Introduction
The staining of live cells enables the observation
and separation of cells following direct co-culture to analyse any
alterations in the phenotypes of co-cultured cells. The application
of lipophilic cell labelling dyes from the DiO-family is a powerful
tool used for the staining of live cells. These lipophilic
fluorescent carbocyanine dyes have been used since the 1980s in
vitro and in vivo due to their high quantum efficiency,
the simplicity of staining protocols and reduced cytotoxicity
compared with hydrophilic dyes (1,2). The
excitation and emission spectra of lipophilic fluorescent dyes
enables multiple staining (2).
Dyes from the DiO-family label the plasma membrane and migrate to
cellular organelles as a consequence of membrane turnover (3).
The fluorescence emission spectral overlap between
two (or more) fluorochromes may be an issue when performing
multicolour experiments. However, modern instruments effectively
separate the collected fluorescent signals from various fluorescent
dyes by fluorescence overlap compensation (4). Other problems, for example spectral
overlap between the emission of one fluorochrome and the excitation
of another, are not so readily solved. This overlap is described as
the bleed-through effect. To avoid it, care must be taken when
selecting fluorochromes and filter sets, or bleed-through must be
measured and subtracted from measurements (5). Furthermore, differences in dye
stability and/or mobility within the cell or other effects
independent of the dye fluorescence emission may influence the
results of multicolour experiments. These multicolour experiments
followed by flow cytometry may be used to estimate nucleic acid
migration between cells. For this, one co-cultured cell population
is stained with lipophilic dyes from the DiO family and other cell
population is stained with hydrophilic dyes coupled with nucleic
acid to monitor their migration (6). Impediments resulting from variations
in fluorochrome dynamics require consideration when designing
multicolour experiments.
DiO (green) and DiD (red) are used in flow cytometry
and confocal microscopy (7–11).
It is recommended that various factors be considered when staining
with lipophilic dyes, including dye concentration, duration of
staining and temperature (12).
Our previous study demonstrated the asymmetry of DiO and DiD
distribution in a heterotypic cell co-culture (13). Data concerning the transfer of DiO
or DiD between cells are contradictory; certain authors suggest
that lipophilic dyes undergo very low intercellular transfer,
whereas others report very high transfer (14–19).
As the stable retention of dyes in cells is in question, it is
uncertain whether two populations of cells prestained with DiO and
DiD may be separated following co-culture. The size of the
co-stained population following co-culture remains to be
elucidated.
The aim of the present study was to measure the
intercellular migration of dyes in multicolour experiments and
quantify their asymmetrical distribution in homotypic co-cultures,
following detection by flow cytometry. The optical, chemical and
cellular factors involved in the asymmetrical distribution of DiO
and DiD in co-culture experiments were investigated. The results of
the present study suggested an application of 1:1 premix of DiO and
DiD to estimate intensity of intercellular contact in co-culture
systems.
The data indicating retention of DiO and DiD in
cultured cells are ambiguous, which precludes the interpretation of
results from a number of previous studies (14–19).
Due to poor retention and the intercellular migration of lipophilic
dyes, separation of cells by cell sorting following co-culture may
be hindered. In the present study, two cell lineages were stained
separately with DiO and DiD, before they were mixed and co-cultured
in single Petri dishes (direct co-culture system), or in two dishes
separated by a 1-µm pore membrane (a Transwell indirect co-culture
system). By quantifying and comparing the intercellular migration
of DiO and DiD in the present study, the observed difference in the
passive transfer of these two lipophilic dyes demonstrated that the
use of these dyes may interfere with cell sorting following
co-culture experiments or during dye co-localisation studies.
Materials and methods
Materials
CHX and CB were purchased from Sigma-Aldrich; Merck
Millipore (Darmstadt, Germany). Vybrant® Cell-Labeling
solution was obtained from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA) and contained the lipophilic dyes, DiO [DiOC18(3); 3,3′-dioctadecyloxacarbocyanine
perchlorate] and DiD [DiIC18(5);
1,1′-dioctadecyl-3,3,3′, 3′-tetramethylindodicarbocyanine
4-chlorobenzenesulfonate salt], with the following spectral maxima:
DiO excitation, 484 nm/emission, 501 nm; and DiD excitation, 644
nm/emission, 663 nm.
Patients and tissues
Human nucleus pulposus cells (NPCs) and bone marrow
mesenchymal stem cells (MSCs) were collected using an anterior
approach from four patients undergoing treatment to correct
thoracolumbar or lumbar scoliosis during routine preparation of the
site for anterior spondylodesis. All patients were recruited into
the study consecutively.
The following exclusion criteria were adopted: i)
Use of analgesic, antibiotic or steroid medication prior to
hospital admission; ii) previous surgery in the spinal area.
Patients received in-depth information on the aim of the present
study and were assured of anonymity. Informed consent from the
legal guardians of each patient was obtained prior to the request
to collect NPCs from donors being made. The design of the present
study was approved by the Ethics Committee of Poznan University of
Medical Sciences (Poznan, Poland; approval number 838/09) and was
performed in accordance with universal ethical principles.
The SW-1353 human bone chondrosarcoma cell line was
purchased from CLS Cell Lines Service GmbH (Eppelheim,
Germany).
