and FasL
expression in ovarian cancer cells. Oncoscience. 1:262–271. 2014.
View Article : Google Scholar
|
|
4
|
Sun ZL, Tang YJ, Wu WG, Xing J, He YF, Xin
DM, Yu YL, Yang Y and Han P: AZD1480 can inhibit the biological
behavior of ovarian cancer SKOV3 cells in vitro. Asian Pac J Cancer
Prev. 14:4823–4827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaughan S, Coward JI, Bast RC Jr, Berchuck
A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R,
Etemadmoghadam D, et al: Rethinking ovarian cancer: Recommendations
for improving outcomes. Nat Reviews Cancer. 11:719–725. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Jiang W, Zhang Z, Qian M and Du B:
Nitidine chloride inhibits LPS-induced inflammatory cytokines
production via MAPK and NFkappab pathway in raw 264.7 cells. J
Ethnopharmacol. 144:145–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Del Poeta M, Chen SF, Von Hoff D, Dykstra
CC, Wani MC, Manikumar G, Heitman J, Wall ME and Perfect JR:
Comparison of in vitro activities of camptothecin and nitidine
derivatives against fungal and cancer cells. Antimicrob Agents
Chemother. 43:2862–2868. 1999.PubMed/NCBI
|
|
9
|
Fang Z, Tang Y, Jiao W, Xing Z, Guo Z,
Wang W, Xu Z and Liu Z: Nitidine chloride induces apoptosis and
inhibits tumor cell proliferation via suppressing ERK signaling
pathway in renal cancer. Food Chem Toxicol. 66:210–216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Wang J, Lin L, He L, Wu Y, Zhang
L, Yi Z, Chen Y, Pang X and Liu M: Inhibition of STAT3 signaling
pathway by nitidine chloride suppressed the angiogenesis and growth
of human gastric cancer. Mol Cancer Ther. 11:277–287. 2012.
View Article : Google Scholar
|
|
11
|
Liao J, Xu T, Zheng JX, Lin JM, Cai QY, Yu
DB and Peng J: Nitidine chloride inhibits hepatocellular carcinoma
cell growth in vivo through the suppression of the JAK1/STAT3
signaling pathway. Int J Mol Med. 32:79–84. 2013.PubMed/NCBI
|
|
12
|
Sun M, Zhang N, Wang X, Cai C, Cun J, Li
Y, Lv S and Yang Q: Nitidine chloride induces apoptosis, cell cycle
arrest and synergistic cytotoxicity with DOX in breast cancer
cells. Tumour Biol. 35:10201–10212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang Z, Tang Y, Jiao W, Xing Z, Guo Z,
Wang W, Shi B, Xu Z and Liu Z: Nitidine chloride inhibits renal
cancer cell metastasis via suppressing AKT signaling pathway. Food
Chem Toxicol. 60:246–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan X, Han H, Wang L, Yang L, Li R, Li Z,
Liu J, Zhao Q, Qian M, Liu M and Du B: Nitidine chloride inhibits
breast cancer cells migration and invasion by suppressing c-Src/FAK
associated signaling pathway. Cancer Lett. 313:181–191. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai Q, Lin J, Wei L, Zhang L, Wang L, Zhan
Y, Zeng J, Xu W, Shen A, Hong Z and Peng J: Hedyotis diffusa willd
inhibits colorectal cancer growth in vivo via inhibition of STAT3
signaling pathway. Int J Mol Sci. 13:6117–6128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomasset SC, Berry DP, Garcea G, Marczylo
T, Steward WP and Gescher AJ: Dietary polyphenolic
phytochemicals-promising cancer chemopreventive agents in humans? A
review of their clinical properties. Int J Cancer. 120:451–458.
2007. View Article : Google Scholar
|
|
19
|
Zhang Y, Zhuang Z, Meng Q, Jiao Y, Xu J
and Fan S: Polydatin inhibits growth of lung cancer cells by
inducing apoptosis and causing cell cycle arrest. Oncology lett.
7:295–301. 2014.
|
|
20
|
Reed JC: Bcl-2: Prevention of apoptosis as
a mechanism of drug resistance. Hematol Oncol Clin North Am.
9:451–473. 1995.PubMed/NCBI
|
|
21
|
Antonsson B: Bax and other pro-apoptotic
Bcl-2 family 'killer-proteins' and their victim the mitochondrion.
Cell Tissue Res. 306:347–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Crompton M: Bax, Bid and the
permeabilization of the mitochondrial outer membrane in apoptosis.
Curr Opin Cell Biol. 12:414–419. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baffy G, Miyashita T, Williamson JR and
Reed JC: Apoptosis induced by withdrawal of interleukin-3 (IL-3)
from an IL-3-dependent hematopoietic cell line is associated with
repartitioning of intracellular calcium and is blocked by enforced
Bcl-2 oncoprotein production. J Biol Chem. 268:6511–6519.
1993.PubMed/NCBI
|
|
24
|
Precht TA, Phelps RA, Linseman DA, Butts
BD, Le SS, Laessig TA, Bouchard RJ and Heidenreich KA: The
permeability transition pore triggers Bax translocation to
mitochondria during neuronal apoptosis. Cell Death Differ.
12:255–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu Q: Restoring p53-mediated apoptosis in
cancer cells: New opportunities for cancer therapy. Drug Resist
Updat. 9:19–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Somanath PR, Vijai J, Kichina JV, Byzova T
and Kandel ES: The role of PAK-1 in activation of MAP kinase
cascade and oncogenic transformation by Akt. Oncogene.
28:2365–2369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gardai SJ, Hildeman DA, Frankel SK,
Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL and
Henson PM: Phosphorylation of Bax Ser184 by Akt regulates its
activity and apoptosis in neutrophils. J Biol Chem.
279:21085–21095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan KH, Blanco-Codesido M and Molife LR:
Cancer therapeutics: Targeting the apoptotic pathway. Crit Rev
Oncol Hematol. 90:200–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Octavia Y, Tocchetti CG, Gabrielson KL,
Janssens S, Crijns HJ and Moens AL: DOX-induced cardiomyopathy:
From molecular mechanisms to therapeutic strategies. J Mol Cell
Cardiol. 52:1213–1225. 2012. View Article : Google Scholar : PubMed/NCBI
|