Introduction
Artemisia argyi
Folium has long been used as an herbal treatment or
moxibustion in the traditional medicine of East Asian countries. It
is used widely to treat various chronic diseases, including
osteoarthritis, asthma, gastrointestinal disorders, dysmenorrhea
and insomnia (1–5). Several studies have reported that
compounds isolated from Artemisia argyi Folium have
antitumor, anti-inflammatory and anti-allergic effects (6–14);
however, to the best of our knowledge, a study using whole
Artemisia argyi Folium extract (AAFE) has not yet been
performed.
Atopic dermatitis (AD) is a common relapsing
inflammatory skin disease, which is associated with the following
symptoms: Erythema, eczema, pruritus, xerosis and lichenification
(15). AD is characterized by
several immune disorders, and patients with AD present high levels
of histamine and immunoglobulin (Ig)E. The cytokine milieu consists
of T helper (Th)2 cytokines, including interleukin (IL)-4, IL-6 and
IL-13; Th1 cytokines, including transforming growth factor (TGF)-β
and interferon (IFN)-γ; and non-Th proinflammatory cytokines,
including IL-1β and tumor necrosis factor (TNF)-α throughout the
acute and chronic phases of AD (16–18).
Overproduction of soluble mediators, including
histamine, IgE and cytokines, is associated with activation of cell
signaling molecules, including Lck/yes-related novel tyrosine
kinase (Lyn), spleen tyrosine kinase (Syk), mitogen-activated
protein kinases (MAPKs), phosphoinositide 3-kinase (PI3K)/AKT and
IκB/nuclear factor kappa-light-chain-enhancer of activated B cells
(NF-κB) in AD pathogenesis (19–22).
The present study aimed to investigate whether AAFE is able to
alleviate the pathological symptoms of multiplex immune disorders
through the regulation of intracellular signaling pathways in an
animal model of 2,4-dinitrochlorobenzene (DNCB)-induced AD.
Materials and methods
Animals
Female BALB/c mice were purchased from Hyochang
Science (Daegu, South Korea) and were 8 weeks old at the initiation
of the present study. Mice were maintained in a
temperature-controlled room (23±1°C) with relative humidity
(50±10%) and underwent a 12 h light/dark cycle. The mice were
housed in polystyrene cages at Dong-Eui University (Busan, South
Korea) and were given ad libitum access to standard rodent
chow and water. The mice used in the present study were cared for
according to the Guide for the Care and Use of Laboratory Animals
(23). The experimental protocol
was approved by the Institutional Animal Research Committee of
Dong-Eui University on Animal Care and Use (Approval number:
DEU-R2014-015), and all efforts were made to minimize animal
suffering and reduce the number of animals used in the
experiments.
Preparation of AAFE
AAFE was isolated from Artemisia argyi Folium
purchased from Omniherb Co., Ltd. (Daegu, South Korea). A total of
100 g Artemisia argyi Folium was mixed with 1 L 75% ethanol
at 60°C, and was incubated for 24 h with agitation (90 rpm). The
extract was filtered and evaporated using a rotary evaporator under
a reduced pressure. The extract was subsequently lyophilized, and
the extract yield was ~20.5%. A voucher specimen (DKMP-201203-AAFE)
was deposited at Korean Medical Physiology Laboratory, Dong-Eui
University. The extracted powder was stored at −20°C until further
use.
Induction of AD-like skin lesions and
administration of AAFE
The backs of the BALB/c mice were shaved using an
electric clipper and depilatory cream, and were washed with
sterilized phosphate-buffered saline (PBS)-gauze 1 day prior to
sensitization. During the sensitization process, 100 μ1 2%
DNCB (dissolved in 100% ethanol; Sigma-Aldrich, St. Louis, MO, USA)
or vehicle (100% ethanol) was applied to the shaved backs of the
DNCB-immunized or non-immunized mice on days 0, 3 and 6. On days 13
