Introduction
Osteosarcoma is the most common malignant primary
bone tumor in adolescents and children, comprising ~20% of all
types of primary bone cancer (1–3).
Complete radical surgical resection is the current treatment for
local osteosarcoma, typically followed by chemotherapy to improve
the prognosis and long-term survival rate. However, due to distal
metastases, osteosarcoma has a poor prognosis. It has been
previously reported that <30% of patients with osteosarcoma
survive for 5 years following the detection of lung metastasis
(4). Due to the poor prognosis and
high mortality rate attributed to the metastatic ability of
osteosarcoma, identification of novel agents to obtain improved
treatment outcomes is a major clinical challenge in osteosarcoma
therapy.
Osteosarcoma cells are highly invasive, so the
suppression of their invasive ability maybe effective in the
treatment of osteosarcoma. A series of complex mechanisms,
including migration, invasion, adhesion to endothelial cells, and
degradation of the extracellular matrix (ECM), are involved in the
process of osteosarcoma metastasis (5,6).
Evidence has demonstrated the crucial role of matrix
metalloproteinases (MMPs) in the degradation of the ECM and cancer
metastasis (7,8). MMP-2 and -9 can degrade the main
component of the ECM, type IV collagen, so are considered to be
involved in cancer metastasis (9,10).
Previous studies have demonstrated that mitogen-activated protein
kinase (MAPK) family members, including extracellular
signal-regulated kinases (ERK) 1/2, p38 and c-Jun N-terminal kinase
(JNK), are important for the regulation of MMP-2 and -9 production
(11,12).
Due to the high motility rate and increasing drug
resistance of osteosarcoma, increasing numbers of studies have
focused on natural products as novel anti-tumor therapeutics for
its treatment (13,14). Trillium tschonoskii Maxim
has long been used in the treatment of neurasthenia, hypertension,
cancer and as an analgesic (15).
As a type of steroidal saponin, Paris saponin VII (PS VII; Fig. 1A) is extracted from Trillium
tschonoskii Maxim. Previous reports have demonstrated that PS
VII exhibits anti-tumor capacity in different types of tumor. Li
et al (16,17) have demonstrated that PS VII
suppresses the growth of colorectal cancer cells via the Ras
pathway in vitro and in vivo. Fan et al
(18) demonstrated that PS VII
suppresses colorectal cancer cell metastasis by regulating MMP-2
and -9 production. PS VII has also been reported to suppress the
proliferation and induce the apoptosis of cervical and breast
cancer cells (19,20). Furthermore, a recent study reported
that PS VII inhibits the migration and invasion of lung cancer
cells (21). However, to the best
of our knowledge, no evidence has reported whether PS VII exerts
any direct effect on osteosarcoma migration and invasion, or the
mechanisms that mediate this effect.
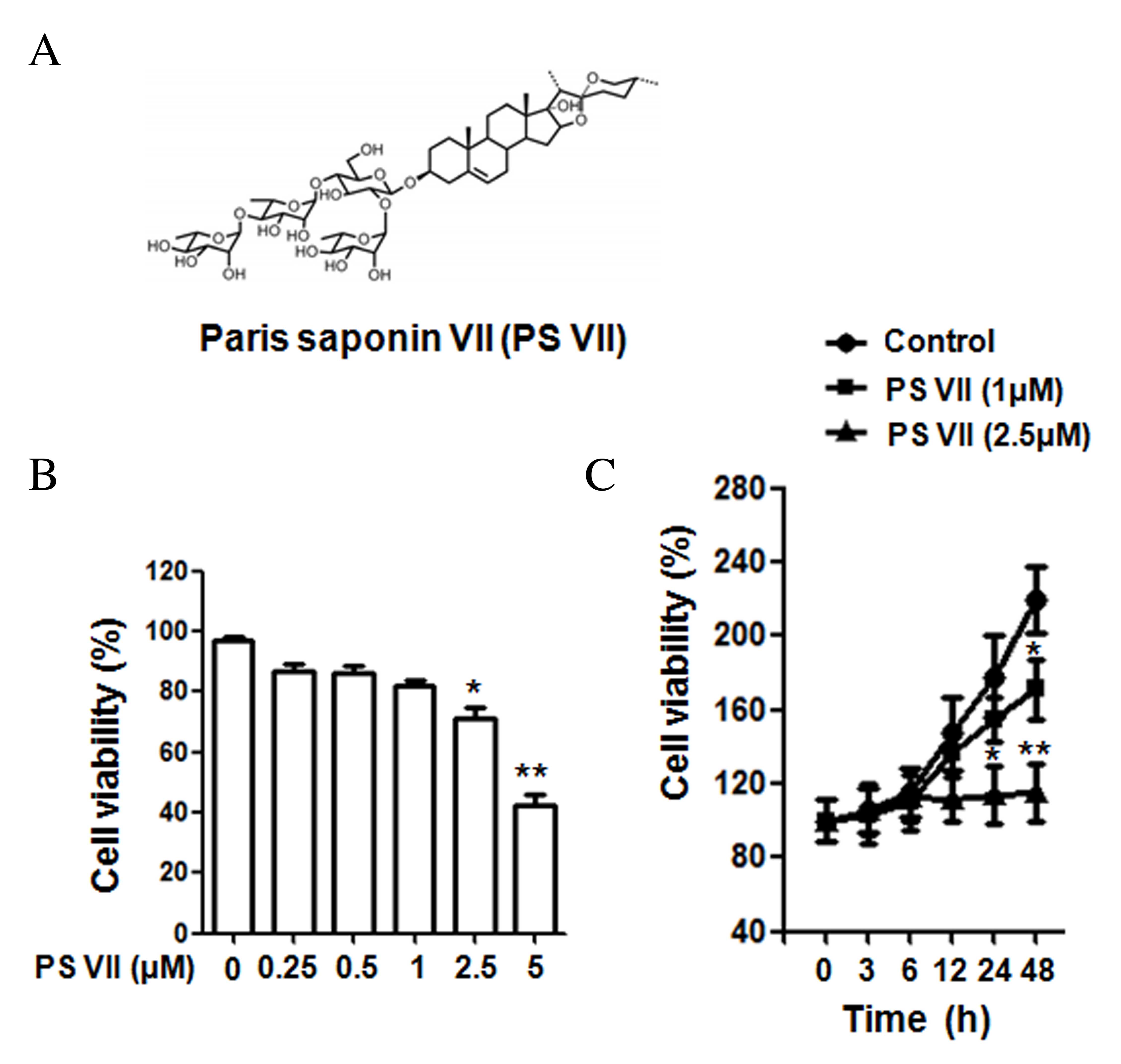 | Figure 1Chemical structure of PS VII and the
effect of PS VII on U2OS osteosarcoma cell proliferation. (A)
Chemical structure of PS VII. (B) Viability of U2OS cells was
examined by MTT assay following 24 h treatment with 0, 0.25, 0.5,
1, 2.5 and 5 μM PS VII. (C) Viability of U2OS cells was
examined by MTT assay following 0, 3, 6, 12, 24 and 48 h treatment
with 0 (control), 1 and 2.5 μM PS VII. Values are presented
as the mean ± standard deviation from three independent
experiments. *P<0.05, **P<0.01 vs.
