Introduction
Lung cancer is one of the most common malignancies
worldwide, and approximately 85% new cases are non-small cell lung
cancer (NSCLC). Despite therapeutic advances, the majority of
patients with lung cancer undergo locally advanced and distant
metastasis, with an overall five-year survival rate less than 20%
(1). Thus, it is imperative to
further investigate the molecular mechanisms underlying the
progression of NSCLC.
The protein phosphatase wild-type p53 induced
phosphatase 1 (Wip1) is a major serine/threonine phosphatase of the
protein phosphatase 2C family encoded by the protein phosphatase,
Mg2+/Mn2+ dependent 1D gene (2). The overexpression and amplification
of Wip1 had been previously identified in numerous types of human
cancer including medulloblastomas (3), clear cell renal cell carcinoma
(4), colorectal cancer (5) and breast cancer (6). In addition, Bulavin et al
(7) demonstrated that Wip1
functioned as a proto-oncogene in breast cancer. ATM
serine/threonine kinase is one of the master regulators of the DNA
damage-induced response signaling pathway (8), which was previously identified to be
inactivated by Wip1 expression and potentially reduced the rate of
tumor evolution (9). In addition,
p38 mitogen-activated protein kinase (MAPK) was considered as a
downstream target of Wip1, which regulated the cell cycle by
inhibiting cyclin D1 under stress (10) and directly suppressed p53
activities through Ser33 and Ser46 dephosphorylation by controlling
cellular apoptosis (8). An
additional study further confirmed that Wip1 overexpression in
mesenchymal stem cells diminished the p38MAPK activity and reduced
the levels of p16 (11).
Accordingly, Wip1 overexpression was identified to suppress
checkpoint kinase 1 (CHK1) activity, whereas downregulation of Wip1
elevated CHK1 to activate the G2/M checkpoint following
DNA damage (12).
However, the expression and functions of Wip1 in
NSCLC remain unclear. In the current study, the expression of Wip1
was identified in NSCLC, and its clinical significance was
determined.
Materials and methods
Tissue collection
Between April and September 2014, a total of 60
NSCLC tissues were obtained from patients who underwent tumor
resection at the First Affiliated Hospital of China Medical
University (Heping, China). The patients were comprised of 34 males
and 26 females with a mean age of 58.6 years (range, 42–72 years).
None of the 60 patients received any chemotherapy or radiotherapy
prior to the operation. All cases were pathologically diagnosed and
staged according to the TNM classification system (13). The 60 fresh specimens of tumor
tissue and 20 adjacent normal lung tissues (5 cm distance from the
cancer) were immediately obtained during the surgery: One part was
stored in 4% paraformaldehyde solution and then embedded in 10%
paraffin for immunohistochemistry, and the other part was preserved
in liquid nitrogen for western blot analysis.
In the cancer group, 34 cases were identified with
lymph node involvement (N+).
As for TNM stage, 44 cases were at stage I~II and 16
were at stage III~IV. In addition, 16 cases were highly
differentiated and 44 cases exhibited with moderate or low
differentiation. There were 40 cases, which presented with a tumor
length larger than 3 cm. In addition, there were 6 cases who
presented with distant metastasis. The experimental protocol was
established, according to the ethical guidelines of the Helsinki
Declaration and was approved by the Human Ethics Committee of the
First Affiliated Hospital of China Medical University. Written
informed consent was obtained from individual participants.
Main reagents and
immunohistochemistry
The rabbit anti-human Wip1 monoclonal antibody was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA;
cat. no. sc-130655). The monoclonal mouse antibodies against p16
(cat. no. 6598), p53 (cat. no. 9284), phosphorylation of p38 (cat.
no. 9215) and GAPDH (cat. no. 2118) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Immunohistochemistry
kit and Bicinchoninic Acid (BCA) Protein Extraction kit were
obtained from OriGene Technologies, Inc. (Beijing, China). RIPA
buffer was purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany).