and 16, 20 μ1 0.2% DNCB or vehicle was used to challenge the
ears of the mice.
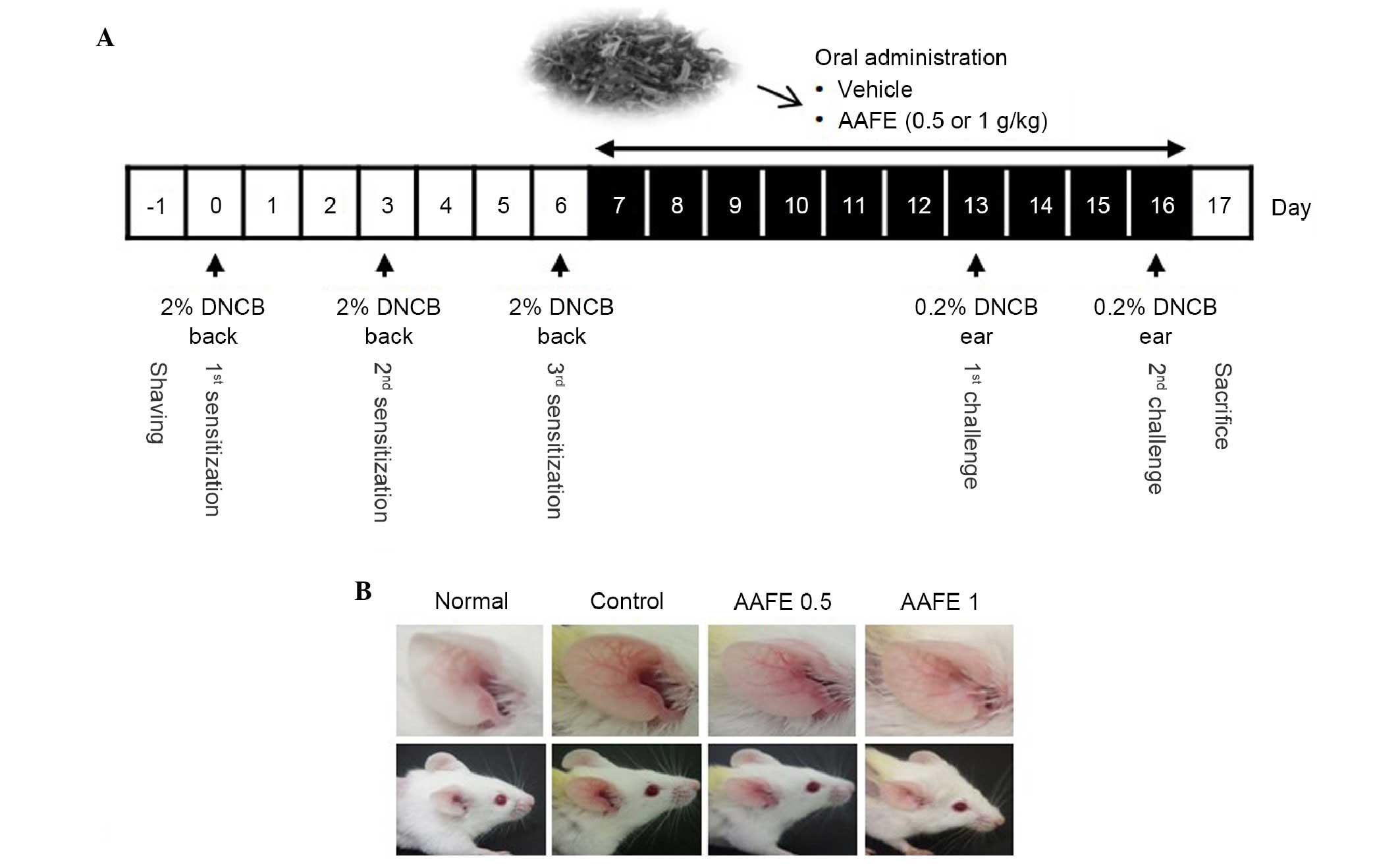
The BALB/c mice (age, 8 weeks) were randomized into
four groups (n=5/group): Non-immunized (normal), DNCB-immunized
(control), and DNCB-immunized AAFE (AAFE 0.5 and AAFE 1)-treated
groups. Mice in the AAFE 0.5 and AAFE 1 groups were orally
administered 0.5 or 1 g/kg AAFE dissolved in saline, respectively,
once daily for 10 days. Normal and control groups were administered
the same volume of saline for 10 days (Fig. 1A). Following completion of the
experiments, the mice were anesthetized with diethyl ether (2 g/kg)
and whole blood samples were collected from the mice by cardiac
puncture. The mice were sacrificed by extended diethyl ether (2
g/kg) inhalation, and the ear tissues and lymph nodes were removed
and stored at −70°C until further analysis.
Histopathology
A total of 16 days after AD induction, the mice were
anesthetized with diethyl ether, and the ear tissues were excised.
Part of the excised tissue was fixed in 4% paraformaldehyde
(Sigma-Aldrich) for 24 h, embedded in paraffin, and used to prepare
5-μm sections. The sections were subsequently deparaffinized
in xylene, rehydrated, and stained with hematoxylin and eosin
(H&E). The sections were examined under a light microscope
(Olympus Corporation, Tokyo, Japan).
Immunohistochemistry
Immunohistochemistry was performed using the
floating method. The deparaffinized sections were then treated with
10% normal goat serum (ab138478; Abcam, Cambridge, UK) for 1 h at
4°C, in order to prevent unspecified immune reactions, followed by
incubation with mouse anti-TGF-β1 (cat. no. ab9758; 1:100 dilution;
rabbit polyclonal; Abcam) overnight at 4°C. The sections were then
incubated for 1 h at room temperature with a biotinylated
anti-mouse IgG antibody (Vectastatin ABC kit; Vector Laboratories,
Inc., Burlingame, CA, USA), and were finally incubated with the ABC
complex (1:100) for 1 h. Peroxidase was visualized using 0.05%
3,3′-diaminobenzidine and 0.01% hydrogen peroxide in Tris-buffered
saline (TBS). The specificity of the immunoreaction was tested by
incubating sections without primary antibody. The expression was
calculated from the percentage of immunopositive cells in
105 μm2.
Histamine assay
Serum histamine levels were measured according to
the o-phthaldialdehyde spectrofluorometric method (24). Blood samples from the mice were
centrifuged for 10 min at 4°C and 400 × g, and the extracted serum
was used to measure histamine levels. Briefly, a mixture of 22.5
μl 0.1 N HCl and 2.5 μl 70% HClO4 was
added to 25 μl serum and centrifuged at 350 × g for 10 min
at 4°C. The upper phase (40 μl) was then transferred to
tubes containing 25 μl 5 N NaOH, 0.06 g NaCl and 500
μl n-butanol, and the sample was centrifuged at 350 × g for
10 min at 4°C to remove contaminated materials from the upper
phase. The lower phase (400 μl) was transferred to tubes
containing 150 μl 0.1 N HCl and 500 μl n-heptane, and
the sample was centrifuged at 350 × g for 10 min at 4°C. The lower
phase (100 μl) was subsequently transferred to tubes
containing 200 μl 1 N NaOH and 5 μl 1%
ο-phthaldialdehyde, and incubated for 3 min at room
temperature. The reaction was terminated by the addition of 10
μl 3 N HCl, and fluorescence intensity was measured at an
excitation wavelength of 360 nm and an emission wavelength of 450
nm using the Fluorescence Spectrometer Spectramax M2 (Molecular
Devices, LLC, Sunnyvale, CA, USA). The percentage of histamine
release was calculated as follows: Histamine release (%) = (AAFE or
Control / Normal) × 100.
Enzyme-linked immunosorbent assay
(ELISA)
Total serum IgE and cytokine (IL-1β, IL-4, IL-6 and
IFN-γ) levels were quantified using the mouse IL-1β, (cat. no.
