Introduction
The aberrant and accelerated proliferation of
vascular smooth muscle cells (VSMCs) is the major biological
process underlying certain pathological conditions, including
in-stent restenosis, the pathological re-narrowing of the vessel
lumen following surgical intervention for vascular stenosis
(1). Such vascular lesions are
typically multifactorial and are most often dependent on the
release of growth factors, including platelet-derived growth
factor-BB (PDGF-BB). The expression of PDGF-BB is known to be
increased following vascular injury, which further activates cell
proliferation signaling by binding to PDGF receptor β (2). Under physiological conditions, VSMCs
remain in a quiescent state and express α-smooth muscle actin,
desmin and smoothelin. However, in response to various mitogenic
stimuli, including PDGF-BB, VSMCs may switch to a state of high
proliferation, resulting in decreased expression levels of these
markers (3). Furthermore, cell
cycle progression and the expression levels of cell
cycle-associated proteins have been found to be upregulated by
PDGF-BB in VSMCs (4). To overcome
VSMC proliferation-mediated restenosis, drug-eluting stents have
been developed, aimed at inhibiting VSMC growth through the release
of antiproliferative substances, including paclitaxel and rapamycin
(5–7). However, unresolved problems with
these compounds include impaired re-endothelialization and the
subsequent induction of thrombosis (8), which makes the characterization of
other compounds with the ability to suppress VSMC proliferation of
clinical relevance. In accordance, the implication of natural
plant-derived compounds in controlling the proliferation of VSMCs
in diseased arteries has been widely investigated in the last
decade.
The fruit of ‘Wu-Zhu-Yu’ (Evodiae fructus) of
Evodia rutaecarpa Benth (Rutaceae) is one of the most
popular and multi-purpose herbs traditionally used in China for the
treatment of headaches, abdominal pain, menstrual problems,
vomiting, diarrhea and other diseases (9). Phytochemical studies have shown the
presence of evodiamine (Fig. 1A),
which is an indole alkaloid present in high levels in the Chinese
medicine, evodia. Evodiamine has a wide variety of bioactivities
with antinociceptive, anti-obesity, vasodilatory, antitumor and
anti-inflammatory effects (10).
Of note, evodiamine exhibits antitumor properties by inhibiting the
proliferation of various cancer cell lines. The molecular
mechanisms through which evodiamine suppresses proliferation rates
involve cell cycle progression arrest (G2/M phase) and the
induction of apoptosis (11). Of
note, evodiamine has a beneficial effect in cardiovascular
diseases. For example, evodiamine causes vasodilation in mesenteric
arteries isolated from rats and its effect is endothelium-dependent
(12). Evodiamine also has a
significant diuretic effect due to the inhibition of aldosterone
release, which can control blood volume (13). In addition, evodiamine inhibits
light-induced production of reactive oxygen species (ROS) and
pro-inflammatory cytokines, phosphorylation of mitogen-activated
protein kinases (MAPKs) p38 and extracellular signal-regulated
kinases 1/2 (Erk1/2), and activation of NADPH oxidase in human
monocytes (14). These findings
suggest that evodiamine has the potential to treat cardiovascular
diseases.
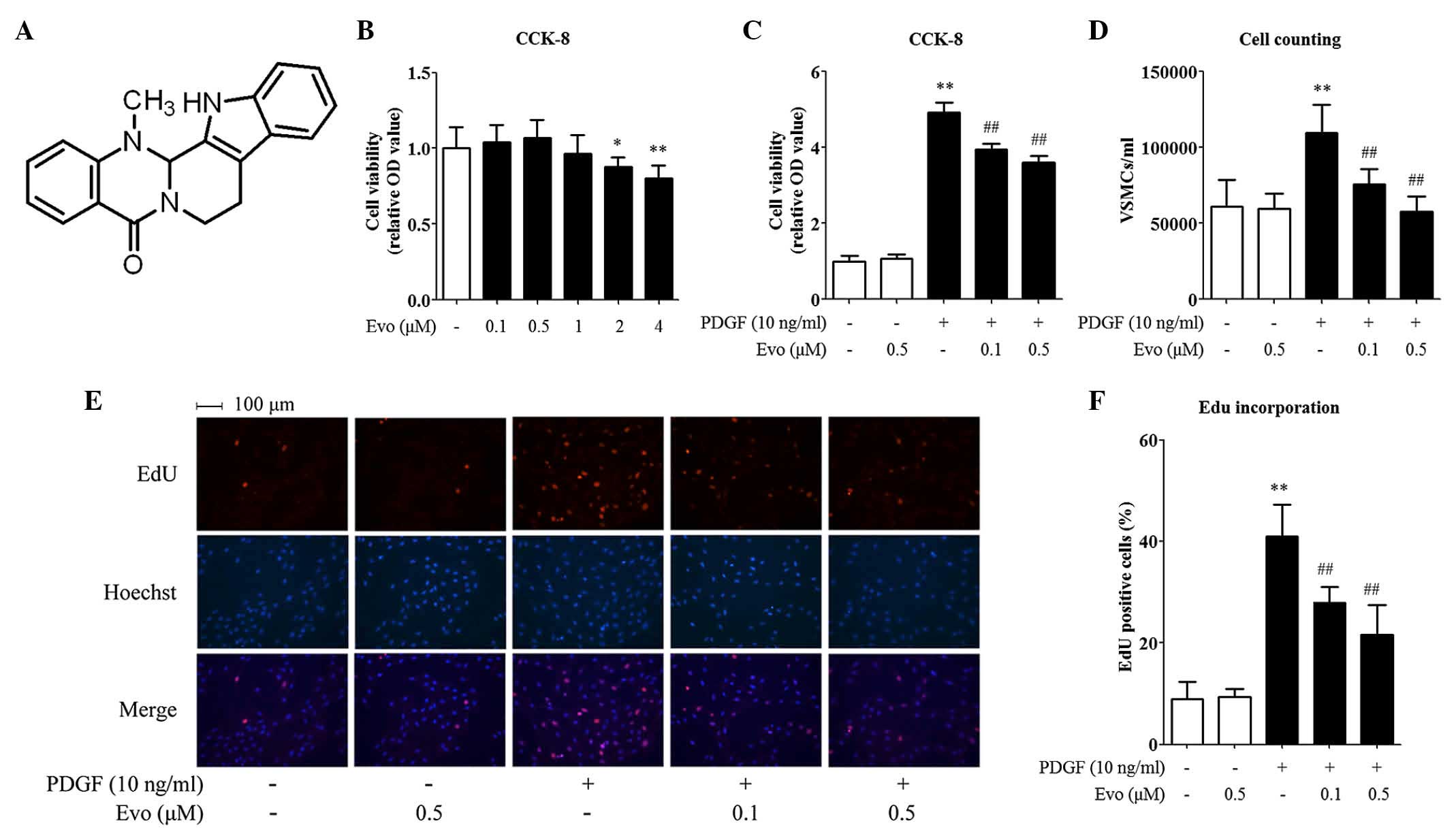 | Figure 1.Evodiamine inhibits PDGF-BB-induced
VSMC proliferation. (A) Chemical structure of evodiamine. To
measure cell toxicity, (B) VSMCs were treated with 0.1, 0.5, 1, 2
or 4 µM evodiamine for 30 h, followed by a CCK-8 analysis. To
measure cell proliferation, VSMCs were pretreated with 0.1 or 0.5
µM evodiamine for 6 h and then stimulated with 10 ng/ml PDGF-BB for
24 h. Cell proliferation was determined using a (C) CCK-8 assay,
(D) direct cell counting and an (E and F) EdU incorporation assay.