A 4-µm section was prepared from the
paraffin-embedded block and dehydrated, and incubated in 3%
hydrogen peroxide for 12 min to block endogenous peroxidase,
followed by trypsin treatment for 18 min. A 10% goat serum was
introduced at room temperature for 20 min, and the Wip1 antibody
(1:100) was added to the tissues and incubated at 4°C overnight. As
for the negative control, the primary antibody was replaced with
PBS. The secondary antibodies in a ready to use kit (SP-9000;
ZSGB-BIO, Beijing, China) were added as appropriate and
3,3′-diaminobenzidine staining was visualized using the hematoxylin
stain. Two pathologists scored the slides respectively. For Wip1
staining assessment, samples with more than 10% cells that were
yellow/brown-stained were classified as positive.
Western blot analysis
Western blot was performed to detect the expression
of Wip1 in fresh tissues. The RIPA buffer was used to extract the
total protein. Next, the protein concentrations were quantified by
the BCA Protein Assay kit. A total of 50 µg total protein of each
sample was loaded onto the 10% SDS-PAGE and transferred to PVDF
membranes (EMD Millipore, Billerica, MA, USA). Following blocking
for 1 h in 5% non-fat milk, the membranes were incubated with
primary antibodies overnight at 4°C (Wip1, 1:200; GAPDH, 1:1,000).
On the second day, the membranes were incubated with the secondary