559603) IL-4 (cat. no. 555232), IL-6 (cat. no. 555240) and IFN-γ
(cat. no. 551866) OptEIA™ sandwich ELISA Quantitation kits (BD
Biosciences, San Diego, CA, USA) according to the manufacturer's
protocol. The total levels in the blood serum were calculated using
a linear regression equation obtained from standard absorbance
values.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the skin-draining lymph
nodes using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. The primers used to amplify IL-1β, IL-4, IL-6, IL-13,
TNF-α, IFN-γ, granulocyte-macrophage colony-stimulating factor
(GM-CSF) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are
shown in Table I and were
purchased from Bioneer, Inc. (Seoul, Korea). Total RNA (1
μg) and total reagents (20 μl) were used with
One-step RT-PCR PreMix (iNtRON Biotechnology, Inc., Seongnam,
Korea) for RT-PCR. The reverse transcription reaction was performed
at 45°C for 30 min and 94°C for 2 min, then the PCR reaction was
performed at 94°C for 30 sec (denaturation), 55-56°C for 30 sec
(annealing), and 72°C (extention) for 1 min, and repeated for 30
cycles followed by incubation at 72°C for 7 min. The PCR reaction
was performed using a GeneAmp PCR system 9700 (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The PCR products were subsequently
separated by 2% (w/v) agarose gel electrophoresis and were stained
with ethidium bromide. GAPDH was used as a housekeeping gene for
each experimental condition.
 | Table IOligonucleotide reverse
transcription-polymerase chain reaction primers used in the present
study. |
Table I
Oligonucleotide reverse
transcription-polymerase chain reaction primers used in the present
study.
| Gene | Sequences
(5′→3′) | Size (bp) | Accession no. |
|---|
| IL-1β | Sense AAG CTC TCC
ACC TCA ATG GAC A | 453 | NM_008361.3 |
| Anti-sense GTC TGC
TCA TTC ACG AAA ABB GAG |
| IL-4 | Sense ACC TTG CTG
TCA CCC TCT TC | 351 | NM_021283 |
| Anti-sense TTG TGA
GCG TGG ACT CAT TC |
| IL-6 | Sense TCC AGT TGC
CTT CTT GGG AC | 100 | NM_031168.1 |
| Anti-sense GTG TAA
TTA AGC CTC CGA CTT G |
| IL-13 | Sense GCT CTC GCT
TGC CTT GGT GGT C | 218 | NM_008355.3 |
| Anti-sense CAT CCG
AGG CCT TTT GGT TAC AG |
| TNF-α | Sense GCG ACG TGG
AAC TGG CAG AAG | 340 | NM_013693.3 |
| Anti-sense TCC ATG
CCG TTG GTT AGG AGG |
| IFN-γ | Sense TCA AGT GGC
ATA GAT GTC GAA GAA | 92 | NM_008337.3 |
| Anti-sense TGG CTC
TGC AGG ATT TTC ATG |
| GM-CSF | Sense GCA TGT AGA
TGC CAT CAA AGA AGC | 342 | X03019.1 |
| Anti-sense CAT TTC
TGG ACC GGC TTC CAG C |
| GAPDH | Sense CCA CAG TCC
ATG CCA TCA C | 568 | NM_008084.3 |
| Anti-sense TCC ACC
ACC CTG TTG CTG TA |
Western blotting
The skin-draining lymph nodes were homogenized with
ice-cold lysis buffer, which consisted of 20 mmol/l Tris-HCl (pH
8.0), 150 mmol/l NaCl, 2 mmol/l ethylenediaminetetraacetic acid, 1
mmol/l NaF, 1% Igepal CA-630, 1 mmol/l phenylmethylsulfonyl
fluoride, 1 mmol/l Na3VO4, and protease
inhibitor cocktail. Following a 10 min incubation on ice, the
homogenized suspension was centrifuged at 2,000 × g for 15 min at
4°C, and the supernatant was used to determine protein
concentrations. The protein concentration in the tissue lysates was
determined using a protein assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Total proteins (25 μg from each sample)
were then separated by l0% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and were transferred to nitrocellulose transfer
membranes (Whatman; GE Healthcare Europe GmbH, Freiburg, Germany).
The membranes were blocked with 5% skim milk in TBS-Tween (TBST)
buffer [10 mmol/l Tris-HCl (pH 7.5), 150 mmol/l NaCl and 0.05%
Tween 20) for 1 h, and were then incubated with phospho-specific or
total antibodies to Lyn; Syk; MAPK family members: Extracellular
signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK) and
p38; PI3K; Akt; IκBα; and β-actin. The membranes were incubated
with the primary antibodies (diluted in 5% skim milk in TBST)
overnight at 4°C and were then washed. The membranes were
subsequently incubated for 1 h at room temperature with horseradish
peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG antibodies
(diluted in 5% skim milk in TBST). Immunoreactive bands were
developed using enhanced chemiluminescence (ECL) regents (Pierce
ECL Western Blotting Substrate; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The primary and secondary
antibodies used in the present study and the dilutions used are
listed in Table II.
 | Table IIPrimary and secondary antibodies used
in the present study. |
Table II
Primary and secondary antibodies used
in the present study.
