1. Introduction
The most common debilitating disorders affecting
society at large are pain and depression, which are the most
prevalent among neurological and psychiatric disorders. Depression
and pain co-exist in almost 80% of patients (1) and are associated with impaired
health-related quality of life, often contributing to high
mortality (2,3). It has been observed that patients
suffering from inflammatory and neuropathic pain are almost 5 times
more prone to develop depression or anxiety disorder as compared to
the general population (4–6). However, the majority of patients who
suffer from comorbid depression and pain are not responsive to
pharmacological treatments that address the pain or depression,
making this comorbidity disorder a heavy burden on patients and
society (7). These clinical
observations on the association of pain and depression have been
confirmed in several animal models of depression and chronic pain
based on genetics, stress, lesion, and pharmacological manipulation
that show altered nociceptive response (8,9).
Considering the significance of the complex interaction between
pain and depression and its societal impact, a better understanding
of the molecular basis for this association is needed for
developing more effective therapeutics.
In ancient times, this depression-pain comorbidity
was treated through the use of extracts of the Cannabis
sativa plant, commonly known now as marijuana. Use of marijuana
for addressing pain due to various reasons has become a hot topic
in terms of possible addiction, drug abuse as well as regulatory
issues. Although historically, the use of marijuana dates back to
over 2000 BC, the biological action of the main psychoactive
ingredient of marijuana, Δ9-tetrahydrocannabinol
(Δ9-THC) has only recently been identified. The
biological receptor of Δ9-THC on the cell surface has
recently been identified and described (10,11).
Characterization of this receptor led to understanding of the mode
of action of Δ9-THC that underlies its wide spectrum of
pharmacological effects, which encompass euphoria, calmness,
appetite stimulation, sensory alterations and analgesia (10,11).
Identification of the first endogenous cannabinoid-like substance,
anandamide, in pig brain reiterated the significance of the
so-called cannabinoid receptor and its endogenous ligands in the
control of a wide variety of biological activities (12). The name 'anandamide', derived from
Sanskrit ('ananda' meaning bliss) is given to
N-arachidonoylethanolamine, for its cannabinomimetic
effects. Another endogenous cannabinomimetic compound known as
2-arachidonoylglycerol (2-AG) was identified (13,14).
Of note, the two endocannabinoids were derivatives of arachidonic
acid. Considering that these compounds are cannabinomimetic and
endogenous, acting on the cannabinoid receptors, they are known as
endocannabinoids.
2. The endocannabinoid system
Besides anandamide and 2-AG, there are other
endogenously produced molecules that also likely influence the
function of CB receptors. These molecules include oleamide
(15), O-arachidonoyl
ethanolamine, also termed virodhamine (16), 2-AG ether or noladin ether
(17), and
N-arachidonoyldopamine (18). However, their physiological role is
not clear and thus whether they are true endocannabinoids has yet
to be ascertained. In addition to Δ9-THC, almost 80
other phytocannabinoids are found in the cannabis extracts, with a
structure similar to that of THC. Of these, THC is the most studied
and was shown to activate cannabinoid receptor type 1 (CB1) and CB2
and affect many pathophysiological processes, including
anti-nociception (19). However,
because of its CB1-mediated unwanted CNS effects, the clinical
utility of THC is limited (19).
Subsequent studies revealed that another phytocannabinoid,
cannabidiol, with very low affinity to bind to CB1 and CB2
receptors, exerts positive pharmacological effects, such as
anti-anxiety, anti-epileptic, anti-bacterial, anti-inflammatory,
anticancer and also anti-diabetic properties without any
psychoactivity (20). Nabiximols,
a cannabis extract containing THC and cannabidiol at a 1:1 ratio,
has been approved for the treatment of neuropathic pain, spasticity
associated with multiple sclerosis and intractable cancer pain
(21). In addition to the natural
cannabinoids, synthetic cannabinoids, such as dronabinol, and its
analogue nabilone, have been developed to address various types of
pain. For instance, dronabinol and nabilone are currently used for
chemotherapy-associated emesis in Canada and USA and nabilone is
indicated for anorexia associated with AIDS-related weight loss
(22). In addition, findings of a
clinical trial showed the efficacy of nabilone in diabetic
neuropathy (23). Another
synthetic drug, an antagonist/inverse agonist of CB1 receptor,
rimonabant, initially approved for obesity and smoking cessation,
was found to have depressive effects and was subsequently
withdrawn.
