Introduction
Chronic ethanol consumption-induced alcoholic liver
disease is one of the most common causes of liver cancer and
cancer-associated mortality (1).
Ethanol generates several harmful products, including reactive
oxygen species (ROS), acetaldehyde (ADH) and cytochrome P450 2E1
(CYP2E1) during metabolism (2).
The CYP2E1 generated during ethanol metabolism
generates reactive oxygen species and ADH (3). Ethanol consumption-induced liver
pathology is correlated with the expression of CYP2E1 (4). The overexpression of CYP2E1 promotes
lipid oxidation and oxidative stress in the liver (5).
ROS have been reported to affect protein oxidation,
lipid oxidation, damage to DNA, enzyme inactivation and the
depletion of various antioxidant enzymes (6–8).
These results can lead early stages of the liver disease and
dysfunction (9).
Glutathione (GSH), glutathione peroxidase (GSH-px)
and catalase (CAT) are antioxidants, which affect the
anti-oxidative system (10). The
enzymatic antioxidant system includes superoxide dismutase (SOD),
CAT and GSH-px (11). Nonenzymatic
antioxidants consist of GSH, and vitamins A, C and E (12). The antioxidant system can assist in
eliminating ROS and oxidative stress (13).
To date, several studies have shown that the
mitogen-activated protein kinase (MAPK) family is crucial in
cellular systems, including cell proliferation, cell
differentiation, development, apoptosis and inflammatory responses
(14–17). MAPKs consist of c-jun N-terminal
kinase (JNK), p38 MAP kinase and extracellular signal-regulated
kinase (ERK). Ethanol affects MAPKs in various cells and organ
systems, which consequently show different pathologic consequences
(14).
Chronic ethanol consumption upregulates the levels
of inducible nitric oxide (NO) synthase (iNOS) and the protein
expression levels of cyclooxygenase (COX)-2 in liver tissues
(18). This protein leads to
induction of the inflammatory response and oxidative stress
(19). The increased expression of
iNOS promotes the production of NO and the COX-2 protein, which
subsequently leads to the release of pro-inflammatory cytokines
(20).
Pyropia yezoensis, a species of marine algae,
has long been consumed in Korea, Japan and China. It has a range of
biological activities, including cell proliferation (21), antioxidation (22) and antiinflammatory effects
(23). In the present study, the
in vivo protective effect of P. yezoensis on chronic
ethanol consumption-induced liver injury was investigated in
mice.
Materials and methods
Preparation of P. yezoensis
glycoprotein (PYGP)
Dried P. yezoensis was purchased in
the Republic of Korea in 2014 (Suhyup, South Korea) and was
homogenized using a blender. The P. yezoensis powder
(40 g) was diluted 1:l with distilled water and stirred for 4 h at
room temperature. The solution was centrifuged (3,000 × g at
4°C for 20 min) and vacuum filtered, following which triple volumes
of ethanol (extract:ethanol, 1:3) were added. After 24 h at 4°C,
the solution was filtered and concentrated using rotary evaporation
at 40°C. The concentrated solution was divided into 1.5 ml tubes,
freeze-dried, and stored at −70°C until use.
Experimental animals
Male Sprague-Dawley rats (n=20; 6 weeks old) were
purchased from Samtaco (Osan, South Korea). The rats were allowed
to adapt to laboratory conditions for 1 week (temperature: 23±3°C,
12 h light/12 h dark cycle, 50% humidity) with free access to water
and food. Animal studies were conducted in accordance with the
Animal Ethics Committee of Pukyong National University (Busan,
South Korea).
Experimental design
The animals were randomly divided into four groups
of five rats, as follows: Control rats, which received distilled
water only (CON group); rats administered with 20% ethanol (3.7
g/kg/BW; EtOH group); rats administered with 20% ethanol (3.7
g/kg/BW)+PYGP (150 mg/kg/BW; EtOH+150 group); and rats administered
with 20% ethanol (3.7 g/kg/BW)+PYGP (300 mg/kg/BW; EtOH+300 group).
The PYGP and ethanol were administered orally once per day for 30
days. The animals in all groups were sacrificed for blood and liver
collection at the end of experimental period (day 31). Blood was
collected immediately, and the livers were frozen in liquid
nitrogen and stored at −70°C until use.
Biochemical indicators of liver
function
Blood samples were centrifuged at 3,000 × g
for 20 min at 4°C to collect serum, which was stored at −20°C until
further analysis. Chronic hepatic damage was measured by detecting
the serum levels of GOT and GPT using an enzymatic analysis kit
(Asan Pharmaceuticals Co., Ltd., Hwasung, South Korea) according to
the manufacturer's protocol. The absorbance was measured using a UV
spectrometer (Ultrospec 2100 pro; GE Healthcare Life Sciences,
Cambridge, UK).