Cell culture
For NPCs, the non-degenerate intervertebral disc
tissue was dissected primarily from Th12 to L3 to separate the
nucleus pulposus from the annulus fibrosus tissue, as described in
our previous study (13). Briefly,
the nucleus pulposus was enzymatically digested overnight at 37°C
with 0.02% collagenase type II (Sigma-Aldrich; Merck Millipore) in
serum-free Dulbecco's modified Eagle's medium/Nutrient F-12 Ham
(DMEM/F-12; Sigma-Aldrich; Merck Millipore) containing 100 U/ml
penicillin, 100 µg/ml streptomycin and 25 ng/ml amphotericin B
(ABAM; Sigma-Aldrich; Merck Millipore). The digested tissue/cell
suspension was filtered through sterile nylon fabric to remove
remaining tissue debris. The cells were centrifuged at 300 × g for
5 min at room temperature, seeded into a tissue culture flask and
cultured at 37°C in 5% CO2/95% air, in (1:1 v/v) DMEM/F-12
(Sigma-Aldrich; Merck Millipore), supplemented with 10% foetal
bovine serum (Sigma-Aldrich; Merck Millipore), ABAM solution and
vitamin C (5 mg/ml).
For MSCs, the non-degenerative vertebrae were
extracted primarily from Th12 to L3. The vertebrae were minced
mechanically and enzymatically digested overnight in identical
conditions as described for NPCs. The digested tissue/cell
suspension was filtered and resuspended in a RosseteSep buffer
(Stemcell Technologies, Inc., Vancouver, BC, Canada). Subsequent
steps of stem cell enrichment were performed according to the Stem
Cells kit procedure (RosetteSep Human Mesenchymal Stem Cell
Enrichment Cocktail; Stemcell Technologies, Inc.). Briefly,
following overnight digestion, the total lysate was resuspended in
the serum-containing medium. Following incubation for 20 min, the
cells were placed in Ficoll solution and centrifuged at 300 ×
g for 25 min at room temperature. The intermediate layer of
cells was transferred to a cell culture dish and placed in the
incubator in medium, as for NPCs except of vitamin C. For the first
4 days of culture the cells were washed daily to enable selection
of adhesive cells only. The random subpopulation of MSCs obtained
was subcultured in a chondrocyte selective cell culture medium
(Human Mesenchymal Stem Cell Functional Identification kit, R&D
Systems, Inc, Minneapolis, MN, USA). The chondrocyte phenotype was
confirmed by quantitative polymerase chain reaction, as described
in our previous study (13). MSCs
destined for co-culture with NPCs were stained with lipophilic dyes
and were subsequently cultured in an insert system or in direct
co-culture with NPCs.
SW-1353 cells were seeded onto a tissue culture
flask and cultured at 37°C in 5% CO2/95% air, in (1:1
v/v) DMEM/F-12 supplemented with 10% foetal bovine serum and
ABAM.
Staining cells for flow cytometry and
microscopy
For flow cytometry and microscopy, the cells were
cultured and stained with DiO or DiD lipophilic dyes. The cells
were removed from the culture dish using trypsin (Sigma-Aldrich;
Merck Millipore) and were counted. Cells (1×106) were incubated for
30 min at room temperature with 10 µl 1 mM DiO or 1 mM DiD dye
solutions in 1 ml cell suspension in culture medium [DiO, 10 µM
(8.8 µg/ml); DiD, 10 µM (10.52 µg/ml)] and washed twice with
phosphate-buffered saline. The cells were subsequently cultured
overnight in the culture medium appropriate for the cell type. The
following day, the stained cells were subcultured into new culture
dishes. Staining of cells directly on culture dishes is recommended
by the manufacturer; however, our modified procedure with trypsin
detachment was applied to enable mixing of the cells directly
following labelling. This was independent of whether trypsin was
applied prior to staining or not, and the application of lower
concentrations of dyes, fluorescent dyes were observed in the cells
rather than in the plasma membranes. A similar protocol was
followed for staining of cells by equimolar premixed DiO and DiD;
10 µl 1 mM DiO and 10 µl 1 mM DiD dye solutions were added to 1 ml
of cell suspension in culture medium. These cells were cultured and
analysed by flow cytometry.
Cells were stained with either DiD or DiO and were
cultured in the indirect co-culture system using 1 µm inserts
(translucent PET membrane; BD Biosciences, Franklin Lakes, NJ, USA)
and 6-well culture dishes (BD Biosciences). Equal numbers of
unstained cells were cultured beneath the inserts. In the direct
co-culture system of the double staining experiment, the cells were
stained with DiO or DiD and cultured together in 6-well culture
plates at a ratio of 1:1. Following culture, the cells were
detached from the plates using trypsin and analysed using a BD
FACSAria™ III (BD Biosciences). The cells designated for
observation under a fluorescent microscope (EVOS® FL
Imaging system; Thermo Fisher Scientific, Inc.) were cultured in
6-well culture plates. Video recordings from this microscope were
recorded and are available on request from the corresponding
author.
Flow cytometric analysis
The BD FACSAria™ III flow cytometer is equipped with
four lasers (375, 405, 488 and 633 nm), 11 fluorescence detectors,
and forward scatter (FSC) and side scatter (SSC) detectors. The
instrument setup (optical alignment), stability and performance
test was performed using the Cytometer Setup and Tracking system
(BD Biosciences). FACSFlow solution (BD Biosciences) was used as
sheath fluid. The configuration of the flow cytometer was as
follows: A 100 µm nozzle and 20 psi (0.138 MPa) sheath fluid
pressure. The cells were characterized by two non-fluorescent
parameters, FSC and SSC, and two fluorescent parameters, green
fluorescence from the DiO reagent was collected using a 530/30 band
pass filter (fluorescein isothiocyanate-A detector) and red
fluorescence from the DiD reagent was collected using a 660/20 band
pass filter (allophycocyanin-A detector). The excitation of the DiO
and DiD fluorescent reagents was achieved with 488 and 633 nm
lasers, respectively. Flow cytometric analyses were performed using
logarithmic gains and specific detector settings. The threshold was
set at the FSC signal. The data were acquired in a four-decade
logarithmic scale as area signals and analysed with FACSDiva
software (version 6.1.3; BD Biosciences). Compensation was
performed to ensure that the crossover between the emission spectra
of DiO and DiD caused no effect on the results. Samples for
compensation (compensation controls) were cells demonstrating
bright emission of individual fluorochromes. The populations were
defined by gating in the plots of DiO vs. DiD fluorescence signals.