antibodies followed by ECL chemiluminescence (Qihai Biotec,
Shanghai, China). The results were analyzed using Gel-Pro-Analyzer
software version 6.3 (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
All statistical analyses were performed using SPSS
software, version 16.0 (SPSS, Inc., Chicago, IL, USA). Fisher's
exact test was used to determine the differences of the Wip1
protein in cancer tissues and normal tissues. The χ2 test was used
to assess the association between the clinicopathological
parameters. Spearman's rank correlation coefficient was applied to
see the correlation between Wip1 and p53, Wip1 and p16, and Wip1
and p38MAPK, respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
Wip1 protein expression in NSCLC and
normal tissues
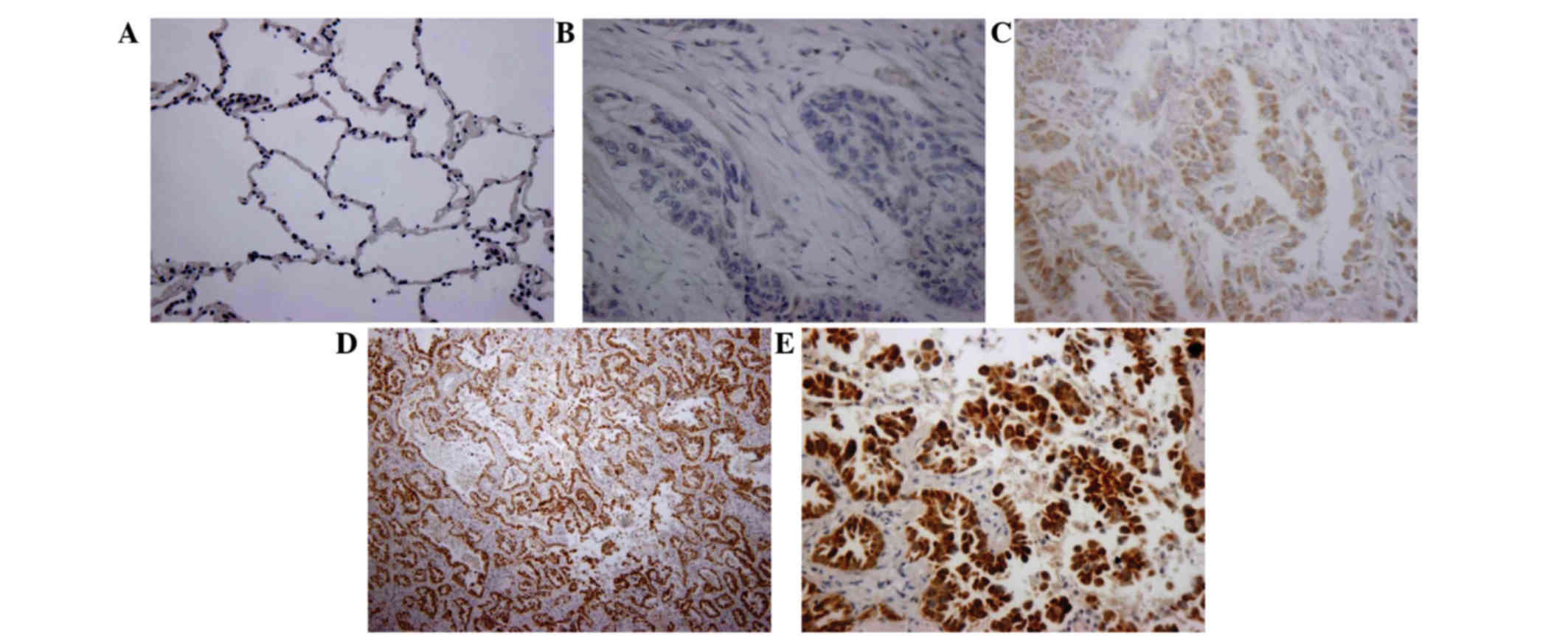
According to the immunohistochemistry data, the Wip1
expression was not detected in normal tissues (Fig. 1A). In NSCLC tissues, Wip1 was
stained negative (Fig. 1B), light
yellow (Fig. 1C) or
yellowish-brown (Fig. 1D and E).
Collectively, Wip1 was expressed in 63.3% (38/60) of NSCLC tissue,
which was significantly greater than in normal tissues (0%;
P<0.01; Table I). Furthermore,
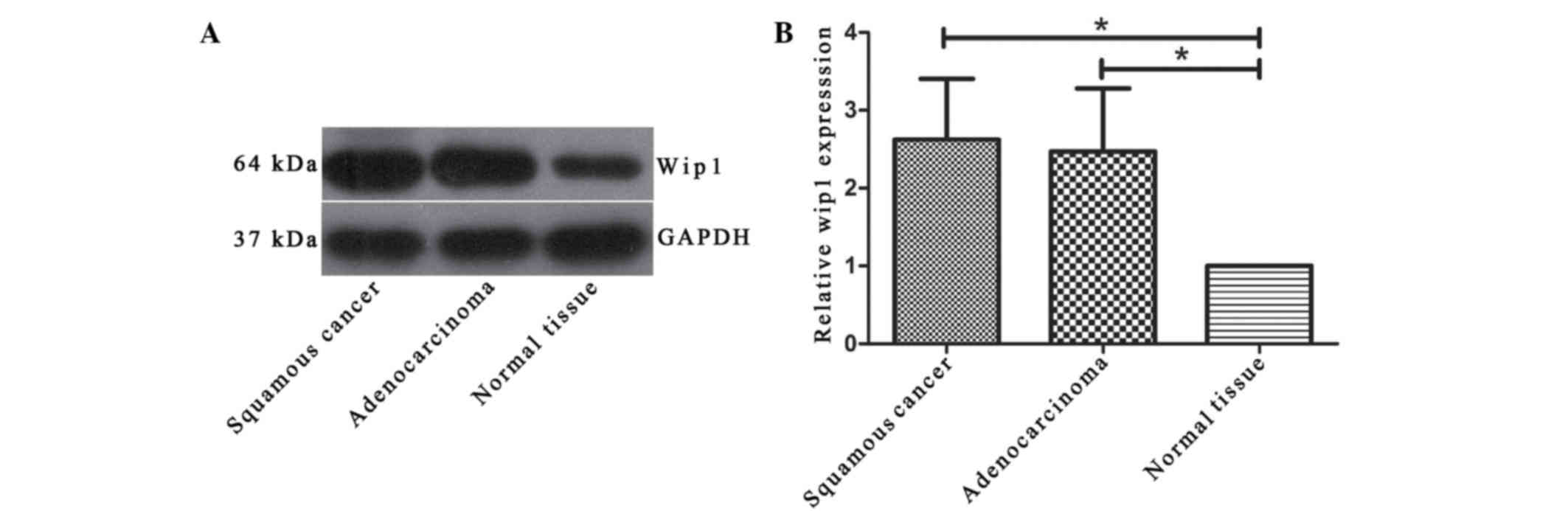
the expression of Wip1 was investigated using western blot
analysis. The data indicated that there was a significant increase
of Wip1 expression in the NSCLC tissues compared with the normal
tissues (P<0.05; Fig. 2).