Biosynthesis of endocannabinoids
Endocannabinoids are lipophilic molecules
synthesized 'on demand' from membrane phospholipids, and released
immediately, without storage in vesicles. Anandamide and 2-AG are
produced at post-synaptic neurons. Anandamide is produced in a
two-step process involving N-arachidonoylation of the
membrane phospholipid, phosphatidylethanolamine, to form
N-arachidonoyl phosphatidylethanolamine (NAPE) by a
calcium-dependent N-acyltransferase, followed by hydrolysis
by a NAPE-selective phospholipase D (NAPE-PLD) to form
N-arachidonoylethanolamine (anandamide) (24,25).
Anandamide levels are regulated by its breakdown through the action
of fatty acid amide hydrolase (FAAH) (26). 2-AG is synthesized in a two-step
process, in which diacylglycerol (DAG) is first produced by the PLC
from inositol phospholipids, followed by the hydrolysis of DAG to
2-AG by plasma membrane-associated sn1-DAG lipase (DAGL) (14). Once formed, 2-AG levels are
regulated by monoacylglycerol lipase (MAGL), which accounts for
~85% of the hydrolysis and by α/β hydrolase domain containing 6
(ABHD6) and ABHD12, which also hydrolyze 2-AG to arachidonic acid
and glycerol (27). In addition to
hydrolysis, 2-AG is acted on by cyclooxygenase-2 (28) and lipoxygenase (29), to form prostaglandin glyceryl
esters and other related bioactive compounds (Fig. 1).
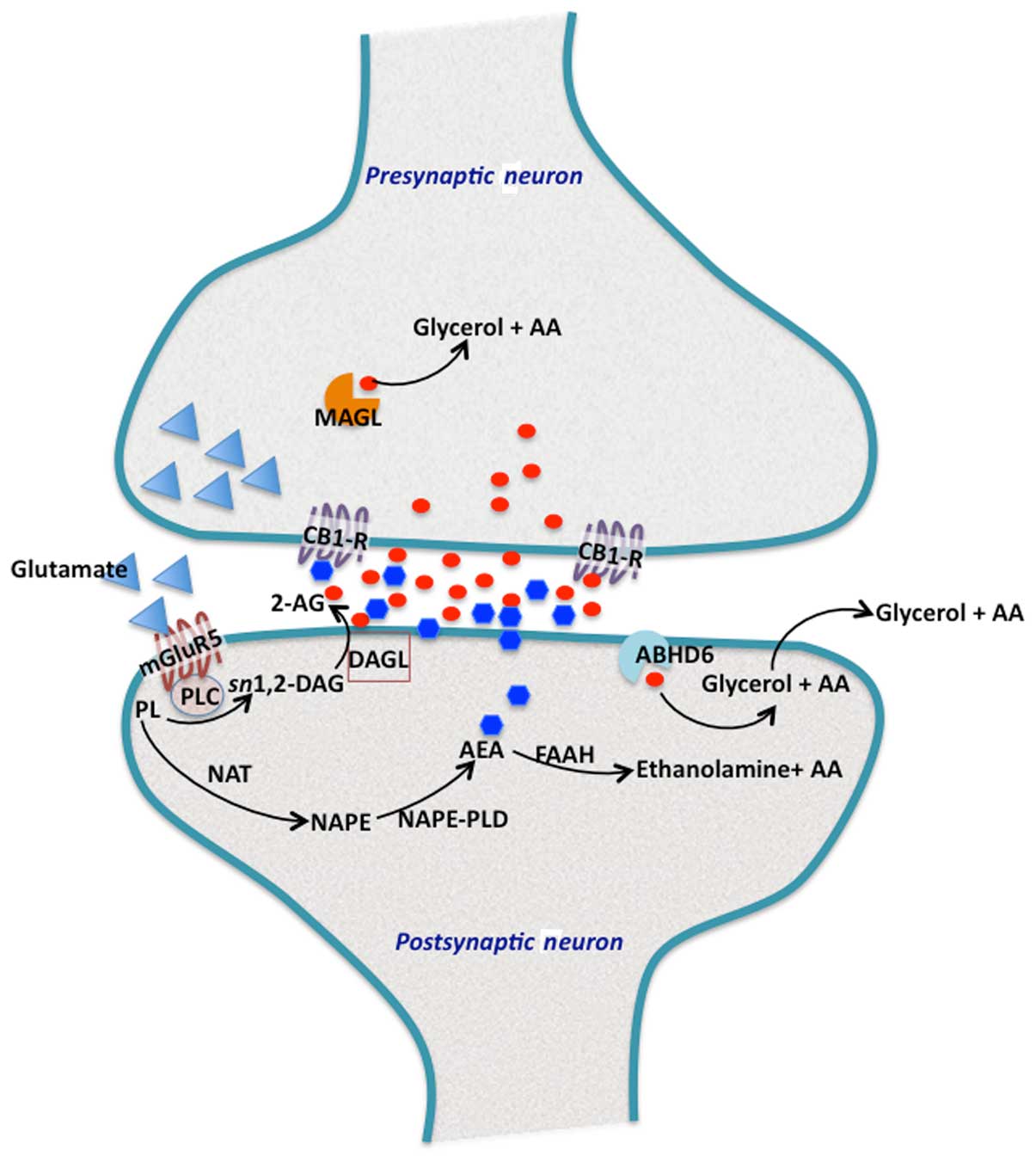 | Figure 1Endocannabinoid biosynthesis and
signalling at synapse. In the perisynaptic zone of the dendritic
spine, the three main proteins involved in 2-arachidonoylglycerol
(2-AG) production are located in the postsynaptic neurons (28,29).
Activation of mGluR5 metabotropic glutamate receptors, leads to the
hydrolysis of membrane phosphatidylinositols (PL) by phospholipase
