Introduction
Rheumatoid arthritis (RA) is a chronic progressive
autoimmune disease characterized by multi-joint synovial
inflammation and the presence of a large number of T lymphocytes
within the synovial tissue infiltrate. CD4+ T
lymphocytes exert the predominant pathogenic role in the
development of RA (1). Previous
studies have identified that, in the limb joints of a mouse RA
model, the mice have the same T-cell receptor (TCR) Vβ clone type T
cells. However, as the development of the disease progresses, the
numbers of this type of clone T cell increase gradually, whereas
those of other TCR Vβ clone type T cells are gradually reduced:
Therefore, this T cell clone type may be associated with the
pathological changes of arthritis (1–3).
Collagen-induced arthritis (CIA) is associated with pathogenetic
and pathological changes that are similar to those of RA, and so
CIA is often used as a model of human RA (4).
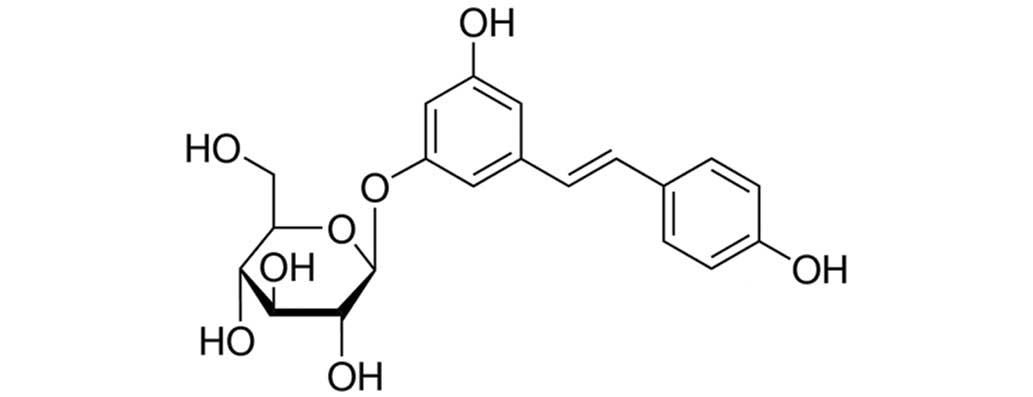
The chemical name of polydatin is 3,4,5-trihydroxy
stilbene-3-β-D-glycosidase; since there are three hydroxyl phenolic
groups in the structure of polydatin, the compound readily reacts
with oxidizing material and functions as an effective antioxidant,
scavenging the effects of free radicals (5). Polydatin is also a monomer, exerting
antiviral and antibacterial effects, and it is a natural extract
used in traditional Chinese medicine [it has been extracted from
Japanese knotweed, Reynoutria Japonica (Houtt), in our
School]. Post-lab studies of giant knotweed have determined that
this plant possesses evident curative properties for the treatment
of blood loss, burns and septic shock, and it may improve the
survival rate of animals in a state of shock by improving the
microcirculation perfusion and enhancing the animals' myocardial
contractile force (5,6).
The present study aimed to determine whether an
effective treatment of polydatin ameliorates the symptoms of CIA,
and also to explore the potential mechanisms involved. The results
have revealed, to our knowledge for the first time, that the
effective treatment of polydatin ameliorates the symptoms of CIA
through an exertion of its antioxidative and anti-inflammatory
effects, and also via activation of the expression of matrix
metalloproteinase-9 (MMP-9), in mice.
Materials and methods
Materials
Polydatin (purity ≥95%, as determined by
high-pressure liquid chromatography; structure shown in Fig. 1), bovine collagen type II (CII),
complete Freund's adjuvant (CFA), incomplete Freund's adjuvant
(IFA), malondialdehyde (MDA), glutathione (GSH), tumor necrosis
factor-α (TNF-α), interleukin-6 (IL-6), specific enzyme-linked
immunosorbent assay kits and the caspase-3/9 fluorometric assay kit
were acquired from Sigma-Aldrich (St. Louis, MO, USA).
Animals and induction of CIA
Male DBA/1J mice (age, 6–7 weeks) were purchased
from the laboratory of Shandong University (Shandong, China),
housed in a controlled environment (22±2°C, 12 h light/dark cycle)
and provided with standard rodent chow and tap-water. Experiments
involving the mice were performed in accordance with the Guide for
the Care and Use of Laboratory Animals, adopted by The Second
People's Hospital of Liaocheng City (Liaocheng, Shandong, China).
Bovine CII (2 mg/ml), an equal volume of CFA and 2 mg/ml
Mycobacterium tuberculosis H37Ra were mixed together. The
mice were intradermally injected with 100 µl of the emulsion
containing 100 µg CII, and subsequently were administered booster
injections with 100 µg CII in IFA after 21 days of primary
immunization.
