Introduction
Pulmonary arterial hypertension (PAH), defined as a
mean pulmonary arterial pressure (mPAP) ≥25 mm Hg at rest, is a
clinical syndrome of heart-lung circulation disorder, and can
ultimately result in right heart failure with higher morbidity and
mortality rates (1,2). Various types of PAH may affect up to
100,000,000 individuals worldwide (3). The estimated prevalence of PAH is
~15/1,000,000 individuals, with a mean age of 50±15 years, and
women constitute 75% of those diagnosed (4,5). The
average duration between the onset of symptoms and diagnosis is
>2 years (5), and the 5-year
mortality rate has reached 34% (6), reinforcing the importance of
diagnosis, treatment and prognosis of PAH, which depends on
investigations of the pathogenesis and etiology of the disease.
PHA is characterized by endothelial dysfunction and
structural remodeling of the pulmonary vasculature, mediated
initially by reduced oxygen availability in the lungs (7,8).
Cell sensing and rapid response to oxygen deprivation are essential
for survival of the organisms, in which the regulation of oxygen
homeostasis becomes an important physiological system (9). As a result of evolution, adaptation
to hypoxia involves a number of genes, in which hypoxia inducible
factor (HIF) is considered to be a core regulator (10).
The first HIF, HIF-1, is a highly conserved
transcription factor in almost all cells, and is involved in
pathological processes associated with hypoxia, including pulmonary
and systemic hypertension, cancer and ischemic myocardial injury
(11–14). HIF-1 is a heterodimeric protein
comprised of an oxygen-regulated HIF-1α subunit and a
constitutively expressed HIF-1β subunit, also termed aryl
hydrocarbon receptor nuclear translocater (15,16).
HIF-1α is a master regulator of transcription in hypoxic cells and
forms a dimer with HIF-1β, further activating genes involved in
energy metabolism, cell proliferation and extracellular matrix
reorganization (17,18). It has been reported that hypoxia
mediates vascular remodeling through the induction of HIF-1α. In
particular, HIF-1α in smooth muscle cells was demonstrated to be
important in hypoxia-induced PAH in mice (19–21).
However, the upstream signaling events responsible for hypoxia, and
its effects on the proliferation of vascular smooth muscular and
endothelial cells, remain to be fully elucidated.
Certain reports have shown that the expression and
activity of HIF-1α are regulated by several protein kinase
signaling pathways, in which extracellular signal-regulated kinase
(ERK) and the serine/threonine kinase, Akt, have been identified as
potent modulators of the expression of HIF-1α (22–25).
ERK is a subfamily member of the mitogen-activated protein kinase
(MAPK) family, and its pathway has been recognized to mediate cell
growth, proliferation and survival (26,27).
Li et al (28) found that
the activation of ERK signaling induces the expression of HIF-1α
and stimulates its transcriptional activity in the developing rat
brain following hypoxia-ischemia, and an increase in the
phosphorylation of ERK1/2 has been observed in retinal
neovascularization and vein occlusion (29). In addition, Akt is activated by the
phosphoinositide 3-kinase (PI3K)-dependent pathway, which is
crucial in cell differentiation, proliferation and survival
(30,31). Numerous studies have revealed the
PI3K/Akt pathway to be critical for ischemia and angiogenesis
(32,33), for example, the PI3K/Akt pathway is
required for the upregulation of HIF-1α in a rat model of focal
cerebral ischemia (34,35). However, whether these two pathways
account for the occurrence of PAH induced by hypoxic conditions
remains to be elucidated.
In the present study, the genes coding HIF-1α
proteins were cloned from the lung tissues of human patients with
PAH, and then were investigated by immunofluorescent techniques and
bioinformatic methods. In addition, the expression and
phosphorylation levels of the HIF-1α pathway components, including
PI3K, Akt, ERK1/2 and HIF-1β, were examined using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses, and the association between target genes and
the development of PAH were examined. The present clinical study
aimed to contribute to the elucidation of the role of HIF-1α and
its intracellular pathway in the occurrence of PAH, and provide a
reference for further functional investigations of the pathogenesis
of PAH.
Materials and methods
Collection of clinical samples
Human lung tissues were collected from participants
during palliative surgery at the Affiliated Hospital of Guangdong
Medical College (Guangdong, China). The participants comprised
patients with PAH (mPAP >30 mmHg; n=5) and a control group of
individuals with mPAP ≤20 mmHg (n=4). A total of 9 patients
including 4 male and 5 female patients aged 15–53 years old (mean,
33.1±15.9 years old) were recruited. According to the updated
clinical classification of pulmonary hypertension, and the
guidelines of the American College of Cardiology and American Heart
Association, PAH was diagnosed using right heart catheterization
(36). The lung tissues collected
from the inferior lobes of left lungs were stored at −80°C for
further manipulation. All clinical protocols and experimental
procedures were approved by the ethics committee of the Affiliated
Hospital of Guangdong Medical College, and a written informed
consent form was obtained from each individual participant.