control. PS VII, Paris saponin VII; MTT,
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide. |
The current study evaluated the effects of PS VII on
osteosarcoma cell migration and invasion, and the underlying
mechanisms.
Materials and methods
Cell lines and reagents
The U2OS osteosarcoma cell line was obtained from
the American Type Culture Collection (ATCC, Manassas, VA, USA) and
was routinely cultured in Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, and 100 μg/ml
streptomycin in 5% CO2 at 37°C. Rabbit antibodies
recognizing p38 (catalog no. 8690), phospho (p)-p38 (catalog no.
4631), ERK1/2 (catalog no. 4695), p-ERK1/2 (catalog no. 4370), JNK
(catalog no. 9252), p-JNK (catalog no. 4668), MMP-2 (catalog no.
13132) and MMP-9 (catalog no. 13667) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Rabbit anti-GAPDH
(catalog no. G9545) was purchased from Sigma-Aldrich (St. Louis,
MO, USA). The horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody (catalog no. ZDR-5306) was obtained
from ZSBG-BIO (Beijing, China). PS VII with a purity of >98% was
purchased from PureOne Biotechnology Co. (Shanghai, China).
SB203580, a selective inhibitor of p38, was purchased from
Sigma-Aldrich.
Cell viability and proliferation
assays
Cell viability and proliferation were examined with
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The U2OS osteosarcoma cells (5,000 cells/well) in 100
μl medium were seeded into 96-well plates. Following
treatment with PS VII of various concentrations (0, 0.25, 0.5, 1,
2.5 and 5 μM), for various time points (0, 3, 6, 12, 24 and
48 h), 20 μl MTT (5 mg/ml) was added into each well.
Following incubation for 4 h, 100 μl of dimethyl sulfoxide
was added to each well for another 15 min. Finally, the absorbance
values were determined by microplate luminometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 490 nm.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from U2OS osteosarcoma cells
using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
and then reverse transcribed to cDNA with RevertAid First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The qPCR analysis was performed
using a LightCycler (Bio-Rad Laboratories, Inc.), with
SYBR® Fast qPCR Mix (catalog no. RR430S; Takara
Biotechnology Co., Ltd., Dalian, China). The primer sequences used
for qPCR were as follows: MMP-9, forward 5′-GACGCAGACATCGTCATCCA-3′
and reverse 5′-CACAACTCGTCATCGTCGAAA-3′; MMP-2, forward
5′-TTGATGGCATCGCTCAGATC-3′ and reverse 5′-TTGTCACGTGGCGTCACAGT-3′;
and GAPDH, forward 5′-TGTGGGCATCAATGGATTTGG-3′ and reverse
5′-ACACCATGTATTCCGGGTCAAT-3′. An initial denaturation step at 95°C
for 10 sec was followed by 40 cycles of denaturation at 95°C for 5
sec, annealing at 60°C for 30 sec, extension at 72°C for 30 sec,
and a final extension step at 72°C for 3 min. Melting curves were
assessed to confirm the specificity of the products generated for
each set of primers. Subsequent to the amplification, ΔΔCq
comparative method was used to normalize the relative levels of
gene expression to GAPDH (22).
Experiments were performed in triplicate.
Western blotting
Following incubation with PS VII, the U2OS
osteosarcoma cells were collected and protein was extracted using
protein lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China). Lysed proteins were centrifuged at 10,000 × g, 4°C for 15
min, and protein concentrations were determined using the
bicinchoninic acid assay (Beyotime Institute of Biotechnology).
Equal quantities of protein (10 μg) from cell lysates were
loaded onto 12% gels and separated by sodium dodecyl
sulphate-polyacrylamide gel electrophoresis. Following separation,
proteins were transferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA) and then blocked with 5% fat-free
milk in Tris-buffered saline with 0.1% Tween-20 (TBST) at room
temperature for 1 h, and incubated with primary antibodies (diluted
1:1,000) overnight at 4°C. The membranes were then washed with TBST
and incubated with an HRP-conjugated secondary antibody (diluted
1:10,000) for 1 h at room temperature. Immune complexes were
detected with enhanced chemiluminescence reagents (EMD Millipore),
and the blots were quantified by densitometric analysis using the
Alpha Imager 2200 (ProteinSimple, San Jose, CA, USA).
Scratch wound healing assay
A scratch wound healing assay was applied to
evaluate the migration of the U2OS osteosarcoma cells. In brief,
the U2OS cells (1×106/well) were seeded in 6-well plates
cultured with DMEM supplemented with 10% FBS. When reaching
confluency, each well was straight scratched with a 200 μl
pipette tip. To evaluate the effect of PS VII on the migration of
U2OS cells, 1 μM PS VII was added to the plates. Following
24 h of incubation, the wound healing areas were imaged and the
distance between two cell edges was analyzed by ImageJ software
version 1.48 (National Institutes of Health, Bethesda, MD, USA).