Division into groups
Animals were randomly divided into five groups: i)
Control group, in which the mice were intradermally injected with
0.5 ml normal saline; ii) CIA group, in which CIA mice were
intradermally injected with 0.5 ml normal saline; iii) ‘polydatin
15’ group, in which CIA mice were intradermally injected with 15
mg/kg polydatin for 24 h; iv) ‘polydatin 30’ group, in which CIA
mice were intradermally injected with 30 mg/kg polydatin for 24 h;
v) and ‘polydatin 45’ group, in which CIA mice were intradermally
injected with 45 mg/kg polydatin for 24 h.
Evaluating CIA progression
Paw swelling was evaluated by measuring the
thickness of two hind paws using a digital caliper. The severity of
the arthritis was evaluated using a clinical scoring system on a
scale of 0–4 for each paw: 0, no signs of arthritis; 1, swelling
and/or redness in one joint; 2, swelling and/or redness in more
than one joint; 3, swelling and/or redness in the entire paw; and
4, severe swelling of the entire paw with deformity and/or
ankylosis. The total arthritis score produced a maximum score of
16, as the sum of each score of the four limbs.
Evaluating oxidative damage and
reactive inflammation
The blood samples were collected from an
intracardiac puncture and subsequently were centrifuged for 20 min
at 2,000 × g at 4°C (Heraeus Pico 17/21; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The levels of MDA, GSH, TNF-α and IL-6
were analyzed using specific enzyme-linked immunosorbent assay kits
in accordance with the manufacturer's protocol (Sigma-Aldrich).
Western blot analysis for MMP-9 and
B-cell lymphoma 2 (Bcl-2)/ Bcl-2-associated X protein (Bax)
The arthritic tissue samples were collected and
homogenized with 100 µl tissue lysis buffer (Beyotime Institute of
Biotechnology, Nanjing, China) for 30 min on ice. Protein
concentrations were determined using the bicinchoninic acid (BCA)
method (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). An equal
quantity of protein was dissolved in a 10% sodium dodecyl sulfate
polyacrylamide gel for electrophoresis, and subsequently
transferred onto fluoride membranes (0.22 mm; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The fluoride membranes were blocked with
5% skim milk powder in Tris-buffered saline-Tween 20 for 1 h at
room temperature and incubated with mouse monoclonal anti-MMP-9
(1:1,000; cat. no. sc-13520), mouse monoclonal anti-Bcl-2 (1:1,000;
cat. no. sc-7382), mouse monoclonal anti-Bax (1:1,000; cat. no.
sc-23959) and mouse monoclonal anti-β-actin (1:1,000; cat. no.
sc-47778) antibodies (1:1,000; all purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) overnight at 4°C.
Subsequently, the membranes were incubated with horseradish
peroxidase (HRP)-conjugated goat anti-mouse secondary antibody
(1:5,000; Beyotime Institute of Biotechnology; cat. no. A0216) and
the bands were visualized using an enhanced chemiluminescence kit
(Tianjin Sungene Biotech Co., Ltd.). Relative protein expression
was determined using Quantity One software (version 4.62; Bio-Rad
Laboratories, Inc.).
Evaluating caspase-3/9 activity
The arthritic tissue samples were collected and
homogenized in 100 µl tissue lysis buffer (Beyotime Institute of
Biotechnology) for 30 min on ice. Protein concentrations were
determined using the BCA method (Nanjing KeyGen Biotech Co., Ltd.).
An equal quantity of protein was evaluated using caspase-3/9
fluorometric assay kits, in accordance with the manufacturer's
protocol (Sigma-Aldrich).