 | Table I.The expression of Wip1 in cancer
tissues and matched normal tissues. |
Table I.
The expression of Wip1 in cancer
tissues and matched normal tissues.
| Group | Total | Wip1 +ve | Wip1 -ve | P-value |
|---|
| Cancer tissue | 60 | 38 | 22 | <0.001 |
| Normal tissue | 20 | 0 | 20 |
|
Association between Wip1 expression
and clinicopathological factors of patients
The associations between Wip1 expression and
clinicopathological factors are summarized in Table II. The increased expression of
Wip1 indicated a significant association with tumor length
(P<0.01) and degree of differentiation (P<0.05), which may
serve as a necessary prognostic marker for patients. However, no
significant correlation was detected between Wip1 and other
clinicopathological features including gender, lymph node
metastasis, smoking history, age, pathological type, TNM stage and
distant metastasis. In addition, western blot analysis demonstrated
that were was no correlation between Wip1 and pathological types
(data not shown; Fig. 2).
 | Table II.Association between Wip1 expression
and clinicopathological factors in 60 patients with non-small cell
lung cancer. |
Table II.
Association between Wip1 expression
and clinicopathological factors in 60 patients with non-small cell
lung cancer.
| Characteristics | Total | Wip1 positive
(n=38) | Wip1 negative
(n=22) | P-value |
|---|
| Gender |
|
|
|
|
| Male | 34 | 20 | 14 |
|
|
Female | 26 | 18 | 8 | 0.407 |
| Lymph node
metastasis |
|
|
|
|
| NO | 26 | 16 | 10 |
|
