Introduction
Cervical cancer is one of the major causes of cancer
morbidity and mortality in women globally, particularly in
developing countries. According to the World Health Organization
International Agency for Research on Cancer (GLOBOCAN 2012),
>500,000 women worldwide were diagnosed with cervical carcinoma
each year, of which >80% were in less developed countries
(1). In China, cervical cancer
cases are increasingly prevalent in young patients (2). Currently, the established treatment
for cervical cancer, particularly in the early stages, is resection
and radiotherapy. However, these mainstream approaches may be
associated with subsequent pregnancy complications, which is
problematic particularly for young or nulliparous women who wish to
remain fertile. Improvements in treatment are required for patients
with cervical cancer and photodynamic therapy (PDT), a treatment
that preserves the cervix, may be a suitable option.
PDT is an innovative technique that is widely used
for local treatment of various solid tumors (3–5) and
certain non-malignant diseases (6–8). PDT
involves using a specific wavelength of light to activate a
photosensitizer that is selectively accumulated in the target
tissue. PDT causes destruction of stained cells through the
production of singlet oxygen or superoxide, and induces
irreversible cell damage through direct and indirect cytotoxicity
(9). 5-Aminolaevulanic acid
(ALA)-mediated PDT (ALA-PDT) is an approved therapeutic option for
local therapy of various human precancerous and cancerous lesions
with encouraging clinical outcomes (10). However, its specific mechanisms are
not fully understood and its efficacy is remarkably varied. A
complete understanding of the molecular mechanisms of PDT-mediated
cell destruction may lead to improvements in its therapeutic
efficacy. Epigenetic regulation of transcription and histone
modification might be involved in PDT-mediated cell destruction
(11). The upregulation of the
histone deacetylases HDAC-1 and HDAC-11 has also been demonstrated
in the mouse cerebral cortex following ALA-PDT (11). Retinoblastoma-associated protein 48
(RbAp48), a highly abundant component of HDACs (12), is required for negative regulation
of E2F transcription factor activity through its interaction with
HDAC1 and HDAC3 (13). A previous
study has also demonstrated that RbAp48 is a critical mediator
controlling human papilloma virus (HPV)-16 transforming activity in
HPV-induced cervical carcinogenesis (14). RbAp48 suppresses the growth of
cervical cancer and reverts transformed phenotypes of cervical
cancer in vitro and in vivo. Furthermore, RbAp48 was
identified as a radiosensitive gene in a microarray analysis used
for selecting radiosensitivity prediction molecules (15). Finally, another previous study
indicated the involvement of RbAp48 in radiation-response and
overexpression of RbAp48 enhanced the radiosensitivity of cervical
cancer (16).
The present study demonstrated that PDT induces
RbAp48, which leads to cancer cell destruction through upregulation
of tumor suppressor protein 53 (p53) and retinoblastoma protein
(Rb) expression, and downregulation of the HPV genes, E6 and E7.
The current study provides evidence for the involvement of RbAp48
in anti-proliferative responses to ALA-PDT.
Materials and methods
Cell culture and PDT
SiHa and HeLa cervical cancer cells were obtained
from the American Type Culture Collection (Manassas, VA, USA) and
cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.), under standard culture conditions. Cells, 80–90% confluent,
were used in all experiments. ALA hydrochloride, obtained from
Sigma-Aldrich (Merck Millipore; Darmstadt, Germany), was dissolved
in DMEM. Various concentrations (0, 50, 100 and 150 µM) of ALA
dissolved in DMEM were added to cells, which were then incubated
for 6 h in the dark at 37°C and 5% CO2. Following
administration of ALA, all samples went through 3 washing steps
with PBS. Fresh culture medium (DMEM supplemented with 10% FBS) was
then added to each well, followed by light-emitting diode laser
irradiation (Wuhan Yage Optic and Electronic Technology Co., Ltd.,
Wuhan, China) at a wavelength of 630 nm for 5 min and the laser
energy was 10 J/cm2. Then, cells were incubated in the
dark at 37°C, 5% CO2 for 24 h.
Cell viability and cell counting
assay
Cells were seeded 2×103 per well in a
96-well culture plate for the cell viability assay and
1×104 per well in 24-well plates for the cell counting
assay. Cells were then treated with various concentrations (0, 50,
100 or 150 µM) of ALA. Cell viability was evaluated by Cell
Counting Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) according to the manufacturer's protocol as described, 24 h
after ALA-PDT treatment. For the cell counting assay, cells were
treated as above and detected as described previously (14). Experiments were performed in
triplicate to ensure the reproducibility of the results. And the
half-maximal inhibitory concentration was derived from the
dose-response curve.
