Introduction
Lung cancer was the most common type of cancer in
men and women in 2012, and it continues to be the most common cause
of cancer-associated mortality. Lung cancer is expected to account
for >25% of male and female cancer deaths, although there has
been a slight decrease in mortality and incidence (1). Approximately 85% of lung cancer
patients have non-small cell lung cancer (NSCLC). In early stage
NSCLC, adjuvant chemotherapy is effective at improving patient
disease-free survival and overall survival (OS). For advanced stage
NSCLC, chemotherapy is the primary first-line treatment, however,
the response rate is only ~30%, and the median OS of metastatic
NSCLC is ~12 months (2).
The epidermal growth factor receptor (EGFR) is a
proto-oncogene regulating cell proliferation, metastasis, and
angiogenesis (3). Abnormalities in
EGFR induce a marked oncogenic potential in NSCLC (4). Tyrosine kinase inhibitors (TKIs)
specific to EGFR are used in second-line and even first-line
therapy in patients with metastatic NSCLC, however, use of this
treatment is limited by the EGFR gene mutation status (5). Gefitinib and erlotinib are
first-generation EGFR TKIs, which block the EGFR signaling pathway
via reversible binding to EGFR (6). In patients with EGFR mutations,
including the exon 19 in-frame deletion or exon 21 L858R point
mutation, the initial response to first-generation EGFR TKIs is
~80%. However, almost all patients acquire resistance to these
agents. In 50% of these patients, resistance is derived by the
occurrence of a secondary T790M mutation in exon 20 of EGFR
(7). Second-generation EGFR TKIs
that inhibit EGFR activity by irreversibly binding to EGFR have
been clinically developed and have indicated promising anti-tumor
activity in NSCLC (8). However,
these irreversible EGFR TKIs are >100-fold less potent in NSCLC
cells with the EGFR T790M mutation than in NSCLC cells with the
EGFR exon 19 in-frame deletion mutation (9,10). A
previous clinical study also demonstrated a limitation of these
agents that suggested the necessity for developing a novel strategy
to conquer the resistance to EGFR TKIs in NSCLC (11).
It has been clearly established in the last few
decades that chronic bacterial infections may contribute to
carcinogenesis. Conversely, another application of bacteria and
bacteria-derived products is their use to protect human beings from
various malignant diseases. It is well known that bacteria mediate
antitumor activities not only by indirect immune activation but
also by direct tumoricidal effects (12–14).
For example, genetically modified strains of Salmonella
typhimurium A1-R, which is auxotrophic for Leu-Arg and has high
anti-tumor virulence, is able to infect tumor cells and directly
result in destruction of the nucleus. This bacterium has been
successfully used to eradicate metastases in orthotopic models of
prostate, breast, and pancreatic cancer, following local and
systemic administration (15–18).
Another important example of bacterial anti-tumor action is
Streptococcus. pyogenes, which binds to target cells via
fibronectin or collagen. Such direct tumor cell contact is
necessary for S. pyogenes infection and leads to induction
of the apoptotic process in tumor cells (19). A single application of live S.
pyogenes into established pancreatic tumors resulted in
complete tumor regression. Side effects included marked leukocyte
infiltration and elevation of pro-inflammatory cytokines, however,
S. pyogenes also exhibited direct lytic activity against
tumor cells (19).
Pseudomonas aeruginosa injection is a type of
therapeutic biological product approved in China for adjuvant
treatment of patients with malignant tumors. This product is made
from an inactivated mutant strain of P. aeruginosa (PA-MSHA)
that is characterized by rich mannose-sensitive hemagglutination
pili (type 1 fimbriae). PA-MSHA has been successfully used in
clinical cancer therapy for a number of years, although its
detailed mechanism of action remains to be elucidated. In previous
studies, PA-MSHA has been demonstrated to directly inhibit tumor
cell proliferation in vitro and induce apoptosis in human
hepatocarcinoma, nasopharyngeal cancer and breast cancer cells
(20,21). Notably, an in-depth study by Liu
et al (22) demonstrated
that the mannose-mediated EGFR signaling pathway was involved in
the apoptosis of MDA-MB-231HM and MDA-MB-468 breast cancer cells
and that it was induced by PA-MSHA (22). These results suggest the potential
therapeutic value of PA-MSHA in tumors typically associated with
overexpression and mutation of EGFR.
In the present study, the direct tumoricidal effect
of PA-MSHA on NSCLC cell lines was tested to evaluate whether P.
aeruginosa injection is a possible adjuvant tool for NSCLC
treatment, particularly in patients with EGFR TKI resistance. The
three NSCLC cell lines were selected for their different gene
expression statuses, as follows: i) A549, an EGFR wild-type cell
line with primary EGFR TKI resistance; ii) PC-9, an EGFR
TKI-sensitive cell line with the exon 19 deletion mutation; and
iii) NCI-H1975, an acquired EGFR TKI-resistant cell line with the
T790M mutation. The cell growth inhibition, apoptosis induction,
and cell cycle redistribution of these three cell lines in response
to PA-MSHA was observed to investigate the potential of PA-MSHA in
treating NSCLC resistant to EGFR TKIs.