| N+ | 34 | 22 | 12 | 0.801 |
| Smoking history |
|
|
|
|
|
Positive | 28 | 18 | 10 |
|
|
Negative | 32 | 20 | 12 | 0.886 |
| Age (years) |
|
|
|
|
| ≥60 | 30 | 18 | 12 |
|
|
<60 | 30 | 20 | 10 | 0.592 |
| Pathological
type |
|
|
|
|
| Squamous
cancer | 22 | 12 | 10 |
|
|
Adenocarcinoma | 38 | 26 | 12 | 0.282 |
| Tumor length
(cm) |
|
|
|
|
| >3
cm | 40 | 30 | 10 |
|
| ≤3
cm | 20 | 8 | 12 | 0.008 |
| TNM stage |
|
|
|
|
| I~II | 44 | 26 | 18 |
|
|
III~IV | 16 | 12 | 4 | 0.408 |
| Differentiation |
|
|
|
|
| High | 16 | 6 | 9 |
|
| Moderate
+ low | 44 | 32 | 13 | 0.03 |
| Distant
metastasis |
|
|
|
|
|
Present | 6 | 4 | 2 |
|
|
Absent | 54 | 34 | 20 | 1 |
Association between Wip1, p53, p16 and
p38MAPK
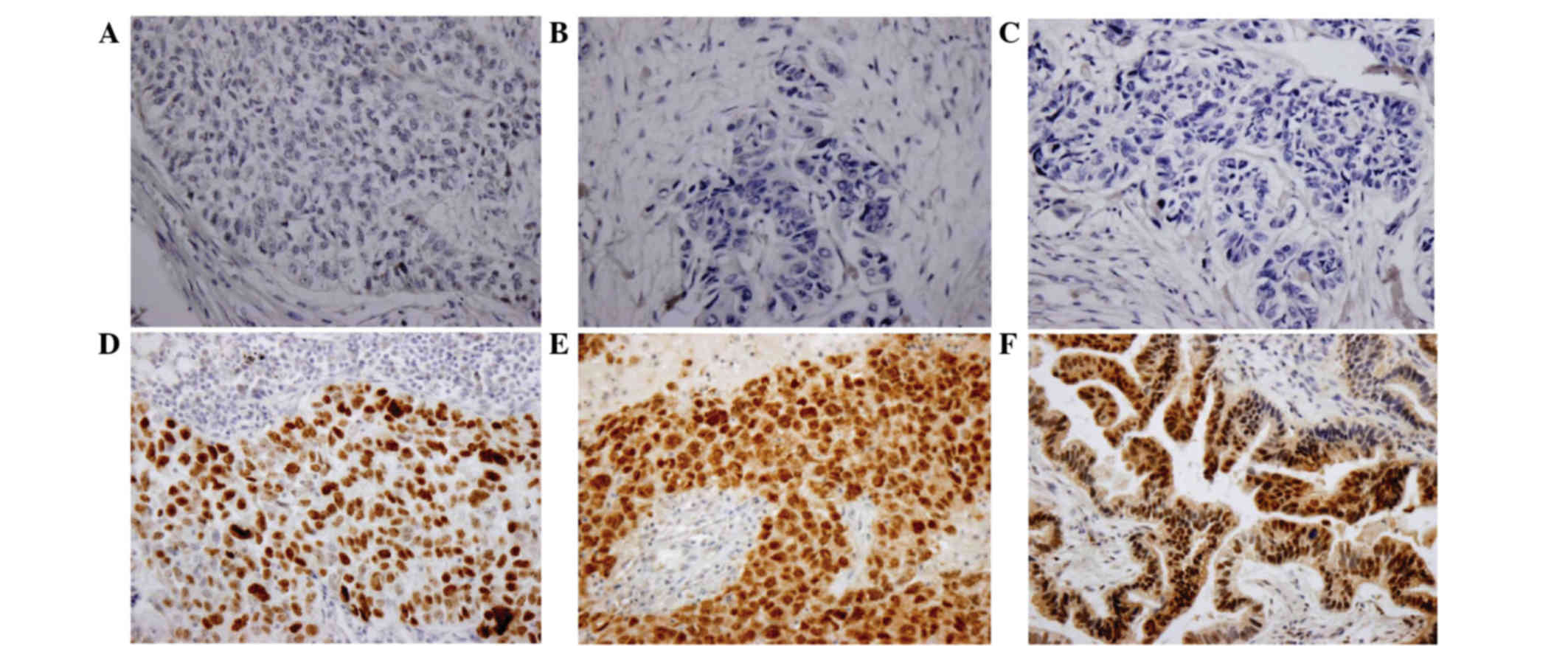
According to the immunohistochemistry data, the
expression of p53 was inversely correlated with Wip1 (r=−0.352). A
total of 16/25 (64%) tumors with negative Wip1 exhibited strong
staining for p53. In contrast, 25/35 (71.4%) cases with positive
Wip1 exhibited weak staining for p53.
Subsequently, whether Wip1 was correlated with the
levels of p38MAPK in NSCLC was investigated. Out of 29
Wip1-positive tumors, 21 (72.4%) did not stain for p38MAPK. On the
contrary, only 61.3% (19/31) of tumors expressing negative Wip1
displayed strong staining for p38MAPK (Table III; r=−0.284). In addition, 15
cases (15/26, 57.7%) of negative Wip1 exhibited high p16 expression
(r=−0.348; Fig. 3).
 | Table III.Associations between Wip1, p53, p16
and p38 mitogen-activated protein kinase. |
Table III.