Statistical analysis
Data are expressed as the mean ± standard deviation,
and compared by one-way analysis of variance using the least
significant difference multiple-comparison test. SPSS 19.0
statistical software (IBM SPSS, Armonk, NY, USA) was used for the
data analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effective treatment of polydatin on
CIA in mice
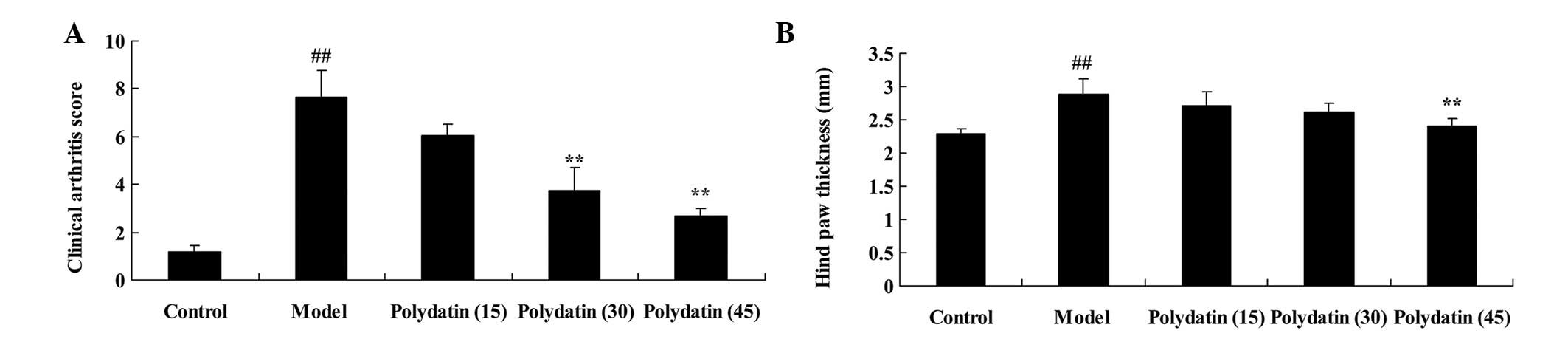
To evaluate the effects of polydatin on CIA in mice,
the clinical arthritis score and hind-paw thickness were evaluated
to determine the effectiveness of the treatment on CIA. These
indexes were markedly higher compared with those of the control
group, although the increases were reduced on treatment with
polydatin in a dose-dependent manner. The difference was revealed
to be statistically significant (P<0.01) following the treatment
with 30 or 45 mg/kg polydatin for the measurement of the clinical
arthritis score, and for the 45 mg/kg polydatin treatment alone in
the case of the hind-paw thickness (Fig. 2).
Effective treatment of polydatin
against oxidative damage in CIA mice
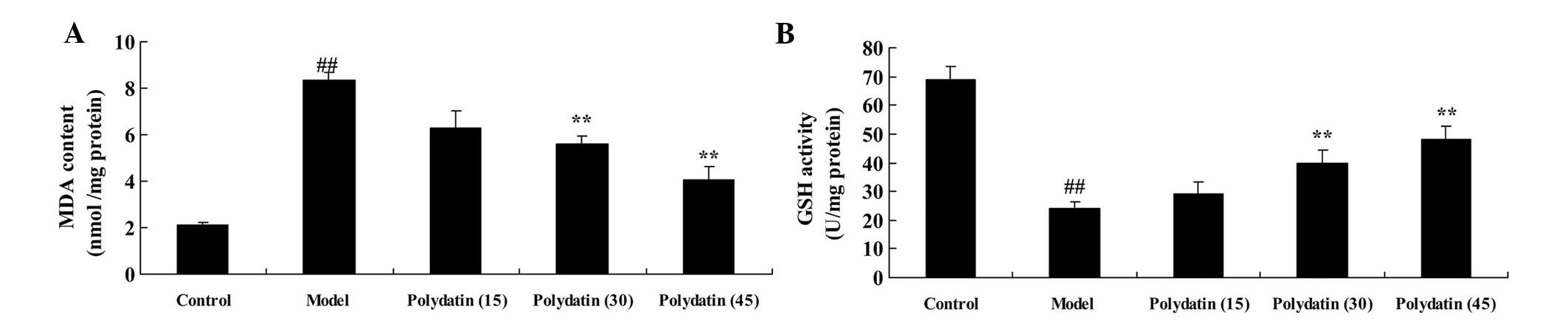
Subsequently, the effectiveness of polydatin as an
antioxidant, working against oxidative damage in CIA mice, was
examined. The levels of MDA and GSH were analyzed to evaluate the
effectiveness of the treatment on CIA. In the CIA group mice, the
MDA level was markedly increased, and that of GSH was markedly
decreased, compared with the control group (Fig. 3). However, the levels of MDA and
GSH in CIA mice were markedly suppressed and promoted by
pretreatment with 30 or 45 mg/kg polydatin compared with the model
group (Fig. 3).
Effective treatment of polydatin on
reactive inflammation in CIA mice
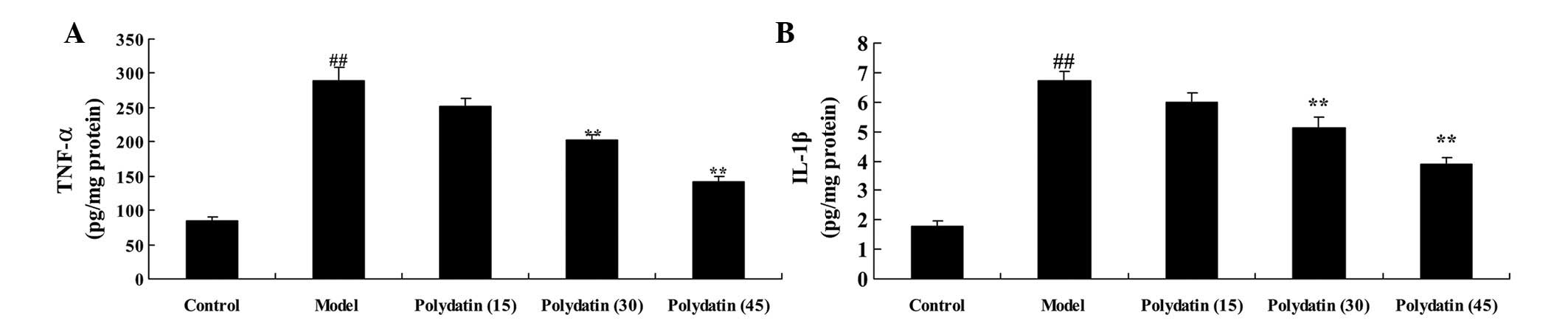
The effectiveness of polydatin in preventing
reactive inflammation in CIA mice was subsequently investigated.