Materials and methods
Cell lines, materials, and
antibodies
Human non-small-cell lung cancer cell lines (PC-9,
A549, NCI-H1975) and the BEAS-2B normal lung epidermal tissue cell
line were used in the current study. The PC-9 cell line has a high
sensitivity for EGFR-TKIs and an exon 19 deletion, A549 is a
primary cell line resistant to EGFR-TKIs with wild-type EGFR, and
NCI-H1975 has an acquired resistance to EGFR-TKIs with T790M (exon
20) and L858R (exon 21) point mutations. All cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and cultured in DMEM medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% heat-inactivated
(56°C for 30 min) fetal calf serum (GE Healthcare Life Science,
Chalfont, UK), 2 mmol/l glutamine from Gibco (Thermo Fisher
Scientific, Inc.), penicillin (100 U/ml) and streptomycin (100
µg/ml). The cell culture was maintained in a humidified atmosphere
at 37°C with 5% CO2.
The strain of PA-MSHA used in the present study was
kindly provided by Beijing Wanter Biopharmaceutical Co., Ltd.
(Beijing, China). The PA-MSHA was scale-cultured at 37°C for 24 h,
inactivated using a chemical method and purified by centrifugation.
The following primary antibodies used (all from Cell Signaling
Technology, Inc., Danvers, MA, USA): Rabbit polyclonal anti-caspase
3 (cat. no. 9662), mouse monoclonal anti-caspase 8 (cat. no. 9746),
rabbit polyclonal anti-caspase 9 (cat. no. 9502), and rabbit
monoclonal anti-GAPDH (cat. no. 2118).
Cell proliferation
The effects of PA-MSHA on the survival of NSCLC
cells were determined using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells were seeded in 96-well plates (1×104
cells/well) to be treated in a concentration- or time-dependent
manner. Various concentrations of PA-MSHA (10, 5, 2.5, 1.25, 0.625,
0.313 and 0.156×109/ml) were then added to BEAS-2B,
PC-9, A549 and NCI-H1975 cells for different time points (0, 24,
48, 72, 96, 120 and 144 h), followed by the addition of MTT for
another 4 h. Following the removal of the culture medium, the
remaining MTT formazan crystals were dissolved with dimethyl
sulfoxide and measured at 490 nm with a microplate reader. The
percentage of inhibition was calculated as follows: Inhibition
ratio (%) = (1 - ODsample / ODcontrol) ×
100%. Experiments were conducted in triplicate, and the half
maximal inhibitory concentration (IC50) values were
determined.
Flow cytometry with Annexin
V-fluorescein isothiocyanate and propidium iodide (PI)
staining
Cells (106/ml) were seeded in 6-well
plates and allowed to reach 70–80% confluence following 6 h in
culture. Without changing the FBS-supplemented media, cells were
treated with the indicated concentration of PA-MSHA (0.156, 0.313,
0.625, and 1.25×109/ml) for 12 h. Cells were then
assessed by Annexin V-PI dual staining assay according to the
manufacturer's protocol. Stained cells were analyzed by
fluorescence activating cell sorting (BD Biosciences, Franklin
Lakes, NJ, USA), and the percentage of apoptotic cells was
determined using the ModFit LT 3.0 software (Verity Software House
Inc., Topsham, ME, USA).
Western blot analysis
Cells were lysed in lysis buffer containing 2 M
NaCl, 10% NP-40, 10% SDS, 1 M Tris-Cl, 1 g/l phenylmethylsulfonyl
fluoride, 0.1 g/l aprotinin and 0.01 g/l leupeptin. Protein samples
were quantified using the bicinchoninic acid method (cat. no.
23227; Thermo Fisher Scientific, Inc.). Samples (40 µg/20 µl) were
loaded for 10% SDS-PAGE electrophoresis and then blotted onto
polyvinylidene fluoride membranes. Subsequently, the membrane was
blocked with bovine serum albumin (Thermo Fisher Scientific, Inc.)
for 1 h and the expression of the target proteins was detected
using primary antibodies (1:1,000). After washing in Tris-buffered
saline containing 0.05% Tween-20 the membranes were incubated with
horseradish peroxidase-conjugated horse anti-mouse (cat. no. 7076)
and goat anti-rabbit (cat. no. 7074) immunoglobulin G secondary
antibodies (1:800; Cell Signaling Technology, Inc.). Pierce
Enhanced Chemiluminescence (ECL) Western Blotting Substrate (cat.
no. 32209; Thermo Fisher Scientific, Inc.) was used to detect the
protein bands, and digital imaging was conducted using the Thermo
Scientific myECL Imager (Thermo Fisher Scientific, Inc.).
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three experiments. The statistical analysis
was performed using SPSS 13.0 software for Windows (SPSS, Inc.,
Chicago, IL, USA). The significance of differences between
experimental conditions was determined using the two-tailed
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
PA-MSHA inhibits growth of NSCLC cell
lines and IC50 values of PA-MSHA differ in NSCLC
lines
The MTT assay demonstrated that PA-MSHA treatment
had concentration- and time-dependent cytotoxic effects on A549,
PC-9, and NCI-H1975 cells, however, not on BEAS-2B cells, which
served as the normal control. The IC50 values are
presented in Table I. As shown in
Fig. 1, exposure of tumor cells to
PA-MSHA for up to 144 h had a cumulative effect on A549 (Fig. 1A and B), PC-9 (Fig. 1C and D), and NCI-H1975 (Fig. 1E and F) cell proliferation in a
concentration- and time-dependent manner. This effect was not
observed in BEAS-2B cells (Fig. 1G and
H). All three NSCLC cell lines were sensitive to PA-MSHA,
however, it was not effective in the BEAS-2B normal lung tissue
cell line. Thus, in the following experiments, the three NSCLC cell
lines were focused on.