Each sample was analysed in triplicate. Cell sorting preceded the
doublet discrimination procedure with the use of height vs. width
scatter signal measurements in order to discriminate single cells
from conglomerates. The cells were sorted into 5 ml cytometric
tubes.
The doublet discrimination procedure involves the
use of height vs. width scatter signals measurement in order to
discriminate single events (single cells) from conglomerates
(agglomerated cells). Flow cytometric data are the result of
instrument settings prior to, during and following acquisition of
the sample. The doublet discrimination procedure uses dot plots for
non-fluorescent parameters: Laser-scattered light collected at the
front and at a 90° angle (perpendicular) to the laser beam.
Therefore, doublet exclusion may be performed during analysis, even
using unstained cells if the condition of the cells is not affected
by the staining procedure. Employed prior to sorting, the doublet
discrimination procedure provides an element of pure sorting
protocol.
Determination of cell viability
SW-1353 cells were stained with DiO or DiD and
cultured for 1, 4 or 7 days in 24-well plates (3×104 cells/well).
Control cells were unstained. Following stimulation, the cells were
incubated in serum free DMEM/F-12 containing 0.5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich; Merck Millipore) for 4 h. The cell culture medium
was then removed and a solvent was added (0.1 M hydrochloric acid
in isopropanol anhydride; Avantor Performance Materials, Gliwice,
Poland). The absorbance was read following gentle mixing using the
Stat-Fax 2100 microplate reader (Awareness Technology, Inc., Plam
City, FL, USA) at a wavelength of 492 nm (background absorbance was
measured at a wavelength of 630 nm).
Statistical analysis
The data are expressed as the mean ± standard
deviation of at least three biological repeats (staining and
co-culture). The groups were compared using the Student's t-test
and the STATISTICA data analysis software program (version 12.0;
StatSoft, Inc., Tulsa, OK, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Asymmetric distribution of dyes
following direct co-culture
Lassailly et al (15) observed an extensive transfer of
DiO-type lipophilic dyes from leukemic cells to neighbouring cells
in vitro and in vivo, and called this phenomenon
‘microenvironmental contamination’. Strassburg et al
(16), in their study of
co-cultured human MSCs and NPCs, observed that up to 87% of
unlabelled cells demonstrated DiL fluorescence following 7 days.
Therefore, DiO-type lipophilic dyes may be transferred and
incorporated by unlabelled cells. Our previous studies revealed a
similar phenomenon of DiO and DiD transfer between MSCs and NPCs in
heterotypic co-culture (13).
Using a fluorescent microscope, rapid movements of DiO and DiD
particles in SW-1353 cells were detected (data not shown; video
recording available on request from the corresponding author).
In our previous study, a predominance of DiD-stained
cells over DiO-stained cells was observed by flow cytometry, which
we termed ‘Q1-Q4 asymmetry’ (13).
In the present study, homotypic co-cultures of SW-1353 cells were
performed to precisely quantify the Q1-Q4 asymmetry. Unstained
SW-1353 cells were subcultured and digested using trypsin. This was
followed by separate staining with DiD and DiO. The DiD- and
DiO-stained cells were subsequently mixed 1:1 with a combination of
200,000 cells from each coloured population for direct co-culture
in one well of a 6-well culture dish for 4 days. The DiD- or DiO
stained cells were also placed unmixed in the upper dish (200,000
stained cells) of the Transwell insert system for 4 days. There
were 200,000 unstained cells in the lower dish of the Transwell
system. The cells were subsequently analysed by flow cytometry,
with the gating of the system set to cover the mean distribution of
DiD-stained cells (Q1), double-stained cells (Q2), unstained
controls (Q3) and DiO-stained cells (Q4). The properties of the
dyes' interaction with the cells induced a wide dispersion of
events and certain events exceeded the boundaries established for
gating.
Asymmetry of the distribution of events was observed
in the flow cytometric cytograms (Fig.
1A and B). There was double the percentage of events (ratio
2:1) corresponding to DiD-stained cells in the Q1 quartile compared
with DiO-stained in the Q4 quartile following 4 days of direct
co-culture of SW-1353. FACS analysis of SW-1353 cells co-cultured
in the Transwell system revealed that without direct contact of
cells the transfer of DiO and DiD was <50% of the transfer in
the direct co-culture (Fig. 1C and
F).
