Introduction
Annually, ~500,000 people are diagnosed with
laryngeal cancer worldwide (1).
The prevalence of laryngeal cancer differs widely between different
geographic areas. In Europe, ~52,000 new cases are diagnosed
annually, and an estimated 13,560 new cases appear in the United
States (2). The yearly incidence
ratio is ~1.4/100,000, which is standardized in China (3). Laryngeal cancer accounts for 2–3% of
all cancer and 25% all head and neck cancer. The majority (90%) of
laryngeal cancers occur in men, and 95% are squamous cell
carcinoma, which is the second most common head and neck squamous
cell cancer. Patients have a good prognosis when laryngeal cancer
is discovered and treated timely. At the initial evaluation, ~40%
of patients exhibit stage III or IV disease (4). Although the 5-year overall survival
rate has risen for the last 30 years due to intensive research and
advances in conventional cancer treatments, terminal laryngeal
cancer remains a troublesome problem for otolaryngologists. Thus,
it is necessary to develop novel therapies to diagnose this cancer
early and to rapidly treat the disease.
Certain studies have demonstrated that laryngeal
cancers are closely associated with oxidative stress (5,6). The
body has developed a system to scavenge oxidants in response to
harmful stimulations. Recent studies identify the role of the
kelch-like ECH-associated protein-1 (KEAP1)/nuclear factor
(erythroid-derived 2)-like 2 (NRF2) system in this process, and
these proteins have central functions in cellular protection
against oxidative stress. Under normal conditions, NRF2 couples
with the anchor protein KEAP1 in the cytoplasm via Cul3-based E3
ligase, which is a negative regulator of NRF2 and promotes
continuous degradation of NRF2 via ubiquitination and proteasomal
degradation systems. NRF2 separates from KEAP1 following exposure
to oxidative stress and translocates to the nucleus for the
transactivation of AU-rich element (ARE)-bearing genes and phase II
detoxifying enzymes via heterodimerization with small Maf proteins.
Evidence indicates that the loss of KEAP1 genes leads to NRF2
upregulation and expression of its downstream genes that encode
cytoprotective proteins, including quinone oxidoreductase-1 (NQO-1)
and heme oxygenase-1 (HO-1), which enhances cancer cell
proliferation. Simultaneous knockdown of NRF2 genes produced the
opposite results. Experiments confirmed this hypothesis in lung and
colorectal cancer (7–9).
To the best of our knowledge, no individual study
has investigated the expression and correlation of NRF2, KEAP1,
NQO-1 and HO-1 and their associations to clinicopathological
features in advanced laryngeal cancer. Thus, the present study
primarily focused on these indicators in a series of laryngeal
cancer and paraneoplastic specimens to improve clinical diagnosis
and treatment of advanced laryngeal cancer.
Materials and methods
Tissue samples
The Animal Ethical Committee of the Eye, Ear, Nose
and Throat Hospital of Fudan University (Shanghai, China) reviewed
and approved the study protocol. Specimens for this study were
collected from 40 patients at the Otorhinolaryngology Head and Neck
Surgery Department of the Eye, Ear, Nose and Throat Hospital of
Fudan University from April 2014 to January 2015. Five cases were
excluded because the tumor cells infiltrated the pericarcinous
tissues. All patients were diagnosed with stage III or IV laryngeal
cancer (10), except for two cases
of stage II cancer. Samples of laryngeal cancer and adjacent normal
tissues were collected from a total of 33 patients with clinical
stage III or IV. All patients were male with an age range from 44
to 81 years (mean 61.7±7.6 years old). No patients had received any
prior treatment for cancer, and there were no other systemic
complications, including heart, hepatic or renal disease. All
tumors were moderate differentiated. All patients underwent primary
total laryngectomy. All surgical excisions of fresh specimens from
the larynx neoplasm and adjacent tissues, which were >1.5 cm
away from the cancer, were used for immunohistochemistry and
western blotting. Two pathologists confirmed that all tumor tissues
were laryngeal squamous cell carcinomas, and all pericarcinous
tissues were confirmed as normal tissues. The clinicopathological
features are summarized in Table
I.
 | Table I.Clinicopathological features of
patients with laryngeal cancer. |
Table I.
Clinicopathological features of
patients with laryngeal cancer.
| Feature | Number of cases |
|---|
| Total | 33 |
| Agea |
|
| ≥62 years
old | 19 |
| <62
years old | 14 |
| Sex |
|
| Male | 33 |
|
Female | 0 |
| Differentiation |
|
| G1 | 5 |
| G2 | 28 |
| G3 | 0 |
| Clinical stage |
|
| III | 16 |
| IV | 17 |
| Lymph node
metastasis |
|
| N0 | 13 |
| N+ | 20 |
| Maximum diameter of
tumor sizeb |
|
| ≥3.2
cm | 17 |
| <3.2
cm | 16 |
Immunohistochemistry
The immunohistochemistry method used was reported
previously (11). The 33 tumor and
pericarcinomatous specimens were fixed in 4% paraformaldehyde and
embedded in paraffin following routine processing. Sections (4 µm
thick) were obtained from each paraffin block. The sections were
cleared in xylene and hydrated through a descending alcohol series
to distilled water. Endogenous peroxidase activities were quenched
with 3% hydrogen peroxide in deionized water for 10 min. An antigen
retrieval step was performed using citrate buffer, pH 6.0, for 10
min, and sections were brought boiled for 10 min in two 5-min
sessions in a microwave. Sections were preincubated with 1% normal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) to block nonspecific binding sites for 1 h at room temperature
and incubated overnight at 4°C with anti-NRF2/KEAP1/HO-1 antibodies
[rabbit polyclonal to NRF2 (cat. no. ab31163), rabbit monoclonal to
KEAP1 (cat. no. ab181144) and HO-1 (cat. no. ab52947); Abcam,
Cambridge, UK] at a 1:100 dilution or an NQO-1 antibody [mouse
monoclonal to NQO-1 (cat. no. 3187); Cell Signaling Technology,
Inc., Danvers, MA, USA] at a 1:25 dilution. The primary antibody
was only added to one of the two sections on each slide, and the
other section was incubated with PBS as a control. The slides were
washed with PBS and incubated with the secondary antibody (part of
ABC complex kit) for 15 min at 37°C, followed by incubation with
the ABC complex kit (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China). Color development and visualization was performed
using 3,3-diaminobenzidine. The tissue sections were lightly
counterstained with hematoxylin, dehydrated, cleared and
coverslipped. The slides were imaged using a light microscope.