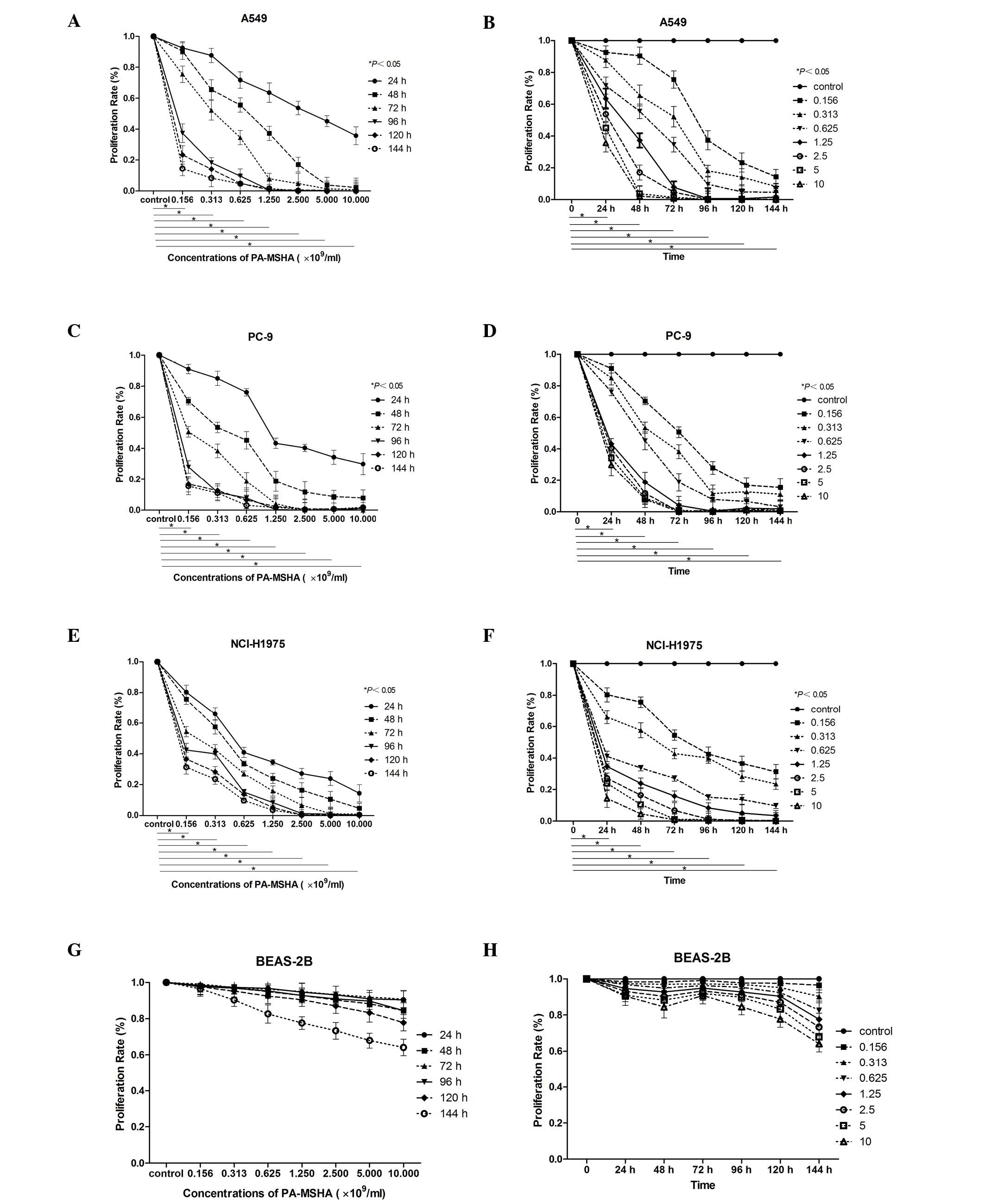 | Figure 1.Effect of PA-MSHA on cell
proliferation. The data are presented as the mean of triplicate
results from a representative experiment, bars indicate the
standard deviation. The effect of PA-MSHA on cell proliferation in
(A and B) A549, (C and D) PC-9, (E and F) NCI-H1975 and (G and H)
BEAS-2B cells was demonstrated to be (A, C, E and G)
concentration-dependent and (B, D, F and H) time-dependent.
P<0.05 for A549, PC-9, NCI-H1975 cells treated with PA-MSHA vs.
the control group. PA-MSHA, Pseudomonas aeruginosa-mannose
sensitive hemagglutinin. |
 | Table I.IC50 values of
Pseudomonas aeruginosa-mannose sensitive hemagglutinin in
different non-small cell lung cancer cell lines. |
Table I.
IC50 values of
Pseudomonas aeruginosa-mannose sensitive hemagglutinin in
different non-small cell lung cancer cell lines.
|
| IC50
(×109/ml) |
|---|
|
|
|
|---|
| Cell line | 24 h | 48 h | 72 h |
|---|
| PC-9 | 2.391 | 1.183 | 0.870 |
| A549 | 2.463 | 1.334 | 0.922 |
| NCI-H1975 | 0.963 | 0.713 | 0.140 |
| BEAS-2B | 22.565 | 16.738 | 6.456 |
PA-MSHA results in a redistribution of
the cell cycle
The underlying mechanism by which PA-MSHA exerted
its anti-proliferative effects was investigated in the present
study. Cells exposed to either PBS or PA-MSHA for 12 h were stained
with PI and analyzed by flow cytometry. The A549, PC-9, and
NCI-H1975 cells were exposed to increasing concentrations of
PA-MSHA (0.156, 0.313, 0.625, and 1.25×109/ml), this was
demonstrated to arrest the A549, PC-9 and NCI-H1975 cells in the
G0/G1 phase of the cell cycle, in a dose-dependent manner, leading
to a reduction in the proportion of cells in the S phase. In the
PC-9 and NCI-H1975 cells, a reduction of cells in the G2/M phase
was observed. In all cell lines, an additional accumulation in the
sub-G1 phase was also observed (Fig.