Antioxidant enzyme measurement
The activities of antioxidant enzymes, including
CAT, GSH and GSH-px, in the homogenized liver samples were measured
using a CAT assay kit, GSH assay kit and GSH-px assay kit,
respectively, according to the manufacturer's protocols (Cayman
Chemical Company, Ann Arbor, MI, USA). The absorbance was measured
using a microplate reader (Benchmark Plus 10730; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
The liver tissue protein was homogenized in lysis
buffer containing 150 mM sodium chloride, 50 mM Tris-HCl (pH 7.5),
0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% triton
X-100 and 2 mM ethylenediaminetetra-acetic acid (Intron
Biotechnology, Inc., Seoul, South Korea) with inhibitors (1 mM
Na3VO4, 1 µg/ml aprotinin, 1 µg/ml leupeptin,
1 µg/ml pepstatin A and 1 mM PMSF; (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany). The protein levels were determined using a
Bichinchominic Acid Assay kit (Pierce; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Equal amounts of protein (20 µg) were
separated via 10–15% SDS-PAGE and then transferred onto a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The transferred membrane was blocked with 1% bovine serum
albumin (BSA) in TBS-T containing 10 mM Tris-HCl (pH 7.5), 150 mM
NaCl and 0.1% Tween 20 (USB, Cleveland, OH, USA). Following
blocking, the membranes were incubated for 4 h at room temperature
with the following primary antibodies: Rabbit anti-rat ERK IgG
polyclonal antibody (diluted 1:1,000 with BSA/TBS-T; cat. no.
sc-94), rabbit anti-rat phosphorylated (p)-ERK IgG polyclonal
antibody (diluted 1:1,000 with BSA/TBS-T; cat. no. sc-7383), mouse
anti-rat JNK IgG monoclonal antibody (diluted 1:1,000 with
BSA/TBS-T; cat. no. sc-7345), mouse anti-rat p-JNK IgG monoclonal
antibody (diluted 1:1,000 with BSA/TBS-T; cat. no. sc-6254), rabbit
anti-rat p38 IgG polyclonal antibody (diluted 1:1,000 with
BSA/TBS-T; cat. no. sc-7149), mouse anti-rat p-p38 IgG monoclonal
antibody (diluted 1:1,000 with BSA/TBS-T; cat. no. sc-7973), mouse
anti-rat iNOS IgG polyclonal antibody (diluted 1:1,000 with
BSA/TBS-T; cat. no. sc-650), goat anti-rat COX-2 IgG polyclonal