Immunohistochemical semiquantitative evaluations of
cytoplasmic and nuclear proteins were performed separately. An
immunohistochemical expression score (0.0 to 4.0 scoring system)
was used, and a final score was obtained by multiplying the
percentage of stained cells and corresponding intensity score. A
score of 0 suggested that there was no detectable staining and 4.0
corresponded to a saturated signal in the tissues. An
immunostaining score ≥0.5 was chosen arbitrarily as overexpression
of cytoplasmic proteins, and positive expression of nuclear
proteins was defined as a score ≥0.1 based on the associated
literature (12). This method of
deriving immunoscores was reported in numerous previous studies
(12,13). Two pathologists evaluated all
immunostaining in a blinded manner, without any clinical
information. Negative controls of nonspecific immunohistochemical
staining were routinely included. A total of 3 randomly selected
fields from each sample were examined.
Western blot analysis
All frozen tissues were lysed in a
radioimmunoprecipitation buffer containing phenylmethylsulfonyl
fluoride with a final concentration of 1 mM/l (CoWin Biotech Co.,
Ltd., Beijing, China), and total protein extracts were obtained
using centrifugation at 4°C and 10,000 × g for 10 min.
Protein concentration was quantified using a Bicinchoninic Acid
Protein Assay kit (Beyotime Institute of Biotechnology, Haimen,
China), and 40 µg total protein were electrophoretically separated
on 12% sodium dodecyl sulfate polyacrylamide gel, transferred to
polyvinylidene fluoride membranes, which were blocked with 5%
non-fat milk in Tris-buffered saline with 0.1% Tween 20 (TBST;
pH=7.4–7.5) at room temperature for 1 h. Membranes were incubated
overnight with anti-NRF2 (cat. no. ab31163), anti-KEAP1 (cat. no.
ab181144), anti-NQO1 (cat. no. 3187), anti-HO1 (cat. no. ab52947)
or anti-β-actin (cat. no. AA128; Beyotime Institute of
