Introduction
Antioxidant-like protein-1 (AOP-1) is an antioxidant
protein with 93.3% homology to SP-22, a mitochondrial antioxidant
protein (1). AOP-1, which is
expressed in the cytoplasm, as well as in the mitochondria, has
been known to reduce intracellular levels of reactive oxygen
species (ROS) by co-operating with mitochondrial thioredoxin and is
important in the maintenance of mitochondrial mass and membrane
potential (1–3). In addition, AOP-1 is associated with
various biological processes, including cell proliferation,
differentiation, apoptosis and gene expression (4).
Aging leads to neuroanatomical and
neurophysiological alterations in the central nervous system
(5–7). In the brain, age-related changes are
closely associated with alterations in DNA damage, synaptic vesicle
transport, autophagic degradation and neuronal activity (8–12).
In particular, oxidative stress, which accumulates with age, is
considered to be one of the major causes of aging and
age-associated cognitive impairment (8,13–16).
Certain previous studies have demonstrated that antioxidant
supplementation, which reduces oxidative stress in the brain,
ameliorates histopathological alterations, as well as learning and
memory deficits in animal models for brain aging (17–19).
In addition, it is well established that marked increases in
oxidative stress occur in the brain during the normal aging process
(16,20). However, age-related changes in
AOP-1 in the brain remain to be fully elucidated. Since the
hippocampus, which is an important region associated with learning
and memory, is the most vulnerable brain region to the aging
process (21–24), the present study aimed to
investigate age-related changes in AOP-1 in the hippocampus of the
Mongolian gerbil, which is a suitable model for research on aging
(20,25).
Materials and methods
Experimental animals
A total of 42 male Mongolian gerbils (Meriones
unguiculatus) were obtained from the Experimental Animal
Center, Kangwon National University (Chuncheon, South Korea). As
previously described (9), the
animals were divided into the following three groups (n=14/group):
Young (3–4 months old; weighing 65–72 g), adult (10–12 months old;
weighing 77–83 g) and aged (18–24 months old; weighing 85–95 g).
The animals were housed in a conventional state under an adequate
temperature (23±3°C) and relative humidity (55±5%) with a 12 h
light/dark cycle, and were allowed free access to food and water.
All experimental procedures for animal handling and use were
approved by the Institutional Animal Care and Use Committee at
Kangwon National University (approval no. KW-130424-3).
Western blot analysis
To examine alterations in the protein expression of
AOP-1 in the hippocampus during normal aging, the animals
(n=7/group) were used, and western blot analysis was performed, as
previously described (9,10,26).
Briefly, the hippocampus was homogenized and centrifuged, at 16,000
× g for 20 min at 4°C and the supernatants were subjected to
western blot analysis. Following centrifugation, the protein level
in the supernatants was determined using a micro bicinchoninic acid
protein assay kit (Sigma-Aldrich, St. Louis, MO, USA) with bovine
serum albumin as a standard (Pierce Biotechnology, Inc., Rockford,
IL, USA). Aliquots containing 20 μg total protein were
boiled in loading buffer containing 150 mM Tris (pH 6.8,
Sigma-Aldrich), 3 mM dithiothreitol, 6% sodium dodecyl sulfate,
0.3% bromophenol blue and 30% glycerol. The aliquots were then
loaded onto a 12% polyacrylamide gel. Following electrophoresis,
the proteins were transferred to nitrocellulose transfer membranes
(Pall Corporation, East Hills, NY, USA). To reduce background
staining, the membranes were incubated with 5% non-fat dry milk
(Sigma-Aldrich) in phosphate-buffered saline (PBS) containing 0.1%
Tween-20 (PBST; Sigma-Aldrich) for 45 min at room temperature.
Membranes were incubated with monoclonal mouse anti-AOP-1 (cat. no.