antibody (diluted 1:1,000 with BSA/TBS-T; cat. no. sc-1745), rabbit
anti-rat CYP2E1 IgG polyclonal antibody (diluted 1:1,000 with
BSA/TBS-T; cat. no. sc-133491) and rabbit anti-rat GAPDH IgG
polyclonal antibody (diluted 1:1,000 with BSA/TBS-T; cat. no.
sc-25778), all from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The membranes were then incubated with peroxidase-conjugated
goat (cat. no. A50-101P), mouse (cat. no. A90-116P) and rabbit
(cat. no. A120-101P) secondary antibodies (1:10,000; GE Healthcare
Life Sciences, Little Chalfont, UK) for 1 h at room temperature.
Antibody binding was visualized using Super Signal West Pico Stable
Peroxide solution and Super Signal West Pico Luminol/Enhancer
solution (Thermo Fisher Scientific, Inc.). The signal was monitored
using X-ray film (Kodak, Rochester, NY, USA), and a developer and
fixer twin pack (Kodak).
Statistical analysis
Values are presented as the mean + standard
deviation and data were analyzed using SPSS version 10.0 software
(SPSS, Inc., Chicago, IL, USA) using one-way analysis of variance
followed by a Duncan's multiple range test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Hepatoprotective effect of PYGP
against chronic ethanol consumption
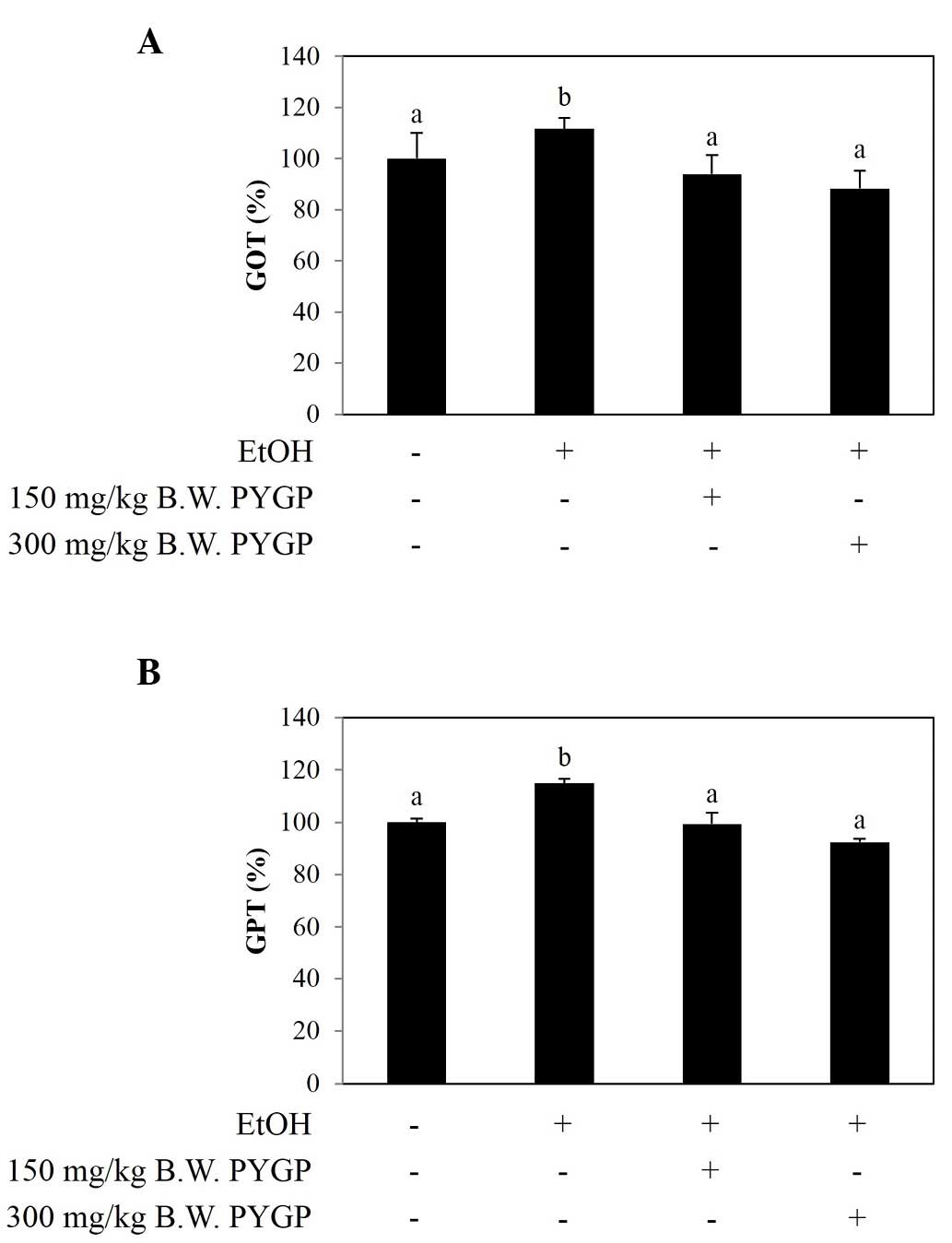
In hepatotoxicity, serum levels of GOT and GPT are
increased by liver injury or liver cell destruction (24). The results of the present study
revealed that the levels of GOT and GPT were significantly
increased in the ethanol group, compared with those of the control
group. However, the groups co-administered with PYGP showed
decreased levels of GOT and GPT (Fig.
1A and B).
Antioxidant enzyme activity in the rat
liver
The activities of the CAT, GSH and GSH-px
antioxidant enzymes were markedly decreased in the ethanol-only
group, compared with the control group. By contrast, the activities
of GSH and GSH-px were restored in the group co-administered with
ethanol and PYGP (300 mg/kg; Fig. 2A
and B). In addition, the activity of CAT was significantly
increased by co-administration with PYGP in a
concentration-dependent manner (Fig.
2C).