2).
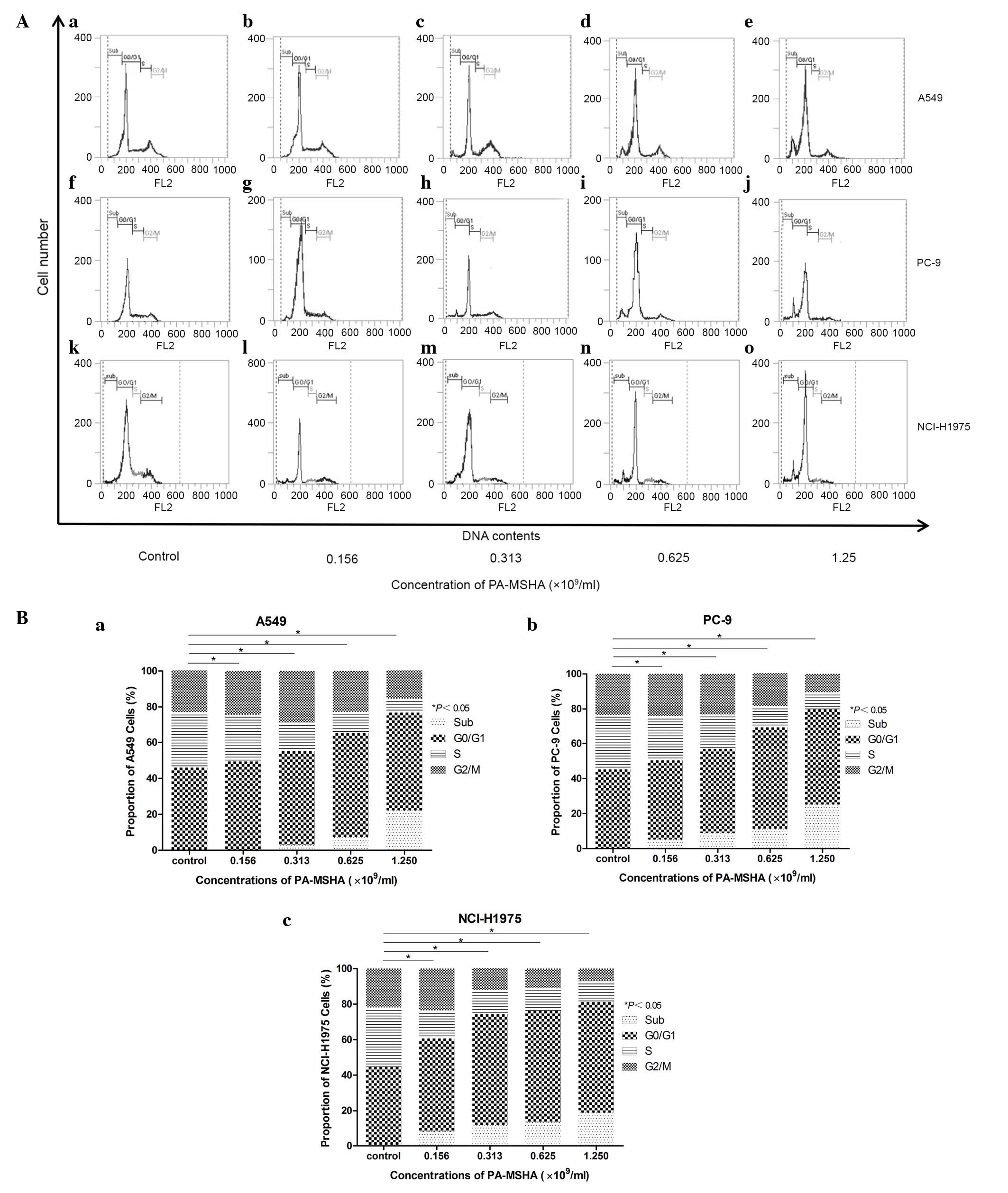 | Figure 2.(A) Effect of PA-MSHA on the
cell-cycle redistribution of (a-e) A549, (f-j) PC-9, and (k-o)
NCI-H1975 cells at different concentrations as follows: (a, f and
k) 0, (b, g and l) 0.156, (c, h and m) 0.313, (d, i and n) 0.625
and (e, j and o) 1.25×109/ml. The percentage of cells in
each phase of the cell-cycle (G0/G1, S, and G2/M) and in the sub-G1
peak was determined by flow cytometry. (B) PA-MSHA redistributed
the cell cycle. Cell cycle distribution of (a) A549, (b) PC-9, and
(c) NCI-H1975 cells in the four phases of the cell cycle are
represented by percentages and representative graphs under these
treatment conditions. *P<0.05 for cells treated with PA-MSHA vs.