C (PLC)-β to form sn1,2-diacylglycerol (sn1,2-DAG), which contains
arachidonic acid at position-2. The sn1,2-DAG is then hydrolyzed by
plasma membrane bound to DAG lipase-α (DAGL), to generate 2-AG. The
concerted action of these protein components located proximal to
each other on the postsynaptic membrane, allows for the rapid
accumulation of 2-AG. 2-AG then enters the the synaptic cleft to
activate cannabinoid receptor type 1 (CB1), present on the
presynaptic axon terminals. 2-AG that reaches into presynaptic
terminals, is hydrolyzed by monoacylglycerol lipase (MAGL). Excess
2-AG in the postsynaptic terminals is degraded by α/β hydrolase
domain containing 6 (ABHD6), which is a MAG hydrolase. By contrast,
arachidonoylethanolamine or anandamide (AEA) is also produced in
the postsynaptic terminals by the action of
N-acyltransferase, which synthesizes N-arachidonoyl
phosphatidylethanolamine (NAPE). NAPE is further hydrolyzed by a
specific PLD (NAPE-PLD) to generate AEA. AEA also traverses the
postsynaptic membrane and reaches the CB1 receptors at the
presynaptic axon terminals. Most of the excess and unused AEA is
rapidly eliminated in postsynaptic terminals by fatty acid amide
hydrolase (FAAH). |
Cannabinoid receptors
Two subtypes of cannabinoid receptors, CB1 and CB2,
have been cloned and characterized (11,30).
CB1 receptors are most abundant in the central nervous system
(CNS), whereas CB2 receptors are present mostly in peripheral
tissues with immune functions, and most densely in the spleen
(31). In the CNS, CB1 receptors
are distributed densely in motor and limbic regions, in areas
involved in pain transmission and modulation (e.g., periaqueductal
grey, rostral ventromedial medulla, and spinal cord dorsal horn),
as well as in the periphery (32).