Gene cloning
The cDNA fragments of HIF-1α and HIF-1β of patients
with PAH were amplified using the Takara RNA LA PCR kit (AMV). PCR
amplification was conducted at 94°C for 4 min, followed by 35
cycles at 94°C for 40 s, at 60°C for 50 s, at 72°C for 3 min, and a
final extension at 72°C for 10 min. The primer sequences of human
HIF-1α were 5′-CGAACGACAAGAAAAAGATAAG-3′ (sense) and
5′-CCACAGAAGATGTTTATTTGATG-3′ (antisense), and HIF-1β were
5′-CCGAAATGACATCAGATGTAC-3′ (sense) and
5′-GTTAGATCAGGGAATTCTTCATTG-3′ (antisense). The PCR products were
sequenced by Invitrogen (Thermo Fisher Scientific, Inc., Shanghai,
China). The sequencing results were used as queries in the BLAST
searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Bioinformatic analyses
The sequences containing the complete coding regions
of the human HIF-1α and HIF-1β genes, and the corresponding amino
acid sequences were obtained from the GenBank (http://www.ncbi.nlm.nih.gov/genbank) and GenPept
(http://www.ncbi.nlm.nih.gov/protein)
databases (HIF-1α, GenBank accession no. U22431; GenPept accession
no. AAC50152; HIF-1β, GenBank accession no. M69238; GenPept
accession no. AAH60838 (37,38).
Comparative bioinformatics analyses of HIF-1α and
HIF-1β were performed online (http://www.ncbi.nlm.nih.gov and http://www.expasy.org). The protein physical and
chemical parameters were circulated using the Protparam tool
(http://web.expasy.org/protparam)
(39). The motifs and structural
domains were searched in the amino acid sequences using the NCBI
conserved domain database (CDD; http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml)
(40–43) and the secondary structures were
predicted using the self-optimized prediction method (SOPMA;
https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html)
(44).
RT-qPCR analysis
The lung tissues were homogenized on ice with a
Teflon-pestle homogenizer in TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), and total RNAs were
isolated following the manufacturer's instructions. A 1 µg sample
of total RNA was reverse-transcribed into cDNA using AMV Reverse
Transcriptase XL (Takara Bio, Inc., Otsu, Japan) and olido (dT)
primers at 42°C for 1 h. The primers for RT-qPCR are listed in
Table I; GAPDH was selected as the
internal control gene for normalization. The qPCR analysis was
performed using 2 µl of cDNA in a total volume of 20 µl containing
10 µl 2X SYBR Premix Ex Taq II (Takara Bio, Inc.,), 0.8 µl forward
primer (10 µM) and 0.8 µl reverse primer (10 µM), in a
LightCycler® 480 System Real-Time PCR system (Roche
Diagnostics GmbH, Mannheim, Germany) using the following thermal
cycling profile: 95°C for 30 sec, followed by 40 cycles of
amplification (95°C for 5 sec and 60°C for 20 sec). The qPCR
reactions were performed in triplicate. Fluorescence was detected
during annealing and extension, and melting curve analysis was
performed immediately following the PCR cycling. The relative
transcript levels were analyzed using the
2−ΔΔCq method (45).
 | Table I.Sequences of primers. |
Table I.
Sequences of primers.
| Gene | Primer
sequence | Size (bp) |
|---|
| GAPDH | Forward
5′-GGCACAGTCAAGGCTGAGAATG-3′ | 143 |
|
| Reward
5′-ATGGTGGTGAAGACGCCAGTA-3′ |
|
| PIK3CA | Forward
5′-TCTGTCTCCTCTAAACCCTG-3 | 103 |
|
| Reward
5′-TTCTCCCAATTCAACCAC-3′ |
|
| Akt1 | Forward
5′-TCTTTGCCGGTATCGTGT-3′ | 150 |
|
| Reward
5′-TGTCATCTTGGTCAGGTGGT-3′ |
|
| Erk1 | Forward
5′-GGGGAGGTGGAGATGGTGA-3′ | 175 |
|
| Reward
5′-GCTGGCAGTAGGTCTGATGTT-3′ |
|
| Erk2 | Forward
5′-TGTTCCCAAATGCTGACT-3′ | 160 |
|
| Reward
5′-AACTTGAATGGTGCTTCG-3′ |
|
| HIF-1α | Forward
5′-GCTCATCAGTTGCCACTTCCAC-3 | 144 |
|
| Reward
5′-CATCTGTGCTTTCATGTCATCTTC-3′ |
|
| HIF-1β | Forward
5′-TGTGGACCCAGTTTCTGTGA-3 | 100 |
|
| Reward
5′-GACCACCACGAAGTGAGGTT-3′ |
|
Western blot analysis
The lung tissues were homogenized in ice-cold cell
lysis buffer for western blot analysis and IP (Beyotime Institute
of Biotechnology, Shanghai, China), and were centrifuged at 10,000
× g for 5 min at 4°C. The supernatants were used for sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western
blot analysis. The concentrations of the proteins in the
supernatants were detected using an Enhanced BCA Protein Assay kit
(Beyotime Institute of Biotechnology). The protein (~50 µg) was
separated using 10% SDS-PAGE and transferred onto PVDF membranes
(EMD Millipore, Billerica, MA, USA). The membranes were blocked
with 5% fat-free milk in Tris-buffered saline with 0.1% Tween-20
(TBS-T) and probed with the following primary antibodies: Mouse
monoclonal HIF-1α (610958; 1:500; BD Biosciences, San Jose, CA,
USA), rabbit polyclonal HIF-1β (bs-1407R; 1:500, BIOSS, Beijing,
China), rabbit monoclonal ERK (#4695; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit monoclonal
phosphorylated (p)-ERK (Thr202/Tyr204; #4370; 1:2,000; Cell
Signaling Technology, Inc.), rabbit monoclonal Akt (#4691; 1:1,000;
Cell Signaling Technology, Inc.) and p-Akt (ser473; #4060; 1:2,000;
Cell Signaling Technology, Inc.) in TBS-T containing 5% bovine
serum albumin (Beyotime Institute of Biotechnology) overnight at
4°C. Following rinsing in TBS-T three times, the membranes were
incubated with goat anti-mouse (#7076) and goat anti-rabbit (#7074)
horseradish-peroxidase-coupled secondary antibodies (Cell Signaling
Technology, Inc.) for 1 h at room temperature. Immunodetection was
performed using BeyoECL Plus (Beyotime Institute of Biotechnology).