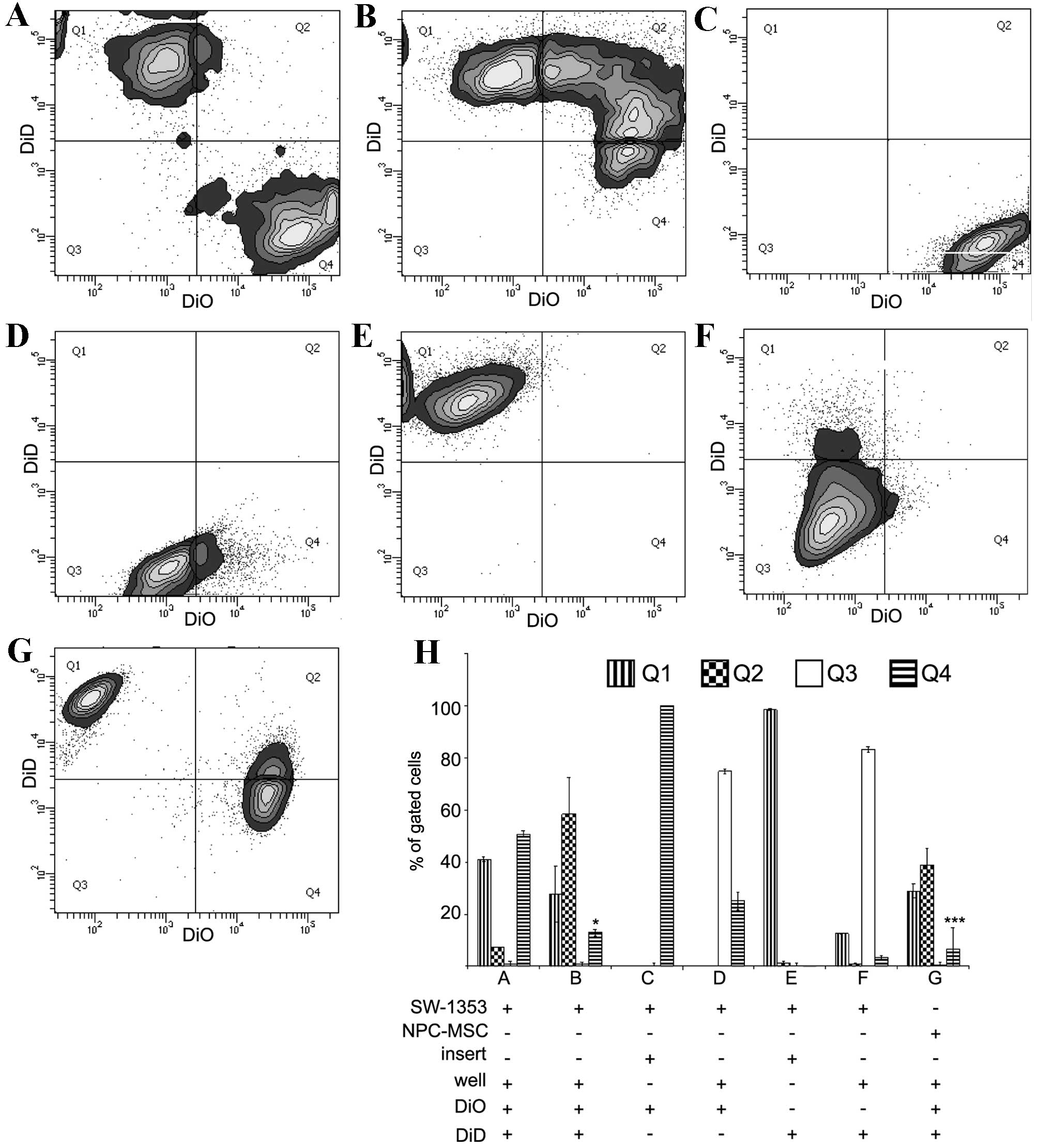 | Figure 1.Transfer of DiO and DiD in co-culture
systems of SW-1353 cells, NPCs and MSCs. The representative contour
plots were obtained by FACS analysis of co-cultured cells.
Harvested cells were excited by blue (488 nm) and red (633 nm)
lasers corresponding to DiO and DiD, respectively. The emission
signals were detected by 530/30 and 660/20 nm detectors. In the
direct co-culture system 200,000 DiO-labelled SW-1353 cells were
mixed with 200,000 DiD-labelled SW-1353 cells. The cells were
measured by FACS (A) immediately following mixing or (B) following
four days of co-culture. In addition, FACS was performed following
4 days of indirect co-culture of (C) 200,000 DiO-labelled cells in
a well insert and (D) 200,000 unstained cells in the bottom well,
and (E) 200,000 DiD-labelled cells in a well insert and (F) 200,000
unstained cells in the bottom well. (G) For comparison, 200,000
DiO-labelled NPCs were mixed with 200,000 DiD-labelled MSCs and
co-cultured in the direct co-culture system for 4 days. (H)
Quantification of three repeats of FACS analyses expressed as the
percentage of gated cells. The gating of the flow cytometry system
was set to cover the mean distribution of DiD-stained cells (Q1),
double-stained cells (Q2), unstained controls (Q3) and DiO-stained
cells (Q4). The data are expressed as the mean ± standard deviation
(*P<0.05 in B showing Q1 vs. Q4; ***P<0.001 in G showing Q1
vs. Q4). NPCs, nucleus pulposus cells; MSCs, mesenchymal stem
cells; FACS, fluorescent-activated cell sorting. |
Direct co-culture of MSCs stained with DiD and NPCs
stained with DiO resulted in Q1-Q4 asymmetry, with the ratio of
Q1:Q4 being 4:1 (Fig. 1G).
Quantification of the percentages of cells in each quadrant from
each experiment is presented in Fig.