Associations between Wip1, p53, p16
and p38 mitogen-activated protein kinase.
|
| p53 | p16 | p38 |
|---|
|
|
|
|
|
|---|
| Wip1 | (+) | (−) | (+) | (−) | (+) | (−) |
|---|
| (+) | 10 | 25 | 8 | 26 | 8 | 21 |
| (−) | 16 | 9 | 15 | 11 | 19 | 12 |
| r | −0.352 | −0.348 | −0.284 |
Discussion
Oncogene activation and cancer suppressor gene
inactivation are the key factors of tumor initiation and
progression. As previously reported, the Wip1 gene has been
identified as one of the p53 target genes induced by ionizing
radiation (2), which inhibits the
activity of p53 (7), downregulated
the expression of p38 mitogen activated protein kinase (14) and reduced the level of p16 protein
levels (15). Increased evidence
suggests that Wip1 serves a vital role in human cancer.
In the current study, it was identified that Wip1
was significantly increased in NSCLC tissues when compared with
normal tissues. Statistical analysis indicated that the
overexpression of Wip1 was notably associated with tumor length and
histological differentiation. By contrast, a previous study
suggested that Wip1 expression was not associated with tumor size
and pathological staging (16). It
was identified that Wip1 expression was increased in the low and
moderate differentiation groups when compared with the high
differentiation group, which indicates that Wip1 may be a novel
prognostic predictor for NSCLC.
Currently, the common treatment for NSCLC is
surgical resection, combined with chemotherapy and/or radiotherapy
prior and subsequent to surgery. However, the survival rate with
this strategy is not satisfactory (17). Therefore, inhibiting positive
regulators of cell proliferation, activating tumor suppressors and
inducing apoptosis are primary interventional strategies in modern
cancer therapy. A previous study indicated that Wip1 overexpression
in transgenic mice promoted cell transformation and accelerated
tumor progression (18).
Consistently, another study demonstrated that small interfering RNA
knockdown of Wip1 in medulloblastoma cells increased the p53
expression and induced apoptosis (19,20).
Further studies demonstrated that Wip1
overexpression disrupted the homeostasis maintained by the
p38MAPK-p53-Wip1 pathway, caused the inactivation of Wnt-p53
through p38MAPK dephosphorylation (15). In the present study, the
correlation between the expression of Wip1 and p53, p38MAPK and p16
was investigated in NSCLC tissues. A clear correlation between Wip1
and p38MAPK was observed. In addition, Wip1 expression was observed
to be concomitant with reduced p53 levels. These data suggest that
Wip1 functionally inactivates p53 and regulates the
p38MAPK-p53-Wip1 signal transduction pathway. Previously, Bulavin
et al (21) reported that
Wip1-null mouse embryonic fibroblasts exhibited significant p16
accumulation and reduced cyclin-dependent kinase 4 activity through
p38MAPK signaling in a p53-independent manner.
In summary, the current study demonstrated that Wip1
was frequently overexpressed and associated with the progression of
NSCLC, which may serve an essential role in the p38MAPK/p53/p16
signaling pathway.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fiscella M, Zhang H, Fan S, Sakaguchi K,
Shen S, Mercer WE, Van De Woude GF, O'Connor PM and Appella E:
Wip1, a novel human protein phosphatase that is induced in response
to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci
USA. 94:6048–6053. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buss MC, Remke M, Lee J, Gandhi K,
Schniederjan MJ, Kool M, Northcott PA, Pfister SM, Taylor MD and
Castellino RC: The WIP1 oncogene promotes progression and invasion
of aggressive medulloblastoma variants. Oncogene. 34:1126–1140.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu S, Qi L, Han W, Wan X, Jiang S, Li Y,
Xie Y, Liu L, Zeng F, Liu Z and Zu X: Overexpression of wip1 is
associated with biologic behavior in human clear cell renal cell
carcinoma. PLoS One. 9:e1102182014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li ZT, Zhang L, Gao XZ, Jiang XH and Sun
LQ: Expression and significance of the Wip1 proto-oncogene in
colorectal cancer. Asian Pac J Cancer Prev. 14:1975–1979. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruark E, Snape K, Humburg P, Loveday C,
Bajrami I, Brough R, Rodrigues DN, Renwick A, Seal S, Ramsay E, et
al: Mosaic PPM1D mutations are associated with predisposition to
breast and ovarian cancer. Nature. 493:406–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bulavin DV, Demidov ON, Saito S,
Kauraniemi P, Phillips C, Amundson SA, Ambrosino C, Sauter G,
Nebreda AR, Anderson CW, et al: Amplification of PPM1D in human
tumors abrogates p53 tumor-suppressor activity. Nat Genet.