A7674; dilution, 1:2,500; Sigma-Aldrich) or monoclonal mouse
anti-β-actin (cat. no. A53161; dilution, 1:2,500; Sigma-Aldrich)
overnight at 4°C. Following washing with PBST three times, the
membranes were incubated with peroxidase-conjugated goat anti-mouse
(cat. no. sc-2031; dilution, 1:1,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) for 1 h at room temperature. An enhanced
chemiluminescence kit (Pierce ECL Western Blotting Substrate;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) The result of the
western blot analysis was scanned and densitometric analysis for
the quantification of the bands was performed using Image J 1.49
software (National Institutes of Health, Bethesda, MD, USA), which
was used to count the relative optical density (ROD). The
expression of AOP-1 was normalized against that of β-actin, which
was used as the internal control protein. A ratio of the ROD was
calibrated as a percentage, with the young group designated as
100%. Western blot analysis was performed with three
replicates.
Immunohistochemistry
To examine age-related changes in AOP-1
immunoreactivity in the gerbil hippocampus, immunohistochemical
staining was performed, as previously described (9,10,26).
In brief, the animals (n=7/group) were anesthetized with Zoletil 50
(30 mg/kg; Virbac, Carros, France) and perfused transcardially with
4% paraformaldehyde (Sigma-Aldrich) in 0.1 M phosphate-buffered
saline (pH 7.4; Sigma-Aldrich). The brains were postfixed with the
same solution for 6 h and sectioned with a cryostat at 30
μm. Immunohistochemical staining for AOP-1 was performed
using monoclonal mouse anti-AOP-1 (cat. no. A7674; dilution, 1:500;
Sigma-Aldrich) as a primary antibody. The brain tissues were
subsequently incubated with biotinylated goat anti-mouse
immunoglobulin G (cat. no. BA-9200; 1:200; Vector Laboratories,
Burlingame, CA, USA) and streptavidin peroxidase complex (cat. no.
SA-5004; 1:200; Vector Laboratories). A negative control test was
performed using pre-immune serum instead of primary antibody in
order to establish the specificity of the immunostaining. The
negative control resulted in the absence of immunoreactivity in all
structures.
A total of six sections with 90 μm intervals
per animal were selected to quantitatively analyze AOP-1
immunoreactivity. Digital images of the hippocampal subregions were
captured with an AxioM2 light microscope (Carl Zeiss, Oberkochen,
Germany) equipped with a digital camera (Axiocam; Carl Zeiss)
connected to a PC monitor. According to our previous method
(27), AOP-1 immunoreactivity was
evaluated using digital image analysis software (MetaMorph 4.01;
Universal Imaging Corp., Bedford Hills, NY, USA). The
immunoreactivity was measured using a 0–255 gray scale system
(white to dark signal between 255 and 0). AOP-1 immunoreactivity
levels of experimental groups were calibrated with the background
in the same section. Subsequently, the ratio of the
immunoreactivity was calibrated as a percentage, with the young
group designated as 100%.