Biotechnology) primary antibodies in blocking buffer at 4°C at a
1:1,000 dilution. Membranes were washed three times in TBST
followed by incubation for 1 h with a horseradish-peroxidase goat
anti-rabbit (cat. no. A0208; Beyotime Institute of Biotechnology)
and goat anti-mouse (cat. no. A0216; Beyotime Institute of
Biotechnology) secondary antibody at a 1:2,500 dilution. Membranes
were washed in TBST, and bands were visualized using enhanced
chemiluminescence kit (beyoECL Plus; Beyotime Institute of
Biotechnology). β-actin was used as the internal control.
Statistical analysis
The significance of intergroup differences in NRF2,
KEAP1, NQO-1, and HO-1 immunostains between laryngeal cancer and
pericarcinomatous specimens were determined using Fisher's exact
test. Mutual correlations on the protein expression levels and
their association with clinicopathological features in carcinoma
tissues were analyzed using Pearson correlation test and
independent-samples t test respectively, prior to which normality
test was applied. The degree of correlation was determined using
Pearson correlation coefficient. P<0.05 (two-sided) was
considered to indicate a statistically significant difference. All
statistical analyses were conducted using the SPSS 17.0 statistical
package (SPSS, Inc., Chicago, IL, USA). All of the statistical
graphs were produced using with GraphPad Prism 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
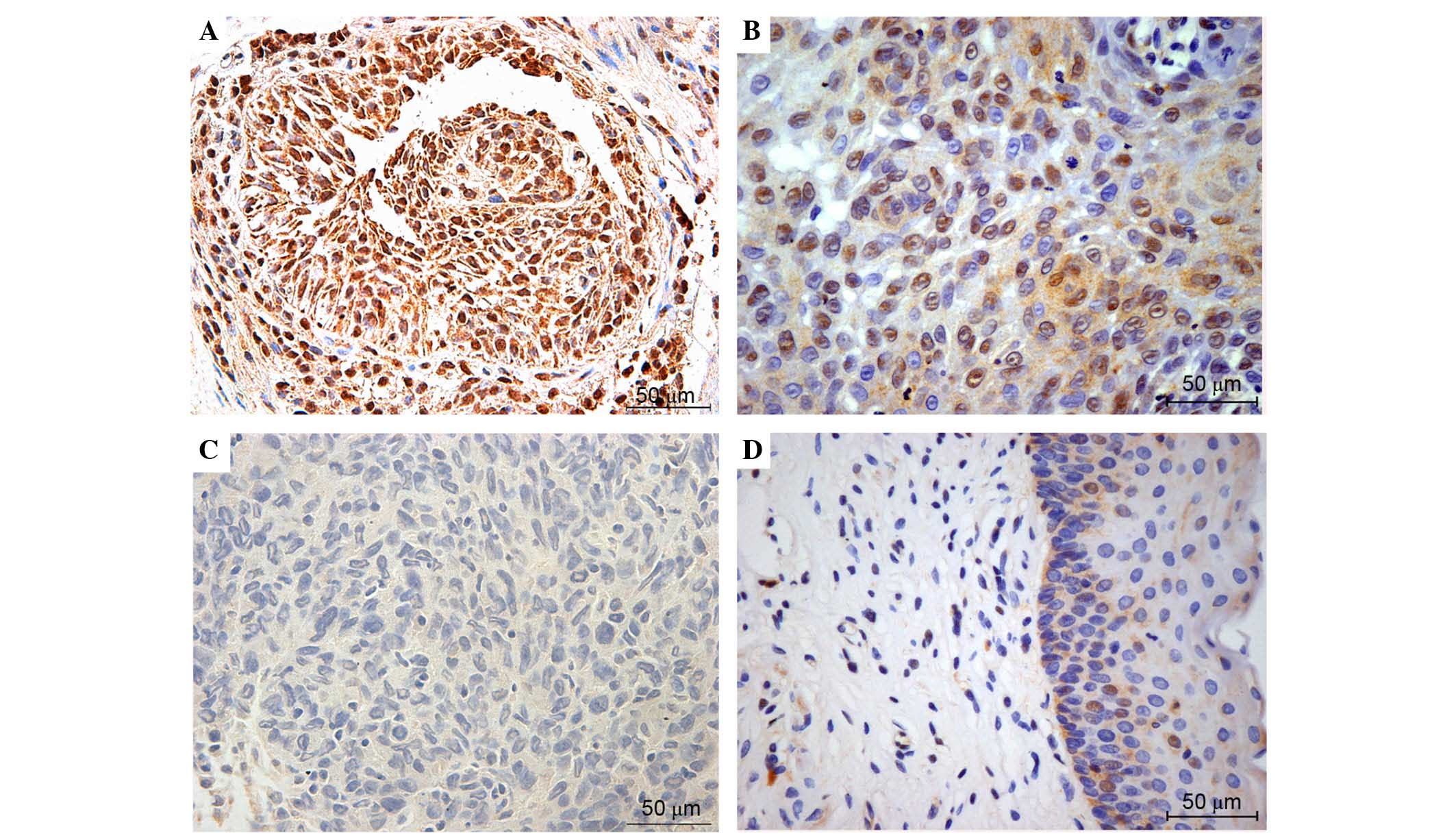
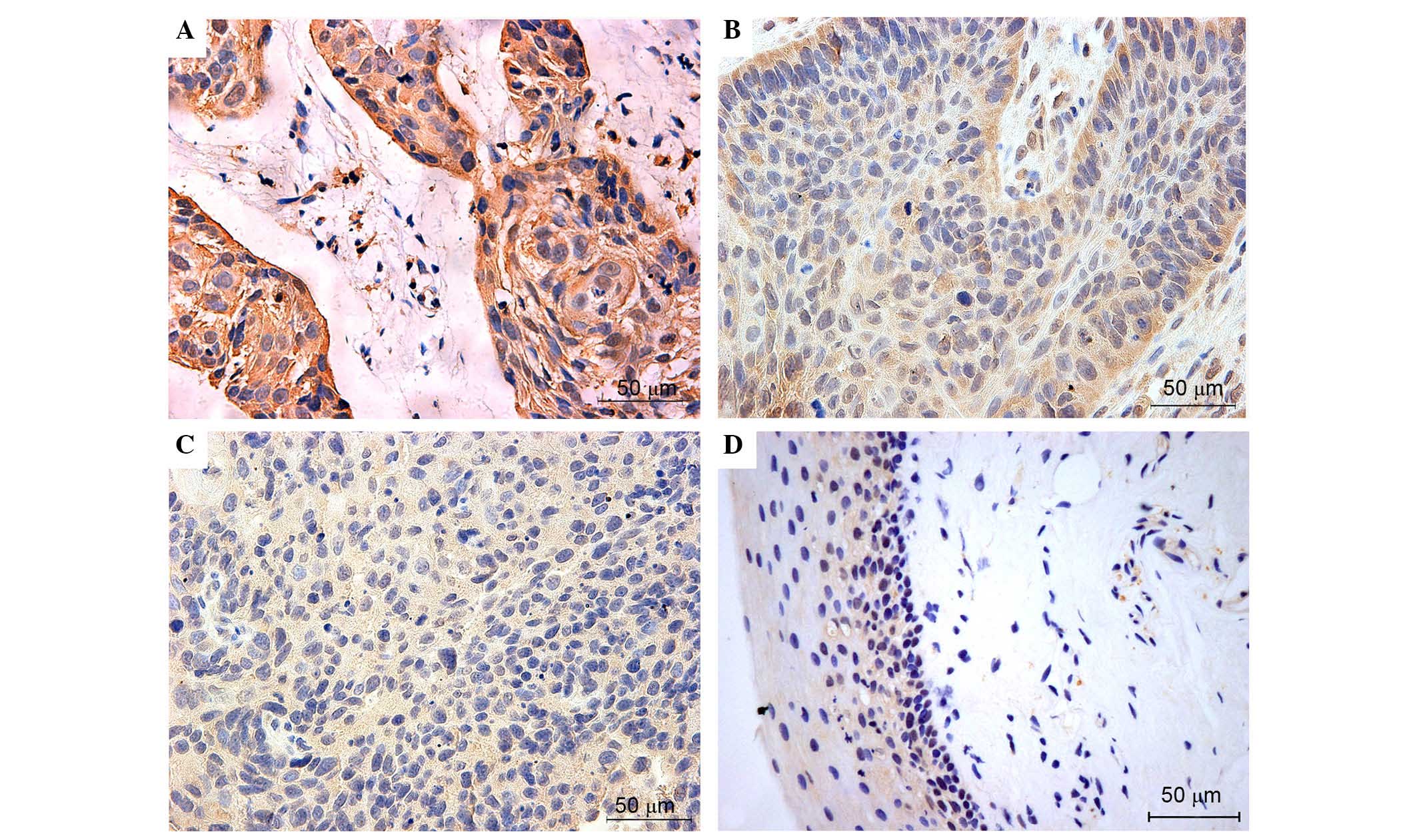
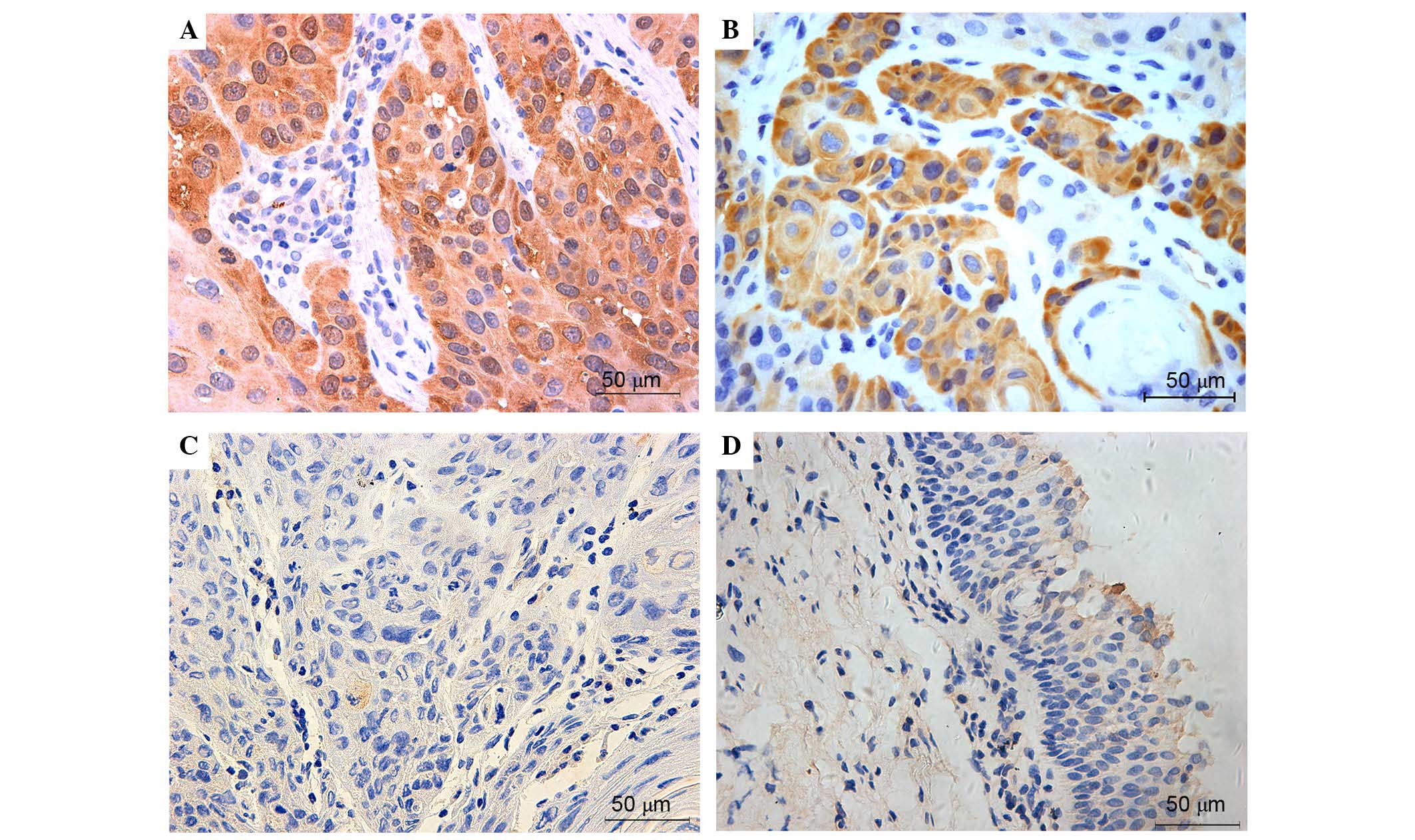
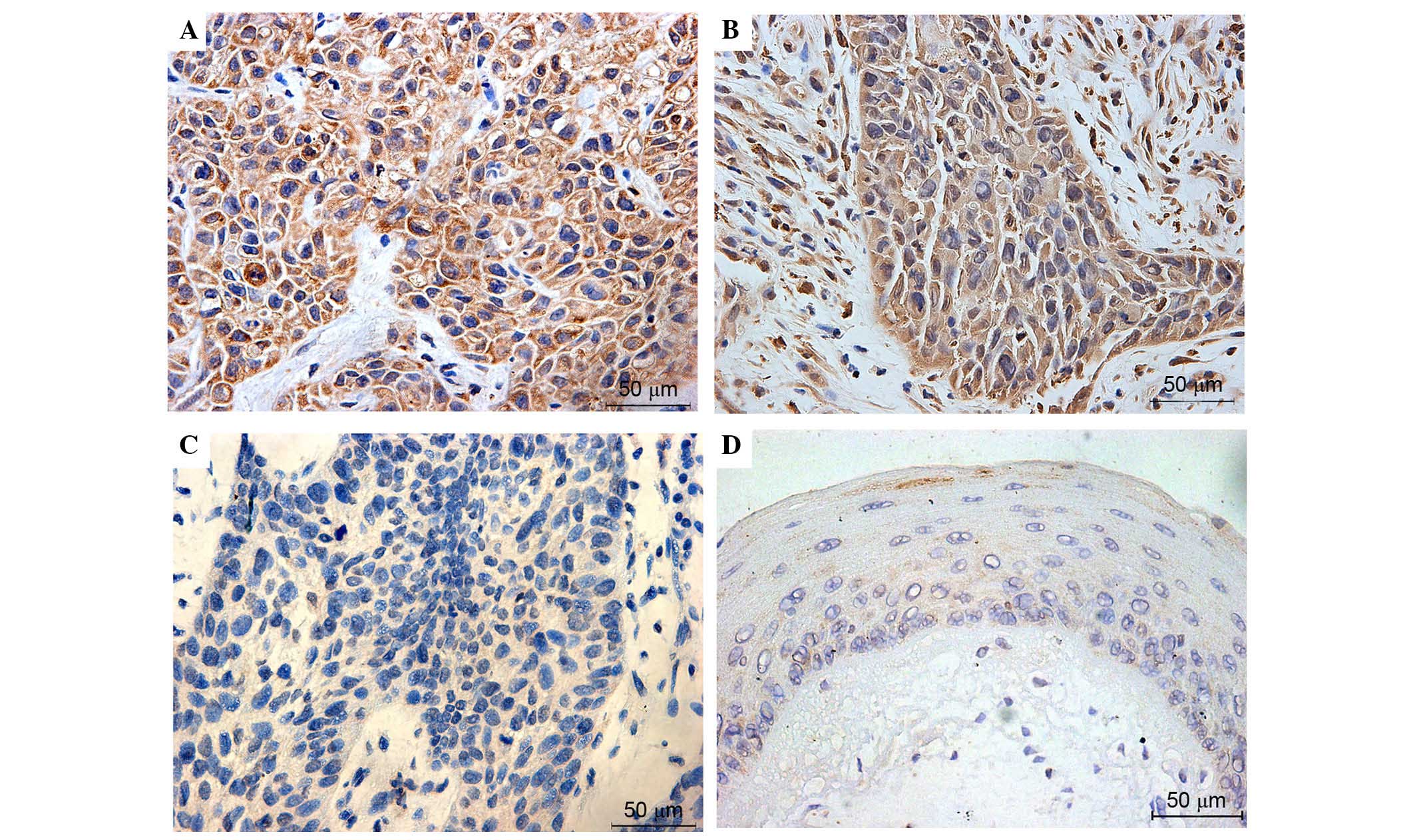
Immunohistochemical detection of NRF2 (Fig. 1), KEAP1 (Fig. 2), NQO-1 (Fig. 3) and HO-1 (Fig. 4) activities assessed the expression
and localization of these proteins in laryngeal carcinoma samples.
High expression levels of NRF2 were common in advanced laryngeal