Bands were visualized using the Bio-Rad ChemiDoc MP system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and analyzed using Quantity
One software (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data were analyzed using an independent-samples
t-test with SPSS 20.0 software (IBM SPSS, Armonk, NY, USA.
The data are presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
Bioinformatics analysis of HIF-1α and
HIF-1β
The cDNA sequences of human HIF-1α and HIF-1β were
aligned using the Basic Local Alignment Search Tool (BLAST) in the
nucleotide database, and the results showed that they were 100%
homologous with homo sapiens HIF-1α and HIF-1β mRNAs,
respectively. The biochemical properties and molecular structures
of human HIF-1α and HIF-1β were analyzed using the online tools,
ProtParam and SOPMA, the results of which are listed in the
Table II. As the dimer of these
two subunits, the HIF protein was found to consist of 1,244 amino
acids with a molecular weight of 138.592 Da; the most frequent
residues were Leu and Ser. The functional domains were scanned in
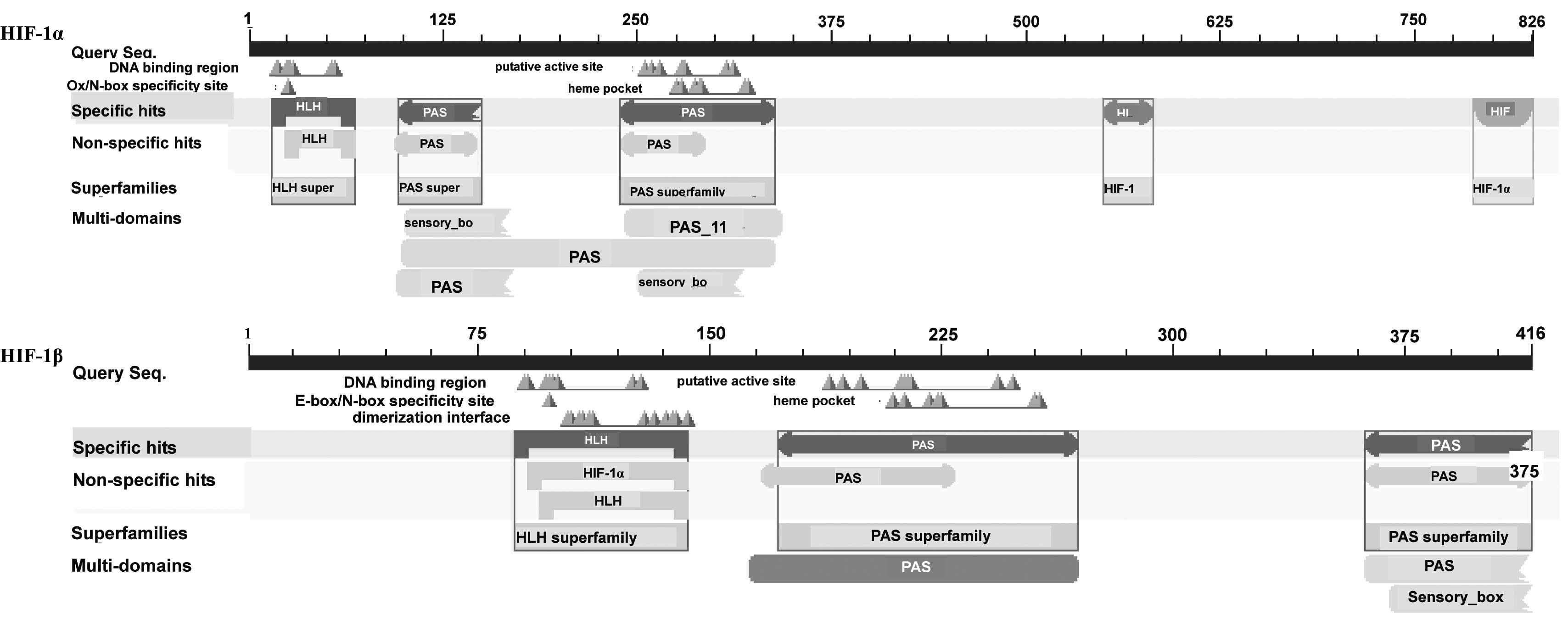
the CDD database (Fig. 1),
following which three motifs were obtained, including a
basic-helix-loop-helix (bHLH) region and two PAS repeat profiles,
which have been previously demonstrated to be transcriptional
activators of HIF-1α and HIF-1β in mammals (46,47).
 | Table II.Biochemical properties and molecular
structures of HIF-1α and HIF-1β. |
Table II.