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. The data were evaluated by a two-way analysis of
variance using SPSS software (version 12.0; SPSS, Inc., Chicago,
IL, USA) and the means were assessed using Duncan's multiple-range
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
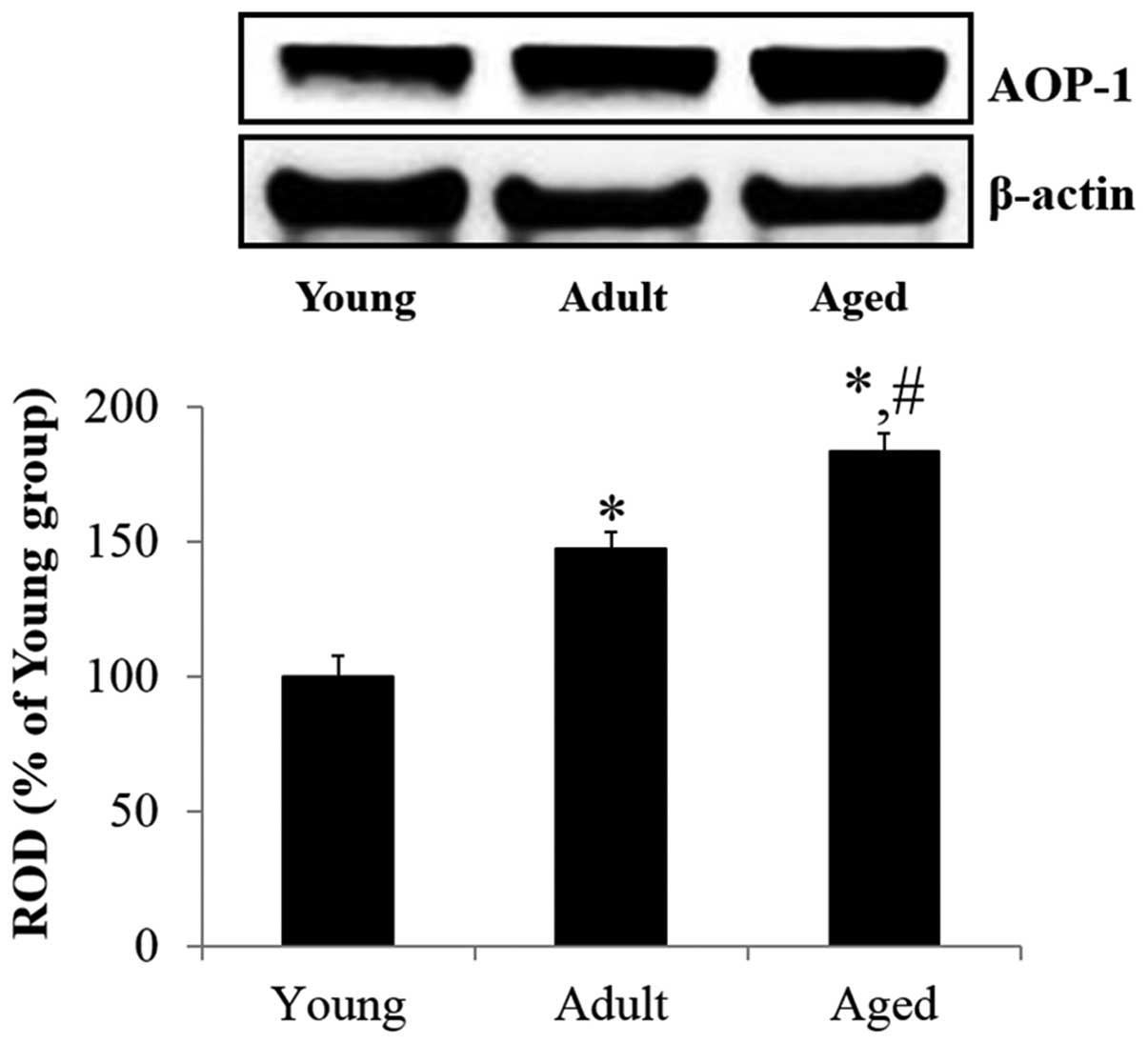
AOP-1 protein level in the
hippocampus
Western blot analysis demonstrated that the protein
expression of AOP-1 was gradually and significantly increased in
the hippocampus with normal aging. The protein expression of AOP-1
in the adult hippocampus was significantly increased (147.0%,
F=9.914) compared with the young group. In the aged group, the
protein expression of AOP-1 in the hippocampus was significantly
higher (124.7%, F=8.107) compared with that in the adult group
(Fig. 1).