In the synapses, CB1 receptors show pre-synaptic localization on
axons and terminals of neurons. The CB1 and CB2 receptors are
G-protein coupled receptors of Gi/Go subtype, and mediate the
inhibition of neurotransmitter release. Once released,
endocannabinoids bind to CB1 receptors located in the presynaptic
membrane. These CB receptors inhibit adenylate cyclase. Only CB1
receptor activation, but not that of CB2 receptors, causes blockage
of voltage-dependent N- and P/Q-type calcium channels through the
activation of potassium channels and mitogen-activated protein
kinase. Although CB2 receptors are mostly localized in immune cells
and peripheral tissues, their presence has been observed in some
subsets of neurons in brain and thus these receptors likely
participate in the modulation of neurotransmission (33). Endocannabinoids also bind to other
receptors including transient receptor potential vanilloid 1,
peroxisome proliferator-activated receptors, GPR55, and GPR119
(34–36) and this non-CB1/2 receptor activity
of endocannabinoids accounts for the differential effects of
certain cannabinoid agonists and pharmacological modulators of
endocannabinoid tone.
Following activation of their receptors,
endocannabinoids are removed from the synaptic
junction/extracellular space by a process of cellular uptake and
then their hydrolysis. It has been suggested that the uptake of
anandamide is probably mediated via a specific 'endocannabinoid
membrane transporter', which is yet to be identified (37,38).
It is not clear how 2-AG uptake is mediated. Anandamide is
hydrolyzed in post-synaptic neurons by FAAH, thus terminating the
anandamide action at the time of its synthesis, whereas 2-AG is
hydrolyzed in pre-synaptic neurons by MAGL, following CB1 receptor
activation. Metabolism of anandamide by lipoxygenase and
cycloxygenase enzymes yields oxygenated products with activity on
non-cannabinoid targets (39).
3. Endocannabinoids in pain and
depression
Pain is an integrative experience that involves
physiological, emotional and cognitive aspects and this experience
varies among individuals. Laboratory animals, on which most of
basic pain research is conducted, cannot report pain and in
animals, pain is generally monitored by differentiating between the
subjective experience and nociception, the measurable neuronal
events underlying the pain (?). Nociceptive pathways are triggered
by the transduction of noxious stimuli, such as heat and mechanical
injury, into neuronal action potentials by sensory afferent
neurons, such as mechanoreceptors in the peripheral nervous system.
These action potentials travel through the axon of the primary
afferent neuron, and the cell body, to a synapse in the superficial
dorsal horn of the spinal cord (41). Inputs, from several cells types
within the spinal cord, are integrated and passed onto ascending
pathways to the brainstem, and subsequently to the thalamus. The
thalamus then transmits the signal to higher brain regions involved
in the sensory (e.g., the somatosensory cortex) and
emotional/affective (e.g., the amygdala and cingulate cortex)
aspects of pain. Due to the cross-talk between supra-spinal
nociceptive regions, incoming nociceptive signals can be either
enhanced or dampened by descending modulatory pathways projecting
from the brain to the spinal cord (40,41).
The endocannabinoid system is distributed throughout the spinal and
supraspinal regions, and thus is able to effectively regulate
neurophysiological activities, including affective and nociceptive
processing (42).
Clinical studies have shown altered endocannabinoid
signaling in patients with chronic pain (43,44)
as well as in psychiatric patients (45,46).
Certain genetic polymorphisms in CB1 and CB2 receptors have been
found to be associated with major depression and bipolar disorder
(47,48) and resistance to treatment was
observed in depression patients having a single nucleotide
polymorphism in the CB1 receptor (49). Elevated components of the
endocannabinoid system, including plasma 2-AG levels and CB1 and
CB2 mRNA levels were observed in the lymphocytes in osteoarthritic
patients, who also exhibited a positive correlation between 2-AG
levels, pain and depression (50).
However, whether these changes are compensatory to tackle the pain
in osteoarthritis patients, is not known. Additional studies are
necessary to better understand the association of endocannabinoid
system and pain and depression.