Magnification, ×200. Data are presented as the mean + standard
deviation of three independently prepared samples, each with six
measurements. *P<0.05 and **P<0.01, compared with the control
group; ##P<0.01, compared with the PDGF-BB-stimulated
group. Evo, evodiamine; PDGF-BB, platelet-derived growth factor-BB;
VSMCs, vascular smooth muscle cells; OD, optical density. |
Although evodiamine has been demonstrated to inhibit
the proliferation of tumor cells and is beneficial for the
cardiovascular system, whether evodiamine regulates the
pathophysiological processes of VSMCs remains to be elucidated.
Therefore, the aim of the present study was to investigate the
antiproliferative activity and the mechanistic target of evodiamine
in PDGF-BB-stimulated VSMCs. The findings provided evidence that
evodiamine suppressed VSMC proliferation and cell cycle progression
via regulating the expression of cell cycle-associated proteins and
the activation of MAPKs p38 and Erk1/2, and inhibiting the
production of ROS.
Materials and methods
Materials
Evodiamine was purchased from Selleck Chemicals
(Houston, TX, USA), and dissolved in DMSO to a 2 mmol/l stock
solution for later use. PDGF-BB was purchased from Sigma-Aldrich;
Merck Millipore (Darmstadt, Germany) and dissolved in 4 mmol/l
hydrochloric acid containing 0.1% bovine serum albumin.
Cell culture
The rat VSMCs were isolated using an explant
technique, as previously described (15). In brief, the thoracic aortas were
isolated from three male Sprague Dawley rats sacrificed by cervical
dislocation at the age of 3–4 weeks (provided by the Laboratory
Animal Center at Nanjing Normal University, Nanjing, China). The
rats were housed on a 12/12 h light/dark cycle at 18–26°C and had
free access to food and water. The middle vascular layers
comprising the major localization of VSMCs were carefully dissected
and cut into small sections for explant. The VSMCs were cultured in
5% CO2 at 37°C using Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.). The cells at passages 4–8 were used in all
experiments. The study was approved by the Laboratory Animal
Welfare and Ethics Committee of Nanjing Normal University (Nanjing,
China).
Cell viability assay
To analyze VSMC viability, a CCK-8 toxicity assay
was used. Briefly, 5×103 VSMCs were seeded into each well of
96-well plates, cultured at 37°C overnight for attachment, and
treated with evodiamine (0.1, 0.5, 1, 2 or 4 µM) in 100 µl medium
for 30 h. Following treatment, 10 µl WST-8 reagent (EnoGene,
Nanjing, China) was added to each well and incubated at 37°C for 2
h. Finally, a microplate reader was used to measure the absorbance
at 450 nm.
Cell proliferation assay
To analyze VSMC proliferation, a CCK-8 proliferation
assay, direct cell counting and an EdU incorporation assay were
used. For the CCK-8 assay, 2×103 VSMCs were seeded into each well
of 96-well plates and incubated at 37°C overnight for attachment.
Subsequently, the cells were pre incubated with 0, 0.1 or 0.5 µM
evodiamine for 6 h, and then challenged with 10 ng/ml PDGF-BB for
24 h in the presence or absence of evodiamine as treated in the
pre-incubation. The cells were incubated in 5% CO2 at
37°C using DMEM without FBS supplementation throughout this
experiment. Following treatment of the cells, WST-8 reagent was
added and processed, as described above.
For the direct cell counting, 5×104 VSMCs
were seeded into each well of 6-well plates. Following a similar
treatment procedure to that described above, the cells were
resuspended with 0.05% trypsin and 0.02% EDTA, and counted using a
hemocytometer.
An EdU incorporation assay was used to analyze cell
proliferation through measuring DNA synthesis. In brief, 50 µM EdU
(Guangzhou RiboBio Co., Ltd., Guangzhou, China) was added to the
medium for 2 h following treatment of the cells, and the cells were
then fixed with 4% paraformaldehyde. EdU incorporation was
determined by incubating the cells with 1X Apollo® 567
reaction reagent (cat. no. C10310-1; Guangzhou RiboBio Co., Ltd.)
at room temperature for 30 min in the dark. The cells were
counterstained with DAPI (Sigma-Aldrich; Merck Millipore). Using a
fluorescence microscope, EdU-positive (pink) cells and DAPI-stained
nuclei (blue) were counted, respectively, and the average ratios
between the were calculated for statistical analysis.
Flow cytometry
Following treatment similar to that described for
the cell proliferation assay, the VSMCs were fixed in 70% ethanol
at −20°C overnight, washed once with PBS and incubated with PBS
containing RNase A (100 µg/ml; Vazyme Biotech, Nanjing, China) at
37°C for 30 min. The cells were then stained with prodium iodide
(Vazyme Biotech) at 4°C for 1 h. Fluorescence was measured and
analyzed using a FACSCalibur flow cytometer (BD Biosciences, San
Jose, CA, USA). The distributions of cells at the G0/G1, S and G2/M
phases were determined using Modfit LT software (version 3.1; BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA (1 µg) was mixed with 2 µl 5X qRT SuperMix
from the HiScript™ Q RT SuperMix kit for qPCR (cat. no. R122-01;
Vazyme Biotech), RNase free water was added to make a total volume
of 10 µl, and then RT was performed at 50°C for 15 min to produce
cDNA. The mRNA levels of heme oxygenase 1 (HO-1), glutathione
peroxidase 1 (GPx-1), superoxide dismutase (SOD) 1 and SOD2 were
quantified by RT-qPCR using AceQ qPCR SYBR Green Master Mix (cat.