cancer tissue, and expression was primarily localized in the nuclei
of these specimens. NRF2 was weakly expressed in the cytoplasm of
the tumor cells. In non-neoplastic samples, NRF2 was barely
expressed. KEAP1, NQO-1 and HO-1 were predominantly observed in the
cytosol of tumor cells, and these proteins were also highly
expressed in majority of laryngeal cancer tissues. Overall, the
expression levels of NRF2, KEAP1, NQO-1 and HO-1 were very low or
negative in the most normal tissues. Figs. 1–4
present the representative expression of NRF2, KEAP1, NQO-1 and
HO-1 in laryngeal cancer and pericarcinomatous normal tissues.
Semiquantitative scoring analyses of
immunohistochemical staining (Fig.
5A) demonstrated that the majority of normal larynx mucosa
exhibited negative or weak expression of NRF2, KEAP1, NQO-1 and
HO-1. By contrast, the expression of these proteins was markedly
enhanced to varying degrees in advanced laryngeal cancer tissues.
Positive staining of nuclear NRF2 (immunoscore ≥0.1) was observed
in 26 cases of laryngeal cancer (79%). Of 33 patient tumors, 17
tumors (52%) exhibited moderate-strong nuclear accumulation (≥2.0).
However, only 4 adjacent normal specimens (12%) exhibited weak NRF2
staining. There was a significant difference in the expression of
NRF2 protein between the tumor and normal tissues (P<0.01).
KEAP1-positive immunostaining (immunoscore ≥0.5) was observed in 23
cancer samples (70%), of which 14 cases (42%) exhibited
moderate-strong KEAP1 expression (≥2.0). The positive rate was
significantly higher in cancerous tissues compared with normal
tissues (5/33, 15%) (P=<0.01). The expression patterns of NQO-1
and HO-1 in all of the laryngeal cancer and normal specimens were
similar. Of the cancerous tissues, 25 (76%) and 27 (82%) samples
exhibited NQO-1- and HO-1-positive staining. This was a significant
increase compared with the neighboring non-tumor specimens
(P<0.01).