the control group in all sub-G1, G0/G1, S and G2/M phases. PA-MSHA,
Pseudomonas aeruginosa-mannose sensitive hemagglutinin. |
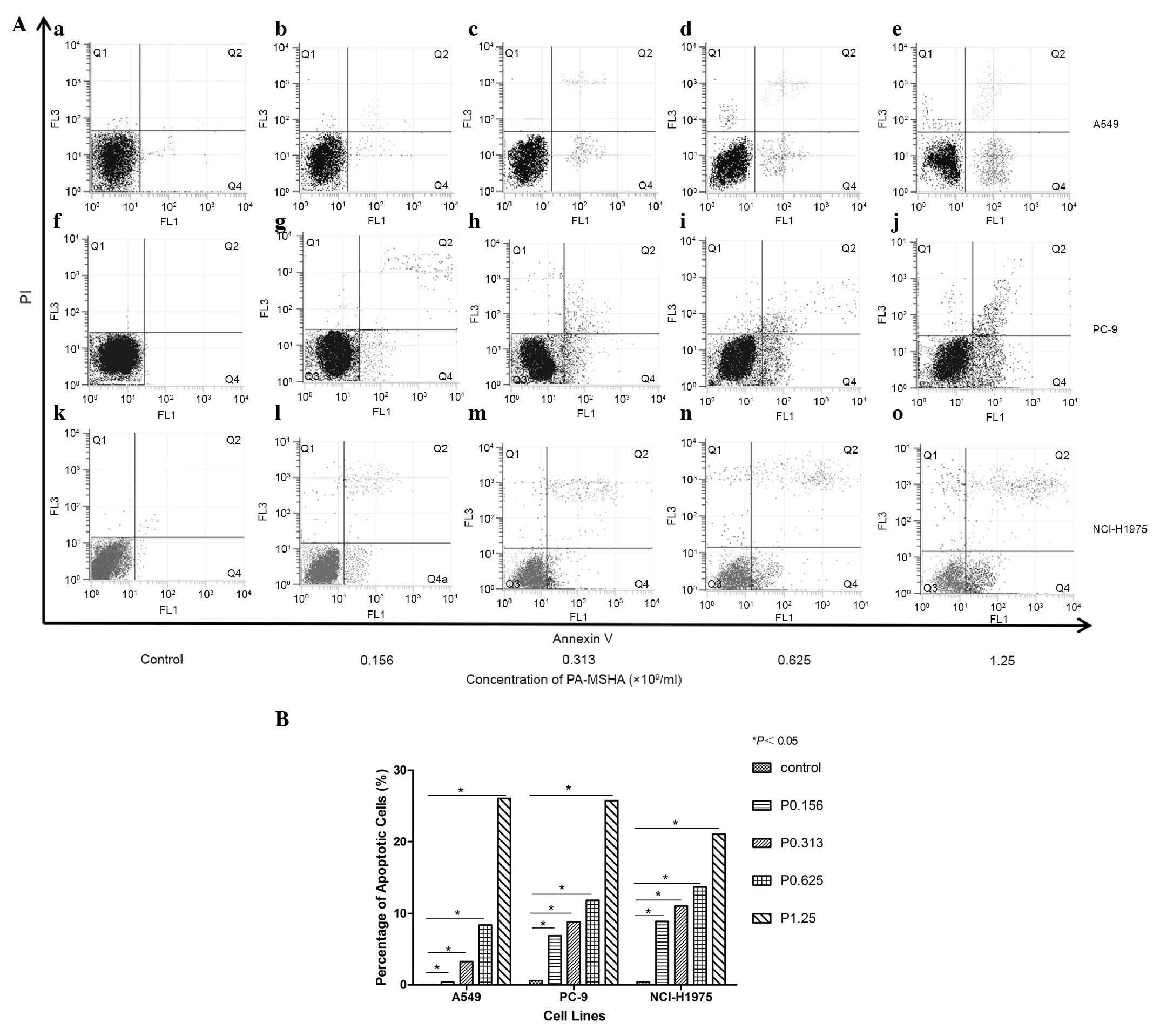
PA-MSHA induces apoptosis
Treatment with PA-MSHA was demonstrated to induce
early and late apoptosis in A549, PC-9 and NCI-H1975 cells based on
flow cytometric analysis. The percentage of apoptotic cells was
0.03% in the A549 cell control group (Fig. 3Aa), this was slightly elevated
following exposure to PA-MSHA (0.156×109/ml; Fig. 3Ab). The number of apoptotic cells
increased in a dose-dependent manner following treatment with high
concentrations of PA-MSHA (0.313, 0.625, and
1.25×109/ml), the percentage of apoptotic cells was
3.30, 8.40, and 26.08% respectively (P<0.05; Fig. 3Ac). The percentage of apoptotic
cells was 6.9% in control PC-9 cells (Fig. 3Af), and treatment with PA-MSHA
(0.156×109/ml) slightly increased the amount (Fig. 3Ag). High concentrations of PA-MSHA
(0.313, 0.625, and 1.25×109/ml) increased the number of
apoptotic cells in a dose-dependent manner, the percentage of
apoptotic cells was 8.83, 11.85, and 25.78%, respectively
(P<0.05; Fig. 3 Ah-j). Control
NCI-H1975 cells indicated an apoptosis rate of 0.39% (Fig. 3Ak), which was increased by
0.156×109/ml PA-MSHA treatment (Fig. 3 Al). High concentrations of PA-MSHA
(0.313, 0.625, and 1.25×109/ml) resulted in a
dose-dependent increase in the number of apoptotic cells, the
percentage of apoptotic cells was 11.04, 13.69, and 21.11%,
respectively (P<0.05; Fig.
3Am-o). Following treatment with PA-MSHA, the three cell lines
exhibited apoptosis in a dose-dependent manner from 0.156
×109/ml (Fig. 3B).