The serum levels of TNF-α and IL-1β were analyzed to evaluate the
effectiveness of polydatin treatment on CIA. A marked elevation in
the serum levels of TNF-α and IL-1β were observed in the CIA group
mice compared with the control group (Fig. 4). As expected, the elevation in the
serum levels of TNF-α and IL-1β were significantly reduced on
treatment with polydatin (30 or 45 mg/kg; P<0.01) compared with
the model group (Fig. 4).
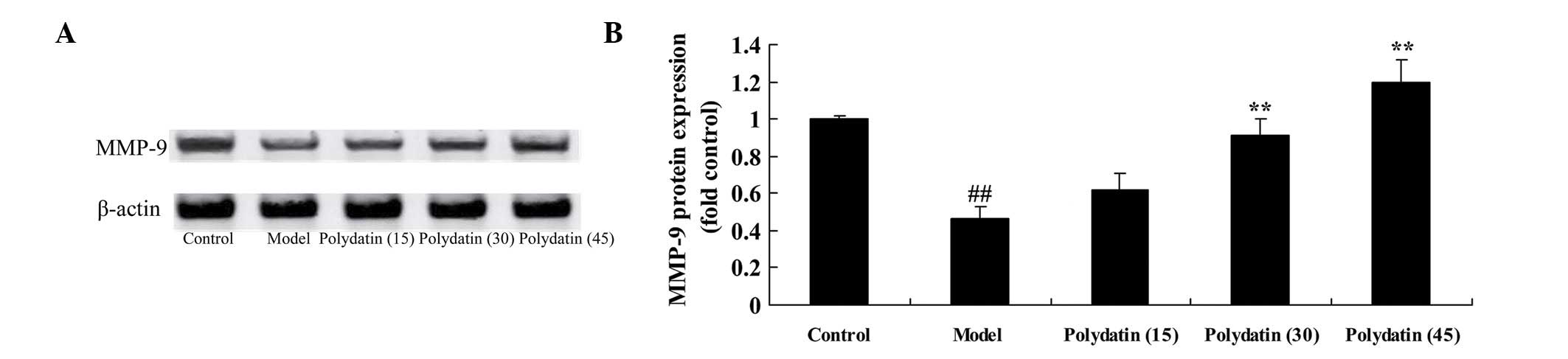
Effective treatment of polydatin on
MMP-9 in CIA mice
Subsequently, the effect of treatment of polydatin
on MMP-9 was investigated in CIA mice. As shown in Fig. 5, the induction of CIA markedly
inhibited the protein expression of MMP-9 in CIA model mice
compared with the control group. However, the addition of 30 or 45
mg/kg polydatin led to a marked increase in the protein expression
of MMP-9 in CIA mice compared with the model group (Fig. 5).
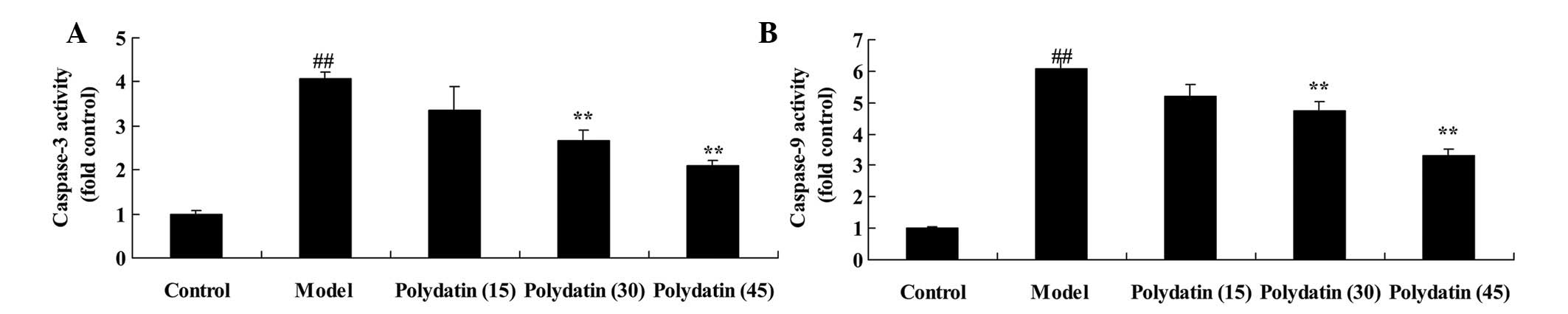
Effective treatment of polydatin on
caspase-3/9 in CIA mice
The effectiveness of the treatment of polydatin
against apoptosis in CIA mice was subsequently analyzed by
measuring the activity of caspases-3 and 9. The induction of CIA
markedly induced the activities of caspases-3 and 9 in the CIA
model mice compared with the control group (Fig. 6). Furthermore, the CIA-induced
activities of caspases-3 and 9 were markedly suppressed on
treatment with 30 or 45 mg/kg polydatin compared with the model
group (Fig. 6).