no. Q111; Vazyme Biotech). In detail, the cDNAs acquired after RT
were diluted 10 times, then 2 µl was mixed with 5 µl AceQ qPCR SYBR
Green Master Mix and 50 µM primers (0.15 µl each), and distilled
deionized water was added to make a total volume of 10 µl. The
samples were amplified using the LightCycler® Nano
system (Roche Diagnostics, Basel, Switzerland). The amplification
conditions were: 95°C for 10 min for initial denaturation, and 45
cycles of amplification consisting of 95°C for 10 sec, and 60°C for
30 sec. 18S ribosomal RNA served as an internal control to
normalize the expression levels of mRNAs. The quantification cycle
(Cq) values were calculated using the instrument software, and the
relative expression levels were calculated using the 2-ΔΔCq method
(16). The primer sequences were
as follows: HO-1, forward 5′-TTTCACCTTCCCGAGCAT-3′ and reverse:
5′-GCCTCTTCTGTCACCCTGT-3′; GPx-1, forward
5′-ACATCAGGAGAATGGCAAGA-3′ and reverse 5′-CCGCAGGAAGGTAAAGAGC-3′;
SOD1, forward 5′-GGTCCACGAGAAACAAGA-3′ and reverse
5′-AGACTCAGACCACATAGGGA-3′; SOD2, forward 5′-GCAAGGTCGCTTACAGAT-3′
and reverse 5′-ATGGCTTTCAGATAGTCAGGTC-3′; 18S, forward
5′-AAACGGCTACCACATCCAAG-3′ and reverse
5′-CCTCCAATGGATCCTCGTTA-3′.
Western blot analysis
The VSMCs were lysed in RIPA buffer containing 50
mmol/l Tris-HCl (pH 8.0), 150 mmol/l NaCl, 1% NP-40, 1% sodium
deoxycholate, 0.1% SDS, 0.1 mmol/l DTT, 0.002 mg/ml leupeptin, 1
mmol/l NaVO3, 0.05 mmol/l PMSF and 0.002 mg/ml aprotinin. The
protein concentrations were quantified using Dc protein assay
reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and 20 µg
proteins were loaded and separated using 10% SDS-PAGE. The proteins
were then transferred onto PVDF membranes (EMD Millipore, Bedford,
MA, USA). After blocking with 5% non-fat milk (blocking buffer) at
room temperature for 1 h, the membranes were incubated overnight at
4°C with appropriate primary antibodies diluted in blocking buffer
as follows: Rabbit monoclonal cyclin-dependent kinase (CDK)2
(1:500; cat. no. 2546; Cell Signaling Technology, Inc., Danvers,
MA, USA), mouse monoclonal CDK4 (1:500; cat. no. 610147; BD
Biosciences), mouse monoclonal CDK6 (1:500; cat. no. 3136; Cell
Signaling Technology, Inc.), mouse monoclonal p21 (1:500; cat. no.
556430; BD Biosciences), rabbit monoclonal p27 (1:500; cat. no.
3,686; Cell Signaling Technology, Inc.), rabbit monoclonal cyclin
D1 (1:500; cat. no. 2978; Cell Signaling Technology, Inc.), mouse
monoclonal cyclin E (1:500; cat. no. 4129; Cell Signaling
Technology, Inc.), mouse monoclonal proliferating cell nuclear
antigen (PCNA; 1:500; cat. no. 2586; Cell Signaling Technology,
Inc.), mouse monoclonal phospho-p38 (Thr180/Try182; 1:500; cat. no.
9216; Cell Signaling Technology, Inc.), rabbit polyclonal total p38
MAPK (1:500; cat. no. 9212; Cell Signaling Technology, Inc.),
rabbit monoclonal phospho-Erk1/2 (1:500; cat. no. 4377; Cell
Signaling Technology, Inc.), rabbit polyclonal total Erk1/2 (1:500;
cat. no. 9102; Cell Signaling Technology, Inc.), rabbit polyclonal
phospho-Akt (Ser473; 1:500; cat. no. 9271; Cell Signaling
Technology, Inc.), rabbit polyclonal total Akt (1:500; cat. no.
9272; Cell Signaling Technology, Inc.) and mouse monoclonal GAPDH
(1:5,000; cat. no. KC-5G5; KangChen Biotech, Inc., Shanghai,
China). Following washing with PBS with 0.1% Tween-20 (PBST) three
times, the membranes were incubated at room temperature for 1 h
with anti-mouse (1:2,000; cat. no. sc-2005) or anti-rabbit
(1:2,000; cat. no. sc-2004) horseradish peroxidase conjugated
secondary antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). Subsequently, the membranes were washed with PBST three
times, and the bands of target proteins were visualized using
Pierce™ ECL Western Blotting Substrate (Thermo Fisher Scientific,
Inc.) and Tanon-5200 Chemiluminescent Imaging System (Tanon Science
& Technology, Ltd., Shanghai, China). The relative intensity of
the target bands were quantified by densitometric scanning using
Image J 1.32j software (National Institutes of Health, Bethesda,
MD, USA).
Measurement of ROS generation
To measure ROS generation in the VSMVs, 2′, 7′
dichlorofluorescin diacetate (DCFH-DA) was used. Briefly, following
pretreatment with 0, 0.1 or 0.5 µM evodiamine for 24 h, the VSMCs
were stimulated with 10 ng/ml PDGF-BB for 1 h, and then loaded with
10 µM DCFH-DA for 1 h (Beyotime Institute of Biotechnology, Inc.,
Nantong, China). These treatments were performed at 37°C. The VSMCs
were then rinsed twice with PBS, and images were captured with a
fluorescence microscope.
Statistical analysis
Graphpad Prism 5 software (GraphPad Software, Inc.,
San Diego, CA, USA) was used to analyze the data in the present
study. Groups of data are presented as the mean + standard
deviation. To compare the data between two groups, Student's
unpaired t-test was used. P<0.05 was considered to
indicate a statistically significant difference.
Results
Evodiamine inhibits PDGF-BB-induced
VSMC proliferation
Safety is of highest priority in drug development.
Therefore, the present study first evaluated the possible cell
toxicity induced by evodiamine using a CCK-8 assay. As shown in
Fig. 1B, treating the VSMCs with
evodiamine alone at concentrations <1 µM for 30 h did not affect
the cell viability. Therefore, doses between 0.1 and 0.5 µM were
considered safe for VSMCs, and were used throughout the present
study. The effect of evodiamine on VSMC proliferation was then
assessed. To ensure maximum accuracy, three methods were used to
quantify the cell proliferation rate. Although the detailed data
differed, the results generated from these methods shared similar
trends. In general, it was found that evodiamine treatment did not
affect the basal level of VSMC proliferation. By contrast, PDGF-BB
significantly accelerated cell growth by 1.79–4.91 fold, compared
with the control, according to the different methods. Furthermore,
evodiamine inhibited PDGF-BB-induced VSMC proliferation in a
dose-dependent manner. For the CCK-8 assay, the rates of inhibition
were 19.65 and 26.77% when the doses of evodiamine were 0.1 and 0.5
µM, respectively (Fig. 1C). For
the cell counting assay, the rates of inhibition were 31.15 and
47.54% (Fig. 1D), and for the EdU
incorporation assay, they were 31.84 and 47.13%, with pink dots
representing EdU-incorporated nuclei in Fig. 1E and F.