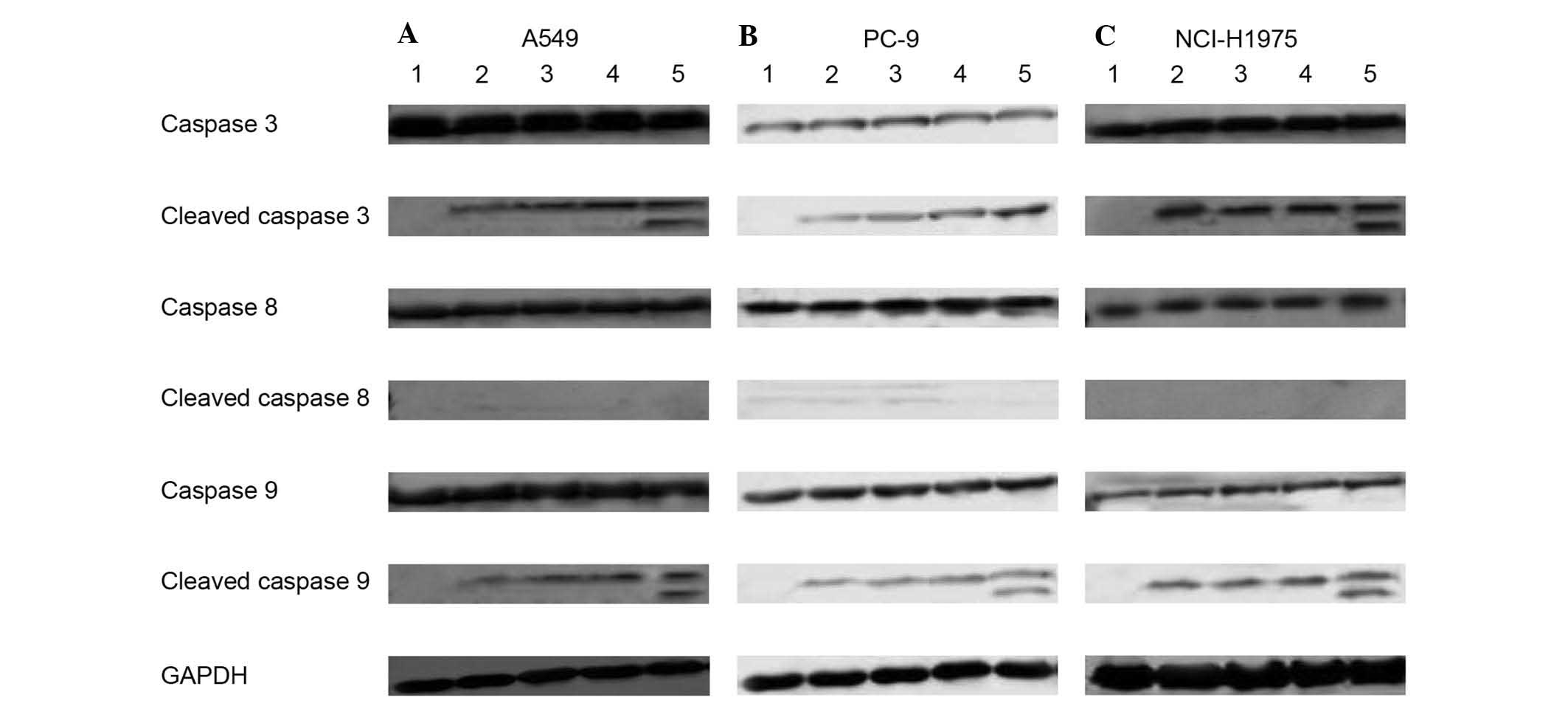
PA-MSHA induced apoptosis via caspase
cascade proteins
A549, PC-9 and NCI-H1975 cells were treated with
PA-MSHA to investigate the underlying mechanism of apoptosis
induction and assess the involvement of caspase-associated
proteins. The lysates were analyzed using antibodies directed
against caspases 3, 8, and 9 and the cleaved forms of the caspase
protein. When A549, PC-9, and NCI-H1975 cells were exposed to
PA-MSHA for >24 h, there was a concentration-dependent increase
in the expression levels of cleaved caspase 3 and caspase 9
proteins (Fig. 4), indicating the
proteolytic processing of the proenzyme to its active enzyme
subunits. However, a concentration-dependent increase in cleaved
caspase 8 was not observed when A549, PC-9, and NCI-H1975 cells
were exposed to PA-MSHA.
Discussion
For the last two decades, PA-MSHA has been used in
China as an adjuvant therapy in the treatment of gastric cancer,
breast cancer, lung cancer, and malignant lymphoma patients to
reduce infection rates associated with chemotherapy, as well as to
improve the sensitivity of chemotherapy, immune function, and
quality of life (23,24).
The present study examined the anti-cancer effects
of PA-MSHA in vitro in three types of NSCLC cell lines,
including two EGFR TKI-resistant lines. The results demonstrate
that PA-MSHA inhibits cell proliferation, redistributes the cell
cycle, and induces cell apoptosis in NSCLC cells with different
genotypes in a concentration-dependent manner. The rate at which
PA-MSHA inhibits proliferation was increased in PC-9, A549 and
NCI-H1975 cells compared with BEAS-2B cells. In addition, the
effects of PA-MSHA on NSCLC were consistent with those previously
observed in breast cancer cells expressing EGFR (21,22).