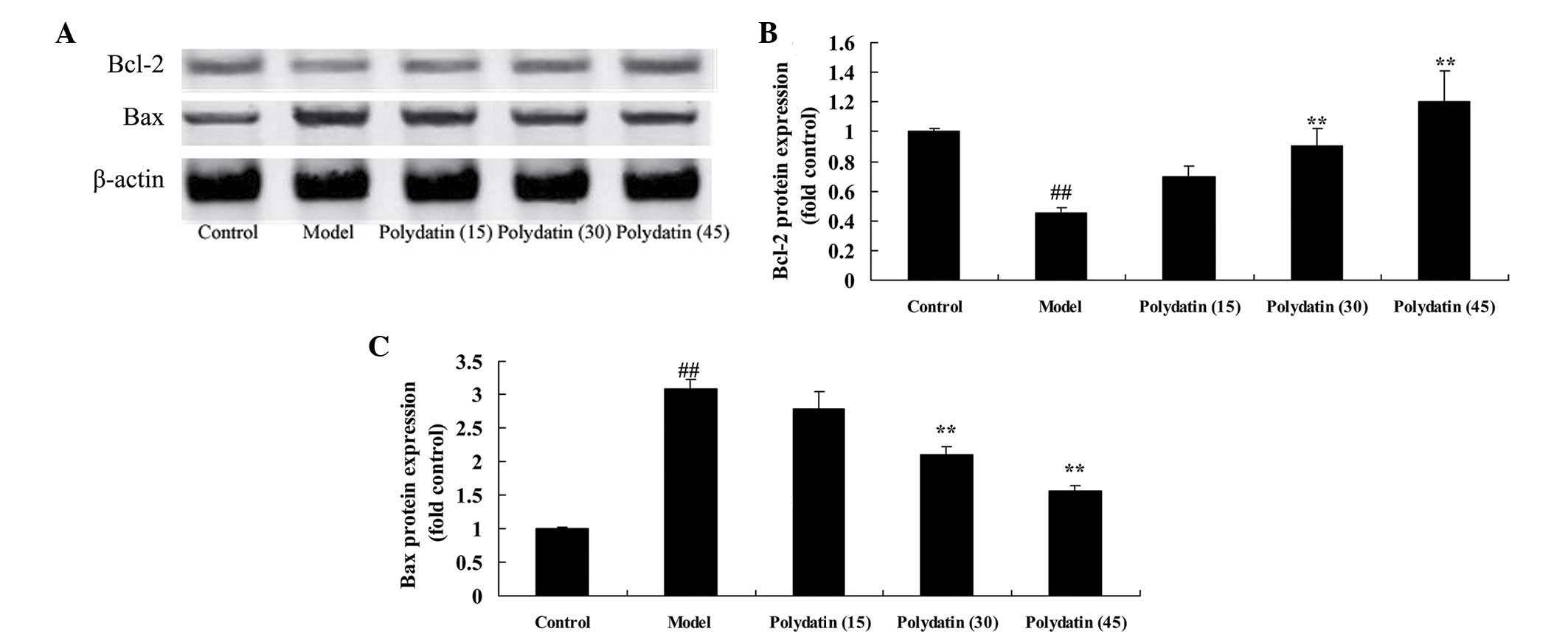
Effective treatment of polydatin on
Bcl-2/Bax in CIA mice
It is well known that the relative levels of
Bcl-2/Bax provide an important indicator of apoptosis. In this
experiment, the protein expression of Bcl-2 and Bax was analyzed by
western blot analysis. Compared with the control group, the protein
expression of Bcl-2 was markedly reduced, and that of the Bax was
markedly increased, in CIA mice (Fig.
7). However, treatment with 30 or 45 mg/kg polydatin
significantly ameliorated the changes in the CIA model mice
compared with the model group (P<0.01; Fig. 7).