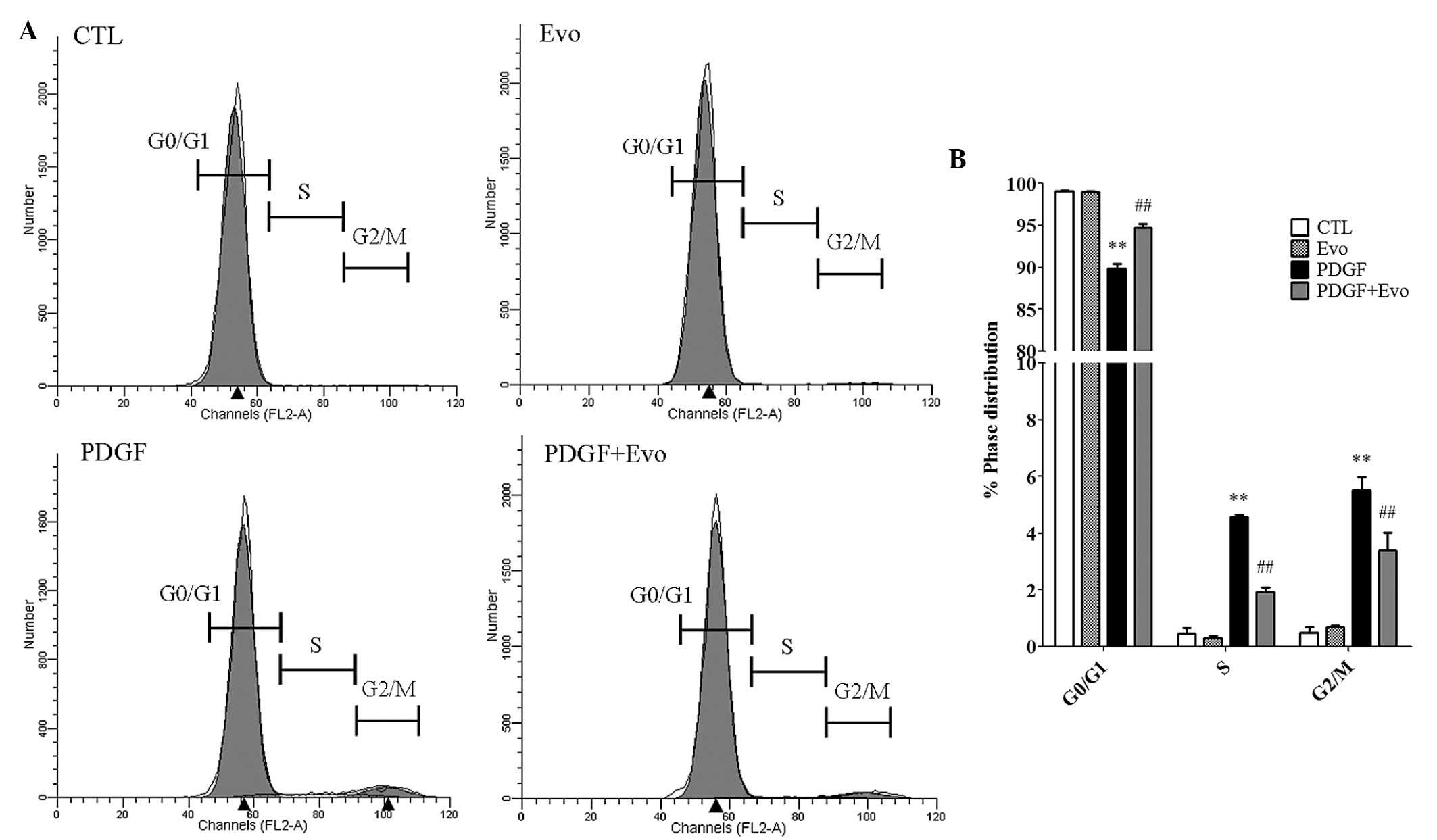
Evodiamine inhibits cell cycle
progression
As cell cycle progression is tightly associated with
accelerated cellular proliferation, the present study used flow
cytometry to investigate how evodiamine affects the cell cycle
phases. As shown in Fig. 2A and B,
PDGF-BB significantly increased the percentage of cells in the
S-phase, from 0.46 to 4.58%, and G2/M phase, from 0.49 to 5.51%.
Correspondingly, the percentage of cells at the G0/G1 phase were
reduced from 99.04 to 89.91%. By contrast, the administration of
evodiamine antagonized the effects of PDGF-BB and caused an
increase in the percentage of cells arrested in the G0/G1 phase
(94.67% of the total cells). These data suggested that the
inhibition of VSMC proliferation by evodiamine may be due to cell
cycle arrest.