| Antibody | Cat. no. | Source | Dilution |
|---|
| Primary
antibodies |
|
TGF-β1 | ab9758 | Abcam, Cambridge,
UK | 1:100 |
|
Lyn/p-Lyn | #2732S/2731S | Cell Signaling
Technologies, Inc., Beverly, MA, USA | 1:1,000 |
|
Syk/p-Syk |
sc-1077/sc293118 | Santa Cruz
Biotechnology, Inc., Dallas, TX, USA | 1:1,000 |
|
ERK/p-ERK | #9102/#9101S | Cell Signaling
Technologies, Inc. | 1:1,000 |
|
JNK/p-JNK | #9151S/#9251S | Cell Signaling
Technologies, Inc., | 1:1,000 |
|
p38/p-p38 | #9212/#9211S | Cell Signaling
Technologies, Inc., | 1:1,000 |
|
PI3K/p-PI3K | #4292S/#4228S | Cell Signaling
Technologies, Inc., | 1:1,000 |
|
Akt/p-Akt | #9272/#9271S | Cell Signaling
Technologies, Inc., | 1:1,000 |
|
IκBα/p-IκBα | #9242/#9246S | Cell Signaling
Technologies, Inc., | 1:1,000 |
|
β-actin | sc-47778 | Santa Cruz
Biotechnology, Inc. | 1:1,000 |
| Secondary
antibodies |
| Goat
anti-rabbit IgG, pAb | ADI-SAB-300-J | Enzo Life Sciences,
Inc., Farmingdale, NY, USA | 1:5,000 |
| Goat
anti-mouse IgG, pAb | ADI-SAB-100-J | Enzo Life Sciences,
Inc. | 1:2,500 |
Statistical analysis
Data from the control or drug-treated groups are
presented as the mean ± standard deviation of three independent
experiments and each experiment includes triplicate sets in
vitro and of five animals per group in vivo. Data were
analyzed using one-way analysis of variance followed by Dunnett's
post-hoc test in the GraphPad Prism 5 package (GraphPad Software
Inc., San Diego, CA, USA). P<0.05 were considered to indicate a
statistically significant difference.
Results
Progression of AD-like skin lesions in
BALB/c mice
The representative clinical features of the
treatment groups are presented in Fig.
1B. Repeated application of DNCB induced skin dryness, followed
by erythema, edema and hyperemia in the ear of the control group.
However, the AAFE 0.5 and AAFE 1 groups exhibited inhibition of
these symptoms of AD (Fig.
1B).
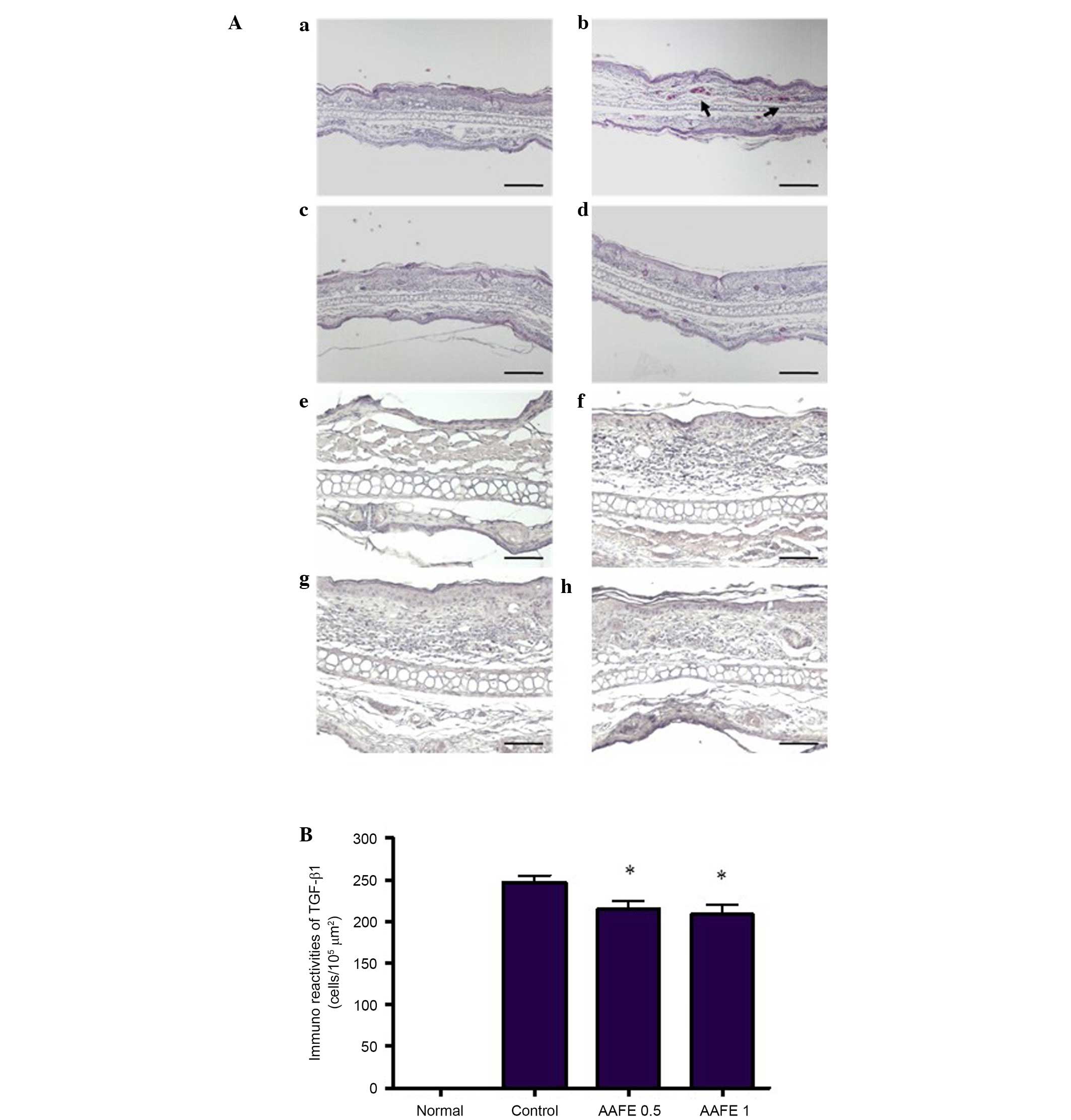
Histopathological and immunohistochemical
features
Histopathological features of the ear skin lesions
are presented in Fig. 2A. In the
normal group, inflammatory cell infiltration was not observed
following H&E staining. However, in the control group
inflammatory cell infiltration of the dermis was observed.