Discussion
RA is a chronic systemic autoimmune disease, and its
pathological basis is joint synovitis (4). RA, of unknown etiology and with
complex and varied clinical manifestations, can cause serious
damage to human health (4). Type
II collagen is a protein that is responsible for organization,
predominantly existing in the articular cartilage and eye tissue
(3,7). It is the protein that is featured
predominantly in the composition of the articular cartilage. Damage
to the joints results in the release of type II collagen, which
stimulates an autoimmune response, and this is considered to be one
of the mechanisms underpinning the development of RA (2,7).
Through the release of type II collagen, mice are able to induce an
autoimmune response, leading to the erosion of cartilage in type II
collagen-mediated multi-arthritis, which is known as CIA (8). The characteristics of CIA are similar
in several ways to those of human RA, including synovitis, the
formation of cartilage and subchondral pannus, inflammatory cell
infiltration, the erosion of cartilage, bone resorption and
remodeling, and so forth (9).
Therefore, CIA is regarded as a unique animal model of human RA,
which is therefore widely used in studies on the mechanism of RA.
In the present study, treatment with 30 or 45 mg/kg polydatin
markedly inhibited the clinical arthritis score and hind-paw
thickness in CIA mice.
Free radicals are involved in the occurrence and
development of RA, in the process of synovial inflammatory lesions
of the joints, and in bone destruction; additionally, free radicals
are directly or indirectly involved in synovial membrane and bone
injuries (8,9). All types of free radicals are
involved in this process: Hydroxyl free radicals biodegrade
proteoglycan, hypochlorous acid leads to the fracture of the
collagen, and the capability of hydrogen peroxide to diffuse
throughout the tissue leads to the inhibition of cartilage
glycoprotein synthesis and obstruction of the synthesis of ATP.
Hydrogen peroxide also exerts an effect on the glycolytic enzyme,
glyceraldehyde-3-phosphate dehydrogenase, in the cartilage cells,
accelerating proteolytic hydrolysis and mediating the degradation
of the cartilage by free radicals (10). The reactivity of hypochlorous acid,
O2- and vitamin C has a great influence on the function
of cartilage, resulting in a reduction in the vitamin C content in
joint synovial fluid. Activated phagocytes produce reactive oxygen
species, which are able to alter the immunofluorescent properties
of immunoglobulin G (IgG), leading to the further activation of
phagocyte fluorescent protein polymers (11,12).
The degeneration of IgG inhibits rheumatoid factor, directly
resulting in the generation of C-reactive protein (12). This type of reaction manifests
itself in the rheumatoid joints in the long term, demonstrating
that free radicals exert an important role in the process of
chronic inflammation (13). In the
present study, the effective treatment of 30 or 45 mg/kg polydatin
markedly reduced oxidative damage in CIA mice. Ji et al
(14) demonstrated that treatment
with polydatin ameliorates blood-brain barrier permeability through
suppression of oxidative stress in the permanent middle cerebral
artery occlusion rat brain (14).
In agreement with previous studies, a study by Miao et al
(15) demonstrated that polydatin
protects against ischemia/reperfusion injury through antioxidative
stress mechanisms (15). The
findings in the present study have also revealed that the
effectiveness of polydatin as an antioxidative agent has an
important role in RA treatment.
RA is a type of chronic, systemic autoimmune disease
which has the predominant feature of symmetrical polyarthritis, and
anti-inflammatory analgesic symptomatic treatments, which would
delay the development of the disease, offer the major therapeutic
options (2,16). Local inflammation of the joints is
a key link in the process of its pathogenesis; bone loss or damage
caused by inflammation is the result of changes to the joints in
RA, and symptoms of pain associated with joint swelling are often
the most severe symptoms of which the patient complains (9). Therefore, inhibiting the inflammation
has become one of the predominant targets of the clinical treatment
(16). In the present study, the
administration of polydatin (30 or 45 mg/kg) markedly ameliorated
reactive inflammation in CIA mice. In a previous study, Chen et
al (17) elucidated that the
effective treatment of polydatin elicited prominent
nephroprotective activities via oxidative stress and inflammatory
responses in fructose-induced urate nephropathic mice. Lou et
al (18) provided evidence
that polydatin inhibits nitric oxide and prostaglandin
E2 production in lipopolysaccharide-stimulated RAW 264.7
cells, and also exerts potent anti-inflammatory activity in
macrophages. These results suggested that the effective treatment
of polydatin against RA is associated with its anti-inflammatory
response in CIA mice.