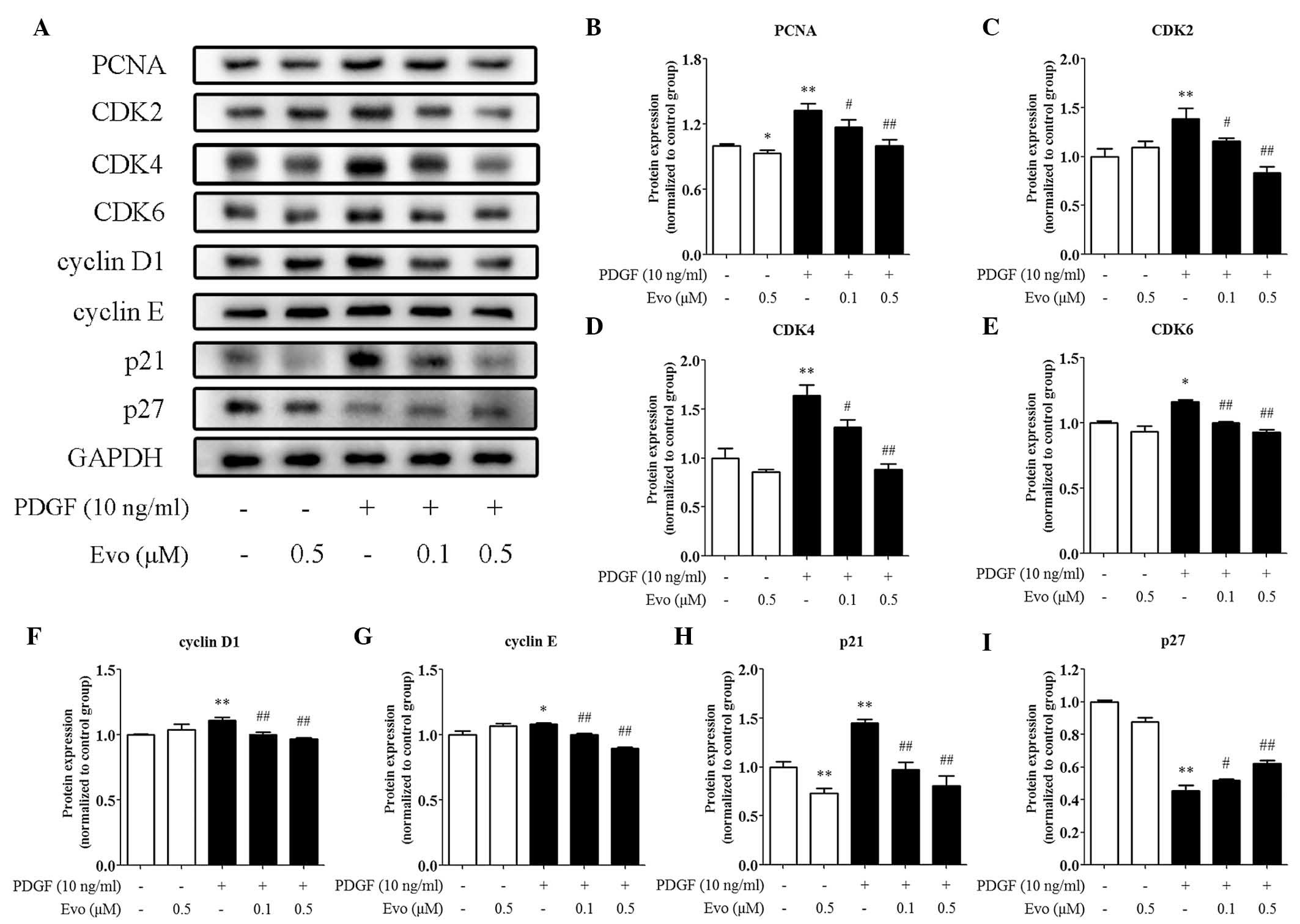
Evodiamine regulates cell
cycle-associated proteins
The cell cycle is finely controlled by a series of
regulatory proteins, therefore, the present study assessed changes
in the expression of these proteins caused by evodiamine. As shown
in Fig. 3A and B, evodiamine
reduced the protein expression level of PCNA, confirming its
inhibitory effects on VSMC proliferation. By contrast, cyclins and
CDKs orchestrate cell cycle progression. It was found that the
PDGF-BB-induced protein expression levels of CDK2/4/6 and cyclin
D1/E, were suppressed by evodiamine in a dose-dependent manner
(Figs. 3C-G). p21 and p27 are
negative regulators of cyclin-CDK complexes and the present study
found that the protein expression of p21 was inhibited by
evodiamine (Fig. 3A and H),
whereas that of p27 was induced by evodiamine (Fig. 3A and I).
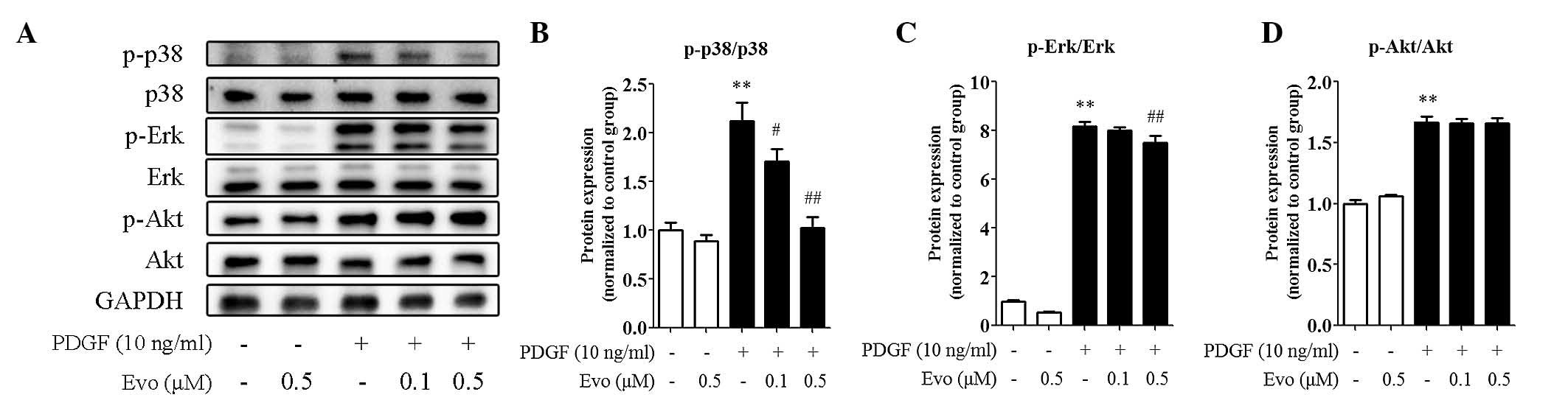
Evodiamine inhibits PDGF-BB-induced
kinase activation
MAPKs, including p38 and Erk1/2, and
serine/threonine kinase Akt are involved in the regulation of
PDGF-BB-induced VSMC proliferation. As shown in Fig. 4A-D, the levels of phosphorylated
(active) p38, Erk1/2 and Akt were all increased by PDGF-BB in the
VSMCs, as expected. However, the administration of evodiamine
markedly decreased the phosphorylation of p38 and Erk1/2,
particularly p38, whereas the effect on Akt phosphorylation was
more modest, indicating the regulation specificity of evodiamine in
kinase activation.
Evodiamine inhibits PDGF-BB-induced
ROS generation in VSMCs
ROS-induced oxidative stress is important in the
initiation of VSMC dysfunction. To directly evaluate the effect of
evodiamine on ROS production, the present study quantified ROS
levels in the PDGF-BB-treated VSMCs. As shown in Fig. 5A and B, treatment with evodiamine
alone did not alter the basal level of ROS generation, whereas
treatment with PDGF-BB (10 ng/ml) for 1 h caused a marked increase
(8.78-fold) in DCFH-DA fluorescence, compared with the control
cells. However, pretreatment with evodiamine for 24 h significantly
inhibited ROS generation. To investigate the possibility that
evodiamine attenuated oxidative stress via the induction of
antioxidant enzymes, the present study analyzed the mRNA expression
levels of genes encoding key antioxidant enzymes, including HO-1,
GPx-1, SOD1 and SOD2. It was found that PDGF-BB stimulation
inhibited the mRNA expression levels of HO-1 and GPx-1, but
increased those of SOD2. Evodiamine increased the basal expression
levels of HO-1, GPx-1 and SOD2. Of note, evodiamine induced all
four antioxidant genes examined in a concentration-dependent manner
in the PDGF-BB-treated VSMCs (Fig.