1H. The Q1-Q4 asymmetry may be produced by various factors,
including an impact of the dyes on cell viability, a rate of dye
degradation, a crossover of emission/excitation spectra, the
intercellular mobility of dyes and chemical structure. The chemical
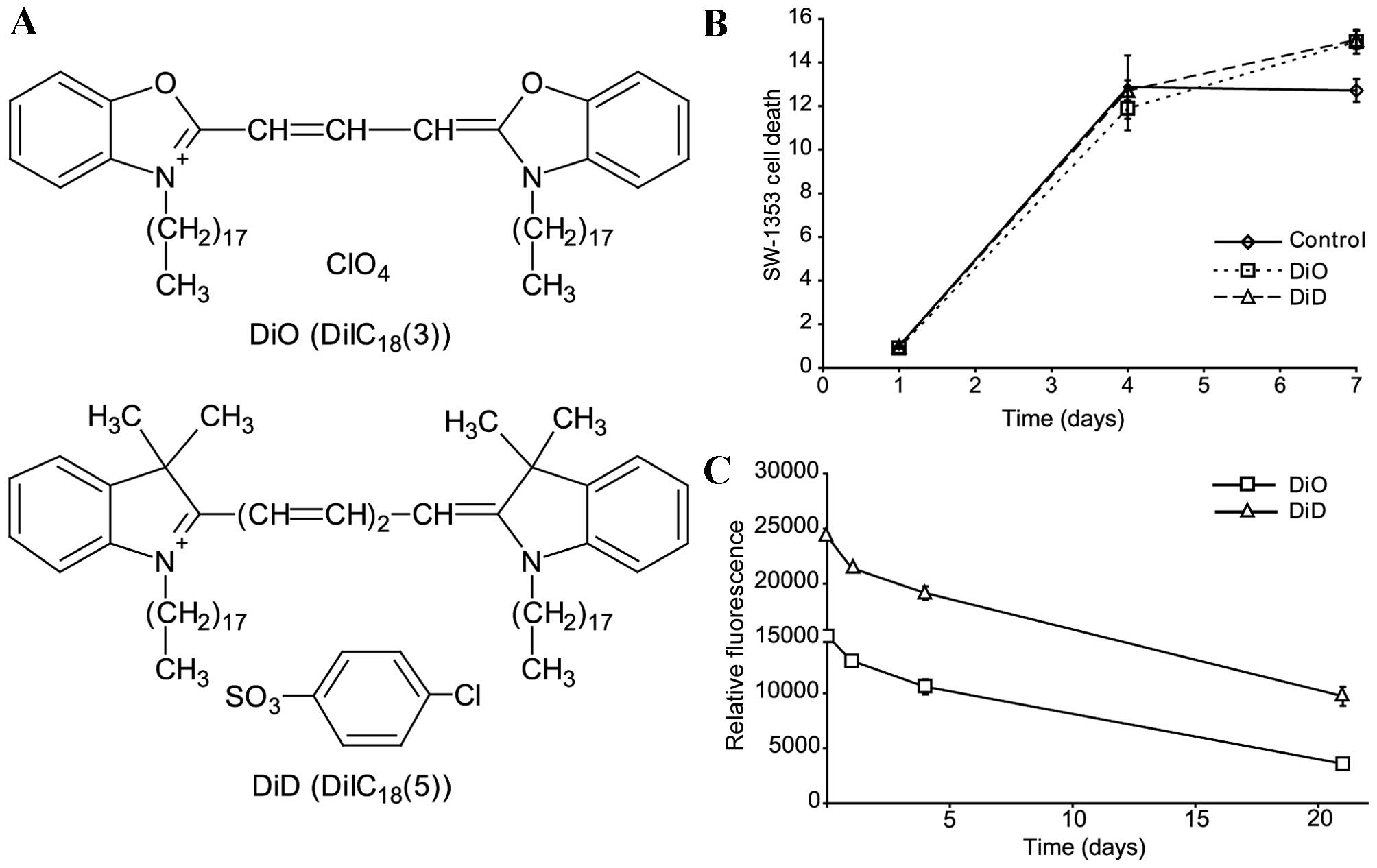
structures of DiD and DiO are presented in Fig. 2A. The following experiments were
performed to address these issues.
Differences in cell viability are not
responsible for asymmetry
SW-1353 cells were stained with DiO or DiD and
cultured (25,000 cells/well) for 1, 4 or 7 days in 24-well plates.
The cells were subsequently analysed using an MTT assay. No
differences in viability were observed between cells stained with
DiO and DiD (Fig. 2B).
Degradation rates of DiO and DiD are
not responsible for asymmetry
To estimate the relative difference in the
degradation rate of DiO and DiD, SW-1353 cells were stained
separately with the lipophilic dyes (Fig. 2C). The stained cells were mixed at
a ratio of 1:1 and cultured for 1, 4 or 21 days. The mean
fluorescence of the cells was measured by flow cytometry. Although
DiO florescence intensity was lower compared with DiD fluorescence
from the beginning of the experiment, the fluorescence decreased at
a similar rate (Fig. 2C).
Therefore, the degradation rates of the fluorescence were similar.
Following 4 days of culture a negligible 3.4% faster decrease of
fluorescence intensity was measured for DiO compared with for DiD
(P=0.0216). Therefore, the Q1-Q4 asymmetry is not explained by the
reduced degradation of DiD.
Crossover of emission-excitation
spectra is not responsible for asymmetry
Compensation using single stained cells was applied
to eliminate any emission-excitation crossover that may have been
responsible for the Q1-Q4 asymmetry in the DiD- and DiO-stained
cells. Additionally the SW-1353 cells were analysed using
independent excitation with 488 nm or 630 nm lasers and routine
application of lasers excitation (Table I). In this experiment, an
unexpectedly high emission of light in the 660/20 detector was
observed. SW-1353 cells stained with DiD and excited by 488 and 630
nm lasers exhibited quadrupled emission in comparison with the
single excitation by the 488 nm laser. However, this optical
effect, which greatly enhanced the emission of DiD, caused no
affect on the counting of events and, therefore, did not explain
Q1-Q4 asymmetry.
 | Table I.Mean fluorescence intensities. |
Table I.