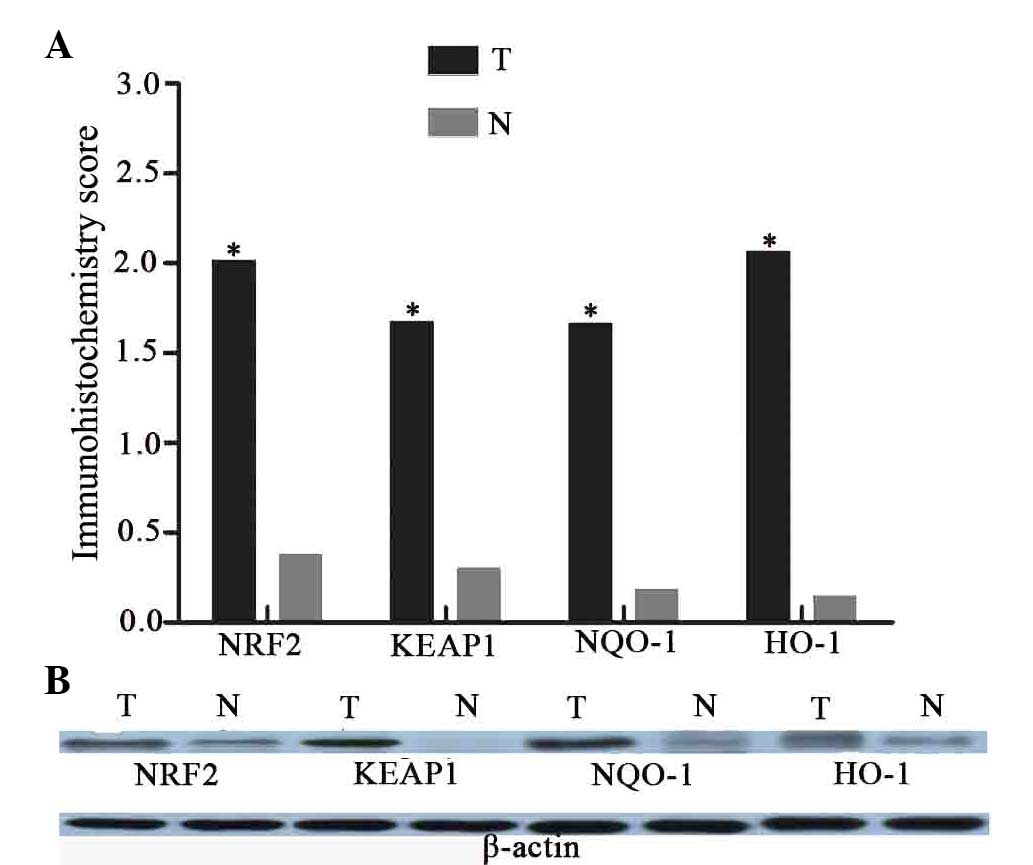 | Figure 5.The expression of NRF2, KEAP1, NQO-1
and HO-1 proteins in laryngeal cancer and adjacent normal tissues.
(A) Mean immunostaining scores of KEAP1, NRF2, NQO-1 and HO-1.
There are significant differences between laryngeal cancer and
adjacent normal tissues on these four proteins expression
(*P<0.01, T vs. N). (B) Immunoblot detection of KEAP1, NRF2,
NQO-1 and HO-1 demonstrates that the protein levels are observably
increased in tumor samples, compared with the normal tissues. T,
laryngeal cancer; N, normal tissues; NRF2, nuclear factor
(erythroid-derived 2)-like 2; KEAP1, kelch-like ECH-associated
protein-1; NQO-1, quinone oxidoreductase-1; HO-1, heme
oxygenase-1. |
Immunoblot analyses were also performed to compare
the expression of NRF2, KEAP1, NQO-1 and HO-1 in laryngeal cancer
and the paired normal tissues (Fig.
5B). Western blot analyses demonstrated that the protein
expression levels of NRF2, KEAP1, NQO-1 and HO-1 were markedly
increased in advanced cancerous tissues compared to with the
adjacent normal tissues.
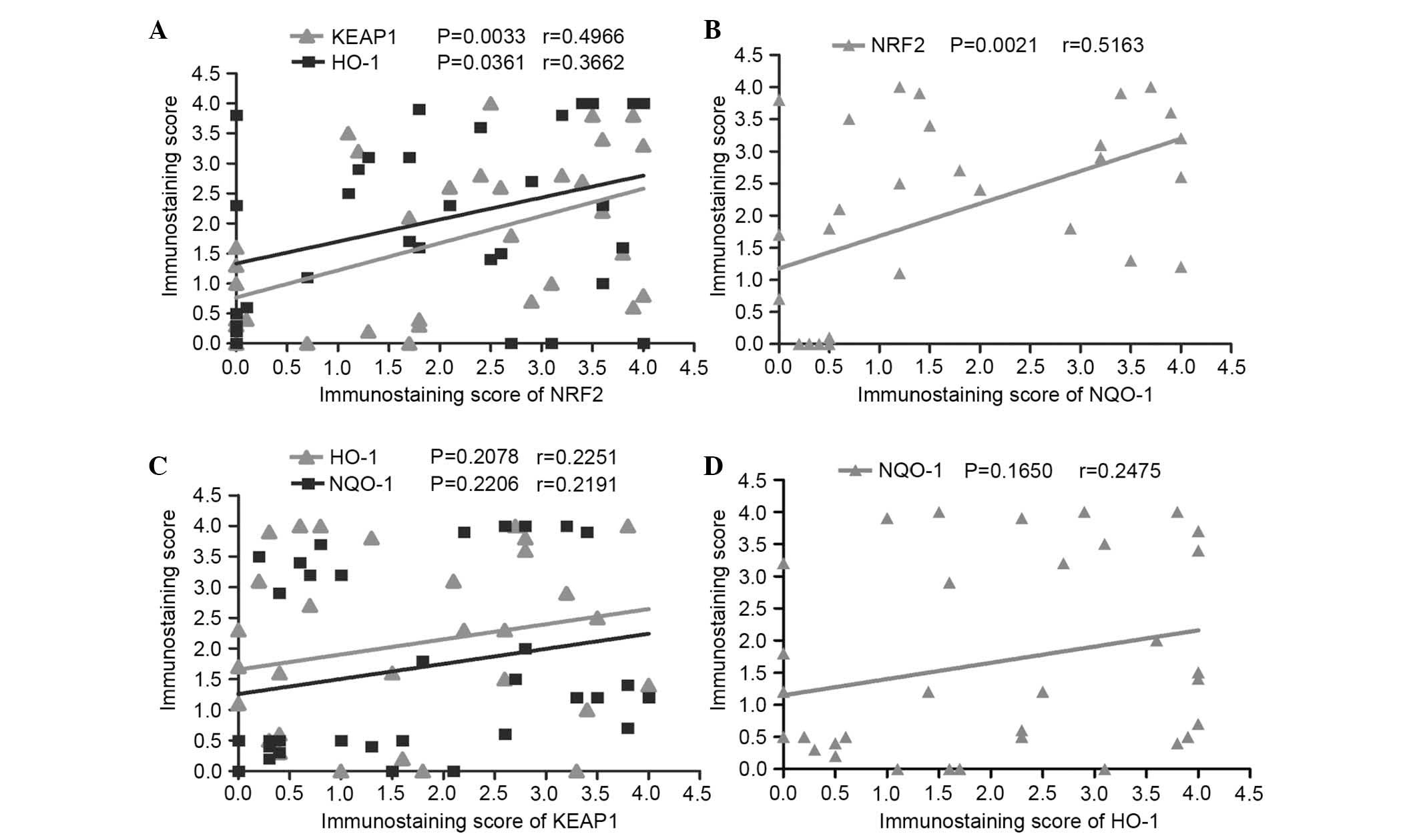
The Pearson correlation coefficient was chosen to
represent associations between the expression of nuclear NRF2 with
the expression of regulatory protein KEAP1 and the phase II
detoxifying enzymes, NQO-1 and HO-1, to determine the biological
effect of KEAP1/NRF2 system in patients with advanced laryngeal
cancer. High KEAP1, NQO-1 and HO-1 expression in laryngeal cancer
tissues were all statistically correlated with nuclear NRF2
(P=0.0033, r=0.4966; P=0.0021, r=0.5163; and P=0.0361, r=0.3662;
respectively; Fig. 6A and B).