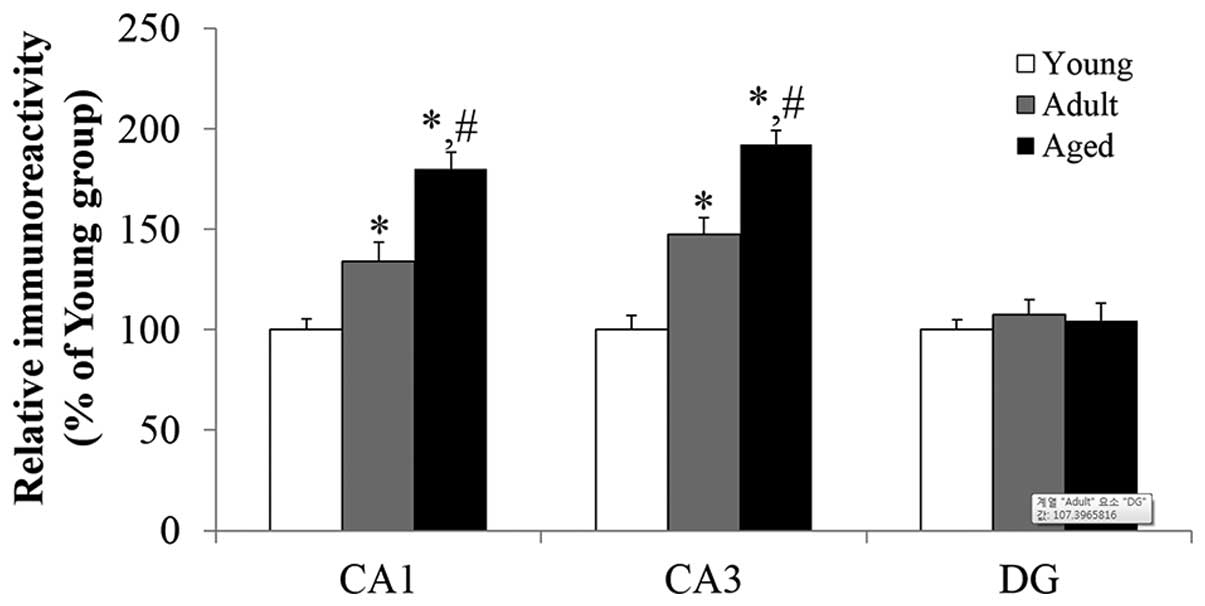
AOP-1 immunoreactivity in the hippocampus
proper
Age-related changes in AOP-1 immunoreactivity
(Fig. 2) in the hippocampus proper
(CA1-3 regions) were found to be generally similar to the result of
western blot analysis. In the hippocampus proper, weak AOP-1
immunoreactivity was primarily detected in pyramidal neurons of the
stratum pyramidale of the young group (Figs. 2A, D, G and 3). In the adult group, AOP-1
immunoreactivity in the pyramidal neurons of the hippocampus proper
was increased, compared with that in the young group (Figs. 2B, E, H and 3; F=34.407 in the CA1 region and F=25.579
in the CA3 region, respectively). In the aged group, AOP-1
immunoreactivity was significantly increased in the pyramidal
neurons of the hippocampus proper (Figs. 2C, F, I and 3; F=24.753 in the CA1 region and F=51.553
in the CA3 region, respectively), compared with the young
group.