Control cells not stimulated with PS VII were analyzed at 0 and 24
h.
In vitro invasion assay
To assess the effect of PS VII on the invasive
ability of U2OS osteosarcoma cells, the Transwell system was
applied. The U2OS cells were cultured in Boyden chambers, with
8-μm pore filter inserts, in 24-well plates. The pore
inserts were pre-coated with BD Matrigel™ Basement Membrane Matrix
(BD Biosciences, San Jose, CA, USA) overnight. The U2OS cells
(1×105 cells/well) were suspended in 100 μl DMEM
supplemented with 1% FBS and were added to the upper chamber. DMEM
with 10% FBS and 1 μM PS VII was added to the lower chamber.
After 24 h of incubation, the cells attaching to the lower surface
were fixed with 100% methanol for 10 min at room temperature and
stained with 0.1% crystal violet. Images of 5 random high-power
fields (magnification, ×200) were captured of each sample and
counted to assess the average number of invasive cells.
Statistical analysis
The data are expressed as the mean ± standard
deviation. All experiments were repeated at least three times.
Comparisons among values for all groups were performed by one-way
analysis of variance. Holm's test was applied for analysis of
differences between two different groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
PS VII suppresses the cell viability of
osteosarcoma cells
The structure of PS VII is presented in Fig. 1A. To determine the effect of PS VII
on the cell viability of U2OS osteosarcoma cells, MTT assays were
performed using doses of 0, 0.25, 0.5, 1, 2.5 and 5 μM PS
VII. As presented in Fig. 1B, at
doses 2.5 and 5 μM, PS VII significantly reduced the
viability of U2OS cells after 24 h treatment, compared with the
untreated control (P=0.042 and P=0.0072, respectively). However, at
doses <2.5 μM (0.25, 0.5 and 1 μM), the
suppression was not significantly different compared with the
untreated control. PS VII at concentrations of 1 and 2.5 μM
were subsequently used to treat the cells at various time points
(0, 3, 6, 12, 24 and 48 h), as presented in Fig. 1C. After 24 h of treatment, PS VII
significantly inhibited the cell proliferation at a dose of 2.5
μM compared with the untreated control (P=0.033), while
proliferation was not inhibited with 1 μM PS VII at 24 h.
However, after 48-h stimulation, 1 (P=0.026) and 2.5 (P=0.0045)
μM PS VII significantly inhibited cell proliferation
compared with the control group. As a result, PS VII at a dose of 1
μM for 24 h was used for subsequent migration and invasion
experiments to exclude the effect on cell proliferation.
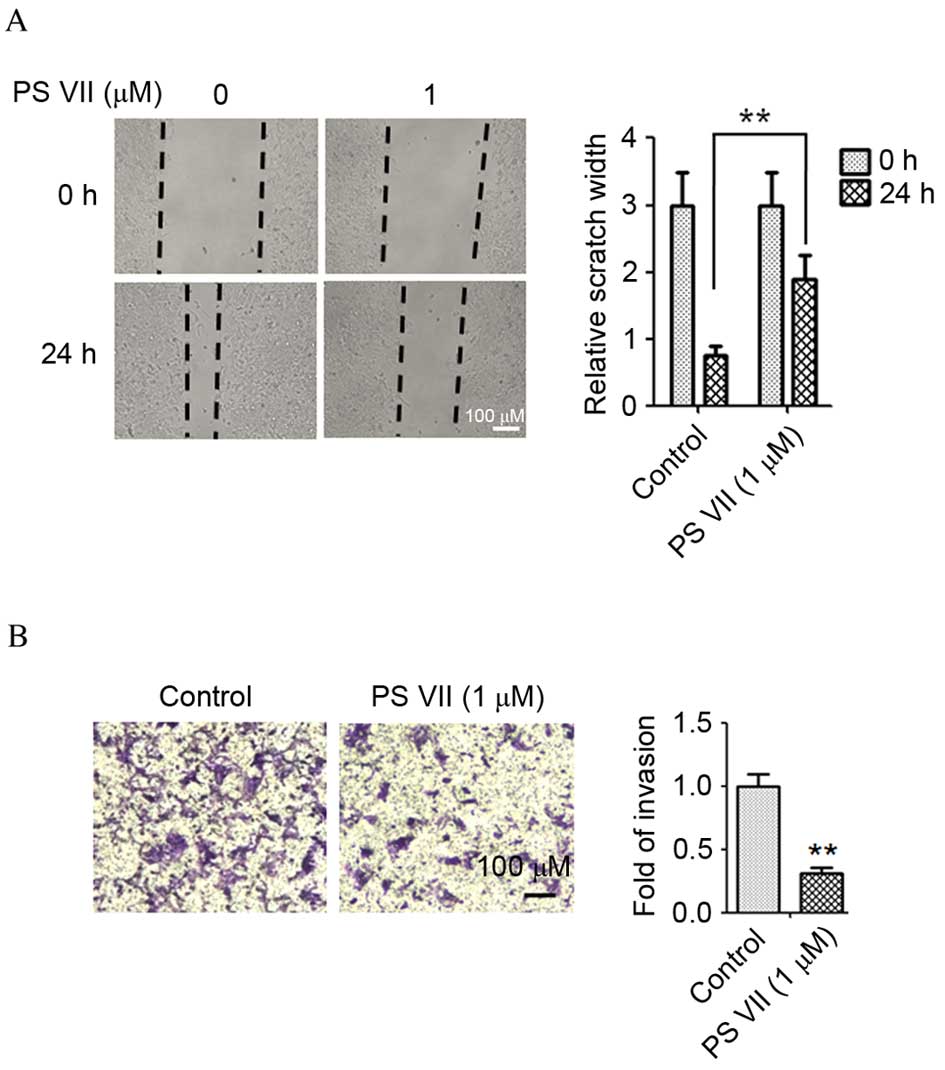
PS VII inhibits the migration and
invasion of osteosarcoma cells
The wound healing assay was performed to evaluate
the effect of PS VII on the migration of osteosarcoma cells. The
U2OS osteosarcoma cells were treated with 1 μM PS VII for 24
h. As presented in Fig. 2A,
migration of U2OS cells was suppressed by 1 μM PS VII
compared with untreated control cells (P=0.0082). The results of
the wound healing assay indicated that healing of the scratch was
significantly reduced following treatment with PS VII. To further
examine the effect of PS VII on cell invasion, a Transwell assay
was used, in which U2OS cells were treated with 1 μM PS VII
for 24 h. As demonstrated in Fig.