However, no significant correlation between the expression of KEAP1
and NQO-1, and HO-1 (Fig. 6C) was
identified in advanced laryngeal carcinoma. No significant
correlation was identified between NQO-1 and HO-1 expression levels
(P=0.165; r=0.24750; Fig. 6D).
Additionally, the association between the expression
of KEAP1/NRF2 and clinicopathological features in advanced
laryngeal cancer tissues. It was revealed that the staining of
NRF2, KEAP1, NQO-1 and HO-1 was independent of age, lymph node
status, tumor stage (clinical stage III and IV) and the tumor size
(Fig. 7).
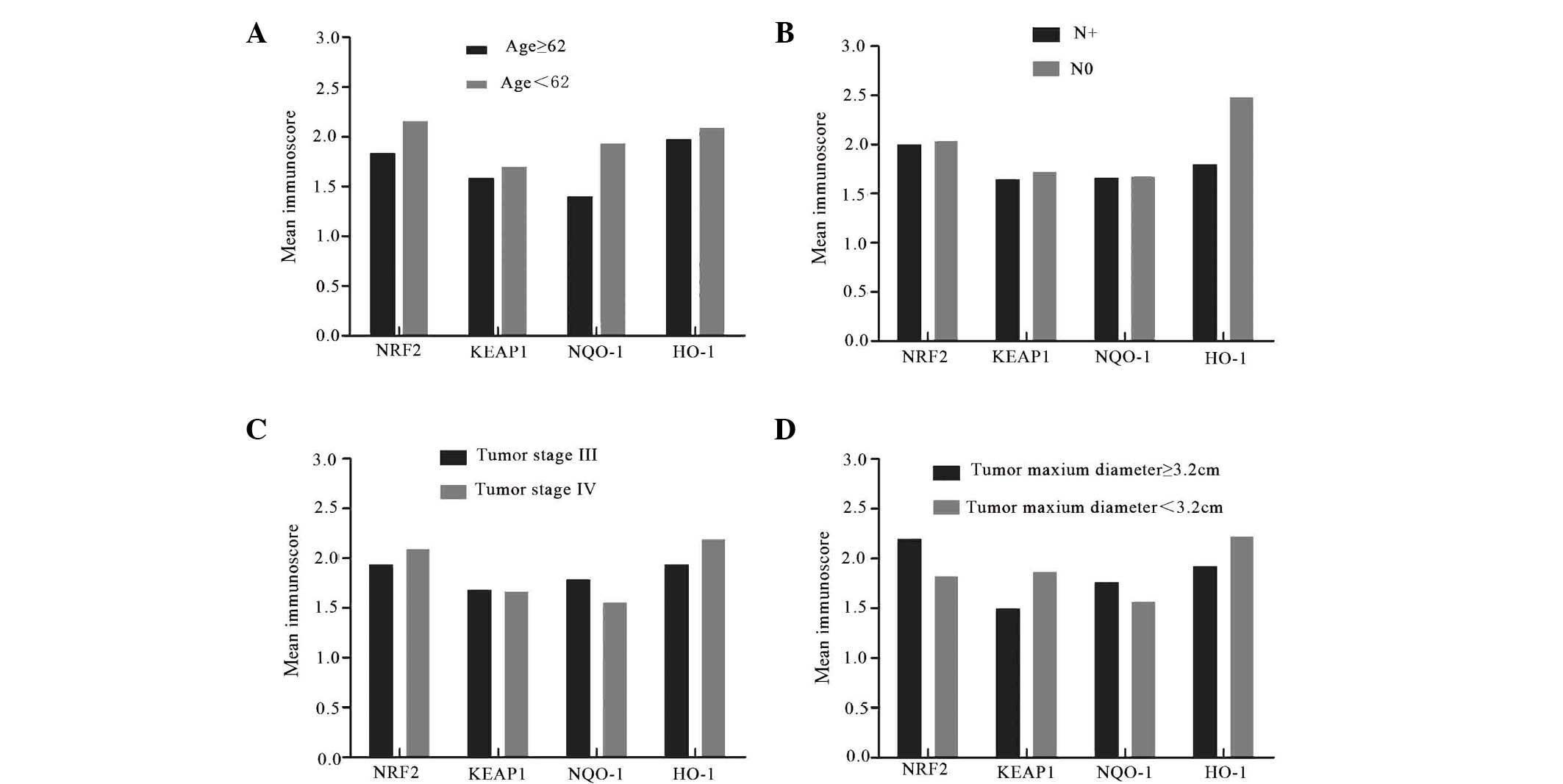 | Figure 7.Association between the expression
NRF2, KEAP1, NQO-1 and HO-1 and clinicopathological features in
advanced laryngeal cancer tissues. (A) The expression levels of
NRF2, KEAP1, NQO-1 and HO-1 in the patients with age <62 years
old are not significantly higher than the patients with age ≥62
(P=0.157, 134, 574 and 0.200, respectively). (B) The expression
levels of these proteins are not obviously associated with lymph
node metastasis (P=0.867, 0.926, 0.957 and 0.266 respectively). (C)
There are no significant differences in the expression levels of
NRF2, KEAP1, NQO-1 and HO-1 between the patients with diagnosed
tumor stage III and IV (P=0.761, 0.972, 0.667 and 0.621
respectively). (D) Tumor size has no significant impact on the
expression levels of NRF2, KEAP1, NQO-1 and HO-1 (P=0.531, 0.809,
0.315 and 0.828 respectively). Data are expressed as the mean
values of patient characteristics. N+, lymph node metastasis; N0,
no lymph node metastasis; NRF2, nuclear factor (erythroid-derived
2)-like 2; KEAP1, kelch-like ECH-associated protein-1; NQO-1,
quinone oxidoreductase-1; HO-1, heme oxygenase-1. |
Discussion
The pathogenesis and etiology of laryngeal carcinoma
is not clear. However, numerous studies demonstrated that oxidative
stress directly or indirectly causes DNA damage, which is a
predisposing factor to malignant lesions (14,15).