Mean fluorescence intensities.
|
| Dye | Excitation | Emission (RFU) |
|---|
|
|
|
|
|
|---|
| Experiment | DiO | DiD | 488 nm | 633 nm | 530/30 | 660/20 |
|---|
| 1 | + | − | + | − | 10,760 | 0 |
| 2 | + | − | − | + | 0 | 152 |
| 3 | − | + | + | − | 116 | 0 |
| 4 | − | + | − | + | 0 | 26,611 |
| 5 | + | − | + | + | 10,245 | 164 |
| 6 | − | + | + | + | 0 | 108,692 |
| 7 | + | + | + | − | 3,337 | 0 |
| 8 | + | + | − | + | 0 | 65,204 |
| 9 | + | + | + | + | 11,364 | 94,900 |
Greater mobility of DiD may be
responsible for Q1-Q4 asymmetry
The above experiments eliminated the most
predictable reasons for Q1-Q4 asymmetry, with the exception of the
unequal transfer rates of DiO and DiD. Following 4 days of
co-culture, two-thirds of the SW-1353 cells, which had formerly
been stained with DiO or DiD and mixed at a ratio of 1:1, were
double-stained (Q2). This occurred due to the intercellular
transfer of dyes, rather than their degradation, which would be
observed as an increase of events in Q3. Therefore, Q1-Q4 asymmetry
may be caused by the greater mobility of DiD compared with DiO.
This unequal rate of transfer greatly complicates the
interpretation of results from experiments involving
double-staining in homotypic and heterotypic co-cultures. This
transfer of dyes maybe cell-dependent or independent.
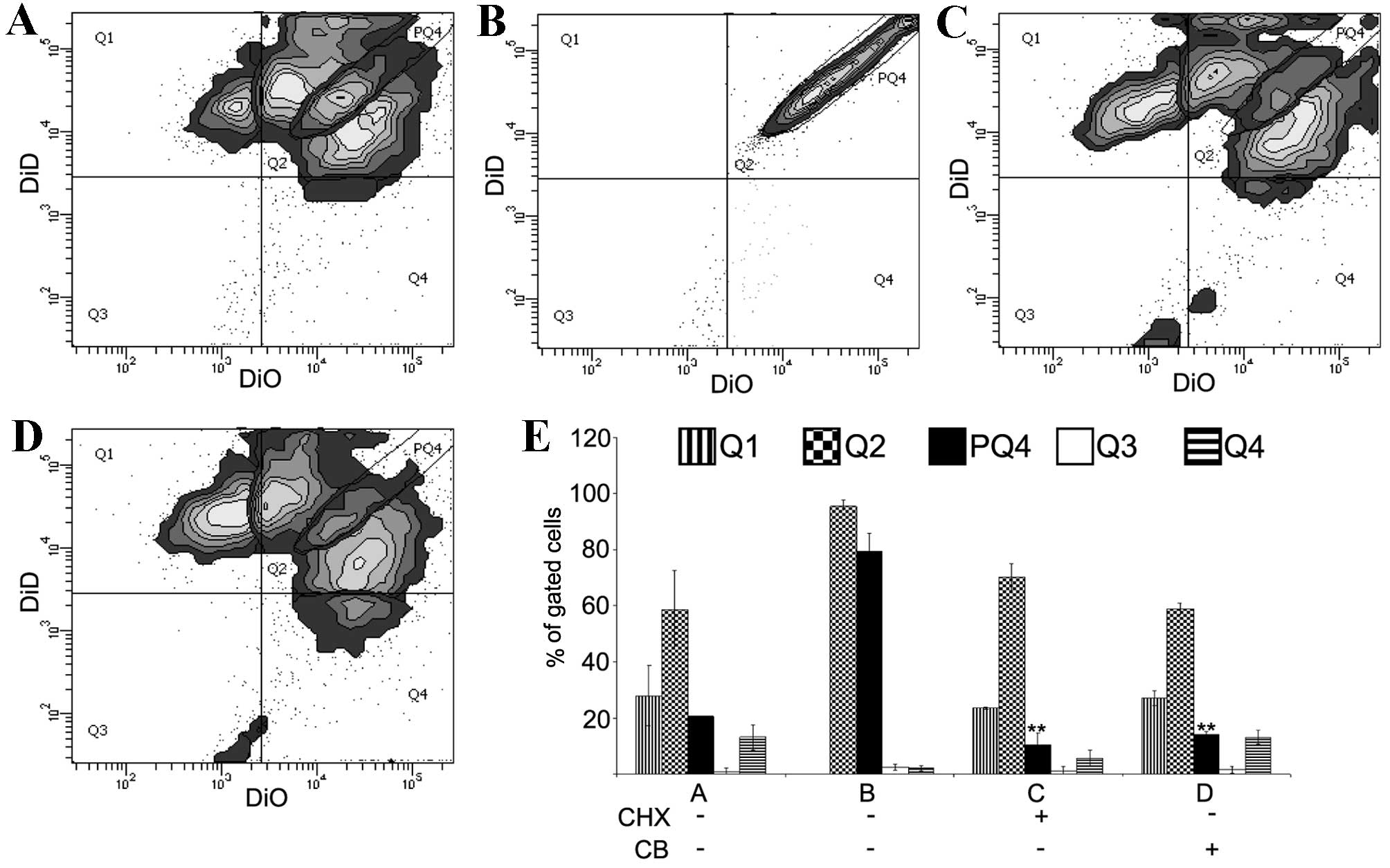
To determine whether the diffusion of lipophilic
dyes is a specific and cell-dependent phenomenon, a protein
synthesis blocker, CHX, and actin filaments polymerisation
inhibitor, CB, were added to the cell culture (Fig. 3). SW-1353 cells stained separately
with DiO and DiD were mixed (200,000 cells from each population)
and co-cultured directly in the absence (control) or presence of
CHX (10 µg/ml) or CB (350 nM). In addition, SW-1353 cells were
stained with an equimolar mix of DiO and DiD, and were cultured
without CHX or CB. Following 4 days, the cells were analysed by
flow cytometry. The controls revealed a similar staining pattern as
shown in Fig. 1 (Fig. 3A). The ‘equimolar population’
served to outline a subpopulation of Q2 labelled PQ4 (Fig. 3B). This narrow subpopulation
displayed a linear distribution of events with a range of two
orders of magnitude of fluorescence intensity. Taking PQ4
subpopulation as a reference, the present study observed that in
cultures treated with CHX and CB PQ4 subpopulations were ~1/3
smaller compared with the control (control 19.7, CHX 12.8 and CB
14.0%, respectively; Fig. 3A, C and
D). Differences in the number of events in control Q2, CHX Q2
and CB Q2 were not significant. The use of cells stained with a
balanced ratio of dyes may be the additional control in flow
cytometry for multicolour experiments that aim to measure dynamics
in intercellular contact.