Inhibition of these histopathological alterations was observed in
the AAFE 0.5 and AAFE 1 groups compared with the control group
(Fig. 2Aa–d). Immunohistochemistry
was performed to evaluate DNCB-induced TGF-β1 expression in the
AD-like lesions. TGF-β1 was not detected in the epidermis and
dermis of the normal group; however, marked TGF-β1 expression was
detected in the epidermis and dermis of the control group.
Conversely, the number of TGF-β1-expressing cells was markedly
lower in the AAFE 0.5 and AAFE 1 groups compared with that of the
control group (Fig. 2Ae–h and
2B).
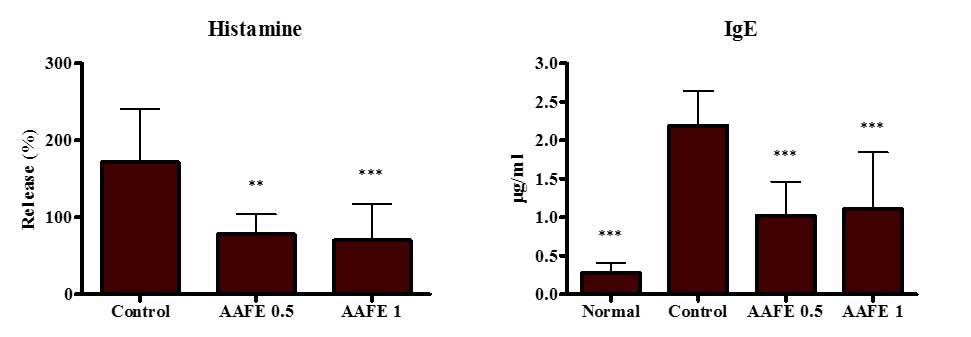
Effects of AAFE on serum levels of
histamine and IgE
To assess the inhibitory effects of AAFE on
histamine and IgE production by DNCB-induced AD-like lesions,
histamine and IgE levels were measured in the serum. The control
group displayed significant increases in histamine and IgE levels
compared with the normal group, whereas the AAFE 0.5 and AAFE 1
groups exhibited suppressed histamine and IgE levels compared with
the control group (Fig. 3).
Effects of AAFE on serum cytokine
production and mRNA expression of cytokines in skin-draining lymph
nodes
To assess the suppressive effects of AAFE on the
production and gene expression of cytokines in DNCB-induced AD-like
lesions, the serum levels of IL-1β, IL-4, IL-6 and IFN-γ cytokines,
and the mRNA expression levels of diverse cytokines were determined
in the skin-draining lymph nodes. The control group exhibited
significant increases in serum levels of IL-1β, IL-4, IL-6 and
IFN-γ compared with the normal group, whereas the AAFE 0.5 and AAFE
1 groups exhibited suppressed serum levels of IL-1β, IL-4, IL-6 and
IFN-γ compared with the control group (Fig. 4A). Furthermore, the skin-draining
lymph nodes of the control group exhibited higher expression levels
of IL-1β, IL-4, IL-6 and IFN-γ compared with in the normal group.
In the AAFE 0.5 and AAFE 1 groups the mRNA expression levels of
IL-4 and IL-6, which are produced by Th2 cells, and IFN-γ, which is
produced by Th1 cells, were suppressed in a dose-dependent manner.
In addition, the AAFE 0.5 and AAFE 1 groups exhibited reduced mRNA
expression levels of IL-1β, TNF-α and GM-CSF (Fig. 4B).
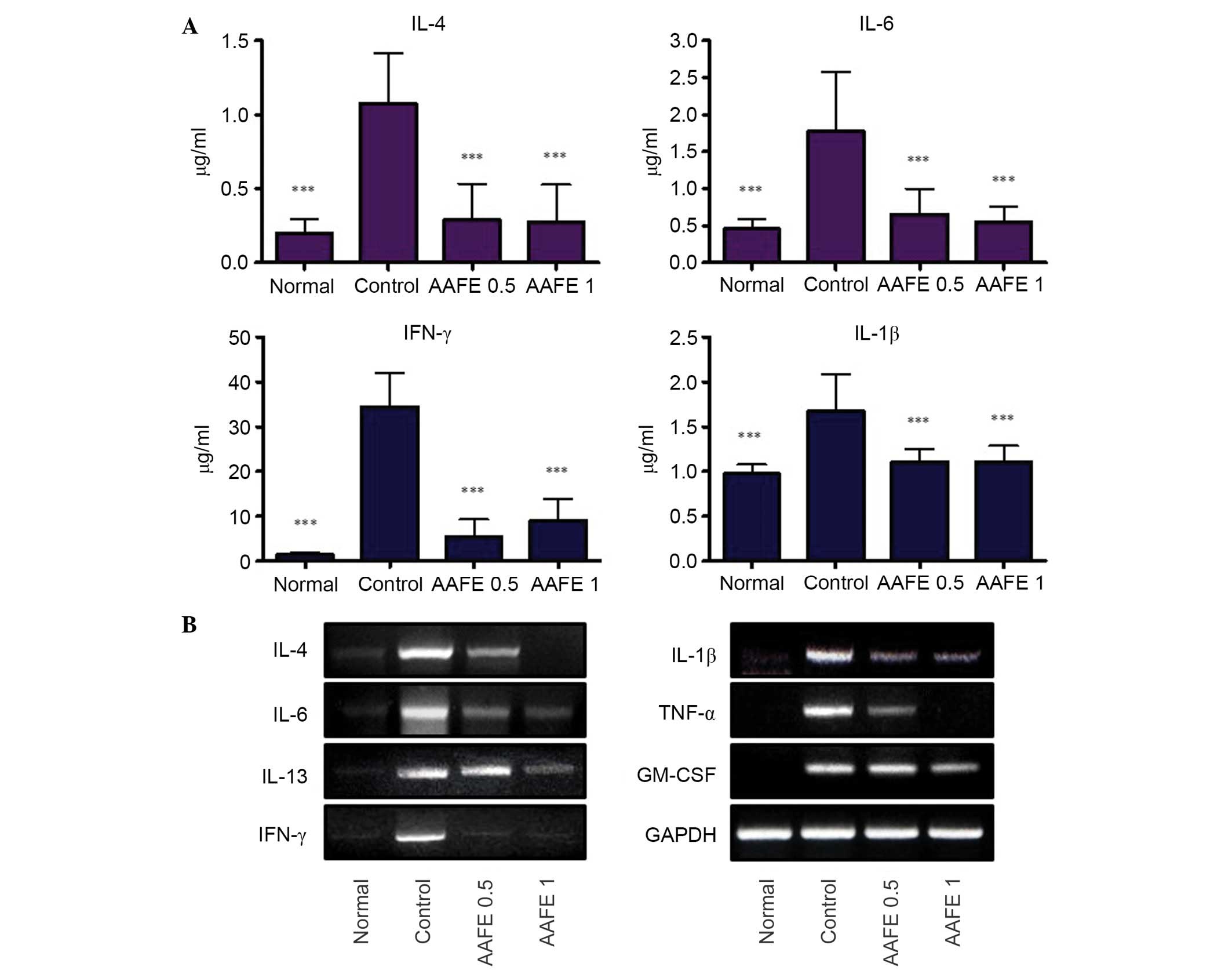 | Figure 4Effects of AAFE on serum levels of
IL-4, IL-6, IFN-γ and IL-1β, and mRNA expression levels of IL-4,
IL-6, IL-13, IFN-γ, IL-1β, TNF-α and GM-CSF in skin-derived lymph
nodes from a mouse model of AD-like lesions. (A) Serum IL-4, IL-6,