MMPs are responsible for the degradation of one or
several types of extracellular matrix, belonging to the
zinc-dependent peptidase enzyme family (19). Previous studies have shown that the
degradation of the extracellular matrix that is associated
predominantly with collagenous connective tissue may promote pannus
hyperplasia, leading to the destruction of the cartilage, ligaments
and bone, and the gelatinases, MMP-2 and MMP-9, are very closely
associated with the collagenous connective tissue reaction
(19,20). MMP-9 acts on elastin and type II
and type V collagens, further degrading the collagen enzyme
degradation products. MMP-9 has been demonstrated to dissolve the
cartilage collagen (21). MMP-9 is
the most widely expressed of all the MMPs, and it is of great
importance to the physiological metabolism of connective tissue
(22). MMP-9 is not only capable
of dissolving collagen types I and II, but it can also degrade
gelatin, an excessive production of which eventually leads to
arthritis (23). Animal
experiments revealed that an inhibitor of the MMPs was able to
prevent destruction of the joints (22). The results in the present study
have shown that treatment with 30 or 45 mg/kg polydatin markedly
increased the protein expression of MMP-9, increased the activities
of caspases-3 and 9, and upregulated the Bcl-2/Bax signaling
pathway in CIA mice. In addition, Zhang et al (24) reported that polydatin was able to
reduce the expression of MMP-9 in the aortas of apolipoprotein E
double-knockout mice. Li et al (25) revealed that polydatin ameliorates
burn-induced lung injury through caspase-3 activity and Bax. The
findings of the present study demonstrated that treatment of the
mice with polydatin against CIA exerted an antiapoptotic effect and
was associated with an increase in the expression of MMP-9,
although the precise mechanism has yet to be fully elucidated.
In conclusion, the effective treatment of polydatin
alleviated symptoms of the disease of CIA, as demonstrated by its
antioxidative and anti-inflammatory properties, the activation of
MMP-9 and suppression of caspases-3/9, and upregulation of the
Bcl-2/Bax pathway. In particular, the present study has identified
a suitable target for the further scientific investigation of
RA.
References
|
1
|
Ohnishi Y, Tsutsumi A, Matsumoto I, Goto
D, Ito S, Kuwana M, Uemura Y, Nishimura Y and Sumida T: Altered
peptide ligands control type II collagen-reactive T cells from
rheumatoid arthritis patients. Mod Rheumatol. 16:226–228. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang JM, Cheng CM, Hung LM, Chung YS and
Wu RY: Potential use of plectranthus amboinicus in the treatment of
rheumatoid arthritis. Evid Based Complement Alternat Med.
7:115–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furuzawa-Carballeda J, Macip-Rodríguez P,
Galindo-Feria AS, Cruz-Robles D, Soto-Abraham V, Escobar-Hernández
S, Aguilar D, Alpizar-Rodríguez D, Férez-Blando K and Llorente L:
Polymerized-type I collagen induces upregulation of
Foxp3-expressing CD4 regulatory T cells and downregulation of
IL-17-producing CD4+ T cells (Th17) cells in
collagen-induced arthritis. Clin Dev Immunol. 2012:6186082012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang H, Chen W, Wang L, Li F, Zhang C and
Xu L: Tumor necrosis factor receptor-associated factor 6 promotes
migration of rheumatoid arthritis fibroblast-like synoviocytes. Mol
Med Rep. 11:2761–2766. 2015.PubMed/NCBI
|
|
5
|
Du QH, Peng C and Zhang H: Polydatin: A
review of pharmacology and pharmacokinetics. Pharm Biol.
51:1347–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu LT, Guo G, Wu M and Zhang WG: The
progress of the research on cardio-vascular effects and acting
mechanism of polydatin. Chin J Integr Med. 18:714–719. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosloniec EF, Ivey RA III, Whittington KB,
Kang AH and Park HW: Crystallographic structure of a rheumatoid
arthritis MHC susceptibility allele, HLA-DR1 (DRB1*0101), complexed
with the immunodominant determinant of human type II collagen. J
Immunol. 177:3884–3892. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu D, Chen J, Zhu H, Xiong XG, Liang QH,
Zhang Y, Zhang Y, Wang Y, Yang B and Huang X: UPLC-PDA
determination of paeoniflorin in rat plasma following the oral
administration of Radix Paeoniae Alba and its effects on rats with
collagen-induced arthritis. Exp Ther Med. 7:209–217.
2014.PubMed/NCBI
|
|
9
|
Li QH, Xie WX, Li XP, Huang KT, Du ZH,
Cong WJ, Zhou LH, Ye TS and Chen JF: Adenosine A2A receptors
mediate anti-inflammatory effects of electroacupuncture on
synovitis in mice with collagen-induced arthritis. Evid Based
Complement Alternat Med. 2015:8095602015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park KH, Mun CH, Kang MI, Lee SW, Lee SK
and Park YB: Treatment of collagen-induced arthritis using immune
modulatory properties of human mesenchymal stem cells. Cell
Transplant. 2015.(Epub ahead of print).
|
|
11
|
Suh SJ, Kim KS, Kim MJ, Chang YC, Lee SD,
Kim MS, Kwon DY and Kim CH: Effects of bee venom on protease
activities and free radical damages in synovial fluid from type II
collagen-induced rheumatoid arthritis rats. Toxicol In Vitro.