Biochemical properties and molecular
structures of HIF-1α and HIF-1β.
| Index | HIF-1α | HIF-1β |
|---|
| Amino acids
(n) | 826 | 416 |
| Molecular weight
(Da) | 92,670.4 | 45,921.6 |
| Theoretical
isoelectric point | 5.17 | 5.79 |
| Formula |
C4027H6410N1108O1309S43 |
C1963H3146N584O637S25 |
| Atoms (n) | 12,897 | 6,355 |
| Extinction
coefficients | 50,155 | 20,690 |
| Estimated half-life
(h) | 30 | 30 |
| Instability
index | 55.97 | 52.65 |
| Aliphatic
index | 74.96 | 71.44 |
| Grand average
hydropathicity | −0.573 | −0.508 |
| Charged amino acids
(%) | 31.72 | 31.97 |
| Acidic amino acids
(%) | 14.29 | 13.46 |
| Basic amino acids
(%) | 10.29 | 11.78 |
| Polar amino acids
(%) | 31.60 | 29.09 |
| Hydrophobic amino
acids (%) | 27.60 | 28.13 |
| Major amino acids
(%) |
|
|
|
| Leu 10.05 | Ser 9.86 |
|
| Ser 9.44 | Leu 7.69 |
|
| Thr 7.99 | Asp 7.69 |
| Secondary structure
(%) |
|
|
|
α-helix | 30.87 | 30.29 |
|
Extended strand | 18.28 | 19.71 |
| Random
coil | 43.83 | 41.11 |
Expression of the PI3K/Akt
pathway
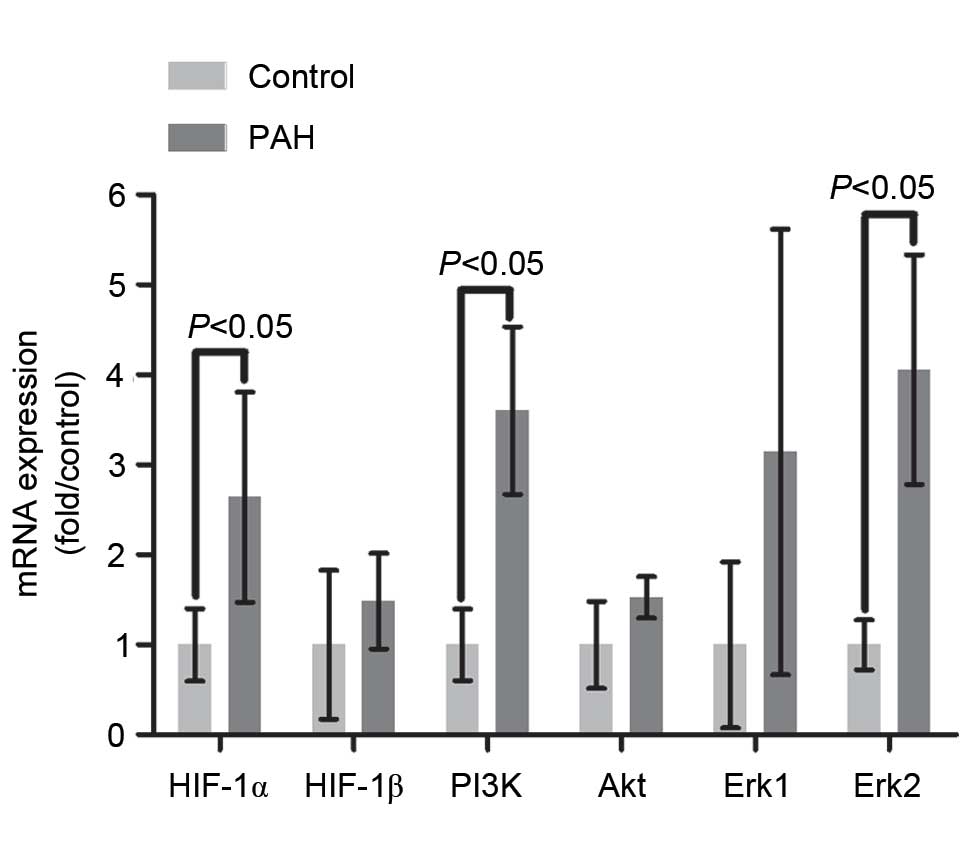
The relative mRNA expression level of PI3K was
significantly elevated (2.6-fold) in the PAH group, compared with
that in the control group (P<0.01; Fig. 2). No significant differences in the
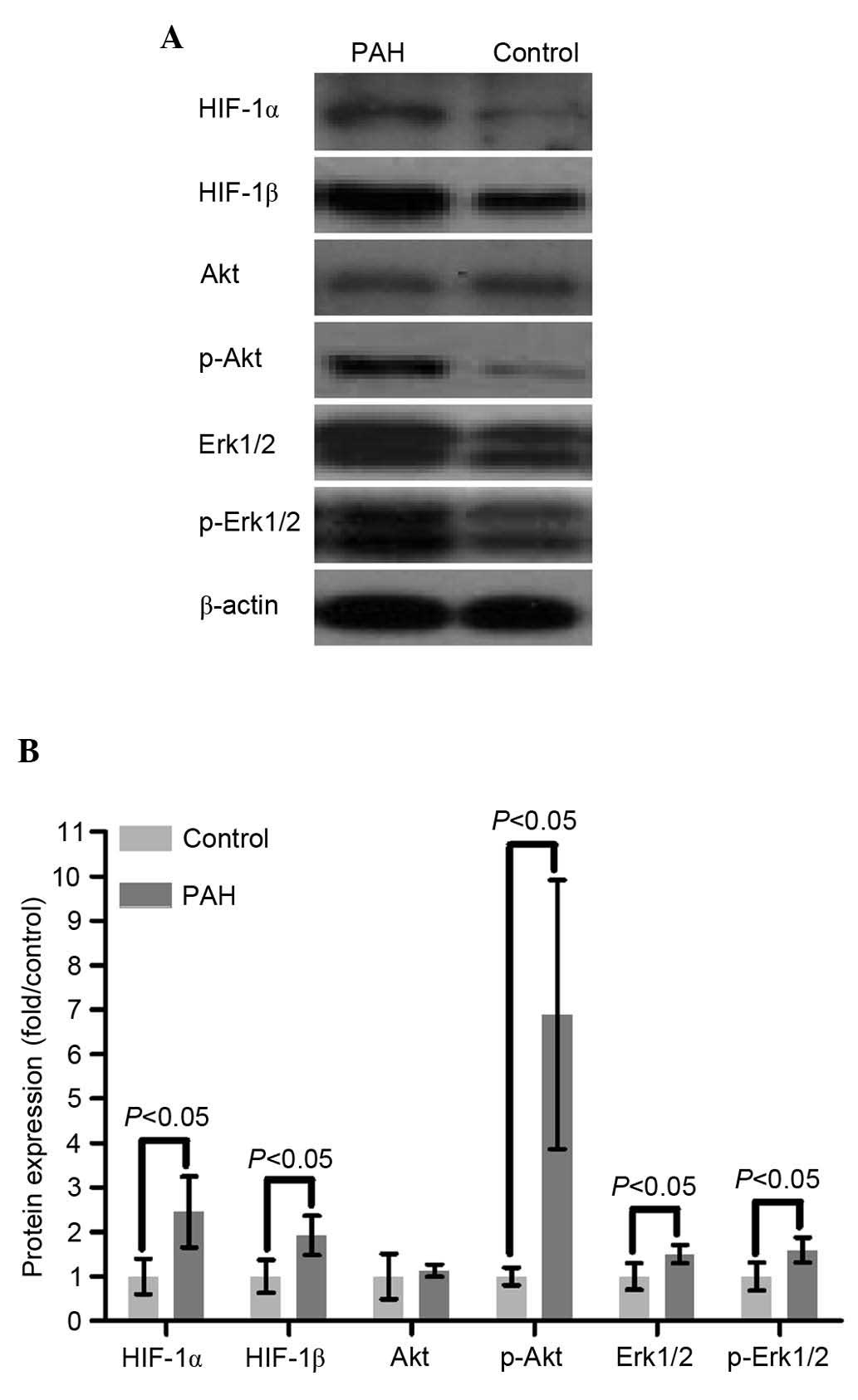
mRNA or protein levels of Akt were found between the two groups
(P>0.05), however, the level of p-Akt in the PAH group was
significantly increased (5.89-fold), compared with that in the
control group, indicating that Akt was activated though
phosphorylation by PI3K (P<0.01; Figs. 2 and 3).
Expression of the Erk1/2 pathway
The mRNA level of Erk2 in the PAH group was
3.06-fold higher, compared with that of control group (P<0.05),
however, no significant difference in the mRNA level of Erk1 was
observed between the two groups (P>0.05; Fig. 2). The results of the western blot