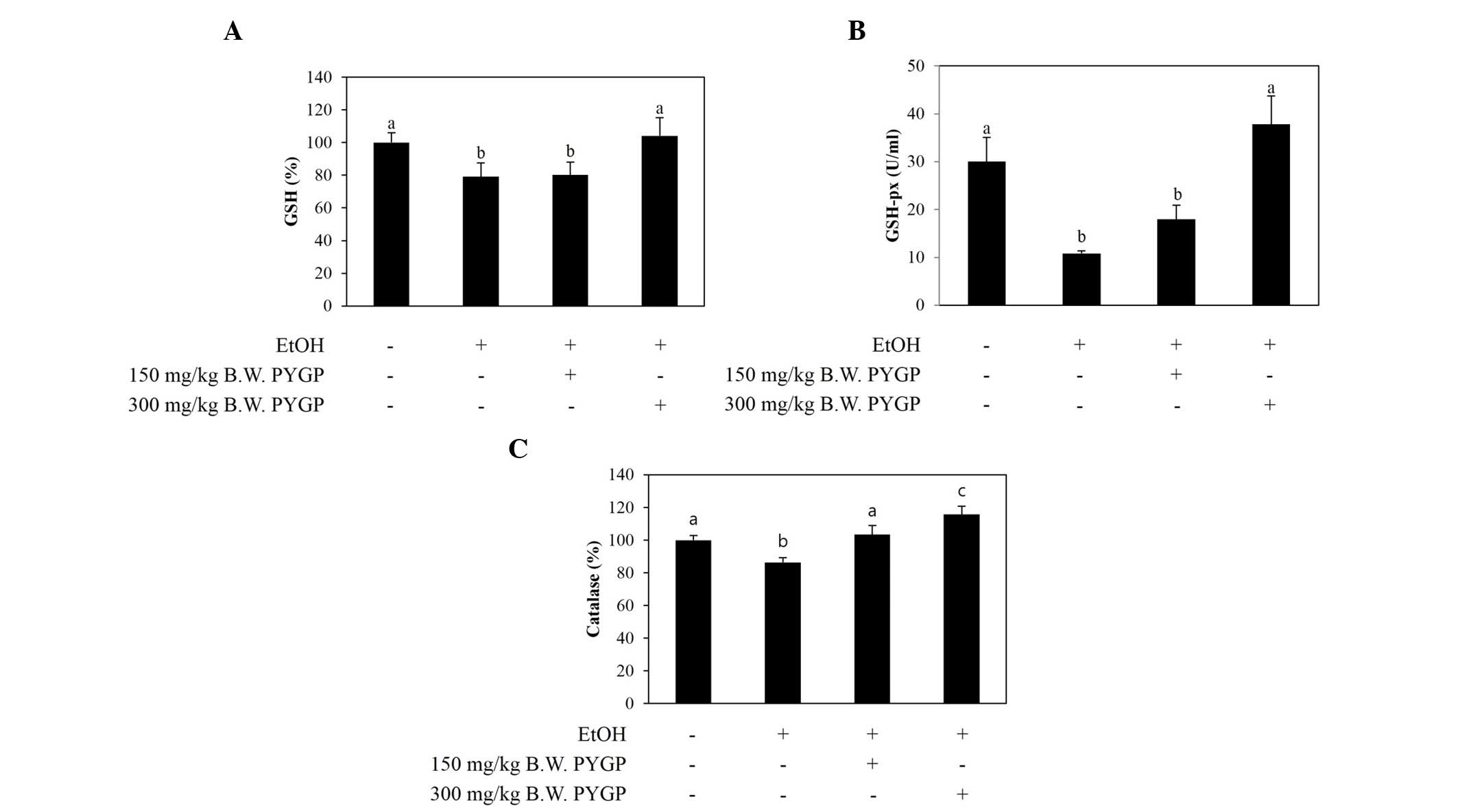 | Figure 2.Levels of the antioxidant enzymes,
GSH, GSH-px and CAT, in the livers of rats in control and
experimental groups. (A) GSH, (B) GSH-px and (C) CAT. Values are
presented as the mean + standard deviation. Groups with different
letters (a, b and c) are significantly different from each other
(P<0.05). EtOH, ethanol; GSH, glutathione; GSH-px, glutathione
peroxidase; CAT, catalase; CON, control; 150, 150 mg/kg Pyropia
yezoensis glycoprotein, 300, 300 mg/kg Pyropia yezoensis
glycoprotein. |
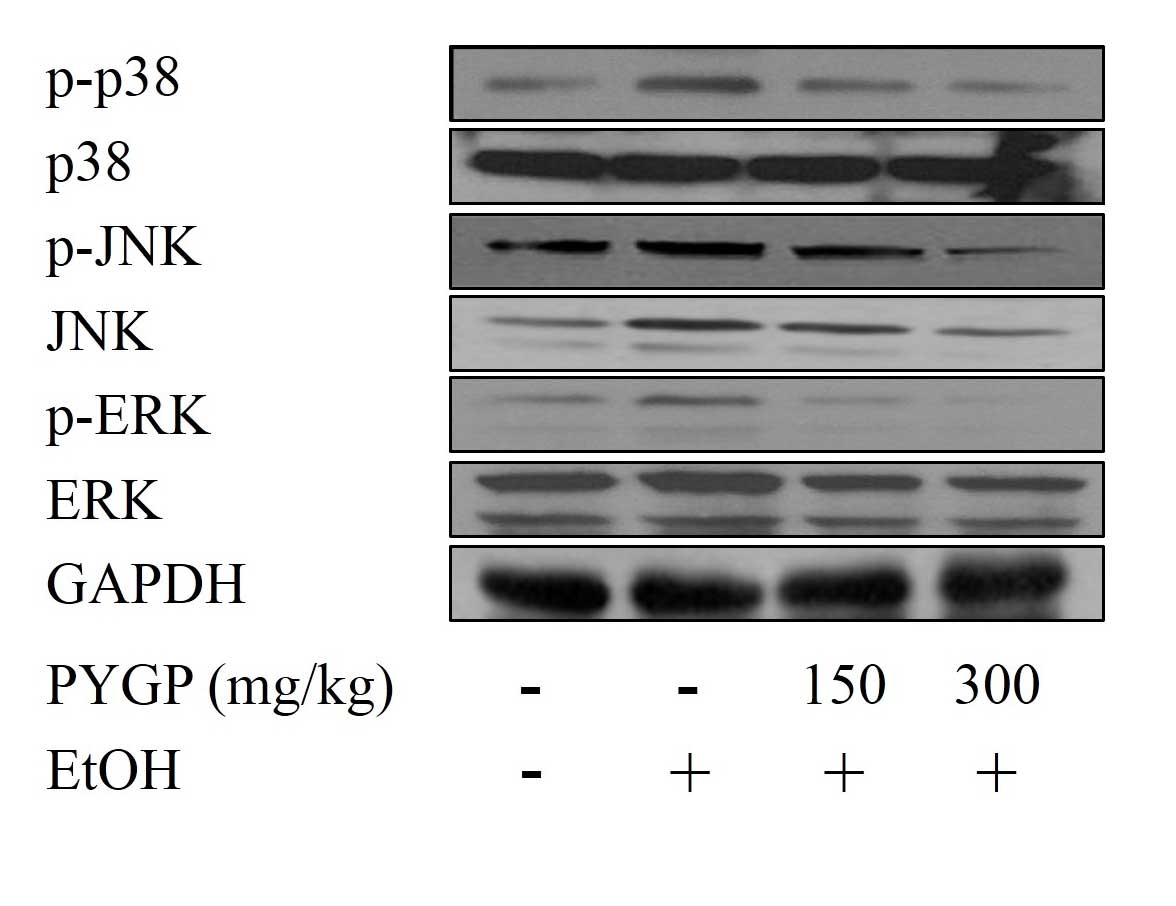
Ethanol-induced phosphorylation of
MAPK is inhibited by PYGP
To examine whether PYGP can inhibit the
phosphorylation of MAPK, the present study used western blot
assays. The results revealed that ethanol induced the
phosphorylation of ERK, JNK and p38, compared with the control
group. PYGP was effective at inhibiting the ethanol-induced protein