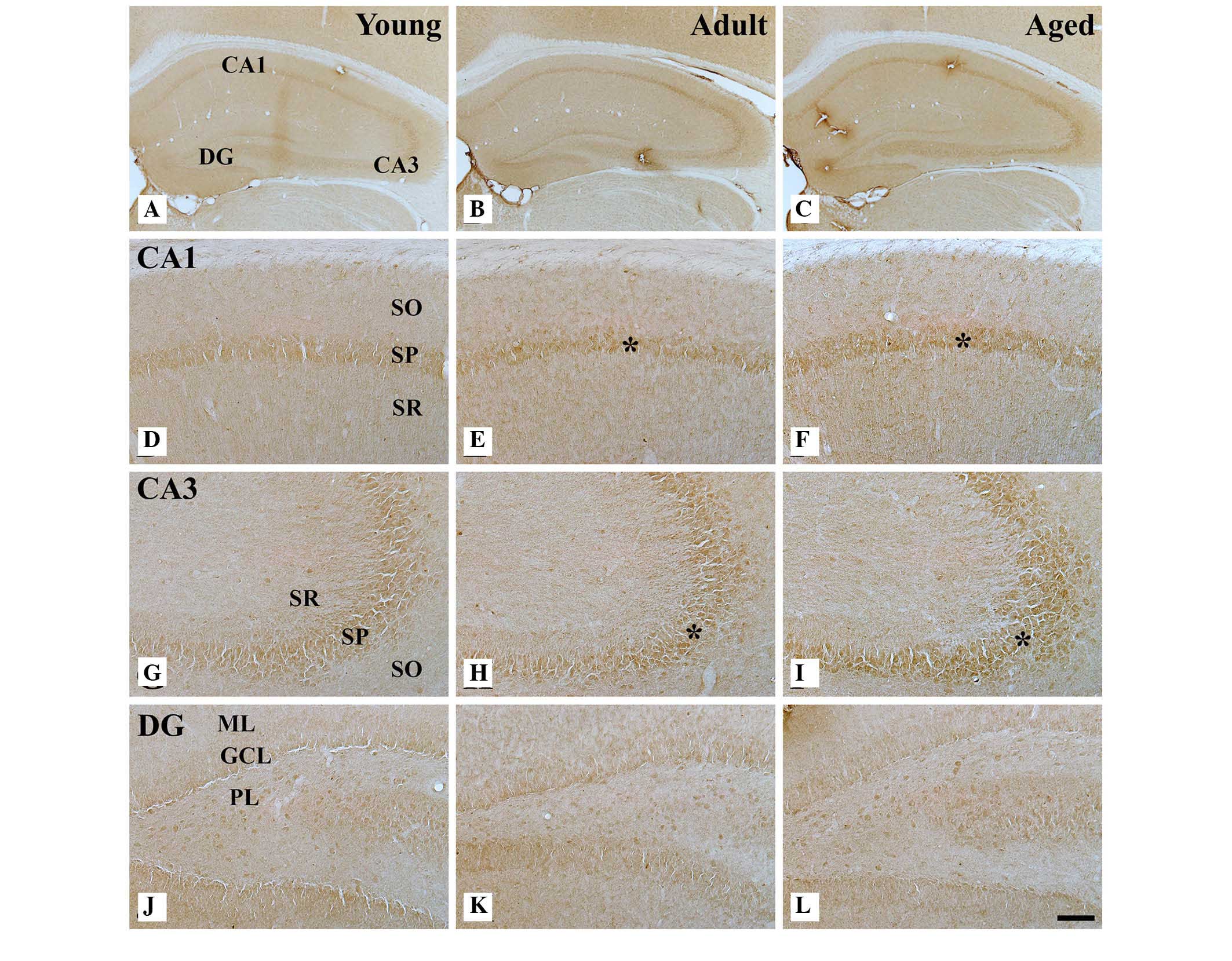 | Figure 2AOP-1 immunohistochemistry in the
(A–C) hippocampus, (D–F) hippocampal CA1 region, (G–I) CA3 region
and (J–L) dentate gyrus of the young (left), adult (middle) and
aged groups (right). AOP-1 immunoreactivity is increased in the
SP (indicated by
asterisks) of the CA1 and CA3 region during the aging process.
However, no marked difference was observed in AOP-1
immunoreactivity in the DG among all groups. Scale bar, 400
μm (A–C) and 50 μm (D–L). GCL, granule cell layer;
ML, molecular layer; PL, polymorphic layer; SO, stratum oriens; SR,
stratum radiatum; SP, stratum pyramidale; DG, dentate gyrus; AOP-1,
antioxidant-like protein-1. |
AOP-1 immunoreactivity in the dentate
gyrus
In the young group, AOP-1 immunoreactivity was
observed predominantly in granule cells of the granule cell layer
and in polymorphic cells of the polymorphic layer (Fig. 2J). AOP-1 immunoreactivity in those
cells of the dentate gyrus was not altered in the adult and aged
groups, compared with that in the young group (Figs. 2K, L and 3).