The body has established appropriate self-defense mechanisms to
cope with these harmful stimuli, including the KEAP1-NRF2-AREs
pathway (16). KEAP1 was
discovered in 1999 (17), and the
KEAP1 system is constantly researched, particularly NRF2. However,
specific research on the association between laryngeal cancer and
this pathway is limited, despite certain notable recent advances
(18). Little is understood about
oxidative stress and the activation of carcinogenesis pathways in
laryngeal cancer. The clinical value of the NRF2, KEAP1, NQO-1 and
HO-1 in advanced laryngeal cancer was investigated in the current
study.
NRF2 is a critical gene that with a core role in
KEAP1/NRF2 system, and NRF2 expression is associated with poor
prognosis in patients with cancer. NRF2 protects normal cells,
however it also promotes the survival of cancer cells (19). As a result, NRF2 has been well
studied. The present study evaluated the expression levels and
correlation of KEAP1, NRF2, NQO-1 and HO-1, and their associations
with clinicopathological features in advanced laryngeal cancer
tissue. The results of the current study demonstrated that KEAP1,
NRF2, NQO-1 and HO-1 were significantly increased in advanced
laryngeal carcinoma samples compared with the normal tissues.
Notably, in tumor samples NRF2 was primarily localized in nuclei,
and KEAP1, NQO-1 and HO-1 were principally expressed in the
cytoplasm. These results were consistent with previous research.
Stacy et al (20) reported
that 91.5% of head and neck tumor tissues (43/47) exhibited a
statistically significant increase in NRF2 expression compared with
normal mucosa. The positive rate of nuclear NRF2 was higher in the
previous study than in the present study, however the previous
study included other cancer tissues in addition to larynx, and no
separate analysis for NRF2 expression in laryngeal carcinoma was
performed. Stacy et al (20) also detected a high expression of
KEAP1 in the cytoplasm of tumor cells. An analysis of the
association between KEAP1 and NRF2 revealed that KEAP1 was not
negatively associated with nuclear NRF2 (20). Another study reported the
significant association of KEAP1 expression with nuclear NRF2 in
oral squamous cell carcinoma (21). This observation was verified in
head and neck cancers and non-small cell lung carcinomas (NSCLC),
in which nuclear NRF2 positivity was as high as 61.8% and its
expression was statistically associated with higher KEAP1
expression (22). The frequency of
positive nuclear NRF2 was significantly higher in pulmonary
squamous cell carcinomas than adenocarcinomas, however, KEAP1
exhibited the opposite expression pattern. However, the present
study only analyzed laryngeal squamous cell carcinoma because
adenocarcinoma of the larynx presents at an extremely low incidence
in the clinic. Nuclear NRF2 overexpression is also observed in
serous cystadenocarcinomas, with a positive rate of 71.1% (27/38)
(23), which is the same level as
the results of the current study in laryngeal carcinoma. The
positive expression of nuclear NRF2 was >30% in one report of
pancreatic adenocarcinoma, however, cytoplasmic NRF2 expression
accounted for 86%, which is higher that the results of the present
study (24). The disparity with
the results of the present study may be due to the different tumor
tissues used.
Various recent studies suggest that KEAP1 mutations
lead to constitutive activation and the nuclear accumulation of
NRF2, which enhances the expression of antioxidative and
detoxifying enzymes (25,26). Certain data demonstrated that KEAP1
mutations occur widely in solid cancers, irrespective of
histological type (27).
Unfortunately, a sequence analysis of KEAP1 was not conducted in
the current study. However, we speculate that this mechanism may be
pervasive in laryngeal cancer, and it may also partially explain
the positive, rather than negative, KEAP1 correlation with nuclear
NRF2 in laryngeal carcinoma tissues. Genetic mutations may give
rise to KEAP1 dysfunction, which leads to the nuclear accumulation
of NRF2 and prompts the expression of downstream proteins. Although
the mechanism of NRF2 nuclear transfer in laryngeal cancer tissues
is not known, the current study demonstrated that high levels of
nuclear NRF2 protein were significantly associated with KEAP1,
NQO-1 and HO-1 overexpression (P=0.0033, r=0.4966; P=0.0021,
r=0.5163 and P=0.0361, r=0.3662; respectively) and the expression
of detoxification and antioxidant enzymes NQO-1 and HO-1 was
markedly elevated in cancer tissue compared with normal mucosa,
which is consistent with previous reports in gallbladder cancer and
NSCLC (28). These results
revealed that NRF2, KEAP1, NQO-1 and HO-1 may be part of a
signaling pathway and have important biological effects on the
occurrence and development of laryngeal cancer. However, due to the
relatively small sample size in the current study, it is necessary
to enlarge the sample numbers and conduct further research. Western
blotting analyses also confirmed that NRF2, KEAP1, NQO-1 and HO-1
were markedly elevated in laryngeal cancer tissue compared with
adjacent normal tissue, which exhibited low expression.
Unfortunately, could not be obtained for western blotting analyses
of nuclear NRF2 expression. However, total NRF2 protein in
laryngeal cancer tissues was markedly higher than in normal
tissues.
Reports regarding the association between the
expression of KEAP1/NRF2 system and clinicopathological features
are not consistent. A previous study reported that Nrf2 and HO-1
expression was associated with tumor stage and metastasis, and not
associated with age in gallbladder cancer (28), whereas in NSCLC no significant
correlation is observed. Liao et al (23) demonstrated that NRF2 expression is
unrelated to age and lymph node metastasis in ovarian epithelial
carcinoma. Furthermore, it was reported that KEAP1 and NRF2 were
not significantly correlated with tumor stage and lymph node status
in oral squamous cell carcinoma, which supports the results of the
current study (21–23,28).
The results of the present indicate that the expression of NRF2,
KEAP1, NQO-1 and HO-1 is independent of age, tumor stage and lymph
node status. Additionally, the association between their expression
and tumor size was analyzed and revealed to not be significant. It
is suggested that their increased expression was common in advanced
laryngeal cancer and further investigations are necessary to
confirm this conclusion.
This report is the first to specifically analyze the
expression levels and correlation of NRF2, KEAP1, NQO-1 and HO-1,
and their association with clinicopathological features in advanced
laryngeal cancer. The present study confirmed that the expression
of NRF2, KEAP1, NQO-1 and HO-1 are increased significantly in
advanced laryngeal squamous cell carcinoma, compared with the
adjacent normal mucosa. Remarkable relevance exists between high
expression of KEAP1, NQO-1, HO-1 and nuclear NRF2. Additionally,
their expression levels were independent of age, tumor stage
(clinical stage III and IV), tumor size and lymph node metastasis.