phosphorylation of ERK, JNK and p38. By contrast, ethanol and PYGP
had no effect on the total protein expression of ERK, JNK or p38.
These result suggested that PYGP inhibited the ethanol-induced
phosphorylation of MAPK (Fig.
3).
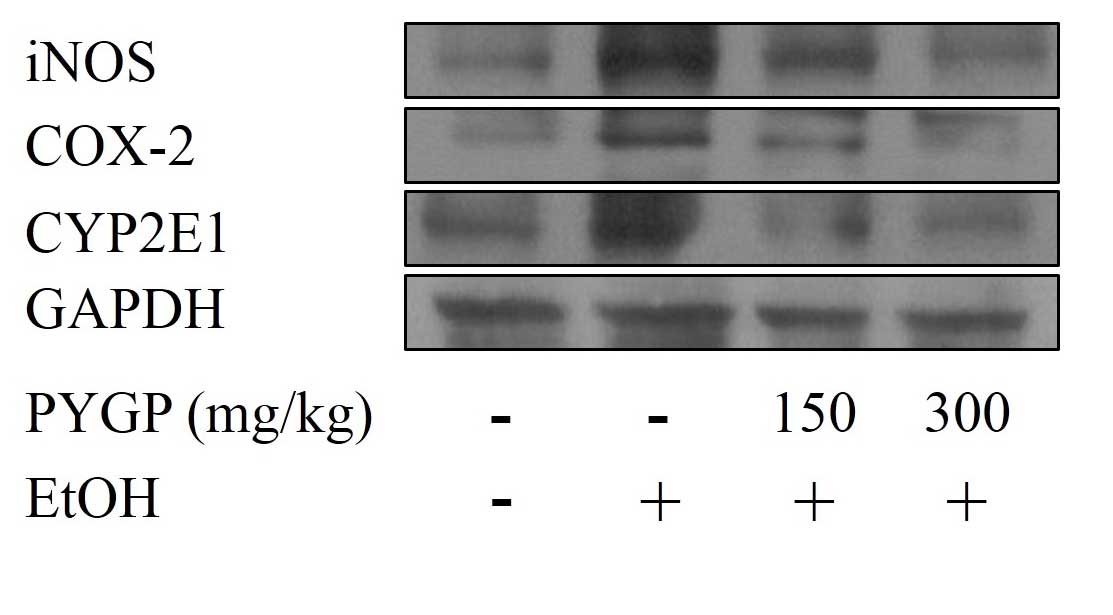
Effects of PYGP on the expression of
COX-2, iNOS and CYP2E1
Chronic ethanol consumption is known to increase the
protein expression levels of iNOS, COX-2 and CYP2E1. These proteins
are associated with liver inflammation and cell injury (19,20).
In the present study, chronic ethanol consumption upregulated the
protein expression levels of iNOS, COX-2 and CYP2E1. By contrast,
when the rats were co-administered with ethanol and PYGP, the
protein expression levels of iNOS, COX-2 and CYP2E1 were markedly
downregulated. These results confirmed that PYGP was important in
the suppression of chronic ethanol-induced protein expression of
iNOS, COX-2 and CYP2E1 (Fig.