Discussion
Our previous study demonstrated no marked
age-related histopathological alterations in the hippocampus
between adult and aged gerbils, as determined by terminal
deoxynucleotidyl transferase dUTP nick end labeling staining and
Fluoro-Jade B histofluorescence staining, although a marked
activation of microglia was observed in the aged group (28). However, in the present, western
blot analysis revealed that the protein expression of AOP-1 was
significantly increased during the normal aging process. It can be
postulated that the significant increase in AOP-1 protein level
occurs due to the age-dependent increases in oxidative stress in
the hippocampus.
Numerous previous studies have reported a
significant increase in oxidative stress in the brain during the
aging process (14,15,20).
On the basis of previous studies (8,14–16,20),
which demonstrated the age-related decrease in antioxidant enzymes
in the brain, the basal levels of oxidative stress markers and the
production of ROS in the hippocampus at various stages of aging was
not measured in the present study. In particular, Selaković et
al (20) reported that basal
levels of certain types of oxidative stress, including superoxide
anion production, superoxide dismutase activity and the index of
lipid peroxidation, were significantly increased in the hippocampus
of the 10-month-old gerbil, compared with those of the 3-month-old
gerbil. It was also reported that the level of lipid peroxidation
was increased and the levels of antioxidant enzymes, including
catalase, superoxide dismutase and glutathione peroxidase, were
decreased in the brain of aged rats compared with those of young
controls (16). The authors
suggested that the age-related decrease in antioxidant enzymes in
the brain is due to deficient antioxidative enzyme defense against
ROS (16).
The present study also found that AOP-1
immunoreactivity was significantly increased in the hippocampus
proper, not in the dentate gyrus and among hippocampal subregions
during the aging process. Therefore, it can be hypothesized that
the age-dependent increase in AOP-1 occurs primarily in the
hippocampus proper. However, it is difficult to explain exactly why
the age-dependent increase in AOP-1 immunoreactivity occurs in the
hippocampus proper and not in the dentate gyrus, since, to the best
of our knowledge, this is the first study regarding the
age-dependent change in AOP-1 protein expression in the
hippocampus. Among the hippocampal subregions, the dentate gyrus is
known as the most sensitive and vulnerable region during the normal
aging process, whereas the hippocampus proper is relatively
resistant (29). In addition, our
previous study demonstrated that FoxO3a, which is strongly
associated with the aging process and senescence (30,31),
was significantly decreased in the dentate gyrus, not in the
hippocampus proper, of the aged gerbil hippocampus (26). On the basis of the present and
other previous studies, it is likely that the expression patterns
of biomarkers for brain aging are different according to brain
areas. In this regard, enhanced AOP-1 expression in the hippocampus
proper, not in the dentate gyrus, may be one of the possible
mechanisms explaining why the dentate gyrus is more sensitive to
oxidative stress compared with the other hippocampal subregions
during the normal aging process.
In conclusion, the present study indicated that
AOP-1 expression is significantly increased in the aged
hippocampus, particularly in the hippocampus proper, during the
normal aging process and suggests that age-related increases in
AOP-1 may be a compensatory process against the elevation of
oxidative stress in the aged hippocampus.
Acknowledgments
The present study was supported by a 2015 Research
Grant from Kangwon National University (no. 520150344), the
National Research Foundation of Korea (NRF) funded by the Ministry
of Education, Science and Technology (no. 2010-0010580) and by the
Basic Science Research Program through the NRF funded by the
Ministry of Education (no. NRF-2014R1A1A2058440).