IFN-γ and IL-1β levels. Data presented as the mean ± standard
deviation from five animals. Data were analyzed using one-way
analysis of variance followed by Dunnett's post-hoc multiple
comparison test. ***P<0.001 vs. the control group.
(B) Representative IL-4, IL-6, IL-13, IFN-γ, IL-1β, TNF-α and
GM-CSF mRNA expression levels. AAFE, Artemisia argyi Folium
extract; IL, interleukin; IFN-γ, interferon-γ; TNF-α, tumor
necrosis factor-α; GM-CSF, granulocyte-macrophage
colony-stimulating factor; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase. |
Effects of AAFE on activation of Lyn,
Syk, MAPKs, PI3K/Akt and IκBα
Lyn, Syk, MAPKs, PI3K/Akt and IκBα/NF-κB signaling
pathways have previously been implicated in the pathogenesis of
allergic inflammatory skin diseases, and proinflammatory cytokines
are known to induce activation of these signaling pathways
(25,26). Therefore, the present study
investigated whether AAFE regulates Lyn, Syk, MAPKs, PI3K/Akt and
IκBα signaling associated with AD-like lesions. As shown in
Fig. 5, in the skin-draining lymph
nodes of the control group the phosphorylation of Lyn, Syk, MAPKs
(ERK, JNK and p38), PI3K/Akt and IκBα were overexpressed compared
with the normal group; however, in the AAFE 0.5 and AAFE 1 groups
the phosphorylation of Lyn, Syk, MAPKs, (ERK, JNK and p38),
PI3K/Akt and IκBα were suppressed in a dose-dependent manner
(Fig. 5).
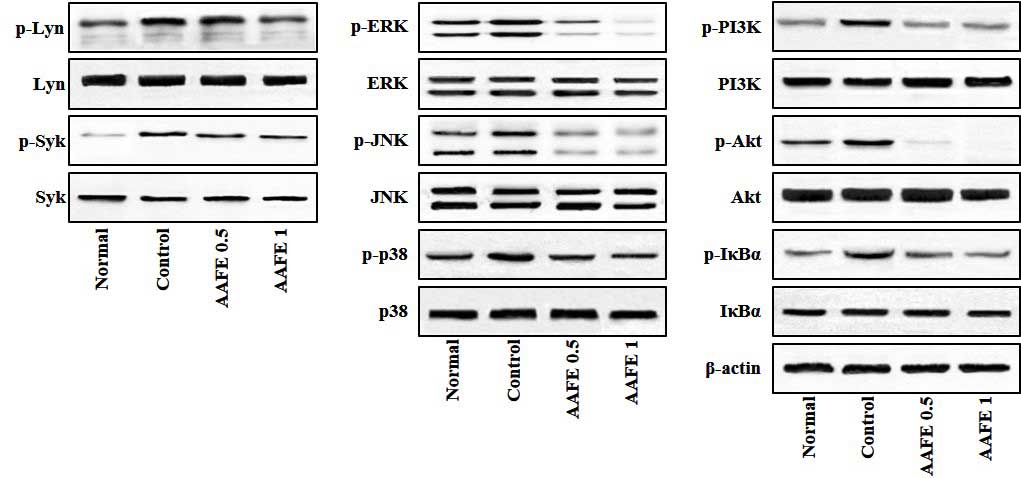 | Figure 5Effects of AAFE on the activation of
Lyn, Syk, MAPKs, PI3K/Akt and IκBα in skin-derived lymph nodes from
a mouse model of AD-like lesions. Representative Lyn, Syk, MAPKs,
PI3K/Akt and IκBα expression levels are presented. Total proteins
were analyzed by western blotting using phospho-specific or total
antibodies against Lyn, Syk, ERK, JNK, p38, PI3K, Akt, IκBα and
β-actin. p-, phosphorylated; AAFE, Artemisia argyi Folium
extract; Lyn, Lck/yes-related novel tyrosine kinase; Syk, spleen
tyrosine kinase; ERK, extracellular signal-regulated kinase; JNK,
c-jun N-terminal kinase; PI3K, phosphatidylinositol 3-kinase. |
Discussion
AD is an inflammatory, relapsing and itchy skin
disorder, which is divided into two phases. Acute AD is
characterized by erythema, eczema, excoriation, exudation and
pruritus, and represents a Th2-biased immune response. Chronic AD
is characterized by dry and thickened skin, lichenification and
fibrotic papules, and represents a Th1/Th2/non Thcomplexed immune
response.