4).
Discussion
Ethanol consumption-induced pathogenesis is
complicated. It is associated with oxidative stress, ROS generation
and alterations in the innate immune response via ethanol
metabolism (25–27). Chronic ethanol consumption in
humans leads to serious liver problems, including fibrosis,
cirrhosis and hepatocellular carcinoma (28). The increased levels of GOT and GPT
as a result of liver injury are commonly used as a measure of
hepatotoxicity. In the present study, chronic ethanol consumption
increased the serum levels of GOT and GPT, whereas
co-administration with PYGP attenuated this increase, resulting in
levels similar to those measured in the control group.
Chronic ethanol consumption induces the loss of
antioxidant or the diminution of enzyme activities, including those
of GSH, GSH-px and CAT (29). GSH
is a tripeptide and effectively scavenges ROS and free radicals
(30). GSH-px acts as a catalyst
in the reduction of H2O2 and diverse
hydroperoxides, with GSH acting as an electron donor (31). CAT is important in the
decomposition of H2O2 and the formation of
H2O and O2 (32). In the present study, the activities
of the antioxidant enzymes, GSH, GSH-px and CAT, were significantly
decreased by chronic ethanol consumption, whereas co-administration
with PYGP increased the activities of these enzymes, compared with
the ethanol-only treatment group.
MAPKs are serine-threonine kinases, which are
essential in intracellular signaling, including cell proliferation,
differentiation, transformation, survival and death (33). Ethanol consumption activates the
MAPK cascade via protein phosphorylation (34). In particular, these proteins
regulate oxidative stress in ethanol-induced hepatotoxicity
(35). In the present study, the
results showed that ethanol consumption caused the phosphorylation
of ERK, JNK and p38 in the rat liver, whereas co-administration
with PYGP attenuated the levels of phosphorylation.
Chronic ethanol consumption increases the protein
expression levels of COX-2 and iNOS in the liver (36). These proteins are associated with
the ethanol-induced liver inflammatory response (37). NO is a reactive oxidizing agent,
and the synthesis of NO is associated with the expression of iNOS
(38). Although predominantly
involved in the protective effect against bacteria, parasites and
tumor cells, the overexpression of NO causes damage to organs
(39). COX-2 is associated with
several biological response, including inflammation, carcinogenesis
and hepatic fibrogenesis (40). In
alcoholic liver disease, the expression of COX-2 is increased in
Kupffer cells (41). The increased
expression of COX-2 promotes lipid peroxidation, endotoxins,
synthesis of tumor necrosis factor-α and thromboxane B2
(TXB2). TXB2, in particular, is associated
with serious alcoholic liver disease (42). In the present study, the protein
expression levels of iNOS and COX-2 were increased by chronic
ethanol consumption, whereas co-administration with PYGP attenuated
the levels of expression, compared with the ethanol only group.
Chronic ethanol consumption promotes the production
of CYP2E1 and generates increased levels of ROS, including
H2O2 and O2 (43,44).
CYP2E1 catalyzes the oxidation of small quantities of ethanol
(~10%) into ADH (45), and ADH is
considered to be a major toxin in ethanol-induced liver injury,
inflammation and extracellular matrix (46). In the present study, the rats
exposed to chronic ethanol consumption showed higher levels of
CYP2E1 enzyme production in the liver, compared with the rats in
the control group. The co-administration of ethanol with PYGP
showed inhibition in the production of CYP2E1.
In conclusion, the present study demonstrated that
chronic ethanol consumption induced hepatotoxicity and inhibited
the levels of antioxidants, including GSH, GSH-px and CAT, in the
liver. In addition, chronic ethanol consumption promoted the
overexpression of iNOS, COX-2 and CYP2E1, and the overactivation of
ERK, JNK and p38. PYGP prevented chronic ethanol
consumption-induced hepatotoxicity in the rats via the
downregulation of MAPKs, iNOS, COX-2 and CYP2E1. These results
suggested that PYGP may offer potential for use as a novel
treatment against chronic ethanol hepatotoxicity.