2B, the invasion of U2OS cells was suppressed by 1 μM PS
VII compared with untreated control cells (P=0.0055), suggesting
that PS VII inhibited the invasive ability of osteosarcoma
cells.
PS VII reduces the production of MMP-2
and -9 in osteosarcoma cells
Previous studies have demonstrated that MMPs,
particularly MMP-2 and -9, are crucial for the migration and
invasion of cancer cells (23,24).
Thus the effect of PS VII on MMP-2 and -9 expression in U2OS
osteosarcoma cells was examined. U2OS cells were stimulated with 0,
0.5, 1 or 2.5 μM PS VII for 24 h. As demonstrated in
Fig. 3A, treatment with 1 and 2.5
μM PS VII significantly reduced the relative mRNA expression
of MMP-2 (P=0.037 and P=0.0067, respectively) and MMP-9 (P=0.0082
and P=0.0071, respectively) in U2OS osteosarcoma cells compared
with control cells. U2OS cells were subsequently treated with 1
μM PS VII for 0, 6, 12 and 24 h (Fig. 3B). Following treatment with 1
μM PS VII for 6 h, the relative mRNA expression of MMP-2 and
-9 did not significantly change. However, when the treatments were
extended to 12 and 24 h, the relative mRNA expression levels of
MMP-2 (P=0.044 and P=0.0062, respectively) and MMP-9 (P=0.0064 and
P=0.0055, respectively) were significantly decreased compared with
the 0 h control. Quantitative western blots were subsequently used
to examine the levels of MMP-2 and -9 protein expression following
identical treatments. As demonstrated in Fig. 3C, following treatment with 1 and
2.5 μM PS VII, the protein expression of MMP-2 (P=0.0034 and
P=0.0042, respectively) and MMP-9 (P=0.0049 and P=0.0036,
respectively) was significantly reduced compared with control
treatment. Similarly to the mRNA levels, the protein expression
also decreased gradually following treatment with 1 μM PS
VII for 6 (MMP-2, P=0.041), 12 (MMP-2, P=0.0062; MMP-9, P=0.0056)
and 24 (MMP-2, P=0.0051; MMP-9, P=0.0046) h compared with at 0 h
(Fig. 3D). These results,
therefore, demonstrated that PS VII suppressed the production of
MMP-2 and -9 in a dose- and time-dependent manner at the mRNA and
protein levels. Furthermore, the results of the present study
indicated that the inhibitory effect of PS VII on U2OS cell
migration and invasion may be associated with MMP-2 and -9