References
|
1
|
Shih SF, Wu YH, Hung CH, Yang HY and Lin
JY: Abrin triggers cell death by inactivating a thiol-specific
antioxidant protein. J Biol Chem. 276:21870–21877. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng Y, Liu DQ, Wang Z, Liu Z, Cao HQ,
Wang LY, Shi N and Meng XM: AOP-1 interacts with cardiac-specific
protein kinase TNNI3K and down-regulates its kinase activity.
Biochemistry (Mosc). 72:1199–1204. 2007. View Article : Google Scholar
|
|
3
|
Wonsey DR, Zeller KI and Dang CV: The
c-Myc target gene PRDX3 is required for mitochondrial homeostasis
and neoplastic transformation. Proc Natl Acad Sci USA.
99:6649–6654. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujii J and Ikeda Y: Advances in our
understanding of peroxiredoxin, a multifunctional, mammalian redox
protein. Redox Rep. 7:123–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gallagher M, Bizon JL, Hoyt EC, Helm KA
and Lund PK: Effects of aging on the hippocampal formation in a
naturally occurring animal model of mild cognitive impairment. Exp
Gerontol. 38:71–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He WB, Zhang JL, Hu JF, Zhang Y, Machida T
and Chen NH: Effects of glucocorticoids on age-related impairments
of hippocampal structure and function in mice. Cell Mol Neurobiol.
28:277–291. 2008. View Article : Google Scholar
|
|
7
|
Himeda T, Mizuno K, Kato H and Araki T:
Effects of age on immunohistochemical changes in the mouse
hippocampus. Mech Ageing Dev. 126:673–677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cardozo-Pelaez F, Brooks PJ, Stedeford T,
Song S and Sanchez-Ramos J: DNA damage, repair, and antioxidant
systems in brain regions: A correlative study. Free Radic Biol Med.
28:779–785. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi HS, Ahn JH, Park JH, Won MH and Lee
CH: Age-dependent changes in expressions of Redd1 and mTOR proteins
in the gerbil hippocampus during normal aging. Mol Med Rep.
13:2409–2414. 2016.PubMed/NCBI
|
|
10
|
Lee CH and Won MH: Increased dynamin-1 and
-2 protein expression in the aged gerbil hippocampus. Cell Mol
Neurobiol. 34:791–796. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rutten BP, Korr H, Steinbusch HW and
Schmitz C: The aging brain: Less neurons could be better. Mech
Ageing Dev. 124:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y
and Fu L: mTOR and autophagy in normal brain aging and caloric
restriction ameliorating age-related cognition deficits. Behav
Brain Res. 264:82–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bagheri M, Joghataei MT, Mohseni S and
Roghani M: Genistein ameliorates learning and memory deficits in
amyloid β (1–40) rat model of Alzheimer's disease. Neurobiol Learn
Mem. 95:270–276. 2011. View Article : Google Scholar
|
|
14
|
Desai KM, Chang T, Wang H, Banigesh A,
Dhar A, Liu J, Untereiner A and Wu L: Oxidative stress and aging:
Is methylglyoxal the hidden enemy? Can J Physiol Pharmacol.
88:273–284. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Finkel T and Holbrook NJ: Oxidants,
oxidative stress and the biology of ageing. Nature. 408:239–247.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haider S, Saleem S, Perveen T, Tabassum S,
Batool Z, Sadir S, Liaquat L and Madiha S: Age-related learning and
memory deficits in rats: Role of altered brain neurotransmitters,
acetylcholinesterase activity and changes in antioxidant defense
system. Age (Dordr). 36:96532014. View Article : Google Scholar
|
|
17
|
Aydin AF, Çoban J, Doğan-Ekici I,
Betül-Kalaz E, Doğru-Abbasoğlu S and Uysal M: Carnosine and taurine
treatments diminished brain oxidative stress and apoptosis in
D-galactose aging model. Metab Brain Dis. 31:337–345. 2016.
View Article : Google Scholar
|
|
18
|
Qu Z, Zhang J, Yang H, Huo L, Gao J, Chen
H and Gao W: Protective effect of tetrahydropalmatine against
d-galactose induced memory impairment in rat. Physiol Behav.