Acknowledgements
This study was supported by the Fishery
Commercialization Technology Development Program through the Korea
Institute of Planning and Evaluation of Technology in Food,
Agriculture, Forestry and Fisheries (iPET) funded by the Ministry
of Oceans and Fisheries (grant no. 2012300734).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Saravanan R, Viswanathan P and Pugalendi
KV: Protective effect of ursolic acid on ethanol-mediated
experimental liver damage in rats. Life Sci. 78:713–718. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cederbaum AI: Role of CYP2E1 in
ethanol-induced oxidant stress, fatty liver and hepatotoxicity. Dig
Dis. 28:802–811. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgan K, French SW and Morgan TR:
Production of a cytochrome P450 2E1 transgenic mouse and initial
evaluation of alcoholic liver damage. Hepatology. 36:122–134. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castillo T, Koop DR, Kamimura S,
Triadafilopoulos G and Tsukamoto H: Role of cytochrome P-450 2E1 in
ethanol-, carbon tetrachloride- and iron-dependent microsomal lipid
peroxidation. Hepatology. 16:992–996. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rouach H, Fatacciolo V, Gentil M, French
SW, Morimoto M and Nordman R: Effect of chronic ethanol feeding on
lipid peroxidation and protein oxidation in relation to liver
pathology. Hepatology. 25:351–355. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernandez-Checa JC, Ookhtens M and
Kaplowitz N: Effect of chronic ethanol feeding on rat hepatocytic
glutathione: Compartmentation, efflux, and response to incubation
with ethanol. J Clin Invest. 80:57–62. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nordmann R, Ribière C and Rouach H:
Implication of free radical mechanisms in ethanol-induced cellular
injury. Free Radic Biol Med. 12:219–240. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu LL, Gong LK, Qi XM, Cai Y, Wang H, Wu
XF, Xiao Y and Ren J: Altered expression of cytochrome P450 and
possible correlation with preneoplastic changes in early stage of
rat hepatocarcinogenesis. Acta Pharmacol Sin. 26:737–744. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matés JM, Pérez-Gómez C and Núñez de
Castro I: Antioxidant enzymes and human diseases. Clin Biochem.
32:595–603. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jurczuk M, Brzóska MM, Moniuszko-Jakoniuk
J, Gałazyn-Sidorczuk M and Kulikowska-Karpińska E: Antioxidant
enzymes activity and lipid peroxidation in liver and kidney of rats
exposed to cadmium and ethanol. Food Chem Toxicol. 42:429–438.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martin KR and Barrett JC: Reactive oxygen
species as double-edged swords in cellular processes: Low-dose cell
signaling versus high-dose toxicity. Hum Exp Toxicol. 21:71–75.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scott RB, Reddy KS, Husain K, Schlorff EC,
Rybak LP and Somani SM: Dose response of ethanol on antioxidant
defense system of liver, lung, and kidney in rat. Pathophysiology.
7:25–32. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Das SK and Vasudevan DM: Alcohol-induced
oxidative stress. Life Sci. 81:177–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Venugopal SK, Chen J, Zhang Y, Clemens D,
Follenzi A and Zern MA: Role of MAPK phosphatase-1 in sustained
activation of JNK during ethanol-induced apoptosis in
hepatocyte-like VL-17A cells. J Biol Chem. 282:31900–31908. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cross TG, Scheel-Toellner D, Henriquez NV,
Deacon E, Salmon M and Lord JM: Serine/threonine protein kinases
and apoptosis. Exp Cell Res. 256:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pearson G, Robinson F, Gibson T Beers, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nanji AA, Jokelainen K, Tipoe GL,
Rahemtulla A, Thomas P and Dannenberg AJ: Curcumin prevents
alcohol-induced liver disease in rats by inhibiting the expression
of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver
Physiol. 284:G321–G327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hseu YC, Wu FY, Wu JJ, Chen JY, Chang WH,
Lu FJ, Lai YC and Yang HL: Anti-inflammatory potential of Antrodia
camphorata through inhibition of iNOS, COX-2 and cytokines via the
NF-kappa B pathway. Int Immunopharmacol. 5:1914–1925. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Surh YJ, Chun KS, Cha HH, Han SS, Keum YS,
Park KK and Lee SS: Molecular mechanisms underlying chemopreventive
activities of anti-inflammatory phytochemicals: Down-regulation of
COX-2 and iNOS through suppression of NF-kappa B activation. Mutat
Res. 480:243–268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee MK, Kim IH, Choi YH, Choi JW, Kim YM
and Nam TJ: The proliferative effects of Pyropia yezoensis peptide
on IEC-6 cells are mediated through the epidermal growth factor
receptor signaling pathway. Int J Mol Med. 35:909–914.
2015.PubMed/NCBI
|
|
22
|
Nakayama R, Tamura Y, Kikuzaki H and
Nakatani N: Antioxidant effect of the constituents of Susabinori
(Porphyra yezoensis). J Am Oil Chem Soc. 76:649–653. 1999.
View Article : Google Scholar
|
|
23
|
Shin ES, Hwang HJ, Kim IH and Nam TJ: A
glycoprotein from Porphyra yezoensis produces anti-inflammatory
effects in liposaccharide-stimulated macrophages via the TLR4
signaling pathway. Int J Mol Med. 28:809–815. 2011.PubMed/NCBI
|
|
24
|
Yamaguchi M, Tsurusaki Y, Misawa H,
Inagaki S, Ma ZJ and Takahashi H: Potential role of regucalcin as a
specific biochemical marker of chronic liver injury with carbon
tetrachloride administration in rats. Mol Cell Biochem. 241:61–67.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bailey SM and Cunningham CC: Contribution
of mitochondria to oxidative stress associated with alcoholic liver
disease. Free Radic Biol Med. 32:11–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hines IN and Wheeler MD: Recent advances
in alcoholic liver disease III. Role of the innate immune response
in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol.