analysis showed that the protein levels of Erk1/2 and p-Erk1/2 in
the PAH group were significantly upregulated, compared with those
in the control group (P<0.01 and P<0.05, respectively;
Fig. 3).
Expression levels of HIF-1α and
HIF-1β
The mRNA and protein levels of HIF-1α in the PAH
group were respectively increased by 1.64- and 1.46-fold, compared
with the control group (P<0.01 and P<0.05, respectively),
suggesting that a higher mRNA level of HIF-1α increased synthesis
of the HIF-1α protein (Figs. 2 and
3). No significant difference in
the mRNA level of HIF-1β was found between the two groups, however,
the protein level of HIF-1β was significantly elevated (by 92%) in
the PAH group, compared with that in the control group (P<0.05;
Figs. 2 and 3).
Discussion
Pulmonary vascular remodeling, including hyperplasia
of pulmonary artery endothelial cells and pulmonary artery smooth
muscle cells is the major pathological change in PAH. Multiple
cytokines, including platelet-derived growth factor, vascular
endothelial growth factor and transforming growth factor-β can
promote cell proliferation and migration in the physiopathological
processes of PAH (48–50).
The MAPK family comprises key factors for regulating
the proliferation, differentiation and apoptosis of cells in
response to certain environmental stresses and cytokines (51,52).
MAPKs usually exist in forms of non-phosphorylated proteins in
mammalian cells. As a member of the MAPK family, Erk1/2 can be
activated though phosphorylation of the Thr185 and Tyr187 residues
to produce a dimer, which is then translocated into the cell
nucleus to activate various transcription factors (53). In the present study, it was found
that Erk1/2 and p-Erk1/2 were upregulated in the patients with PAH,
suggesting that Erk1/2 signaling pathway may be important for
pulmonary vascular remodeling in PAH.