production.
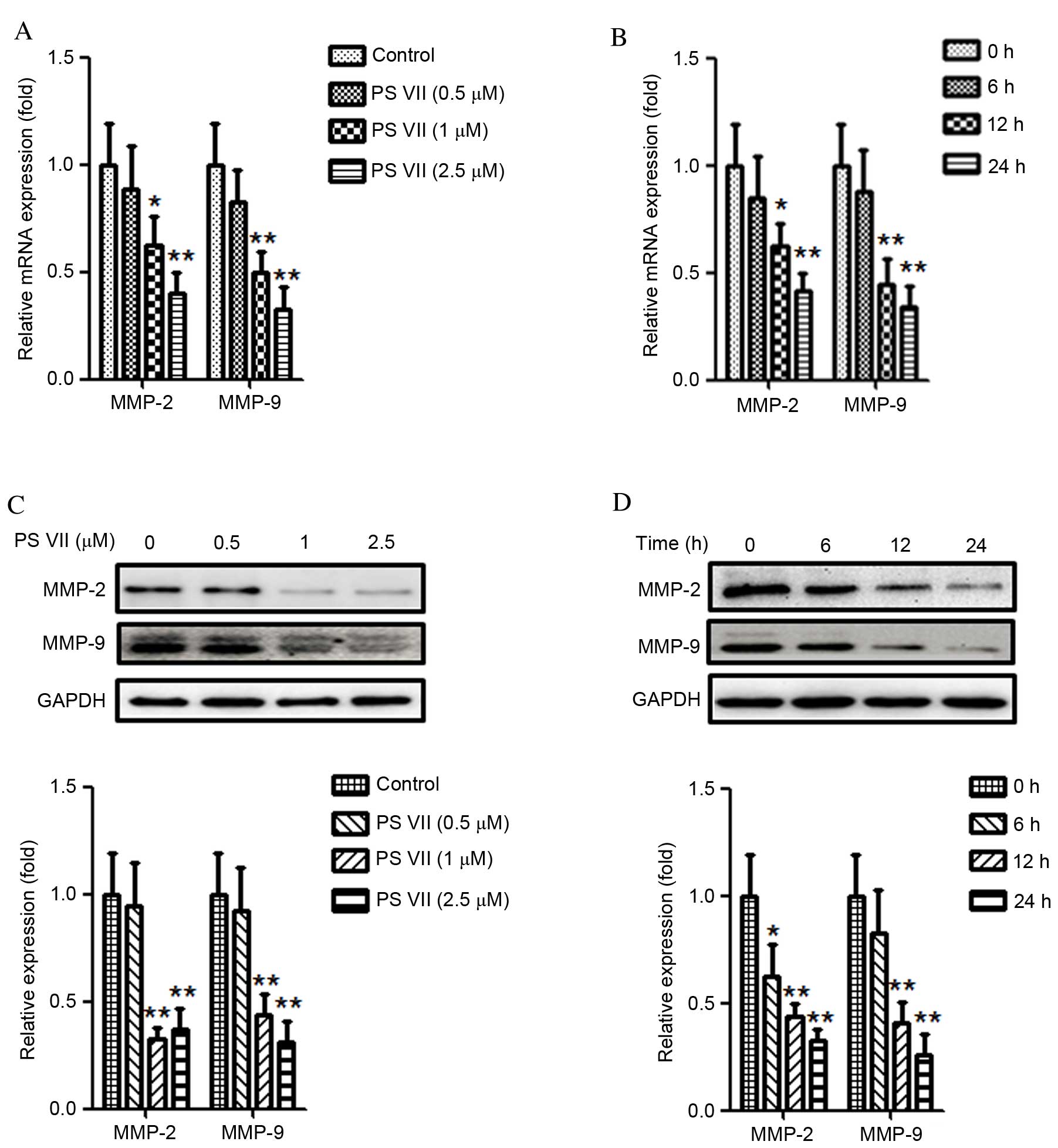 | Figure 3PS VII reduces the expression of MMP-2
and -9 of U2OS osteosarcoma cells. (A) MMP-2 and -9 mRNA levels
were assessed by qPCR following treatment of U2OS cells with 0
(control), 0.5, 1 and 2.5 μM PS VII for 24 h. (B) MMP-2 and
-9 mRNA levels were assessed by qPCR following treatment of U2OS
cells with 1 μM PS VII for 0 (control), 6, 12 and 24 h. (C)
MMP-2 and -9 protein expression was assessed by quantitative
western blot analysis following stimulation of U2OS cells with 0
(control), 0.5, 1 and 2.5 μM PS VII for 24 h. (D) MMP-2 and
-9 protein expression was assessed by quantitative western blot
analysis following stimulation of U2OS cells with 1 μM PS
VII for 0 (control), 6, 12 and 24 h. Values are presented as the
mean ± standard deviation from three independent experiments.
*P<0.05, **P<0.01 vs. control. PS VII,
Paris saponin VII; MMP, matrix metalloproteinase; qPCR,
quantitative polymerase chain reaction; GADPH, glyceraldehyde
3-phosphate dehydrogenase. |
p38 MAPK signaling pathway modulates PS
VII-regulated MMP-2/9 production
MAPK signaling pathways are important for tumor
development. Inactivation of MAPK signaling has previously been
demonstrated to inhibit tumor cell migration and invasion (25). To examine the association between
PS VII and the production of MMP-2 and -9, phosphorylation levels
of three members of the MAPK family, ERK1/2, p38 and JNK, was
detected following treatment of U2OS cells with 1 μM PS VII
for 0, 5, 15, 30 and 60 min (Fig. 4A
and B). The relative level of p-p38 was significantly reduced
compared with the 0 min control following 5 (P=0.0057), 15
(P=0.0044) and 30 (P=0.0061) min of treatment and the effect had
begun to gradually increase by 60 min of treatment (P=0.0076;
Fig. 4B). However, the expression
of p-ERK1/2 and p-JNK remained unchanged compared with the 0 min
control (Fig. 4B). These results
indicated that the p38 MAPK signaling pathway may be involved in
MMP-2 and -9 production in U2OS osteosarcoma cells.
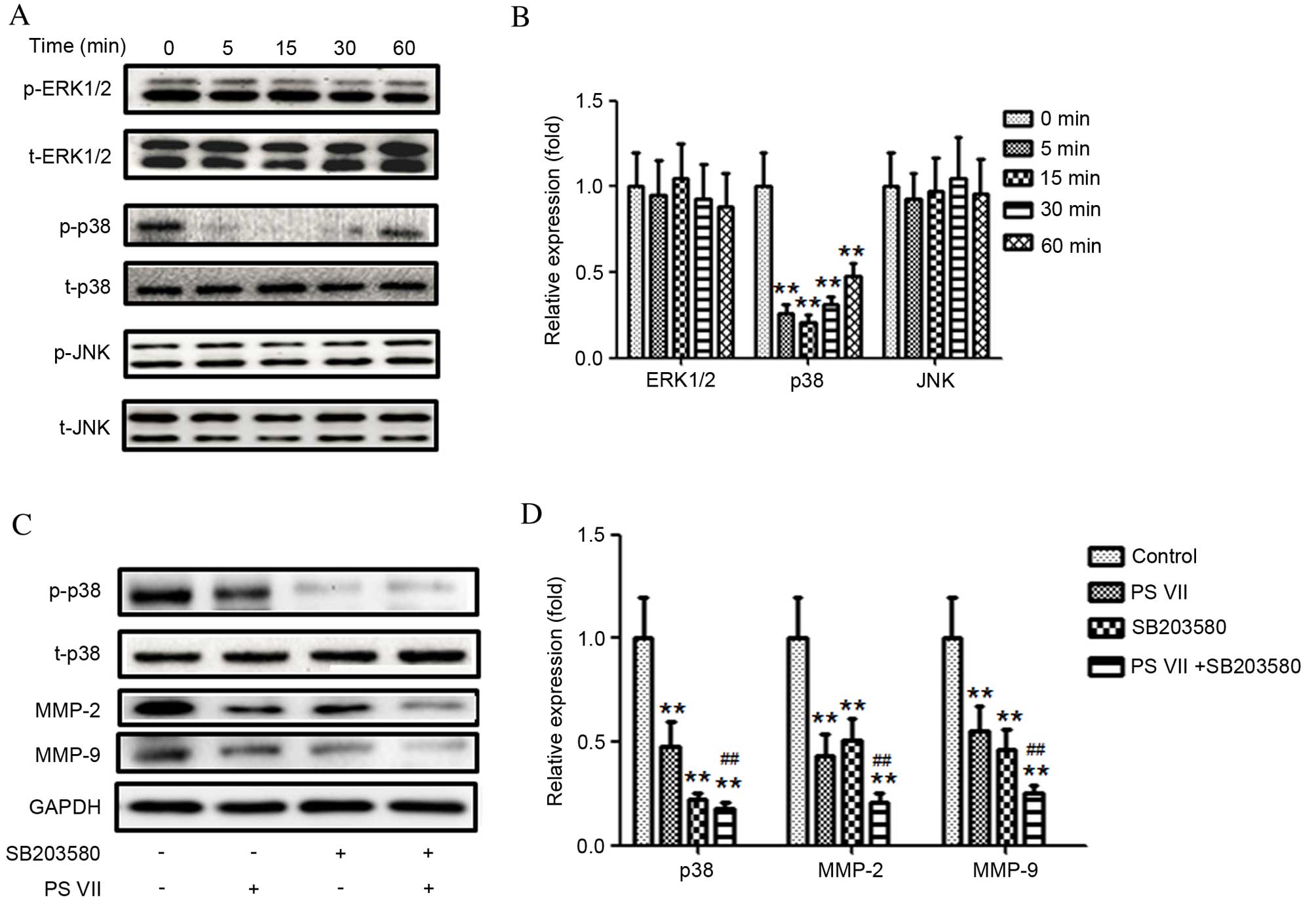 | Figure 4p38 mitogen-activated protein kinase,
but not ERK1/2 or JNK, modulates PS VII-mediated production of
MMP-2 and -9 in U2OS osteosarcoma cells. (A) p-ERK1/2, ERK1/2,