Although, to the best of our knowledge, relatively
few clinical studies have directly addressed the importance of
endocannabinoids in pain-depression interactions, improved muscle
and nerve pain by the intake of cannabis has been reported in HIV
patients, who exhibited improved symptoms of depression and anxiety
(51). In cancer patients, daily
adjunctive administration of Cesamet (nabilone, a Δ9-THC
analogue) for 30 days was found to improve overall anxiety and pain
(52). The therapeutic efficacy of
nabilone for pain management and quality of life improvement was
demonstrated in a randomized, double-blind, placebo-controlled
trial in patients with fibromyalgia (53) (Table
I). Similar results were obtained in studies using
Δ9-THC (dronabinol) in patients with chronic central
neuropathic pain or fibromyalgia (54). The above and other studies
(55–57) together indicate that
depression/anxiety and pain, when present together in a variety of
patients, respond to exogenously administered cannabinoids,
although the underlying mechanism remains to be elucidated. It has
been demonstrated that Δ9-THC-mediated reductions in
pain are associated with enhanced amygdala activity and reduced
functional connectivity between the amygdala and somatosensory
cortex (58). Thus, the amygdala
likely forms the common neural circuit and connecting link between
emotional responding and pain. The precise mechanism(s) by which
the endocannabinoids influence behavioral/emotional and nociceptive
processing remains to be determined. At present, there is
considerable evidence involving the endocannabinoid system in
eliciting potent effects on neurotransmission, neuroendocrine, and
inflammatory processes, which are all known to be deranged in
depression and chronic pain.
 | Table ICannabinoid-based therapies to treat
pain and depression. |
Table I
Cannabinoid-based therapies to treat
pain and depression.
| Condition | Cannabinoid-based
drug | Outcomes for
pain | Outcomes for
depression and anxiety |
|---|
| HIV | Marijuana | ↓Muscle, nerve
pain | ↓Anxiety |
| Cancer | Nabilone | ↓Pain score | ↓Overall
stress |
| Fibromyalgia | Nabilone | ↓Pain | ↓Anxiety |
| Offenders with
psychiatric disorders | Nabilone | ↓Pain | ↓Post-traumatic
stress disorder symptoms |
| Chronic central
neuropathic pain |
Δ9-THC | ↓Pain and pain
intensity | ↓Anxiety |
| Diabetic peripheral
neuropathy | Sativex
(Δ9-THC, cannabidiol) | ↓Pain | ↑Quality of
life |
4. Conclusions
Depression and pain co-exist in the majority of
patients and often contribute to high mortality. Most patients who
suffer from the comorbid depression and pain are not responsive to
pharmacological treatments that address either the pain or
depression, exacerbating this comorbidity disorder. Cannabinoids
present in marijuana are well-known to contain pain and depression,
and Δ9-THC, the active ingredient of marijuana, exerts
its activity by activating CB1 and CB2 receptors. These receptors
are activated by naturally present endocannabinoids, anandamide and
2-AG, which exert cannabinomimetic effects. The endocannabinoid
system is involved in eliciting potent effects on
neurotransmission, neuroendocrine, and inflammatory processes,
which are known to be deranged in depression and chronic pain.
Several synthetic cannabinomimetic drugs are being developed to
treat pain and depression. However, the precise mode of action of
endocannabinoids on different targets in the body and whether their
effects on pain and depression follow the same or different
pathways, remains to be determined in future studies.
References
|
1
|
Poole H, White S, Blake C, Murphy P and
Bramwell R: Depression in chronic pain patients: Prevalence and
measurement. Pain Pract. 9:173–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scholich SL, Hallner D, Wittenberg RH,
Hasenbring MI and Rusu AC: The relationship between pain,
disability, quality of life and cognitive-behavioural factors in
chronic back pain. Disabil Rehabil. 34:1993–2000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hassett AL, Aquino JK and Ilgen MA: The
risk of suicide mortality in chronic pain patients. Curr Pain
Headache Rep. 18:4362014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hawker GA, Gignac MA, Badley E, Davis AM,
French MR, Li Y, Perruccio AV, Power JD, Sale J and Lou W: A
longitudinal study to explain the pain-depression link in older
adults with osteoarthritis. Arthritis Care Res (Hoboken).
63:1382–1390. 2011. View Article : Google Scholar
|
|
5
|
Emery PC, Wilson KG and Kowal J: Major
depressive disorder and sleep disturbance in patients with chronic
pain. Pain Res Manag. 19:35–41. 2014. View Article : Google Scholar :
|
|
6
|
Lin MC, Guo HR, Lu MC, Livneh H, Lai NS
and Tsai TY: Increased risk of depression in patients with
rheumatoid arthritis: A seven-year population-based cohort study.