Similar to the MAPK signaling pathway, PI3K/Akt also
induces cell growth, triggered by certain growth factors (54). Activated PI3K drives the production
of phosphatidyl-inositol-3,4,5-trisphosphate, which can bind to
pleckstrin homology domains of Akt, and promote Akt phosphorylation
at Thr308 and Ser473 residues, which induces the translocation of
Akt into the nucleus to provide signals for cell survival (55). In addition, the second messenger
PIP3 interacts with several cytoskeletal proteins, including
paxilin, profilin, vinculin and filamin, to promote the
polymerization of actin filaments, which can affect cell morphosis
and migration (56–59). The present study showed that the
levels of PI3K and phosphorylated Akt were markedly elevated in the
patients with PAH, suggesting that the PI3K/Akt pathway may be
involved in the pathological lesion of PAH by regulating cell
proliferation, migration and adhesion, and even vascular
stability.
HIF-1α and HIF-1β belong to the bHLH/PAS protein
family, functioning as modulators in cell proliferation and
differentiation (60). As HIF-1α
lacks a transmembrane domain, HIF-1β is recruited to dimerize with
HIF-1α for nuclear translocation. The degradation of the HIF-1α is
suppressed by hypoxia, whereas the expression of HIF-1β in cells is
commonly considered to be oxygen-independent. However, Wolff et
al (61) found that the
regulation of HIF-1β is more complex, and showed that the protein
levels of HIF-1β are affected by hypoxia and hypoxia mimetics
(61). In addition to HIF-1α, the
present study found that the protein levels of HIF-1β were also
elevated in the lungs of patients with PAH, suggesting that these
two molecules may be involved in the pathogenesis of PAH.
Therefore, the present study hypothesized that HIF-1β may exhibit
corresponding responses to the changes of HIF-1α.
The present study demonstrated for the first time,
to the best of our knowledge, changes in the expression levels of
ERK1/2, PI3K, Akt and HIF-1 in the lungs of patients with PAH.
However, the roles of these signaling molecules in the pathogenesis
of PAH and the associations among these signaling molecules require
further investigations in the future.
Acknowledgements
This study was supported by Collaborative Innovation
and Platform Environment Construction Projects of Guangdong
Province (2015A050502049), Natural Science Foundation of Guangdong
Province (2015A030313520), Science and Technology Planning Project
of Guangdong Province (2014A020212739), Research Project of
Traditional Chinese Medicine Bureau of Guangdong Province
(20151259, 20161142) and the National Natural Science Foundation of
China (no. 81300035).
References
|
1
|
Stamm JA, Risbano MG and Mathier MA:
Overview of current therapeutic approaches for pulmonary
hypertension. Pulm Circ. 1:138–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Savai R, Al-Tamari HM, Sedding D,
Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N,
Grimminger F, Seeger W, et al: Pro-proliferative and inflammatory
signaling converge on FoxO1 transcription factor in pulmonary
hypertension. Nat Med. 20:1289–1300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seeger W and Pullamsetti SS: Mechanics and
mechanisms of pulmonary hypertension-Conference summary and
translational perspectives. Pulm Circ. 3:128–136. 2013.PubMed/NCBI
|
|
4
|
Humbert M, Sitbon O, Chaouat A, Bertocchi
M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot
F, et al: Pulmonary arterial hypertension in France: Results from a
national registry. Am J Respir Crit Care Med. 173:1023–1030. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Badesch DB, Raskob GE, Elliott CG,
Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG,
Turner M, et al: Pulmonary arterial hypertension: Baseline
characteristics from the REVEAL Registry. Chest. 137:376–387. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thenappan T, Shah SJ, Rich S, Tian L,
Archer SL and Gomberg-Maitland M: Survival in pulmonary arterial
hypertension: A reappraisal of the NIH risk stratification
equation. Eur Respir J. 35:1079–1087. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCullagh BN, Costello CM, Li L, O'Connell
C, Codd M, Lawrie A, Morton A, Kiely DG, Condliffe R, Elliot C, et
al: Elevated plasma CXCL12α is associated with a poorer prognosis
in pulmonary arterial hypertension. PLoS One. 10:e01237092015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang X, Zou L, Yu X, Chen M, Guo R, Cai
H, Yao D, Xu X, Chen Y, Ding C, et al: Salidroside attenuates
chronic hypoxia-induced pulmonary hypertension via adenosine A2a
receptor related mitochondria-dependent apoptosis pathway. J Mol
Cell Cardiol. 82:153–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimoda LA and Laurie SS: HIF and
pulmonary vascular responses to hypoxia. J Appl Physiol (1985).
116:867–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prabhakar NR and Semenza GL: Adaptive and
maladaptive cardiorespiratory responses to continuous and
intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2.
Physiol Rev. 92:967–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai Z, Luo W, Zhan H and Semenza GL:
Hypoxia-inducible factor 1 is required for remote ischemic
preconditioning of the heart. Proc Natl Acad Sci USA.