31:210–215. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dudgeon C, Shreeram S, Tanoue K, Mazur SJ,
Sayadi A, Robinson RC, Appella E and Bulavin DV: Genetic variants
and mutations of PPM1D control the response to DNA damage. Cell
Cycle. 12:2656–2664. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Filipponi D, Muller J, Emelyanov A and
Bulavin DV: Wip1 controls global heterochromatin silencing via
ATM/BRCA1-dependent DNA methylation. Cancer Cell. 24:528–541. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Casanovas O, Miró F, Estanyol JM, Itarte
E, Agell N and Bachs O: Osmotic stress regulates the stability of
cyclin D1 in a p38SAPK2-dependent manner. J Biol Chem.
275:35091–35097. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JS, Lee MO, Moon BH, Shim SH, Fornace
AJ Jr and Cha HJ: Senescent growth arrest in mesenchymal stem cells
is bypassed by Wip1-mediated downregulation of intrinsic stress
signaling pathways. Stem Cells. 27:1963–1975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liao Q, Guo X, Li X, Xiong W, Li X, Yang
J, Chen P, Zhang W, Yu H, Tang H, et al: Prohibitin is an important
biomarker for nasopharyngeal carcinoma progression and prognosis.
Eur J Cancer Prev. 22:68–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
Internation Association for the Study of Lung Cancer: The IASLC
Lung Cancer Staging Project: Proposals for the revision of the TNM
stage groupings in the forthcoming (seventh) edition of the TNM
classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koom WS, Park SY, Kim W, Kim M, Kim JS,
Kim H, Choi IK, Yun CO and Seong J: Combination of radiotherapy and
adenovirus-mediated p53 gene therapy for MDM2-overexpressing
hepatocellular carcinoma. J Radiat Res. 53:202–210. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito-Ohara F, Imoto I, Inoue J, Hosoi H,
Nakagawara A, Sugimoto T and Inazawa J: PPM1D is a potential target
for 17q gain in neuroblastoma. Cancer Res. 63:1876–1883.
2003.PubMed/NCBI
|
|
16
|
Yang DH, He JA, Li J, Ma WF, Hu XH, Xin SJ
and Duan ZQ: Expression of proto-oncogene Wip1 in breast cancer and
its clinical significance. Zhonghua Yi Xue Za Zhi. 90:519–522.
2010.(In Chinese). PubMed/NCBI
|
|
17
|
Sculier JP: Nonsmall cell lung cancer. Eur
Respir Rev. 22:33–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tarulli GA, De Silva D, Ho V, Kunasegaran
K, Ghosh K, Tan BC, Bulavin DV and Pietersen AM: Hormone-sensing
cells require Wip1 for paracrine stimulation in normal and
premalignant mammary epithelium. Breast Cancer Res. 15:R102013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu H, Liu C, Zhao Z, Gao N, Chen G, Wang Y
and Cui J: Clinical implications of GRHL3 protein expression in
breast cancer. Tumour Biol. 35:1827–1831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang D, Zhang H, Hu X, Xin S and Duan Z:
Abnormality of pl6/p38MAPK/p53/Wipl pathway in papillary thyroid
cancer. Gland Surg. 1:33–38. 2012.PubMed/NCBI
|
|
21
|
Bulavin DV, Phillips C, Nannenga B,
Timofeev O, Donehower LA, Anderson CW, Appella E and Fornace AJ Jr:
Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis
through p38 MAPK-mediated activation of the p16 (Ink4a)-p19 (Arf)
pathway. Nat Genet. 36:343–350. 2004. View
Article : Google Scholar : PubMed/NCBI
|