20:1465–1471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jones NR, Pegues MA, McCrory MA, Singleton
W, Bethune C, Baker BF, Norris DA, Crooke RM, Graham MJ and Szalai
AJ: A Selective inhibitor of human C-reactive protein translation
is efficacious in vitro and in C-reactive protein transgenic mice
and humans. Mol Ther Nucleic Acids. 1:e522012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Sun W, Chen L, Xu X, Wu Y, Zhang J
and Zhang Y: Anti-arthritic activity of Fu-Fang-Lu-Jiao-Shuang on
collagen-induced arthritis in Balb/c mice and its underlying
mechanisms. Pharmacogn Mag. 11:242–249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji H, Zhang X, Du Y, Liu H, Li S and Li L:
Polydatin modulates inflammation by decreasing NF-κB activation and
oxidative stress by increasing Gli1, Ptch1, SOD1 expression and
ameliorates blood-brain barrier permeability for its
neuroprotective effect in pMCAO rat brain. Brain Res Bull.
87:50–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miao Q, Wang S, Miao S, Wang J, Xie Y and
Yang Q: Cardioprotective effect of polydatin against
ischemia/reperfusion injury: Roles of protein kinase C and mito
K(ATP) activation. Phytomedicine. 19:8–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrandiz ML, Maicas N, Garcia-Arnandis I,
Terencio MC, Motterlini R, Devesa I, Joosten LA, van den Berg WB
and Alcaraz MJ: Treatment with a CO-releasing molecule (CORM-3)
reduces joint inflammation and erosion in murine collagen-induced
arthritis. Ann Rheum Dis. 67:1211–1217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen L, Lan Z, Lin Q, Mi X, He Y, Wei L,
Lin Y, Zhang Y and Deng X: Polydatin ameliorates renal injury by
attenuating oxidative stress-related inflammatory responses in
fructose-induced urate nephropathic mice. Food Chem Toxicol.
52:28–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lou T, Jiang W, Xu D, Chen T and Fu Y:
Inhibitory effects of polydatin on lipopolysaccharide-stimulated
RAW 264.7 cells. Inflammation. 38:1213–1220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lou G, Gao Y, Ning XM and Zhang QF:
Expression and correlation of CD44v6, vascular endothelial growth
factor, matrix metalloproteinase-2 and matrix metalloproteinase-9
in Krukenberg tumor. World J Gastroenterol. 11:5032–5036. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Catanzaro R, Marotta F, Jain S, Rastmanesh
R, Allegri F, Celep G, Lorenzetti A, Polimeni A and Yadav H:
Beneficial effect of a sturgeon-based bioactive compound on gene
expression of tumor necrosis factor-α, matrix metalloproteinases
and type-10 collagen in human chondrocytes. J Biol Regul Homeost
Agents. 26:337–345. 2012.PubMed/NCBI
|
|
21
|
Vitlianova K, Georgieva J, Milanova M and
Tzonev S: Blood pressure control predicts plasma matrix
metalloproteinase-9 in diabetes mellitus type II. Arch Med Sci.
11:85–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Babichenko II, Andriukhin MI, Pulbere S
and Loktev A: Immunohistochemical expression of matrix
metalloproteinase-9 and inhibitor of matrix metalloproteinase-1 in
prostate adenocarcinoma. Int J Clin Exp Pathol. 7:9090–9098.
2014.PubMed/NCBI
|
|
23
|
Surlin P, Oprea B, Solomon SM, Popa SG,
Moţa M, Mateescu GO, Rauten AM, Popescu DM, Dragomir LP, Puiu I, et
al: Matrix metalloproteinase −7, −8, −9 and −13 in gingival tissue
of patients with type 1 diabetes and periodontitis. Rom J Morphol
Embryol. 55:(Suppl 3). S1137–S1141. 2014.
|
|
24
|
Zhang JC, Chen KJ and Zheng GJ: Regulatory
effect of Chinese herbal compound for detoxifying and activating
blood circulation on expression of NF-κB and MMP-9 in aorta of
apolipoprotein E gene knocked-out mice. Zhongguo Zhong Xi Yi Jie He
Za Zhi. 27:40–44. 2007.(In Chinese). PubMed/NCBI
|
|
25
|
Li T, Cai S, Zeng Z, Zhang J, Gao Y, Wang
X and Chen Z: Protective effect of polydatin against burn-induced
lung injury in rats. Respir Care. 59:1412–1421. 2014. View Article : Google Scholar : PubMed/NCBI
|