p-p38, p38, p-JNK and JNK protein expression was assessed by
western blot following stimulation of U2OS cells with 1 μM
PS VII for 0 (control), 5, 15, 30 and 60 min, and (B)
quantitatively analyzed (*P<0.05,
**P<0.01 vs. 0 min control). (C) p-p38, p38, MMP-2,
MMP-9 and GADPH (blot control) protein expression was assessed by
western blot following stimulation of U2OS cells with 1 μM
PS VII for 24 h, ± 50 μM SB203580 for 1 h prior to PS VII
treatment and (D) quantitatively analyzed (**P<0.01
vs. no-treatment control; ##P<0.01, vs. PS
VII-treated cells). Data are presented as the mean ± standard
deviation from three independent experiments. PS VII, Paris saponin
VII; p-, phosphorylated; ERK, extracellular signal-regulated
kinase; JNK, c-Jun N-terminal kinase; t-, total; MMP, matrix
metalloproteinase; GADPH, glyceraldehyde 3-phosphate
dehydrogenase. |
To investigate whether the effect of PS VII is
p38-dependent, a p38 inhibitor, SB203580, was used to block p38
activation. As demonstrated in Fig. 4C
and D, inhibition of p38 activity with SB203580 further reduced
the expression of MMP-2 (P=0.0055) and -9 (P=0.0056) compared with
PS VII treatment alone. These results indicate that inhibition of
MMP-2 and -9 production by PS VII may be p38-dependent.
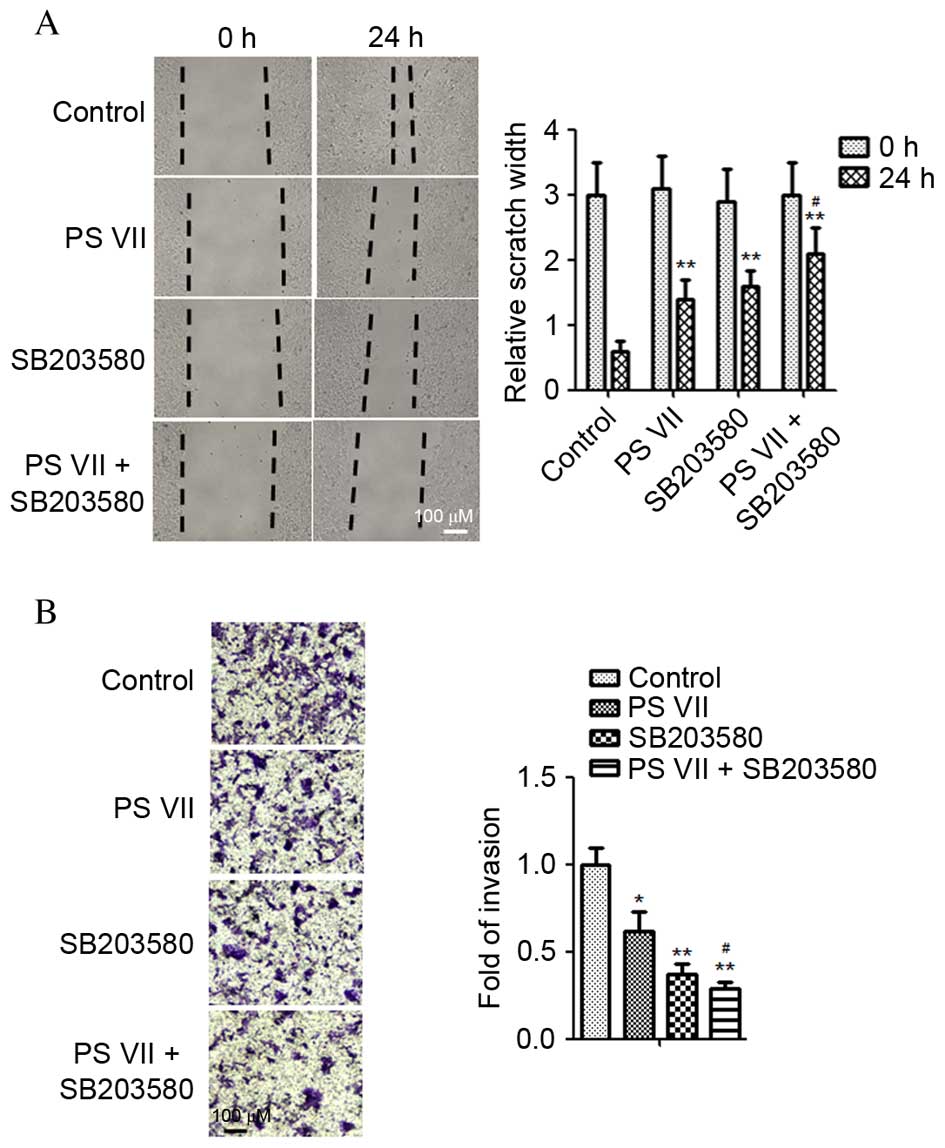
Inhibition of the p38 MAPK signaling
pathway enhances the anti-metastasic effect of PS VII in
osteosarcoma cells
To further investigate whether the anti-metastasic
effect of PS VII was attributable to p38 MAPK signaling
inactivation, the effect of SB203580 on U2OS cell migration in the
presence and absence of PS VII was examined. As demonstrated in
Fig. 5A, the wound healing assay
indicated that the PS VII-induced inhibition of cell migration was
significantly enhanced by the p38 inhibitor compared with PS VII
treatment only (P=0.032). Similarly, Transwell assays indicated
that inhibition of p38 MAPK significantly enhanced the PS
VII-induced inhibition of osteosarcoma cell invasion (P=0.044;
Fig. 5B). Therefore, the present
study supports the hypothesis that inhibition of metastasis in
osteosarcoma cells by PS VII is modulated by suppression of MMP-2
and -9 production via the p38 MAPK signaling pathway.