Clinics (Sao Paulo). 70:91–96. 2015. View Article : Google Scholar
|
|
7
|
Gameroff MJ and Olfson M: Major depressive
disorder, somatic pain, and health care costs in an urban primary
care practice. J Clin Psychiatry. 67:1232–1239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li JX: Pain and depression comorbidity: A
preclinical perspective. Behav Brain Res. 276:92–98. 2015.
View Article : Google Scholar
|
|
9
|
Yalcin I, Barthas F and Barrot M:
Emotional consequences of neuropathic pain: Insight from
preclinical studies. Neurosci Biobehav Rev. 47:154–164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Devane WA, Dysarz FA III, Johnson MR,
Melvin LS and Howlett AC: Determination and characterization of a
cannabinoid receptor in rat brain. Mol Pharmacol. 34:605–613.
1988.PubMed/NCBI
|
|
11
|
Matsuda LA, Lolait SJ, Brownstein MJ,
Young AC and Bonner TI: Structure of a cannabinoid receptor and
functional expression of the cloned cDNA. Nature. 346:561–564.
1990. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Devane WA, Hanus L, Breuer A, Pertwee RG,
Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A and
Mechoulam R: Isolation and structure of a brain constituent that
binds to the cannabinoid receptor. Science. 258:1946–1949. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mechoulam R, Ben-Shabat S, Hanus L,
Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR,
Compton DR, et al: Identification of an endogenous 2-monoglyceride,
present in canine gut, that binds to cannabinoid receptors. Biochem
Pharmacol. 50:83–90. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugiura T, Kondo S, Sukagawa A, Nakane S,
Shinoda A, Itoh K, Yamashita A and Waku K: 2-Arachidonoylglycerol:
A possible endogenous cannabinoid receptor ligand in brain. Biochem
Biophys Res Commun. 215:89–97. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leggett JD, Aspley S, Beckett SR, D'Antona
AM and Kendall DA and Kendall DA: Oleamide is a selective
endogenous agonist of rat and human CB1 cannabinoid receptors. Br J
Pharmacol. 141:253–262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Porter AC, Sauer JM, Knierman MD, Becker
GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB,
et al: Characterization of a novel endocannabinoid, virodhamine,
with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther.
301:1020–1024. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanus L, Abu-Lafi S, Fride E, Breuer A,
Vogel Z, Shalev DE, Kustanovich I and Mechoulam R: 2-arachidonyl
glyceryl ether, an endogenous agonist of the cannabinoid CB1
receptor. Proc Natl Acad Sci USA. 98:3662–3665. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang SM, Bisogno T, Trevisani M,
Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey
JF, Chu CJ, et al: An endogenous capsaicin-like substance with high
potency at recombinant and native vanilloid VR1 receptors. Proc
Natl Acad Sci USA. 99:8400–8405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pertwee RG: Targeting the endocannabinoid
system with cannabinoid receptor agonists: Pharmacological
strategies and therapeutic possibilities. Philos Trans R Soc Lond B
Biol Sci. 367:3353–3363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Starowicz K and Di Marzo V:
Non-psychotropic analgesic drugs from the endocannabinoid system:
'magic bullet' or 'multiple-target' strategies? Eur J Pharmacol.
716:41–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sastre-Garriga J, Vila C, Clissold S and
Montalban X: THC and CBD oromucosal spray (Sativex®) in
the management of spasticity associated with multiple sclerosis.
Expert Rev Neurother. 11:627–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang T, Collet JP, Shapiro S and Ware MA:
Adverse effects of medical cannabinoids: A systematic review. CMAJ.
178:1669–1678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toth C, Mawani S, Brady S, Chan C, Liu C,
Mehina E, Garven A, Bestard J and Korngut L: An enriched-enrolment,
randomized withdrawal, flexible-dose, double-blind,
placebo-controlled, parallel assignment efficacy study of nabilone
as adjuvant in the treatment of diabetic peripheral neuropathic
pain. Pain. 153:2073–2082. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Di Marzo V, Fontana A, Cadas H, Schinelli
S, Cimino G, Schwartz JC and Piomelli D: Formation and inactivation
of endogenous cannabinoid anandamide in central neurons. Nature.