110:17462–17467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gilkes DM, Xiang L, Lee SJ, Chaturvedi P,
Hubbi ME, Wirtz D and Semenza GL: Hypoxia-inducible factors mediate
coordinated RhoA-ROCK1 expression and signaling in breast cancer
cells. Proc Natl Acad Sci USA. 111:E384–E393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang S, Chen P, Shui X, He Y, Wang H,
Zheng J, Zhang L, Li J, Xue Y, Chen C and Lei W: Baicalin
attenuates transforming growth factor-β1-induced human pulmonary
artery smooth muscle cell proliferation and phenotypic switch by
inhibiting hypoxia inducible factor-1α and aryl hydrocarbon
receptor expression. J Pharm Pharmacol. 66:1469–1477. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Semenza GL: Regulation of oxygen
homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda).
24:97–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sutton KM, Hayat S, Chau NM, Cook S,
Pouyssegur J, Ahmed A, Perusinghe N, Le Floch R, Yang J and
Ashcroft M: Selective inhibition of MEK1/2 reveals a differential
requirement for ERK1/2 signalling in the regulation of HIF-1 in
response to hypoxia and IGF-1. Oncogene. 26:3920–3929. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue Y, Li NL, Yang JY, Chen Y, Yang LL and
Liu WC: Phosphatidylinositol 3′-kinase signaling pathway is
essential for Rac1-induced hypoxia-inducible factor-1 (alpha) and
vascular endothelial growth factor expression. Am J Physiol Heart
Circ Physiol. 300:H2169–H2176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Semenza GL: Hypoxia-inducible factors in
physiology and medicine. Cell. 148:399–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim CS, Kiriakidis S, Sandison A, Paleolog
EM and Davies AH: Hypoxia-inducible factor pathway and diseases of
the vascular wall. J Vasc Surg. 58:219–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim YM, Barnes EA, Alvira CM, Ying L,
Reddy S and Cornfield DN: Hypoxia-inducible factor-1α in pulmonary
artery smooth muscle cells lowers vascular tone by decreasing
myosin light chain phosphorylation. Circ Res. 112:1230–1233. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ball MK, Waypa GB, Mungai PT, Nielsen JM,
Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK,
Steinhorn RH, et al: Regulation of hypoxia-induced pulmonary
hypertension by vascular smooth muscle hypoxia-inducible factor-1α.
Am J Respir Crit Care Med. 189:314–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith KA and Yuan JX: Hypoxia-inducible
factor-1α in pulmonary arterial smooth muscle cells and
hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med.
189:245–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mottet D, Michel G, Renard P, Ninane N,
Raes M and Michiels C: ERK and calcium in activation of HIF-1. Ann
N Y Acad Sci. 973:448–453. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim JH, Lee ES, You HJ, Lee JW, Park JW
and Chun YS: Ras-dependent induction of HIF-1alpha785 via the
Raf/MEK/ERK pathway: A novel mechanism of Ras-mediated tumor
promotion. Oncogene. 23:9427–9431. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan L, Santi M, Rushing EJ, Cornelison R
and MacDonald TJ: ERK activation of p21 activated kinase-1 (Pak1)
is critical for medulloblastoma cell migration. Clin Exp
Metastasis. 27:481–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Liu Q, Lu L, Zhao X, Gao X and
Wang Y: Astragaloside IV stimulates angiogenesis and increases
hypoxia-inducible factor-1α accumulation via phosphatidylinositol
3-kinase/Akt pathway. J Pharmacol Exp Ther. 338:485–491. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1alpha and VEGF in
laser-induced rat choroidal neovascularization. Invest Ophthalmol
Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin J, Yuan F, Shen MQ, Feng YF and He QL:
Vascular endothelial growth factor regulates primate
choroid-retinal endothelial cell proliferation and tube formation
through PI3K/Akt and MEK/ERK dependent signaling. Mol Cell Biochem.
381:267–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li L, Xiong Y, Qu Y, Mao M, Mu W, Wang H
and Mu D: The requirement of extracellular signal-related protein
kinase pathway in the activation of hypoxia inducible factor 1
alpha in the developing rat brain after hypoxia-ischemia. Acta
Neuropathol. 115:297–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bullard LE, Qi X and Penn JS: Role for
extracellular signal-responsive kinase-1 and −2 in retinal
angiogenesis. Invest Ophthalmol Vis Sci. 44:1722–1731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coffer PJ, Jin J and Woodgett JR: Protein
kinase B (c-Akt): A multifunctional mediator of
phosphatidylinositol 3-kinase activation. Biochem J. 335:1–13.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kandel ES and Hay N: The regulation and
activities of the multifunctional serine/threonine kinase Akt/PKB.
Exp Cell Res. 253:210–229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ackah E, Yu J, Zoellner S, Iwakiri Y,
Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, et
al: Akt1/protein kinase Balpha is critical for ischemic and
VEGF-mediated angiogenesis. J Clin Invest. 115:2119–2127. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Steinle JJ, Zamora DO, Rosenbaum JT and
Granger HJ: Beta 3-adrenergic receptors mediate choroidal
endothelial cell invasion, proliferation, and cell elongation. Exp
Eye Res. 80:83–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye Z, Guo Q, Xia P, Wang N, Wang E and
Yuan Y: Sevoflurane postconditioning involves an up-regulation of
HIF-1α and HO-1 expression via PI3K/Akt pathway in a rat model of
focal cerebral ischemia. Brain Res. 1463:63–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jeong YJ, Cho HJ, Magae J, Lee IK, Park KG
and Chang YC: Ascofuranone suppresses EGF-induced HIF-1α protein
synthesis by inhibition of the Akt/mTOR/p70S6K pathway in
MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol.