Discussion
Investigation of metastasis and the underlying
molecular mechanisms is important for aiding the development of
effective agents to suppress cancer metastasis. Natural products
are currently receiving increased attention in cancer therapy
research for their anti-tumor properties and reduced side-effects
compared with traditional chemotherapeutics (26,27).
Previous studies have demonstrated that PS VII suppresses cell
proliferation, migration and invasion in colorectal (16–18),
cervical (19), breast (20) and lung (21) cancer cells. Although previous
studies have demonstrated the anti-tumor activity of PS VII, the
effect of PS VII on osteosarcoma cell migration and invasion, and
the molecular mechanisms had not been investigated. The present
study initially evaluated the effect of PS VII on the viability of
osteosarcoma cells, and revealed that 1 μM PS VII
significantly inhibited cell proliferation after 48 h of treatment,
with no difference before 24 h. A stimulation time point of 24 h
and stimulation dose of 1 μM was therefore used in
subsequent experiments, to exclude the influence on proliferation.
To the best of our knowledge, the present study is the first to
have demonstrated that PS VII effectively inhibits osteosarcoma
cell migration and invasion, and that PS VII suppresses
osteosarcoma cell migration and invasion by inhibiting MMP-2 and -9
production via the p38 MAPK signaling pathway. This is, therefore,
the first evidence of the anti-metastatic activity of PS VII on
osteosarcoma cells and a molecular mechanism for such activity.
Metastasis of osteosarcoma is a complicated process
and is often correlated with the hydrolysis of the ECM by a number
of proteolytic enzymes, including MMPs, which are proteinases
involved in the migration and invasion of malignant cells (5,6,23,24).
Among them, MMP-2 and -9 are crucial for the initiation of
metastasis and invasion (9,10).
Previous studies have demonstrated the importance of MMP-2 and -9
in osteosarcoma progression, with high MMP-2 expression associated
with poor prognosis (28–30). The current study examined the
suppressive effect of PS VII on the migration and invasion of U2OS
osteosarcoma cells by using scratch wound healing and Transwell
assays, and established that reduced expression of MMP-2 and -9
following PS VII treatment was associated with the suppression of
migration and invasion in U2OS osteosarcoma cells. This indicates
that metastasis inhibition by PS VII may be associated with MMP-2
and -9 activity.
Previous studies have reported that MAPK family
members, including ERK, p38 and JNK, may be involved in MMP-2 and
-9 production, thus promoting tumor proliferation and metastasis
(11,12,31).
The p38 MAPK signaling pathway is involved in the modulation of
proliferation, apoptosis, migration and invasion of cancer cells
(32–34). In the present study, p38 expression
in osteosarcoma cells was determined by western blot, and the
phosphorylation of p38 was observed to be reduced when treated with
PS VII. To further confirm that the p38 signaling pathway was
involved in PS VII-induced osteosarcoma cells metastasis
suppression, p38 activity was blocked using the p38 inhibitor,
SB203580. Inhibition of p38 phosphorylation by SB203580 was
demonstrated to enhance MMP-2 and -9 downregulation induced by PS
VII. Furthermore, inhibition of p38 activity by SB203580 further
suppressed osteosarcoma cell migration and invasion. PS VII was,
therefore, demonstrated to inhibit osteosarcoma cell metastasis
through suppression of p38 MAPK activity, resulting in
downregulation of MMP-2 and -9, and further suggesting that p38
MAPK is upstream of MMP-2 and -9 in the signaling cascade.
Therefore, to the best of our knowledge, the current
study presents the first evidence that PS VII suppresses the
migration and invasion of osteosarcoma cells by reducing MMP-2 and
MMP-9 production. Furthermore, the effect of PS VII is modulated by
the p38 MAPK signaling pathway. Thus, PS VII is indicated to be a
potential novel therapeutic agent in osteosarcoma therapy, and a
candidate for further study in vivo.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huh WW, Holsinger FC, Levy A, Palla FS and
Anderson PM: Osteosarcoma of the jaw in children and young adults.
Head Neck. 34:981–984. 2012. View Article : Google Scholar
|
|
4
|
Mohseny AB, Machado I, Cai Y, Schaefer KL,
Serra M, Hogendoorn PC, Llombart-Bosch A and Cleton-Jansen AM:
Functional characterization of osteosarcoma cell lines provides
representative models to study the human disease. Lab Invest.
91:1195–1205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC
and Lu KH: Silibinin suppresses human osteosarcoma MG-63 cell
invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of
MMP-2. Carcinogenesis. 28:977–987. 2007. View Article : Google Scholar
|
|
6
|
Lu KH, Yang HW, Su CW, Lue KH, Yang SF and
Hsieh YS: Phyllanthus urinaria suppresses human osteosarcoma cell
invasion and migration by transcriptionally inhibiting u-PA via ERK
and Akt signaling pathways. Food Chem Toxicol. 52:193–199. 2013.