In previous studies by Liu et al (21,22),
PA-MSHA, which is characterized by mannose-sensitive
hemagglutination pili, can specifically conjugate with the
mannose-rich surface of high-mannose tumor cells. EGFR contains a
large number of mannose oligosaccharides, which likely serve as the
binding domain to PA-MSHA on the cell surface. In addition to these
lectin-like activities, further studies have demonstrated that
PA-MSHA can ablate the EGFR signaling cascade by reducing basal
EGFR phosphorylation in breast cancer cells that overexpress EGFR
(22). Growth suppression and cell
death induced by PA-MSHA is mediated by the mannose-sensitive
hemagglutination pili of P. aeruginosa and that this effect
may be independent of EGFR gene mutation status.
However, observed growth inhibition and cell death
may be the result of apoptosis induced by cell cycle
redistribution. Flow cytometric analysis and Annexin V-PI dual
staining were performed to further investigate this. Consistent
with results observed in breast cancer cell lines (21), a concentration-dependent decrease
in the proportion of S phase cells and an increase in the sub-G1
population of the PA-MSHA-treated NSCLC cells was observed,
suggesting that the arrested cells had entered into apoptosis. To
the best of our knowledge, this is the first study to demonstrate
PA-MSHA redistributes the cell cycle of NSCLC cells at the S and
sub-G1 phases. Notably, proliferation inhibition, cell cycle
arrest, and induction of apoptosis were observed in NSCLC cells
treated with PA-MSHA independent of EGFR gene mutation status. This
suggests that PA-MSHA-mediated effects may be a novel strategy to
overcome the resistance to EGFR TKIs in NSCLC patients.
Earlier data has demonstrated that caspase-activated
apoptosis is critical in the carcinogenesis, etiology,
pathogenesis, and therapy of a number of human malignancies,
including breast cancer, nasopharyngeal cancer and hepatocellular
carcinoma (20–22,25).
This apoptosis was triggered by various stimuli, followed by
initiation and execution via two predominant pathways, the
intrinsic mitochondrial pathway and the extrinsic membrane death
receptor pathway (26,27). In the intrinsic mitochondrial
pathway, caspase 9 and the downstream cleavage of caspase 3 was
activated by the loss of mitochondrial membrane potential, which
induces release of mitochondrial components into the cytoplasm,
such as cytochrome c (27).
Alternatively, in the extrinsic pathway, the cell death ligands
have been demonstrated to bind to cell surface death receptors and
subsequently activate caspase 8 and caspase 3 (28).
In breast cancer, the data suggest that PA-MSHA
triggered the extrinsic pathway, by interacting with the
pro-apoptotic caspase cascade via the EGFR pathway, and the
intrinsic apoptosis pathway, by mediating a mitochondrial effect
(21,22). However, the results of the present
study suggest that apoptosis of NSCLC cell lines induced by PA-MSHA
is mediated directly via caspase 3 and 9 and that the intrinsic
pathway mediated by mitochondria may be important in apoptosis.
Contrary to the results reported in breast cancer, the current
study did not observe downregulated expression of caspase 8 and
upregulated expression of cleaved caspase 8 in the three different
NSCLC genotypes. These data suggest that the extrinsic pathway may
not be key in PA-MSHA-induced NSCLC apoptosis. However, the
dominant pathway requires further elucidation. The data from the
present study suggest that PA-MSHA induced apoptosis of human NSCLC
cells is mediated via caspase activation triggered by either
mitochondrial or other pathways, such as EGFR-associated
pathways.
In conclusion, the present study demonstrated, for
the first time, that PA-MSHA treatment induces reduced
proliferation, cell cycle redistribution and apoptosis via caspase
family proteins in different NSCLC genotypes (PC-9, A549 and
NCI-H1975 cells) independent of EGFR resistance. The in
vitro experiments with PA-MSHA indicated that, in addition to
an activated immune system, cytotoxicity may also contribute to
NSCLC treatment. Treatment with PA-MSHA, either alone or in
combination with standard therapeutic options, such as
chemotherapy, radiotherapy, and particularly, targeted therapy, may
be a novel strategy for the management of NSCLC. Further research
is required to support the in vivo findings of the current
study.
Acknowledgements
The present study was supported by the Shanghai
Science and Technology Committee Natural Science Foundation of
Shanghai (grant no. 12ZR 1406400), National Natural Science
Foundation of China (grant no. 81302009), and Clinical Research
Funds of Wu Jieping Medical Foundation (grant no.
320.6750.14278).
Glossary
Abbreviations
Abbreviations:
|
PA-MSHA
|
Pseudomonas aeruginosa-mannose
sensitive hemagglutinin
|
|
NSCLC
|
non-small-cell lung cancer
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
FCM
|
flow cytometry
|
|
PI
|
propidium iodide
|
|
OS
|
overall survival
|
|
EGFR
|
epidermal growth factor receptor
|
|
TKIs
|
tyrosine kinase inhibitors
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Desantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harari PM, Allen GW and Bonner JA: Biology
of interactions: Antiepidermal growth factor receptor agents. J
Clin Oncol. 25:4057–4065. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hynes NE and Lane HA: ERBB receptors and
cancer: The complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mendelsohn J and Baselga J: Status of
epidermal growth factor receptor antagonists in the biology and
treatment of cancer. J Clin Oncol. 21:2787–2799. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Engelman JA and Jänne PA: Mechanisms of
acquired resistance to epidermal growth factor receptor tyrosine
kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res.
14:2895–2899. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li D, Ambrogio L, Shimamura T, Kubo S,
Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A,
Himmelsbach F, et al: BIBW2992, an irreversible EGFR/HER2 inhibitor
highly effective in preclinical lung cancer models. Oncogene.