372:686–691. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugiura T, Kondo S, Sukagawa A, Tonegawa
T, Nakane S, Yamashita A and Waku K: Enzymatic synthesis of
anandamide, an endogenous cannabinoid receptor ligand, through
N-acylphosphatidylethanolamine pathway in testis: Involvement of
Ca(2+)-dependent transacylase and phosphodiesterase activities.
Biochem Biophys Res Commun. 218:113–117. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cravatt BF, Giang DK, Mayfield SP, Boger
DL, Lerner RA and Gilula NB: Molecular characterization of an
enzyme that degrades neuromodulatory fatty-acid amides. Nature.
384:83–87. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blankman JL, Simon GM and Cravatt BF: A
comprehensive profile of brain enzymes that hydrolyze the
endocannabinoid 2-arachidonoylglycerol. Chem Biol. 14:1347–1356.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kozak KR, Rowlinson SW and Marnett LJ:
Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to
glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem.
275:33744–33749. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van der Stelt M, van Kuik JA, Bari M, van
Zadelhoff G, Leeflang BR, Veldink GA, Finazzi-Agrò A, Vliegenthart
JF and Maccarrone M: Oxygenated metabolites of anandamide and
2-arachidonoylglycerol: Conformational analysis and interaction
with cannabinoid receptors, membrane transporter, and fatty acid
amide hydrolase. J Med Chem. 45:3709–3720. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Munro S, Thomas KL and Abu-Shaar M:
Molecular characterization of a peripheral receptor for
cannabinoids. Nature. 365:61–65. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maldonado R, Valverde O and Berrendero F:
Involvement of the endocannabinoid system in drug addiction. Trends
Neurosci. 29:225–232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pertwee RG: Receptors and channels
targeted by synthetic cannabinoid receptor agonists and
antagonists. Curr Med Chem. 17:1360–1381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim J and Li Y: Chronic activation of CB2
cannabinoid receptors in the hippocampus increases excitatory
synaptic transmission. J Physiol. 593:871–886. 2015. View Article : Google Scholar :
|
|
34
|
Overton HA, Babbs AJ, Doel SM, Fyfe MC,
Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM,
Tang-Christensen M, et al: Deorphanization of a G protein-coupled
receptor for oleoylethanolamide and its use in the discovery of
small-molecule hypophagic agents. Cell Metab. 3:167–175. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun Y, Alexander SP, Kendall DA and
Bennett AJ: Cannabinoids and PPARalpha signalling. Biochem Soc
Trans. 34:1095–1097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ryberg E, Larsson N, Sjögren S, Hjorth S,
Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T and
Greasley PJ: The orphan receptor GPR55 is a novel cannabinoid
receptor. Br J Pharmacol. 152:1092–1101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fowler CJ: Anandamide uptake explained?
Trends Pharmacol Sci. 33:181–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jhaveri MD, Richardson D and Chapman V:
Endocannabinoid metabolism and uptake: Novel targets for
neuropathic and inflammatory pain. Br J Pharmacol. 152:624–632.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Woodward DF, Carling RW, Cornell CL, Fliri
HG, Martos JL, Pettit SN, Liang Y and Wang JW: The pharmacology and
therapeutic relevance of endocannabinoid derived cyclo-oxygenase
(COX)-2 products. Pharmacol Ther. 120:71–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Basbaum AI, Bautista DM, Scherrer G and
Julius D: Cellular and molecular mechanisms of pain. Cell.
139:267–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Todd AJ: Neuronal circuitry for pain
processing in the dorsal horn. Nat Rev Neurosci. 11:823–836. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fitzgibbon M, Finn DP and Roche M: High
times for painful blues: The endocannabinoid system in
pain-depression comorbidity. Int J Neuropsychopharmacol.