273:542–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Simonneau G, Robbins IM, Beghetti M,
Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin
MT, Jing ZC, et al: Updated clinical classification of pulmonary
hypertension. J Am Coll Cardiol. 54:(1 Suppl). S43–S54. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Strausberg RL, Feingold EA, Grouse LH,
Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler
GD, Altschul SF, et al: Generation and initial analysis of more
than 15,000 full-length human and mouse cDNA sequences. Proc Natl
Acad Sci USA. 99:16899–16903. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Combet C, Blanchet C, Geourjon C and
Deléage G: NPS@: Network protein sequence analysis. Trends Biochem
Sci. 25:147–150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marchler-Bauer A and Bryant SH: CD-Search:
Protein domain annotations on the fly. Nucleic Acids Res. 32:(Web
Server issue). W327–W331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marchler-Bauer A, Anderson JB, Chitsaz F,
Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales
NR, Gwadz M, et al: CDD: Specific functional annotation with the
conserved domain database. Nucleic Acids Res. 37:(Database issue).
D205–D210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marchler-Bauer A, Lu S, Anderson JB,
Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer
RC, Gonzales NR, et al: CDD: A conserved domain database for the
functional annotation of proteins. Nucleic Acids Res. 39:(Database
issue). D225–D229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marchler-Bauer A, Derbyshire MK, Gonzales
NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI,
et al: CDD: NCBI's conserved domain database. Nucleic Acids Res.
43:(Database issue). D222–D226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gasteiger E, Hoogland C, Gattiker A,
Duvaud S, Wilkins M, Appel R and Bairoch A: Protein identification
and analysis tools on the ExPASy serverJohn MW: The Proteomics
Protocols Handbook. Humana Press; Totowa, NJ: pp. 571–607. 2005,
View Article : Google Scholar
|
|
45
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reyes H, Reisz-Porszasz S and Hankinson O:
Identification of the Ah receptor nuclear translocator protein
(Arnt) as a component of the DNA binding form of the Ah receptor.
Science. 256:1193–1195. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou YD, Barnard M, Tian H, Li X, Ring HZ,
Francke U, Shelton J, Richardson J, Russell DW and McKnight SL:
Molecular characterization of two mammalian bHLH-PAS domain
proteins selectively expressed in the central nervous system. Proc
Natl Acad Sci USA. 94:713–718. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao Y, Lv W, Piao H, Chu X and Wang H:
Role of platelet-derived growth factor-BB (PDGF-BB) in human
pulmonary artery smooth muscle cell proliferation. J Recept Signal
Transduct Res. 34:254–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Voelkel NF and Gomez-Arroyo J: The role of
vascular endothelial growth factor in pulmonary arterial
hypertension. The angiogenesis paradox. Am J Respir Cell Mol Biol.
51:474–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gore B, Izikki M, Mercier O, Dewachter L,
Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, Lebrin F
and Eddahibi S: Key role of the endothelial TGF-β/ALK1/endoglin
signaling pathway in humans and rodents pulmonary hypertension.
PLoS One. 9:e1003102014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
McKay MM and Morrison DK: Integrating
signals from RTKs to ERK/MAPK. Oncogene. 26:3113–3121. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Barsyte-Lovejoy D, Galanis A and Sharrocks
AD: Specificity determinants in MAPK signaling to transcription
factors. J Biol Chem. 277:9896–9903. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
McMullen JR and Jzumo S: Role of
insulin-like growth factor 1 (IGF-1)/phosphoinositide-3-kinase
(PI3K) pathway mediating physiological cardiac hypertrophy.
Novartis Found Symp. 274:90–111; discussion 111–117, 152–155,
272–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang Y, Tseng CC, Tsai YL, Fu X, Schiff R
and Lee AS: Cancer cells resistant to therapy promote cell surface
relocalization of GRP78 which complexes with PI3K and enhances
PI(3,4,5)P3 production. PLoS One. 8:e800712013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Vanhaesebroeck B and Waterfield MD:
Signaling by distinct classes of phosphoinositide 3-kinases. Exp
Cell Res. 253:239–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vanhaesebroeck B, Leevers SJ, Ahmadi K,
Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ and
Waterfield MD: Synthesis and function of 3-phosphorylated inositol
lipids. Annu Rev Biochem. 70:535–602. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Roymans D and Slegers H:
Phosphatidylinositol 3-kinases in tumor progression. Eur J Biochem.
268:487–498. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Greenwood JA, Theibert AB, Prestwich GD
and Murphy-Ullrich JE: Restructuring of focal adhesion plaques by
PI 3-kinase. Regulation by Ptdlns (3,4,5)-p(3) binding to
alpha-actinin. J Cell Biol. 150:627–642. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kietzmann T, Samoylenko A, Roth U and
Jungermann K: Hypoxia-inducible factor-1 and hypoxia response
elements mediate the induction of plasminogen activator inhibitor-1
gene expression by insulin in primary rat hepatocytes. Blood.
101:907–914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wolff M, Jelkmann W, Dunst J and Depping
R: The aryl hydrocarbon receptor nuclear translocator (ARNT/HIF-1β)
is influenced by hypoxia and hypoxia mimetics. Cell Physiol
Biochem. 32:849–858. 2013. View Article : Google Scholar : PubMed/NCBI
|