The present study determined the effects of AAFE on
DNCB-induced AD-like lesions. Macroscopic observations indicated
that AAFE prevented the progression of AD-like pathogenesis through
a reduction of clinical symptoms, including eczema and erythema
(Fig. 1B).
TGF-β1 is a multifunctional cytokine that is
implicated in skin dendritic cell homeostasis, fibroblast growth
and tissue remodeling. TGF-β1 promotes severe skin inflammation,
the formation of lichenification and fibrotic papules in AD
lesions, and also has an important role in structural changes, such
as fibrosis, in chronic lung diseases (27). In the present study,
histopathological analysis indicated that AAFE attenuated
inflammatory symptoms and TGF-β1 immunoreactivities in ear skin
tissue (Fig. 2).
IgE-mediated hypersensitive immune responses to
generally harmless environmental antigens contribute to the
pathogenesis of AD. IgE binds to allergens and induces the
aggregation of FcεRI on the surface of mast cells/basophils,
triggering an allergic cascade through the release of inflammatory
mediators, including histamine, prostaglandins, chemokines and
cytokines (28,29). The results of the present study
suggested that AAFE exerted an inhibitory effect on histamine
release and IgE production in the serum (Fig. 3).
The cytokine milieu of patients with AD is known to
consist of Th2 cytokines, Th1 cytokines and non-Th proinflammatory
cytokines throughout the acute and chronic phases of AD (16–18).
The Th2 cytokines, which include IL-4, IL-6 and IL-13, are
systemically upregulated in AD, and the typical Th1 cytokine,
IFN-γ, is upregulated in chronic AD-skin lesions (30). Non-Th proinflammatory cytokines,
including IL-1β and TNF-α, are produced from resident cells such as
keratinocytes, mast cells and dendritic cells, thus implying their
roles in the initiation and maintenance of atopic inflammation
(30,31). In the present study, treatment with
AAFE attenuated serum levels of IL-4, IL-6, IFN-γ and IL-1β
(Fig. 4A), and the mRNA expression
levels of IL-4, IL-6, IL-13, IL-1β, TNF-α and GM-CSF in
skin-derived lymph nodes (Fig.
4B). These results suggested that oral administration of AAFE
may attenuate the excessive cytokine milieu and inflammation in a
model of AD-like skin lesions.
The Src-family kinase, Lyn, and the protein tyrosine
kinase, Syk, are key regulators in the allergic proximal signaling
pathway; these tyrosine kinases lead to the activation and tyrosine
phosphorylation of MAPKs, PI3K/Akt and IκBα downstream of Syk
(25,26). MAPKs (ERK1/2, p38 and JNK) are
involved in the immune response and transduce extracellular
signals, including inflammatory cytokines, growth factors and
stress stimuli, into intracellular responses such as inflammation,
differentiation and apoptosis (32). The PI3K/Akt pathway regulates the
mast cell response, which is related to allergic diseases (33). Activation of NF-κB signaling by
proinflammatory cytokines mediates the degradation of IκBα and the
nuclear translocation of NF-κB, which consequently induces
inflammation and tissue damage (22). In the present study, AAFE inhibited
DNCB-induced phosphorylation of Lyn/Syk, MAPKs (ERK, JNK and p38),
PI3K/Akt and IκBα (Fig. 5).
In conclusion, the findings of the present study
demonstrated that AAFE exerted ameliorative effects on DNCB-induced
AD-like lesions, by exerting anti-allergenic skin inflammatory
effects related to the recovery of skin barrier dysfunction. These
effects may be associated with suppression of cytokine abundance
via the regulation of crucial factors, including Lyn, Syk, MAPKs,
PI3K/Akt, and IκBα, during the process of AD pathogenesis.
The present study aimed to determine whether AAFE
exerts therapeutic effects in AD. The anti-AD effects of AAFE were
identified, and were shown to be related to suppression of
inflammatory actions and skin barrier damage in a mouse model of
AD-like skin lesions. Therefore, the effects of AAFE may help
improve treatment for various allergic diseases and skin
diseases.
Acknowledgments
The present study was supported by a grant from the
Dong-Eui University Foundation (grant no. 2013AA108).
References
|
1
|
Ren X, Yao C, Wu F, Li Z, Xing J and Zhang
H: Effectiveness of moxibustion treatment in quality of life in
patients with knee osteoarthritis: A randomized, double-blinded,
placebo-controlled trial. Evid Based Complement Alternat Med.
2015:5695232015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen R, Chen M, Xiong J, Chi Z, Zhang B,
Tian N, Xu Z, Zhang T, Li W, Zhang W, et al: Curative effect of
heat-sensitive moxibustion on chronic persistent asthma: A
multicenter randomized controlled trial. J Tradit Chin Med.
33:584–591. 2013. View Article : Google Scholar
|
|
3
|
Park JW, Lee BH and Lee H: Moxibustion in
the management of irritable bowel syndrome: Systematic review and
meta-analysis. BMC Complement Altern Med. 13:2472013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang J, Yu S, Lao L, Yang M, Chen J, Luo
X, Wang Y, Chen X, Li J, Zhu L, et al: Use of moxibustion to treat
primary dysmenorrhea at two interventional times: Study protocol
for a randomized controlled trial. Trials. 16:352015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo J, Wang LP, Liu CZ, Zhang J, Wang GL,
Yi JH and Cheng JL: Efficacy of acupuncture for primary insomnia: A
randomized controlled clinical trial. Evid Based Complement
Alternat Med. 2013:1638502013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeong MA, Lee KW, Yoon DY and Lee HJ:
Jaceosidin, a pharmacologically active flavone derived from
Artemisia argyi, inhibits phorbolester-induced upregulation of
COX-2 and MMP-9 by blocking phosphorylation of ERK-1 and -2 in
cultured human mammary epithelial cells. Ann N Y Acad Sci.