5C-F).
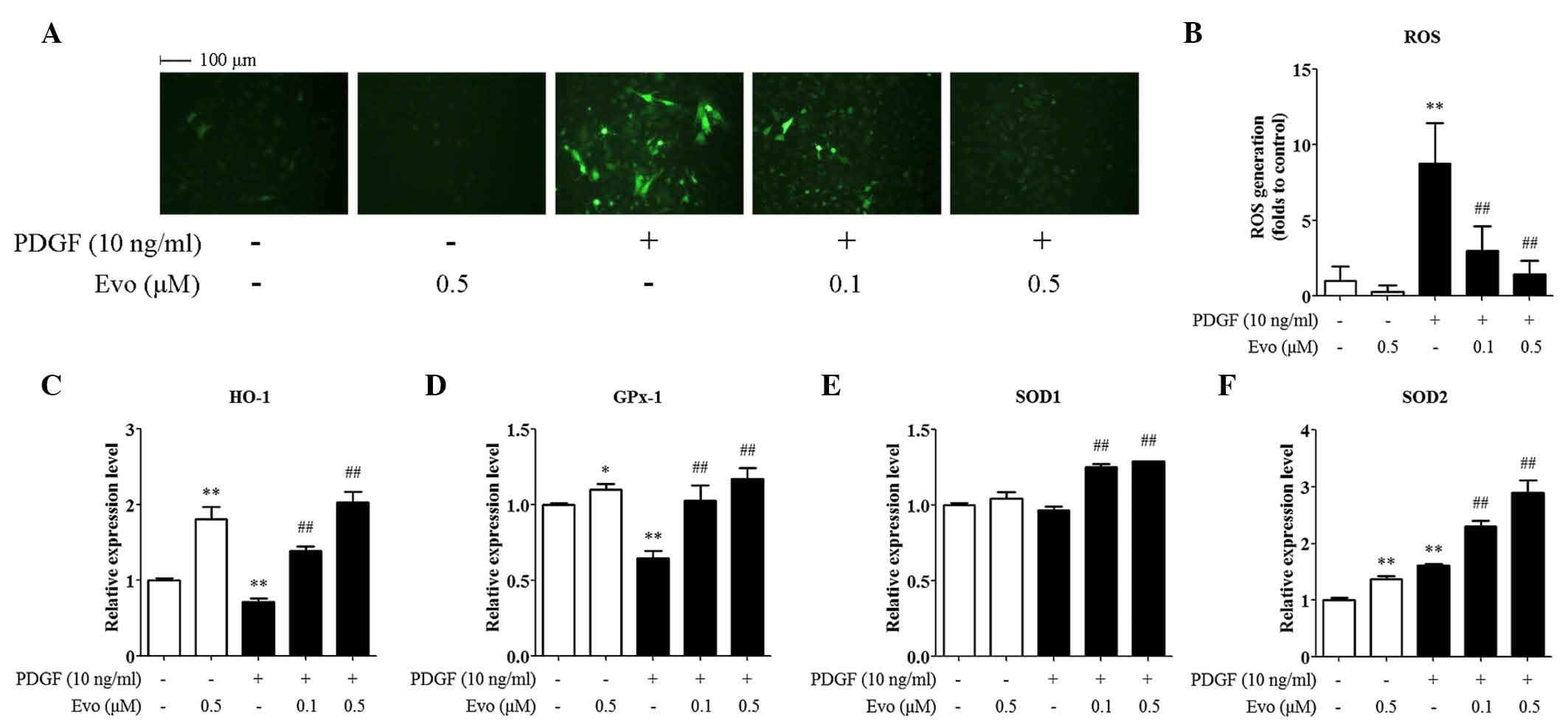 | Figure 5.Evodiamine ameliorates PDGF-BB-induced
oxidative stress in VSMCs. VSMCs were pretreated with 0.1 or 0.5 µM
evodiamine for 24 h, and then stimulated with 10 ng/ml PDGF-BB for
1 h. (A) ROS detection using 2′ 7′ dichlorofluorescin diacetate
staining. Magnification, ×200. (B) Quantitative data of five
independent experiments, expressed as the fold increase, compared
with the control. (C-F) mRNA expression levels of antioxidant genes
were assessed using reverse transcription-quantitative polymerase
chain reaction analysis. Data are presented as the mean+ standard
deviation of three independent experiments. *P<0.05 and
**P<0.01, compared with the control group;
##P<0.01, compared with the PDGF-BB-stimulated group.
Evo, evodiamine; PDGF-BB, platelet-derived growth factor-BB; VSMCs,
vascular smooth muscle cells; ROS, reactive oxygen species; HO-1,
heme oxygenase-1; GPx-1, glutathione peroxidase 1; SOD, superoxide
dismutase. |
Discussion
In the present study, it was demonstrated that
evodiamine inhibited PDGF-BB-induced VSMC proliferation in a
dose-dependent manner without causing cell toxicity. Treatment with
evodiamine inhibited progression of the cell cycle, suppressed
activation of the p38 and Erk MAPK pathways and ameliorated the
generation of ROS. To the best of our knowledge, the present study
is the first to show beneficial effects of evodiamine on the
pathophysiological processes of VSMCs.
Mitogenic signals, including PDGF-BB, regulate VSMC
proliferation through activating diverse pathways, among which the
cell cycle is a common point of convergence. Cell cycle progression
is controlled by a series of protein kinases, including cyclins and
CDKs (17). The activity of these
cyclins and kinases are regulated by their expression levels,
phosphorylation status and the presence of specific inhibitors. p21
and p27 are two negative regulators, which can arrest the cell
cycle at the G0/G1 phase (18).
The findings of the present study indicated that treatment with
evodiamine caused cell cycle arrest in the VSMCs, accompanied with
reduced expression levels of CDK 2/4/6, PCNA (a proliferation
marker protein) and cyclin D1/E. By contrast, the protein levels of
p27 were correspondingly increased. Although p21 has been
traditionally considered to be a cyclin kinase inhibitor, it has
bimodal effects on cell cycle progression and cell proliferation.
The transfection of antisense p21 oligodeoxynucleotides into VSMCs
leads to a decrease in the expression levels of cyclin D1 and CDK4,
and results in the inhibition of PDGF-BB-induced DNA synthesis and
cell proliferation (19).