27:4702–4711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirsch FR, Varella-Garcia M, Bunn PA Jr,
Di Maria MV, Veve R, Bremmes RM, Barón AE, Zeng C and Franklin WA:
Epidermal growth factor receptor in non-small-cell lung carcinomas:
Correlation between gene copy number and protein expression and
impact on prognosis. J Clin Oncol. 21:3798–3807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takezawa K, Okamoto I, Tanizaki J, Kuwata
K, Yamaguchi H, Fukuoka M, Nishio K and Nakagawa K: Enhanced
anticancer effect of the combination of BIBW2992 and thymidylate
synthase-targeted agents in non-small cell lung cancer with the
T790M mutation of epidermal growth factor receptor. Mol Cancer
Ther. 9:1647–1656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miller VA, Hirsh V, Cadranel J, Chen YM,
Park K, Kim SW, Zhou C, Su WC, Wang M, Sun Y, et al: Afatinib
versus placebo for patients with advanced, metastatic
non-small-cell lung cancer after failure of erlotinib, gefitinib,
or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase
2b/3 randomised trial. Lancet Oncol. 13:528–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao M, Yang M, Ma H, Li X, Tan X, Li S,
Yang Z and Hoffman RM: Targeted therapy with a Salmonella
typhimurium leucine-arginine auxotroph cures orthotopic human
breast tumors in nude mice. Cancer Res. 66:7647–7652. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maletzki C, Linnebacher M, Kreikemeyer B
and Emmrich J: Pancreatic cancer regression by intratumoural
injection of live Streptococcus pyogenes in a syngeneic mouse
model. Gut. 57:483–491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baban CK, Cronin M, O'Hanlon D, O'Sullivan
GC and Tangney M: Bacteria as vectors for gene therapy of cancer.
Bioeng Bugs. 1:385–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao M, Geller J, Ma H, Yang M, Penman S
and Hoffman RM: Monotherapy with a tumor-targeting mutant of
Salmonella typhimurium cures orthotopic metastatic mouse models of
human prostate cancer. Proc Natl Acad Sci USA. 104:10170–10174.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crull K and Weiss S: Antibiotic control of
tumor-colonizing Salmonella enterica serovar Typhimurium. ExpBiol
Med (Maywood). 236:1282–1290. 2011. View Article : Google Scholar
|
|
17
|
Hayashi K, Zhao M, Yamauchi K, Yamamoto N,
Tsuchiya H, Tomita K and Hoffman RM: Cancer metastasis directly
eradicated by targeted therapy with a modified Salmonella
typhimurium. J Cell Biochem. 106:992–998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yam C, Zhao M, Hayashi K, Ma H, Kishimoto
H, McElroy M, Bouvet M and Hoffman RM: Monotherapy with a
tumortargeting mutant of S. typhimurium inhibits liver metastasis
in a mouse model of pancreatic cancer. J Surg Res. 164:248–255.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kreikemeyer B, Klenk M and Podbielski A:
The intracellular status of Streptococcus pyogenes: Role of
extracellular matrix-binding proteins and their regulation. Int J
Med Microbiol. 294:177–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Wu D and Chen L: Pseudomonas
aeruginosa vaccine inhibits the proliferation of human
nasopharyngeal cancer cells in vitro. Nan Fang Yi Ke Da Xue Xue
Bao. 32:544–547. 2012.(In Chinese). PubMed/NCBI
|
|
21
|
Liu ZB, Hou YF, Di Min-Dong GH, Wu J, Shen
ZZ and Shao ZM: PA-MSHA inhibits proliferation and induces
apoptosis through the up-regulation and activation of caspases in
the human breast cancer cell lines. J Cell Biochem. 108:195–206.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu ZB, Hou YF, Zhu J, Hu DL, Jin W, Ou
ZL, Di GH, Wu J, Shen ZZ and Shao ZM: Inhibition of EGFR pathway
signaling and the metastatic potential of breast cancer cells by
PA-MSHA mediated by type 1 fimbriae via a mannose-dependent manner.
Oncogene. 29:2996–3009. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Hao D, Zhang H, Ren L, Yang Y, Li L,
Chai J, Zhou X and Fu L: A clinical study on PA-MSHA vaccine used
for adjuvant therapy of lymphoma and lung cancer. Hua Xi Yi Ke Da
Xue Xue Bao. 31:334–337. 2000.(In Chinese). PubMed/NCBI
|
|
24
|
Chen WD, Tang ZH and Xu F: Application of
PA-MSHA vaccine adjuvant therapy and TAC scheme for treatment of
breast carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 29:1204–1207.
2009.(In Chinese). PubMed/NCBI
|
|
25
|
Philchenkov AA: Caspases as regulators of
apoptosis and other cell functions. Biochemistry (Mosc).
68:365–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mathiasen IS and Jäättelä M: Triggering
caspase-independent cell death to combat cancer. Trends Mol Med.
8:212–220. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tretiakova I, Blaesius D, Maxia L,
Wesselborg S, Schulze-Osthoff K, Cinatl J Jr, Michaelis M and Werz
O: Myrtucommulone from Myrtus communis induces apoptosis in cancer
cells via the mitochondrial pathway involving caspase-9. Apoptosis.
13:119–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hegardt C, Andersson G and Oredsson SM:
Different roles of spermine in glucocorticoid- and Fas-induced
apoptosis. Exp Cell Res. 266:333–341. 2001. View Article : Google Scholar : PubMed/NCBI
|