19:192015.
|
|
43
|
Richardson D, Pearson RG, Kurian N, Latif
ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ and
Chapman V: Characterisation of the cannabinoid receptor system in
synovial tissue and fluid in patients with osteoarthritis and
rheumatoid arthritis. Arthritis Res Ther. 10:R432008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kaufmann I, Hauer D, Huge V, Vogeser M,
Campolongo P, Chouker A, Thiel M and Schelling G: Enhanced
anandamide plasma levels in patients with complex regional pain
syndrome following traumatic injury: A preliminary report. Eur Surg
Res. 43:325–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hill MN and Gorzalka BB: Is there a role
for the endocannabinoid system in the etiology and treatment of
melancholic depression? Behav Pharmacol. 16:333–352. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Koethe D, Llenos IC, Dulay JR, Hoyer C,
Torrey EF, Leweke FM and Weis S: Expression of CB1 cannabinoid
receptor in the anterior cingulate cortex in schizophrenia, bipolar
disorder, and major depression. J Neural Transm Vienna.
114:1055–1063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Monteleone P, Bifulco M, Maina G,
Tortorella A, Gazzerro P, Proto MC, Di Filippo C, Monteleone F,
Canestrelli B and Buonerba G: Investigation of CNR1 and FAAH
endocannabinoid gene polymorphisms in bipolar disorder and major
depression. Pharmacol Res. 61:400–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Minocci D, Massei J, Martino A, Milianti
M, Piz L, Di Bello D, Sbrana A, Martinotti E, Rossi AM and Nieri P:
Genetic association between bipolar disorder and 524A>C
(Leu133Ile) polymorphism of CNR2 gene, encoding for CB2 cannabinoid
receptor. J Affect Disord. 134:427–430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Domschke K, Dannlowski U, Ohrmann P,
Lawford B, Bauer J, Kugel H, Heindel W, Young R, Morris P, Arolt V,
et al: Cannabinoid receptor 1 (CNR1) gene: Impact on antidepressant
treatment response and emotion processing in major depression. Eur
Neuropsychopharmacol. 18:751–759. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
La Porta C, Bura SA, Llorente-Onaindia J,
Pastor A, Navarrete F, García-Gutiérrez MS, De la Torre R,
Manzanares J, Monfort J and Maldonado R: Role of the
endocannabinoid system in the emotional manifestations of
osteoarthritis pain. Pain. 156:2001–2012. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Woolridge E, Barton S, Samuel J, Osorio J,
Dougherty A and Holdcroft A: Cannabis use in HIV for pain and other
medical symptoms. J Pain Symptom Manage. 29:358–367. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Maida V, Ennis M, Irani S, Corbo M and
Dolzhykov M: Adjunctive nabilone in cancer pain and symptom
management: A prospective observational study using propensity
scoring. J Support Oncol. 6:119–124. 2008.PubMed/NCBI
|
|
53
|
Skrabek RQ, Galimova L, Ethans K and Perry
D: Nabilone for the treatment of pain in fibromyalgia. J Pain.
9:164–173. 2008. View Article : Google Scholar
|
|
54
|
Weber J, Schley M, Casutt M, Gerber H,
Schuepfer G, Rukwied R, Schleinzer W, Ueberall M and Konrad C:
Tetrahydrocannabinol (Delta 9-THC) treatment in chronic central
neuropathic pain and fibromyalgia patients: Results of a
multicenter survey. Anesthesiol Res Pract.
2009:8272902009.PubMed/NCBI
|
|
55
|
Schley M, Legler A, Skopp G, Schmelz M,
Konrad C and Rukwied R: Delta-9-THC based monotherapy in
fibromyalgia patients on experimentally induced pain, axon reflex
flare, and pain relief. Curr Med Res Opin. 22:1269–1276. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Whiting PF, Wolff RF, Deshpande S, Di
Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K,
Ryder S, Schmidlkofer S, Westwood M and Kleijnen J: Cannabinoids
for medical use: A systematic review and meta-analysis. JAMA.
313:2456–2473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Williamson EM and Evans FJ: Cannabinoids
in clinical practice. Drugs. 60:1303–1314. 2000. View Article : Google Scholar
|
|
58
|
Lee MC, Ploner M, Wiech K, Bingel U,
Wanigasekera V, Brooks J, Menon DK and Tracey I: Amygdala activity
contributes to the dissociative effect of cannabis on pain
perception. Pain. 154:124–134. 2013. View Article : Google Scholar : PubMed/NCBI
|