Therefore, the observation that PDGF-BB increased and evodiamine
decreased the expression of p21 in the present study was not
unusual. Of note, the inhibitory effects of evodiamine on cell
cycle progression have been previously observed in various human
tumor cell lines, including small-cell lung cancer cells (20), gastric cancer cells (11), breast cancer cells (21), gastric adenocarcinoma cells
(22) and cervical carcinoma HeLa
cells (23), and are considered to
be important in the antitumor action of evodiamine. The data
obtained in the present study extends the current recognition of
evodiamine, and suggested that the regulation of cell cycle
progression by evodiamine may be general and function in a broader
range of cell types, including tumor cells and vascular cells.
MAPKs, including p38 and Erk1/2, are a family of
serine/threonine kinases, which respond to various cellular
stimuli, including growth factor stimulation, osmotic stress,
cytokines and extracellular matrix components (24). Protein phosphorylation events are
involved in the regulation of gene expression by activating
transcription factors and through post-transcriptional mechanisms
in MAPK-orientated signal transduction (25). In particular, mitogenesis is
induced in VSMCs via the phosphorylation and activation of MAPKs,
which in turn promotes VSMC proliferation under pathological
conditions (26). In the present
study, it was shown that PDGF-BB stimulation significantly
increased the levels of phosphorylated p38 MAPK and Erk1/2 in
VSMCs, which was inhibited by evodiamine treatment. These findings
were in accordance with previous studies involving tumor cells, and
suggested that the inhibition of mitogenesis and p-38/p-Erk
activity may serve as the molecular basis for the functions of
evodiamine (21,27,28).
However, the regulation of p38 and Erk activity by evodiamine is
cell type-dependent and may be negative or positive. For example,
whereas evodiamine inhibited the phosphorylation and activity of
p38 and Erk in VSMCs in the present study, human monocytes
(14) and certain tumor cells
(29), it has been reported that
evodiamine activates p38 in human melanoma A375-S2 cells,
stimulating the production of ROS and nitrogen oxide (30). Evodiamine also causes sustained
activation of the Erk/MAPK signaling pathway in 3T3-L1 and primary
preadipocytes, leading to a potent inhibitory effect for
adipogenesis (31). As p38 MAPK
and Erk1/2 are important intracellular signaling cascades in the
regulation of several cellular activities, and are orchestrated by
various upstream factors, including hormones, transcriptional
factors and epigenetic regulators, it is reasonable to suggest that
the regulation of p38 MAPK and Erk1/2 by evodiamine is the net
output of the comprehensive actions of these factors and
environmental stimuli.
The activation of phosphatidylinositol 3-kinase
(PI3K) and its downstream target, Akt, is important in triggering
mitogenesis (32). Although
PDGF-BB increased the phosphorylation of Akt in the present study,
evodiamine had a modest effect on its phosphorylation/activation. A
possible explanation for this is that a difference exists in the
regulation of cellular physiology by MAPKs and PI3K/Akt. For
example, previous studies have indicated that Erk is critical in
the regulation of cell growth, proliferation and differentiation,
whereas PI3K/Akt is involved in regulating cell survival and
apoptosis (33,34). This was supported by a previous
study showing that peroxisome proliferator-activated receptor δ
agonist inhibits VSMC proliferation by significantly inhibiting the
phosphorylation of Erk1/2, but not of Akt (35).
In the pathogenesis of various cardiovascular
diseases, oxidative stress is critical in triggering VSMC
dysfunction. Oxidative stress activates several downstream
signaling molecules, including MAPKs, protein tyrosine
phosphatases, protein tyrosine kinases and transcription factors
(36). To protect the body from
oxidative stress, endogenous antioxidant defense is evoked, which
leads to the increased expression of antioxidant enzymes, including
HO-1, GPx-1 and SOD. The induction of these antioxidant enzymes has
anti-atherosclerotic, antidiabetic and renoprotective effects, and
is critical for the maintenance of physiological homeostasis
(37). In particular, these
antioxidant enzymes protect VSMCs from oxidative injury and
antagonize VSMC proliferation (38). The present study showed that
treatment with PDGF-BB caused a more marked increase in DCFH-DA
fluorescence, compared with the control VSMCs, suggesting an
increased oxidative burden is induced by PDGF-BB. By contrast,
evodiamine eliminated ROS generation and induced the mRNA
expression levels of antioxidant enzymes in a dose-dependent
manner. As increased oxidative stress is an upstream event leading
to a diversity of pathophysiological changes in VSMCs, including
cell cycle progression, mitogenesis and proliferation, it is
essential that future investigations investigate whether the
attenuation of oxidative stress by evodiamine is central or causal
in its protective functions.
In conclusion, the results of the present study
demonstrated that evodiamine inhibited VSMC proliferation by
suppressing cell cycle progression, p38 MAPK and Erk1/2 activation,
and ROS generation. These findings suggested that, in addition to
its current pharmacological antitumor effects, evodiamine offers
potential in the prevention and treatment of cardiovascular
diseases associated with the abnormal proliferation of VSMCs.
Acknowledgements
This study was supported by grants from the National
Basic Research Program of China (973 Program; grant nos.
2012CB947600 and 2013CB911600), the National Natural Science
Foundation of China (grant nos. 31422028, 31171137, 31271261,
31401009 and 31171135), the Program for the Top Young Talents by
the Organization Department of the CPC Central Committee, NSF of
Jiangsu Province of China (grant no. BK20140041), the Jiangsu
Planned Projects for Postdoctoral Research Funds (grant no.
1302042C), the Collaborative Innovation Center for Cardiovascular
Disease Translational Medicine (Nanjing Medical University) and the
Priority Academic Program Development of Jiangsu Higher Education
Institutions.
References
|
1
|
Curcio A, Torella D and Indolfi C:
Mechanisms of smooth muscle cell proliferation and endothelial
regeneration after vascular injury and stenting: Approach to
therapy. Circ J. 75:1287–1296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boucher P and Gotthardt M: LRP and PDGF
signaling: A pathway to atherosclerosis. Trends Cardiovasc Med.
14:55–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rensen SS, Doevendans PA and van Eys GJ:
Regulation and characteristics of vascular smooth muscle cell
phenotypic diversity. Neth Heart J. 15:100–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park ES, Lee KP, Jung SH, Lee DY, Won KJ,
Yun YP and Kim B: Compound K, an intestinal metabolite of
ginsenosides, inhibits PDGF-BB-induced VSMC proliferation and
migration through G1 arrest and attenuates neointimal hyperplasia
after arterial injury. Atherosclerosis. 228:53–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palmieri C, Ravani M, Trianni G, Gianetti
J, Vaghetti M, Rizza A, Paradossi U, Beqiri A and Berti S:
Drug-eluting stents versus bare-metal stents in acute ST-segment
elevation myocardial infarction. A single-center experience with
long-term follow up. J Invasive Cardiol. 22:151–158.