Discussion
Various successful co-culture methodologies to
investigate heterotypic cell-cell interactions in mixed co-cultures
are noted in previous studies (20). Certain of these methodologies
employed lipophilic dyes for tracking co-cultured cells (2). These dyes present numerous
advantages; however, they have various properties that limit their
use in the observation and separation of co-cultured cells. One of
the limiting factors is intercellular migration of lipophilic dyes
(15). In our previous study, it
was observed that this migration was asymmetrical in the co-culture
of NPCs and MSCs stained by DiO and DiD (13). However, this previous study did not
demonstrate quantitative differences in the intercellular transfer
of the two lipophilic dyes, DiO and DiD, in homotypic cell
co-culture systems. These differences may be measured by flow
cytometry and are indistinguishable for fluorescent microscopy. In
the present study, using flow cytometry, differences in the
migration of DiO and DiD were measured. The difference was
expressed as a number of events in gates Q1 and Q4, corresponding
to DiD and DiO-stained cells and termed ‘Q1-Q4 asymmetry’. SW-1353
human chondrosarcoma cells were stained with DiO and DiD, and were
subsequently co-cultured directly and indirectly. SW-1353 cells
were selected for the purposes of the present study, despite their
limited similarity to human articular chondrocytes (21). Compared with primary cells, SW-1353
cells exhibited increased genetic and phenotypic homogeneity, which
is required for quantification during flow cytometry analyses.
Comparable asymmetry has been observed in NP-MSC co-culture,
therefore asymmetry may be considered a cell-independent process.
However, this requires further investigation in other cell
types.
Using the direct co-culture system of SW-1353 cells
stained separately by DiO and DiD, mixed at a ratio of 1:1, a 2:1
ratio of DiD-stained cells to DiO-stained cells was observed
following 4 days of co-culture. These results indicated that this
asymmetry was caused by the imbalance in intercellular migration of
DiO and DiD.
The present study supported earlier data
demonstrating the intercellular transfer of the DiO family of dyes
mediated by cell-cell contact and diffusible particles (15,16).
Investigation of DiI transport from labelled to unlabelled cells
following 7 days of co-culture revealed that 80% of the unlabelled
cells became stained (16).
The observed Q1-Q4 asymmetry is unlikely to be
caused by an effect on cell proliferation, as DiD and DiO labelling
did not affect proliferation. The degradation rate of the two dyes
was comparable and crossover effects were negligible. Through the
elimination of these possible explanations, the results of the
present study indicated that the Q1-Q4 asymmetry was caused by
varying diffusion rates of DiD and DiO. These dyes have different
chemical structures, in addition DiD solution contains
4-chlorobenzenesulfonate and ethanol. DiO solution contains
dimethylformamide. It has been identified that in DiD-perchlorate
(DiD without 4-chlorobenzenesulfonate) the diffusion properties are
comparable to those of DiI; however, DiD-perchlorate diffusion was
not evaluated in association with DiO (22).
The Q1-Q4 asymmetry hindered confirmation of whether
the cell-dependent or -independent process induced
microenvironmental contamination. The gating of the PQ4
sub-population obtained by analysis of the double-stained SW-1353
cells by 1:1 DiO/DiD premix enabled this confirmation. By
incubating SW-1353 with CHX and CB, it was revealed that dye
transfer is primarily a cell-independent process.
This double-prestained population is important as a
control; otherwise the Q1-Q4 asymmetry impedes precise
quantification of the intercellular transfer of dyes used in
dual-staining. A strict control must be applied prior to each
experiment involving double staining of cells. The control staining
must be performed by adding the mixed dyes to each cell line.
The applicability of DiO and DiD in the separation
of co-cultured cells by flow cytometry requires additional
investigation. Although DiO and DiD are referred to as ‘lipophilic
dyes’ intense internalization and poor persistence in the cell
membrane was observed. However, these lipophilic dyes may be a
useful tool in quantification of intercellular interactions.
Lipophilic molecules, including DiO and DiD, characterised by
subtle differences in biocompatibility, may be a model of the
intercellular traffic of similar endogenous molecules produced by
two cell populations.
In conclusion, the present study quantified the
asymmetry of DiO and DiD distribution in SW-1353 homotypic
co-cultures, and determined that this asymmetry is
cell-independent, and is not explained by cell proliferation, dye
degradation or crossover. The limitation of the present study is
the undetermined time for the complete double staining of the whole
population of co-cultured cells by DiO and DiD. The uninvestigated
role of 4-chlorobenzenesulfonate may be one of the factors causing
the different rate of release of DiO and DiD from the cells.
Therefore, it will be useful to apply dyes with the same additives
and analyse migration. Future studies are required to confirm the
hypothesis that the speed of DiO and DiD migration is different, by
measuring the movement of dye particles visible under microscope.