These results suggest that increased expression of NRF2, KEAP1,
NQO-1 and HO-1 were common and may possess important clinical value
to diagnose and treat advanced laryngeal cancer.
Acknowledgements
The project was supported by the Science and
Technology Commission of Shanghai Municipality (grant no. KW12010),
Health Bureau of New Pudong District (grant no. PW2012D-4) and
Shanghai Municipal Education Commission (grant no. 13ZZ008).
References
|
1
|
Harrington KJ, Nutting CM and Pandha HS:
Gene therapy for head and neck cancer. Cancer Metastasis Rev.
24:147–164. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
American Society of Clinical Oncology, ;
Pfister DG, Laurie SA, Weinstein GS, Mendenhall WM, Adelstein DJ,
Ang KK, Clayman GL, Fisher SG, Forastiere AA, et al: American
Society of Clinical Oncology clinical practice guideline for the
use of larynx-preservation strategies in the treatment of laryngeal
cancer. J Clin Oncol. 24:3693–3704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho HY, Reddy SP and Kleeberger SR: Nrf2
defends the lung from oxidative stress. Antioxid Redox Signal.
8:76–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inci E, Civelek S, Seven A, Inci F, Korkut
N and Burçax G: Laryngeal cancer: In relation to oxidative stress.
Tohoku J Exp Med. 200:17–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jaramillo MC and Zhang DD: The emerging
role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev.
27:2179–2191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh A, Boldin-Adamsky S, Thimmulappa RK,
Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F,
Watson WH, et al: RNAi-mediated silencing of nuclear factor
erythroid-2-related factor 2 gene expression in non-small cell lung
cancer inhibits tumor growth and increases efficacy of
chemotherapy. Cancer Res. 68:7975–7984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khor TO, Huang MT, Prawan A, Liu Y, Hao X,
Yu S, Cheung WK, Chan JY, Reddy BS, Yang CS and Kong AN: Increased
susceptibility of NRF2 knockout mice to colitis-associated
colorectal cancer. Cancer Prev Res (Phila). 1:187–191. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Edge SB and Compton CC; The American Joint
Committee on Cancer, : The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li CJ, Wang SZ, Wang SY and Zhang YP:
Assessment of the effect of local application of amifostine on
acute radiation-induced oral mucositis in guinea pigs. J Radiat
Res. 55:847–854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manne U, Myers RB, Moron C, Poczatek RB,
Dillard S, Weiss H, Brown D, Srivastava S and Grizzle WE:
Prognostic significance of Bcl-2 expression and p53 nuclear
accumulation in colorectal adenocarcinoma. Int J Cancer.
74:346–358. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Piyathilake CJ, Bell WC, Oelschlager DK,
Heimburger DC and Grizzle WE: The pattern of expression of Mn and
Cu-Zn superoxide dismutase varies among squamous cell cancers of
the lung, larynx, and oral cavity. Head Neck. 24:859–867. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han Y and Chen JZ: Oxidative stress
induces mitochondrial DNA damage and cytotoxicity through
independent mechanisms in human cancer cells. Biomed Res Int
825065. 2013. View Article : Google Scholar
|
|
15
|
McLean LS, Watkins CN, Campbell P, Zylstra
D, Rowland L, Amis LH, Scott L, Babb CE, Livingston WJ, Darwanto A,
et al: Aryl hydrocarbon receptor ligand 5F 203 induces oxidative
stress that triggers DNA damage in human breast cancer cells. Chem
Res Toxicol. 28:855–871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dwivedi R, Raturi D, Kandpal N, Dwivedi R,
Singh R and Puri V: Oxidative stress in patients with laryngeal
carcinoma. Indian J Cancer. 45:97–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dinkova-Kostova AT, Holtzclaw WD and
Kensler TW: The role of Keap1 in cellular protective responses.
Chem Res Toxicol. 18:1779–1791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kensler TW and Wakabayashi N: Nrf2: Friend
or foe for chemoprevention? Carcinogenesis. 31:90–99. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JM and Johnson JA: An important role
of NRF2-ARE pathway in the cellular defense mechanism. J Biochem
Mol Biol. 37:139–143. 2004.PubMed/NCBI
|
|
20
|
Stacy DR, Ely K, Massion PP, Yarbrough WG,
Hallahan DE, Sekhar KR and Freeman ML: Increased expression of
nuclear factor E2 p45-related factor 2 (NRF2) in head and neck
squamous cell carcinomas. Head Neck. 28:813–818. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang CF, Zhang L, Ma SR, Zhao ZL, Wang
WM, He KF, Zhao YF, Zhang WF, Liu B and Sun ZJ: Clinical
significance of KEAP1 and NRF2 in oral squamous cell carcinoma.
PLoS One. 8:e834792013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Solis LM, Behrens C, Dong W, Suraokar M,
Ozburn NC, Moran CA, Corvalan AH, Biswal S, Swisher SG, Bekele BN,
et al: Nrf2 and Keap1 abnormalities in non-small cell lung
carcinoma and association with clinicopathologic features. Clin
Cancer Res. 16:3743–3753. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao H, Zhou Q, Zhang Z, Wang Q, Sun Y, Yi
X and Feng Y: NRF2 is overexpressed in ovarian epithelial carcinoma
and is regulated by gonadotrophin and sex-steroid hormones. Oncol
Rep. 27:1918–1924. 2012.PubMed/NCBI
|
|
24
|
Soini Y, Eskelinen M, Juvonen P, Kärjä V,
Haapasaari KM, Saarela A and Karihtala P: Nuclear Nrf2 expression
is related to a poor survival in pancreatic adenocarcinoma. Pathol
Res Pract. 210:35–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohta T, Iijima K, Miyamoto M, Nakahara I,
Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T,
et al: Loss of Keap1 function activates Nrf2 and provides
advantages for lung cancer cell growth. Cancer Res. 68:1303–1309.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Konstantinopoulos PA, Spentzos D,
Fountzilas E, Francoeur N, Sanisetty S, Grammatikos AP, Hecht JL
and Cannistra SA: Keap1 mutations and Nrf2 pathway activation in
epithelial ovarian cancer. Cancer Res. 71:5081–5089. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoo NJ, Kim HR, Kim YR, An CH and Lee SH:
Somatic mutations of the KEAP1 gene in common solid cancers.
Histopathology. 60:943–952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Zhang M, Zhang L, Cai H, Zhou S,
Zhang J and Wang Y: Correlation of Nrf2, HO-1, and MRP3 in
gallbladder cancer and their relationships to clinicopathologic
features and survival. J Surg Res. 164:e99–e105. 2010. View Article : Google Scholar : PubMed/NCBI
|