154:114–125. 2016. View Article : Google Scholar
|
|
19
|
Tchantchou F, Chan A, Kifle L, Ortiz D and
Shea TB: Apple juice concentrate prevents oxidative damage and
impaired maze performance in aged mice. J Alzheimers Dis.
8:283–287. 2005.PubMed/NCBI
|
|
20
|
Selaković V, Rauš Balind S, Radenović L,
Prolić Z and Janać B: Age-dependent effects of ELF-MF on oxidative
stress in the brain of Mongolian gerbils. Cell Biochem Biophys.
66:513–521. 2013. View Article : Google Scholar
|
|
21
|
Kan H, Hu W, Wang Y, Wu W, Yin Y, Liang Y,
Wang C, Huang D and Li W: NADPH oxidase-derived production of
reactive oxygen species is involved in learning and memory
impairments in 16-month-old female rats. Mol Med Rep. 12:4546–4553.
2015.PubMed/NCBI
|
|
22
|
Liao J, Xia X, Wang GZ, Shi YM and Ge JW:
Naotaifang extract treatment results in increased ferroportin
expression in the hippocampus of rats subjected to cerebral
ischemia. Mol Med Rep. 11:4047–4052. 2015.PubMed/NCBI
|
|
23
|
Sasaki K and Yoshizaki F: Investigation
into hippocampal nerve cell damage through the mineralocorticoid
receptor in mice. Mol Med Rep. 12:7211–7220. 2015.PubMed/NCBI
|
|
24
|
Xu Y, Liu Z, Song X, Zhang K, Li X, Li J,
Yan X, Li Y, Xie Z and Zhang H: Cerebralcare Granule®
attenuates cognitive impairment in rats continuously overexpressing
microRNA-30e. Mol Med Rep. 12:8032–8040. 2015.PubMed/NCBI
|
|
25
|
Cheal ML: The gerbil: A unique model for
research on aging. Exp Aging Res. 12:3–21. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park JH, Lee CH, Yoo KY, Choi JH, Hwang
IK, Lee JY, Kang IJ and Won MH: FoxO3a immunoreactivity is markedly
decreased in the dentate gyrus, not the hippocampus proper, of the
aged gerbil. Exp Gerontol. 46:836–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee CH, Park JH, Cho JH, Kim IH, Ahn JH,
Lee JC, Chen BH, Shin BN, Tae HJ, Bae EJ, et al: Effect of oenanthe
javanica extract on antioxidant enzyme in the rat liver. Chin Med J
(Engl). 128:1649–1654. 2015. View Article : Google Scholar
|
|
28
|
Lee CH, Yoo KY, Choi JH, Park OK, Hwang
IK, Kim SK, Kang IJ, Kim YM and Won MH: Neuronal damage is much
delayed and microgliosis is more severe in the aged hippocampus
induced by transient cerebral ischemia compared to the adult
hippocampus. J Neurol Sci. 294:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Small SA, Chawla MK, Buonocore M, Rapp PR
and Barnes CA: Imaging correlates of brain function in monkeys and
rats isolates a hippocampal subregion differentially vulnerable to
aging. Proc Natl Acad Sci USA. 101:7181–7186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kyoung Kim H, Kyoung Kim Y, Song IH, Baek
SH, Lee SR, Hye Kim J and Kim JR: Down-regulation of a forkhead
transcription factor, FOXO3a, accelerates cellular senescence in
human dermal fibroblasts. J Gerontol A Biol Sci Med Sci. 60:4–9.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li M, Chiu JF, Mossman BT and Fukagawa NK:
Down-regulation of manganese-superoxide dismutase through
phosphorylation of FOXO3a by Akt in explanted vascular smooth
muscle cells from old rats. J Biol Chem. 281:40429–40439. 2006.
View Article : Google Scholar : PubMed/NCBI
|