View Article : Google Scholar
|
|
7
|
Stallings-Mann M and Radisky D: Matrix
metalloproteinase-induced malignancy in mammary epithelial cells.
Cells Tissues Organs. 185:104–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liotta LA and Stetler-Stevenson WG:
Metalloproteinases and cancer invasion. Semin Cancer Biol.
1:99–106. 1990.PubMed/NCBI
|
|
10
|
Himelstein BP, Canete-Soler R, Bernhard
EJ, Dilks DW and Muschel RJ: Metalloproteinases in tumor
progression: The contribution of MMP-9. Invasion Metastasis.
14:246–258. 1994.PubMed/NCBI
|
|
11
|
Kim BS, Park JY, Kang HJ, Kim HJ and Lee
J: Fucoidan/FGF-2 induces angiogenesis through JNK- and
p38-mediated activation of AKT/MMP-2 signalling. Biochem Biophys
Res Commun. 450:1333–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin YJ, Park I, Hong IK, Byun HJ, Choi J,
Kim YM and Lee H: Fibronectin and vitronectin induce AP-1-mediated
matrix metalloproteinase-9 expression through integrin
α(5)β(1)/α(v) β(3)-dependent Akt, ERK and JNK signaling pathways in
human umbilical vein endothelial cells. Cell Signal. 23:125–134.
2011. View Article : Google Scholar
|
|
13
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang P, Yang HL, Yang YJ, Wang L and Lee
SC: Overcome cancer cell drug resistance using natural products.
Evid Based Complement Alternat Med. 2015:7671362015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Xiao M, Guo L, Wang L, Tang L, Xu Y,
Yan F and Chen F: Genetic diversity and genetic structure of an
endangered species, Trillium tschonoskii. Biochem Genet.
43:445–458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Sun Y, Fan L, Zhang F, Meng J, Han
J, Guo X, Zhang D, Zhang R, Yue Z, et al: Paris saponin VII
inhibits growth of colorectal cancer cells through Ras signaling
pathway. Biochem Pharmacol. 88:150–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Liu C, Xiao D, Han J, Yue Z, Sun Y,
Fan L, Zhang F, Meng J, Zhang R, et al: Trillium tschonoskii
steroidal saponins suppress the growth of colorectal Cancer cells
in vitro and in vivo. J Ethnopharmacol. 168:136–145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan L, Li Y, Sun Y, Yue Z, Meng J, Zhang
X, Zhang R, Zhang D, Zhang F and Mei Q: Paris saponin VII inhibits
metastasis by modulating matrix metalloproteinases in colorectal
cancer cells. Mol Med Rep. 11:705–711. 2015.
|
|
19
|
Zhang W, Zhang D, Ma X, Liu Z, Li F and Wu
D: Paris saponin VII suppressed the growth of human cervical cancer
Hela cells. Eur J Med Res. 19:412014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Fan L, Sun Y, Miao X, Zhang F, Meng
J, Han J, Zhang D, Zhang R, Yue Z and Mei Q: Paris saponin VII from
trillium tschonoskii reverses multidrug resistance of
adriamycin-resistant MCF-7/ADR cells via P-glycoprotein inhibition
and apoptosis augmentation. J Ethnopharmacol. 154:728–734. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan L, Li Y, Sun Y, Han J, Yue Z, Meng J,
Zhang X, Zhang F and Mei Q: Paris Saponin VII inhibits the
migration and invasion in human A549 lung cancer cells. Phytother
Res. Jun 24–2015.Epub ahead of print. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
23
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
24
|
Kallakury BV, Karikehalli S, Haholu A,
Sheehan CE, Azumi N and Ross JS: Increased expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinases 1 and 2 correlate with poor prognostic variables
in renal cell carcinoma. Clin Cancer Res. 7:3113–3119.
2001.PubMed/NCBI
|
|
25
|
Rajoria S, Suriano R, Wilson YL, Schantz
SP, Moscatello A, Geliebter J and Tiwari RK: 3,3′-diindolylmethane
inhibits migration and invasion of human cancer cells through
combined suppression of ERK and AKT pathways. Oncol Rep.
25:491–497. 2011.
|
|
26
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomasset SC, Berry DP, Garcea G, Marczylo
T, Steward WP and Gescher AJ: Dietary polyphenolic
phytochemicals-promising cancer chemopreventive agents in humans? A
review of their clinical properties. Int J Cancer. 120:451–458.
2007. View Article : Google Scholar
|
|
28
|
Poudel B and Kim DK, Ki HH, Kwon YB, Lee
YM and Kim DK: Downregulation of ERK signaling impairs U2OS
osteosarcoma cell migration in collagen matrix by suppressing MMP9
production. Oncol Letters. 7:215–218. 2014.
|
|
29
|
Liu F and Zhang Q: Questions about XY Wen
et al. Entitled 'Matrix metalloproteinase 2 expression and survival
of patients with osteosarcoma: A meta-analysis'. Tumour Biol.
36:557–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wen X, Liu H, Yu K and Liu Y: Matrix
metalloproteinase 2 expression and survival of patients with
osteosarcoma: A meta-analysis. Tumour Biol. 35:845–848. 2014.
View Article : Google Scholar
|
|
31
|
Thompson N and Lyons J: Recent progress in
targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug
discovery. Curr Opin Pharmacol. 5:350–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Wang X, Wu T, Li B, Liu T, Wang
R, Liu Q, Liu Z, Gong Y and Shao C: Isoliensinine induces apoptosis
in triple-negative human breast cancer cells through ROS generation
and p38 MAPK/JNK activation. Sci Rep. 5:125792015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xia P, Zhang R and Ge G: C/EBPβ mediates
TNF-α-induced cancer cell migration by inducing MMP expression
dependent on p38 MAPK. J Cell Biochem. 116:2766–2777. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gupta J and Nebreda AR: Roles of p38α
mitogen-activated protein kinase in mouse models of inflammatory
diseases and cancer. FEBS J. 282:1841–1857. 2015. View Article : Google Scholar : PubMed/NCBI
|