This approach of using software to analyse video recordings may
confirm the differences in the speed of DiO and DiD transport.
Finally, the type of cellular activity responsible for active dye
transfer requires clarification.
Acknowledgements
The present study was supported solely by the Polish
National Science Centre (grant no. NN403600538). The authors would
like to thank Ms. Beata Raczak and Ms. Bogumila Ratajczak
(Department of Biochemistry and Molecular Biology, Poznan
University of Medical Sciences, Poznan, Poland) for their
indispensable assistance during the preparation of this paper.
References
|
1
|
Honig MG and Hume RI: Fluorescent
carbocyanine dyes allow living neurons of identified origin to be
studied in long-term cultures. J Cell Biol. 103:171–187. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Progatzky F, Dallman MJ and Lo Celso C:
From seeing to believing: Labelling strategies for in vivo
cell-tracking experiments. Interface Focus. 3:201300012013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sezgin E, Chwastek G, Aydogan G, Levental
I, Simons K and Schwille P: Photoconversion of bodipy-labeled lipid
analogues. Chembiochem. 14:695–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shapiro HM: Practical Flow Cytometry.
7362003.
|
|
5
|
Rietdorf J and Stelzer EHK: Special
optical elementsHandbook of Biological Confocal Microscopy. New
York: Springer Verlag; 2006, View Article : Google Scholar
|
|
6
|
Thayanithy V, Dickson EL, Steer C,
Subramanian S and Lou E: Tumor-stromal cross talk: Direct
cell-to-cell transfer of oncogenic microRNAs via tunneling
nanotubes. Transl Res. 164:359–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fritzsch B, Muirhead KA, Feng F, Gray BD
and Ohlsson-Wilhelm BM: Diffusion and imaging properties of three
new lipophilic tracers, NeuroVue Maroon, NeuroVue Red and NeuroVue
Green and their use for double and triple labeling of neuronal
profile. Brain Res Bull. 66:249–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huerta L, López-Balderas N, Larralde C and
Lamoyi E: Discriminating in vitro cell fusion from cell aggregation
by flow cytometry combined with fluorescence resonance energy
transfer. J Virol Methods. 138:17–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lo Celso C, Fleming HE, Wu JW, Zhao CX,
Miake-Lye S, Fujisaki J, Côté D, Rowe DW, Lin CP and Scadden DT:
Live-animal tracking of individual haematopoietic stem/progenitor
cells in their niche. Nature. 457:92–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maklad A and Fritzsch B: The developmental
segregation of posterior crista and saccular vestibular fibers in
mice: A carbocyanine tracer study using confocal microscopy. Brain
Res Dev Brain Res. 135:1–17. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Plotnikov EY, Khryapenkova TG, Galkina SI,
Sukhikh GT and Zorov DB: Cytoplasm and organelle transfer between
mesenchymal multipotent stromal cells and renal tubular cells in
co-culture. Exp Cell Res. 316:2447–2455. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jensen EC: Use of fluorescent probes:
Their effect on cell biology and limitations. Anat Rec (Hoboken).
295:2031–2036. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lehmann TP, Filipiak K, Juzwa W,
Sujka-Kordowska P, Jagodziński PP, Zabel M, Głowacki J, Misterska
E, Walczak M and Głowacki M: Co-culture of human nucleus pulposus
cells with multipotent mesenchymal stromal cells from human bone
marrow reveals formation of tunnelling nanotubes. Mol Med Rep.
9:574–582. 2014.PubMed/NCBI
|
|
14
|
Burguera EF, Bitar M and Bruinink A: Novel
in vitro co-culture methodology to investigate heterotypic
cell-cell interactions. Eur Cell Mater. 19:166–179. 2010.PubMed/NCBI
|
|
15
|
Lassailly F, Griessinger E and Bonnet D:
‘Microenvironmental contaminations’ induced by fluorescent
lipophilic dyes used for noninvasive in vitro and in vivo cell
tracking. Blood. 115:5347–5354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strassburg S, Hodson NW, Hill PI,
Richardson SM and Hoyland JA: Bi-directional exchange of membrane
components occurs during co-culture of mesenchymal stem cells and
nucleus pulposus cells. PLoS One. 7:e337392012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yumoto K, Berry JE, Taichman RS and
Shiozawa Y: A novel method for monitoring tumor proliferation in
vivo using fluorescent dye DiD. Cytometry A. 85:548–555. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cirulis JT, Strasser BC, Scott JA and Ross
GM: Optimization of staining conditions for microalgae with three
lipophilic dyes to reduce precipitation and fluorescence
variability. Cytometry A. 81:618–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fonseca PC, Nihei OK, Savino W, Spray DC
and Alves LA: Flow cytometry analysis of gap junction-mediated
cell-cell communication: Advantages and pitfalls. Cytometry A.
69:487–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goers L, Freemont P and Polizzi KM:
Co-culture systems and technologies: Taking synthetic biology to
the next level. J R Soc Interface. 11(pii): 20140065.
2014.PubMed/NCBI
|
|
21
|
Gebauer M, Saas J, Sohler F, Haag J, Söder
S, Pieper M, Bartnik E, Beninga J, Zimmer R and Aigner T:
Comparison of the chondrosarcoma cell line SW1353 with primary
human adult articular chondrocytes with regard to their gene
expression profile and reactivity to IL-1beta. Osteoarthritis
Cartilage. 8:697–708. 2005. View Article : Google Scholar
|
|
22
|
Agmon A, Yang LT, Jones EG and O'Dowd DK:
Topological precision in the thalamic projection to neonatal mouse
barrel cortex. J Neurosci. 15:549–561. 1995.PubMed/NCBI
|