2010.PubMed/NCBI
|
|
6
|
Rosner D, McCarthy N and Bennett M:
Rapamycin inhibits human in stent restenosis vascular smooth muscle
cells independently of pRB phosphorylation and p53. Cardiovasc Res.
66:601–610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen KT, Shaikh N, Wawro D, Zhang S,
Schwade ND, Eberhart RC and Tang L: Molecular responses of vascular
smooth muscle cells to paclitaxel-eluting bioresorbable stent
materials. J Biomed Mater Res A. 69:513–524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao FH, Chen YD, Jin ZN and Lu SZ: Are
impaired endothelial progenitor cells involved in the processes of
late in-stent thrombosis and re-endothelialization of drug-eluting
stents? Med Hypotheses. 70:512–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fei XF, Wang BX, Li TJ, Tashiro S, Minami
M, Xing DJ and Ikejima T: Evodiamine, a constituent of evodiae
fructus, induces anti-proliferating effects in tumor cells. Cancer
Sci. 94:92–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang J and Hu C: Evodiamine: A novel
anti-cancer alkaloid from Evodia rutaecarpa. Molecules.
14:1852–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Liu X, Wu D, Zhang M, Ran G, Bi Y
and Huang H: Growth inhibition and induction of apoptosis in
SGC7901 human gastric cancer cells by evodiamine. Mol Med Rep.
9:1147–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiou WF, Chou CJ, Shum AY and Chen CF:
The vasorelaxant effect of evodiamine in rat isolated mesenteric
arteries: Mode of action. Eur J Pharmacol. 215:277–283. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hung PH, Lin LC, Wang GJ, Chen CF and Wang
PS: Inhibitory effect of evodiamine on aldosterone release by Zona
glomerulosa cells in male rats. Chin J Physiol. 44:53–57.
2001.PubMed/NCBI
|
|
14
|
Heo SK, Yun HJ, Yi HS, Noh EK and Park SD:
Evodiamine and rutaecarpine inhibit migration by light via
suppression of nadph oxidase activation. J Cell Biochem.
107:123–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gordon D, Mohai LG and Schwartz SM:
Induction of polyploidy in cultures of neonatal rat aortic smooth
muscle cells. Circ Res. 59:633–644. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abukhdeir AM and Park BH: P21 and p27:
Roles in carcinogenesis and drug resistance. Expert Rev Mol Med.
10:e192008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weiss RH, Joo A and Randour C: p21
(Waf1/Cip1) is an assembly factor required for platelet-derived
growth factor-induced vascular smooth muscle cell proliferation. J
Biol Chem. 275:10285–10290. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang C, Zhang J, Qi D, Fan X, Luo J, Liu L
and Tan Q: Evodiamine induces G2/M arrest and apoptosis via
mitochondrial and endoplasmic reticulum pathways in H446 and human
small-cell lung cancer cells. PLoS One. 9:e1152042014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du J, Wang XF, Zhou QM, Zhang TL, Lu YY,
Zhang H and Su SB: Evodiamine induces apoptosis and inhibits
metastasis in MDAMB-231 human breast cancer cells in vitro and in
vivo. Oncol Rep. 30:685–694. 2013.PubMed/NCBI
|
|
22
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and
Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric
adenocarcinoma cells via simultaneous induction of apoptosis and
autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI
|
|
23
|
Yang J, Wu LJ, Tashino S, Onodera S and
Ikejima T: Protein tyrosine kinase pathway-derived ROS/NO
productions contribute to G2/M cell cycle arrest in
evodiamine-treated human cervix carcinoma HeLa cells. Free Radic
Res. 44:792–802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cuevas BD, Abell AN and Johnson GL: Role
of mitogen-activated protein kinase kinase kinases in signal
integration. Oncogene. 26:3159–3171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao
T, Miyazaki H and Iwao H: Role of JNK, p38 and ERK in
platelet-derived growth factor-induced vascular proliferation,
migration and gene expression. Arterioscler Thromb Vasc Biol.
23:795–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang L, Liu X, Wu D, Zhang M, Ran G, Bi Y
and Huang H: Growth inhibition and induction of apoptosis in
SGC7901 human gastric cancer cells by evodiamine. Mol Med Rep.
9:1147–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen MC, Yu CH, Wang SW, Pu HF, Kan SF,
Lin LC, Chi CW, Ho LL, Lee CH and Wang PS: Anti-proliferative
effects of evodiamine on human thyroid cancer cell line ARO. J Cell
Biochem. 110:1495–1503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang J, Cai X, Lu W, Hu C, Xu X, Yu Q and
Cao P: Evodiamine inhibits STAT3 signaling by inducing phosphatase
shatterproof 1 in hepatocellular carcinoma cells. Cancer Lett.
328:243–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Nitric oxide activated by p38 and NF-kappaB facilitates
apoptosis and cell cycle arrest under oxidative stress in
evodiamine-treated human melanoma A375-S2 cells. Free Radic Res.
42:1–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang T, Wang Y, Kontani Y, Kobayashi Y,
Sato Y, Mori N and Yamashita H: Evodiamine improves diet-induced
obesity in a uncoupling protein-1-independent manner: Involvement
of antiadipogenic mechanism and extracellularly regulated
kinase/mitogen-activated protein kinase signaling. Endocrinology.
149:358–366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao N, Zhang Z, Jiang BH and Shi X: Role
of PI3K/AKT/mTOR signaling in the cell cycle progression of human
prostate cancer. Biochem Biophys Res Commun. 310:1124–1132. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Duronio V: The life of a cell: Apoptosis
regulation by the PI3K/PKB pathway. Biochem J. 415:333–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lim HJ, Lee S, Park JH, Lee KS, Choi HE,
Chung KS, Lee HH and Park HY: PPAR delta agonist L-165041 inhibits
rat vascular smooth muscle cell proliferation and migration via
inhibition of cell cycle. Atherosclerosis. 202:446–454. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Conour JE, Graham WV and Gaskins HR: A
combined in vitro/bioinformatic investigation of redox regulatory
mechanisms governing cell cycle progression. Physiol Genomics.
18:196–205. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pisoschi AM and Pop A: The role of
antioxidants in the chemistry of oxidative stress: A review. Eur J
Med Chem. 97:55–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen S, Ding Y, Tao W, Zhang W, Liang T
and Liu C: Naringenin inhibits TNF-alpha induced VSMC proliferation
and migration via induction of HO-1. Food Chem Toxicol.
50:3025–3031. 2012. View Article : Google Scholar : PubMed/NCBI
|