1095:458–466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee SH, Bae EA, Park EK, Shin YW, Baek NI,
Han EJ, Chung HG and Kim DH: Inhibitory effect of eupatilin and
jaceosidin isolated from Artemisia princeps in IgE-induced
hypersensitivity. Int Immunopharmacol. 7:1678–1684. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim MJ, Han JM, Jin YY, Baek NI, Bang MH,
Chung HG, Choi MS, Lee KT, Sok DE and Jeong TS: In vitro
antioxidant and anti-inflammatory activities of Jaceosidin from
Artemisia princeps Pampanini cv. Sajabal. Arch Pharm Res.
31:429–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Min SW, Kim NJ, Baek NI and Kim DH:
Inhibitory effect of eupatilin and jaceosidin isolated from
Artemisia princeps on carrageenan-induced inflammation in mice. J
Ethnopharmacol. 125:497–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin Y, Sun Y, Gu L, Zheng W, Gong F, Wu X,
Shen Y and Xu Q: Jaceosidin inhibits contact hypersensitivity in
mice via down-regulating IFN-γ/STAT1/T-bet signaling in T cells.
Eur J Pharmacol. 651:205–211. 2011. View Article : Google Scholar
|
|
11
|
Bao X, Yuan H, Wang C, Liu J and Lan M:
Antitumor and immunomodulatory activities of a polysaccharide from
Artemisia argyi. Carbohydr Polym. 98:1236–1243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, Li J, Sun J, Zeng KW, Cui JR,
Jiang Y and Tu PF: NO inhibitory guaianolide-derived terpenoids
from Artemisia argyi. Fitoterapia. 85:169–175. 2013. View Article : Google Scholar
|
|
13
|
Nam Y, Choi M, Hwang H, Lee MG, Kwon BM,
Lee WH and Suk K: Natural flavone jaceosidin is a neuroinflammation
inhibitor. Phytother Res. 27:404–411. 2013. View Article : Google Scholar
|
|
14
|
Zeng KW, Wang S, Dong X, Jiang Y and Tu
PF: Sesquiterpene dimer (DSF-52) from Artemisia argyi inhibits
microglia-mediated neuroinflammation via suppression of NF-κB,
JNK/p38 MAPKs and Jak2/Stat3 signaling pathways. Phytomedicine.
21:298–306. 2014. View Article : Google Scholar
|
|
15
|
Spergel JM and Paller AS: Atopic
dermatitis and the atopic march. J Allergy Clin Immunol. 112(Suppl
6): S118–S127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akdis CA, Akdis M, Trautmann A and Blaser
K: Immune regulation in atopic dermatitis. Curr Opin Immunol.
12:641–646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng W and Novak N: Pathogenesis of atopic
dermatitis. Clin Exp Allergy. 45:566–574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Martinez O, Overbergh L, Mathieu
C, Prabhakar BS and Chan LS: Early up-regulation of Th2 cytokines
and late surge of Th1 cytokines in an atopic dermatitis model. Clin
Exp Immunol. 138:375–387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dickinson RJ and Keyse SM: Diverse
physiological functions for dual-specificity MAP kinase
phosphatases. J Cell Sci. 119:4607–4615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sabio G and Davis RJ: TNF and MAP kinase
signalling pathways. Semin Immunol. 26:237–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fruman DA and Bismuth G: Fine tuning the
immune response with PI3K. Immunol Rev. 228:253–272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wullaert A, Bonnet MC and Pasparakis M:
NF-κB in the regulation of epithelial homeostasis and inflammation.
Cell Res. 21:146–158. 2011. View Article : Google Scholar
|
|
23
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington (DC), USA: 2011
|
|
24
|
Shore PA, Burkhalter A and Cohn VH Jr: A
method for the fluorometric assay of histamine in tissues. J
Pharmacol Exp Ther. 127:182–186. 1959.PubMed/NCBI
|
|
25
|
Sanderson MP, Wex E, Kono T, Uto K and
Schnapp A: Syk and Lyn mediate distinct Syk phosphorylation events
in Fcε RI-signal transduction: Implications for regulation of
IgE-mediated degranulation. Mol Immunol. 48:171–178. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siraganian RP, de Castro RO, Barbu EA and
Zhang J: Mast cell signaling: The role of protein tyrosine kinase
Syk, its activation and screening methods for new pathway
participants. FEBS Lett. 584:4933–4940. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee KS, Park SJ, Kim SR, Min KH, Lee KY,
Choe YH, Hong SH, Lee YR, Kim JS, Hong SJ, et al: Inhibition of
VEGF blocks TGF-beta1 production through a PI3K/Akt signalling
pathway. Eur Respir J. 31:523–531. 2008. View Article : Google Scholar
|
|
28
|
Gilfillan AM and Beaven MA: Regulation of
mast cell responses in health and disease. Crit Rev Immunol.
31:475–529. 2011. View Article : Google Scholar
|
|
29
|
Kalesnikoff J and Galli SJ: New
developments in mast cell biology. Nat Immunol. 9:1215–1223. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leung DY, Boguniewicz M, Howell MD, Nomura
I and Hamid QA: New insights into atopic dermatitis. J Clin Invest.
113:651–657. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schuepbach-Mallepell S, Philippe V,
Brüggen MC, Watanabe H, Roques S, Baldeschi C and Gaide O:
Antagonistic effect of the inflammasome on thymic stromal
lymphopoietin expression in the skin. J Allergy Clin Immunol.
132:1348–1357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peti W and Page R: Molecular basis of MAP
kinase regulation. Protein Sci. 22:1698–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim MS, Rådinger M and Gilfillan AM: The
multiple roles of phosphoinositide 3-kinase in mast cell biology.
Trends Immunol. 29:493–501. 2008. View Article : Google Scholar : PubMed/NCBI
|