287:G310–G314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar KJ, Chu FH, Hsieh HW, Liao JW, Li
WH, Lin JC, Shaw JF and Wang SY: Antroquinonol from ethanolic
extract of mycelium of Antrodia cinnamomea protects hepatic cells
from ethanol-induced oxidative stress through Nrf-2 activation. J
Ethnopharmacol. 136:168–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leung MT, Lu Y, Yan W, Morón-Concepción
JA, Ward SC, Ge X, de la Rosa L Conde and Nieto N:
Argininosuccinate synthase conditions the response to acute and
chronic ethanol-induced liver injury in mice. Hepatology.
55:1596–1609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ostrowska J, Łuczaj W, Kasacka I, Różański
A and Skrzydlewska E: Green tea protects against ethanol-induced
lipid peroxidation in rat organs. Alcohol. 32:25–32. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu G, Fang YZ, Yang S, Lupton JR and
Turner ND: Glutathione metabolism and its implications for health.
J Nutr. 134:489–492. 2004.PubMed/NCBI
|
|
31
|
Chang TS, Cho CS, Park S, Yu S, Kang SW
and Rhee SG: Peroxiredoxin III, a mitochondrion-specific
peroxidase, regulates apoptotic signaling by mitochondria. J Biol
Chem. 279:41975–41984. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vimal V and Devaki T: Hepatoprotective
effect of allicin on tissue defense system in
galactosamine/endotoxin challenged rats. J Ethnopharmacol.
90:151–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park HM, Kim SJ, Mun AR, Go HK, Kim GB,
Kim SZ, Jang SI, Lee SJ, Kim JS and Kang HS: Korean red ginseng and
its primary ginsenosides inhibit ethanol-induced oxidative injury
by suppression of the MAPK pathway in TIB-73 cells. J
Ethnopharmacol. 141:1071–1076. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aroor AR and Shukla SD: MAP kinase
signaling in diverse effects of ethanol. Life Sci. 74:2339–2364.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tahir M, Rehman MU, Lateef A, Khan R, Khan
AQ, Qamar W, Ali F, O'Hamiza O and Sultana S: Diosmin protects
against ethanol-induced hepatic injury via alleviation of
inflammation and regulation of TNF-α and NF-κB activation. Alcohol.
47:131–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murakami A and Ohigashi H: Targeting NOX,
INOS and COX-2 in inflammatory cells: Chemoprevention using food
phytochemicals. Int J Cancer. 121:2357–2363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gardner CR, Heck DE, Yang CS, Thomas PE,
Zhang XJ, DeGeorge GL, Laskin JD and Laskin DL: Role of nitric
oxide in acetaminophen-induced hepatotoxicity in the rat.
Hepatology. 27:748–754. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Quan J, Yin X and Xu H: Boschniakia
rossica prevents the carbon tetrachloride-induced hepatotoxicity in
rat. Exp Toxicol Pathol. 63:53–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu KQ: Cyclooxygenase 2 (COX2)-prostanoid
pathway and liver diseases. Prostaglandins Leukot Essent Fatty
Acids. 69:329–337. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nanji AA, Miao L, Thomas P, Rahemtulla A,
Khwaja S, Zhao S, Peters D, Tahan SR and Dannenberg AJ: Enhanced
cyclooxygenase-2 gene expression in alcoholic liver disease in the
rat. Gastroenterology. 112:943–951. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nanji AA, Khettry U, Sadrzadeh SM and
Yamanaka T: Severity of liver injury in experimental alcoholic
liver disease. Correlation with plasma endotoxin, prostaglandin E2,
leukotriene B4, and thromboxane B2. Am J Pathol. 142:367–373.
1993.PubMed/NCBI
|
|
43
|
Zima T and Kalousová M: Oxidative stress
and signal transduction pathways in alcoholic liver disease.
Alcohol Clin Exp Res. 29:(Suppl 11). S110–S115. 2005. View Article : Google Scholar
|
|
44
|
Lu Y and Cederbaum AI: CYP2E1 and
oxidative liver injury by alcohol. Free Radic Biol Med. 44:723–738.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ceni E, Mello T and Galli A: Pathogenesis
of alcoholic liver disease: Role of oxidative metabolism. World J
Gastroenterol. 20:17756–17772. 2014.PubMed/NCBI
|
|
46
|
Seth D, Haber PS, Syn WK, Diehl AM and Day
CP: Pathogenesis of alcohol-induced liver disease: Classical
concepts and recent advances. J Gastroenterol Hepatol.
26:1089–1105. 2011. View Article : Google Scholar : PubMed/NCBI
|