Western blotting
Cells were harvested 24 h after ALA-PDT treatment
and then washed 3 times with cold PBS. Total protein was extracted
from cells by the use of NP-40 lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol, separated on 12% polyacrylamide-SDS gels and transferred
onto a 0.2 µm polyvinylidene fluoride membrane
(Immobilon®; EMD Millipore, Billerica, MA, USA). After
blocking with TBS (0.1% Tween-20) containing 5% non-fat milk for 1
h at room temperature, the blot was washed with TBST (10 mM TBS,
100 mM NaCl, 0.1% Tween-20) 3 times for 10 min. The blot was then
incubated with mouse anti-RbAp48 antibodies (1:1,000; cat. no.
ab55778; Abcam, Cambridge, UK) or glyceraldehyde phosphate
dehydrogenase antibodies (GAPDH; 1:2,000; cat. no. ab8245; Abcam,
UK) overnight at 4°C. The blot was washed again 3 times for 10 min
and then incubated with a goat anti-mouse horseradish
peroxidase-conjugated secondary antibody (1:1,000; cat. no. ab6789;
Abcam) for 1 h at room temperature. The blot was washed 3 times for
10 min and immune complexes were visualized by chemiluminescence
using an ECL kit (GE Healthcare Life Sciences, Chalfont, UK) and
imaged using ImageQuant LAS 4000 (GE Healthcare Life Sciences). The
images were analyzed and quantified using Image J software version
1.44 (National Institutes of Health, Bethesda, MD, USA). The
intensity of bands was normalized in relative to GAPDH signals.
Each experiment was repeated 3 times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and then 5 µg
total RNA was purified and reversely transcribed to cDNA using
random primers with the PrimeScript™ RT Reagent Kit with gDNA
Eraser (Takara Bio, Inc., Otsu, Japan) following the manufacturer's
protocol. Amplification of PCR products was quantified using
SYBR® Premix Ex Taq™ (Takara Bio, Inc., Otsu, Japan) and
performed on the LightCycler 2.0 Instrument (Roche Diagnostics,
Basel, Switzerland) equipped with a LightCycler Software version
4.0 (Roche Diagnostics, Basel, Switzerland) using the following PCR
conditions: 40 cycles, 95°C for 15s, 60°C for 1 min. Primers used
for RT-qPCR were as follows (forward and reverse, respectively):
RbAp48, forward 5′-ATGCCCCAGAACCCTTGTATC-3′ and reverse
5′-GCCCATAGCCTTCCTTCTGAT-3′; HPV-16 E6, forward
5′-AATGTTTCAGGACCCACAGG-3′ and reverse 5′-TCACGTCGCAGTAACTGTTG-3′;
HPV-16 E7, forward 5′-AGTGTGACTCTACGCTTCGG-3′ and reverse
5′-TGTGCCCATTAACAGGTCTT-3′; p53, forward 5′-GGCAGCTGGTTAGGTAGAGG-3′
and reverse 5′-AGGTCGACCAAGAGGTTGTC-3′; Rb, forward
5′-ACCCAGAAGCCATTGAAATC-3′ and reverse 5′-TCTGGGTGCTCAGACAGAAG-3′;
capase-3, forward 5′-TAAATGAATGGGCTGAGCTG-3′ and reverse
5′-ATGGAGAAATGGGCTGTAGG-3′; β-actin, forward
5′-CTCCAAATGCAAACTGGATG-3′ and reverse 5′-TGTTGATTTGGGCACAGACT-3′,
as described previously (14).
Target gene expression levels were normalized to β-actin levels in
the same reaction. The ΔΔCq method was used to normalize mRNA
levels (17).
Small interfering RNA (siRNA)
transfection
Cells were plated in 6-well culture plates at
1×105 cells/well and incubated at 37°C until they
reached 80% confluency. Lipofectamine® 2000 reagent
(Invitrogen, Thermo Fisher Scientific, Inc.) was used to transfect
siRNA oligonucleotides that targeted RbAp48 (siRbAp48;
Sigma-Aldrich; Merck Millipore) and mammalian expression pSUPER
vector (OligoEngine, Seattle, WA, USA) into SiHa and HeLa cells as
described previously (14). The
sequences of siRNAs were as follows: 5′-CAGGGCATACGGCAGTAGT-3′ for
si-RbAp48 (1) and
5′-CGAGGAATACAAAATATGG-3′ for si-RbAp48 (2), as previously described (14). Cells were transfected with the
pSUPER vector to generate a control line. The sequences of control
siRNA contained in the pSUPER vector was 5′-GACTCCAGTGGTAATCTAC-3′.
At 48 h post-transfection, cells were harvested and used for
further experiments. Expression of RbAp48 was determined by RT-qPCR
and western blot assay.
Apoptosis rate analysis by flow
cytometry
Cells were plated in 35 mm dishes and then received
100 µM ALA-PDT treatment or irradiation as described earlier, and
were incubated at 37°C in the dark for 24 h. Then, the medium was
removed, cells were washed twice with PBS and collected in tubes
(1×106) and were then stained using an Annexin V-FITC/PI
Apoptosis Detection kit (BestBio, Co., Shanghai, China) according
to the manufacturer's protocols. Analysis was carried out at
activating wavelength 488 nm and fluorescence emission was
monitored at 623 nm by a FACS Calibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, CA, USA) and the data analyzed
using by FACS Calibur flow cytometer instrument equipped with BD
CellQuest Pro software version 5.1 (BD Biosciences).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical comparisons between means were assessed by Student's
t-test or two-way analysis of variance using GraphPad Prism
(version 6.0; GraphPad Software, Inc., La Jolla, CA, USA).
Bonferroni's post hoc test was used for post hoc comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ALA-PDT induces RbAp48 expression in
SiHa and HeLa cells
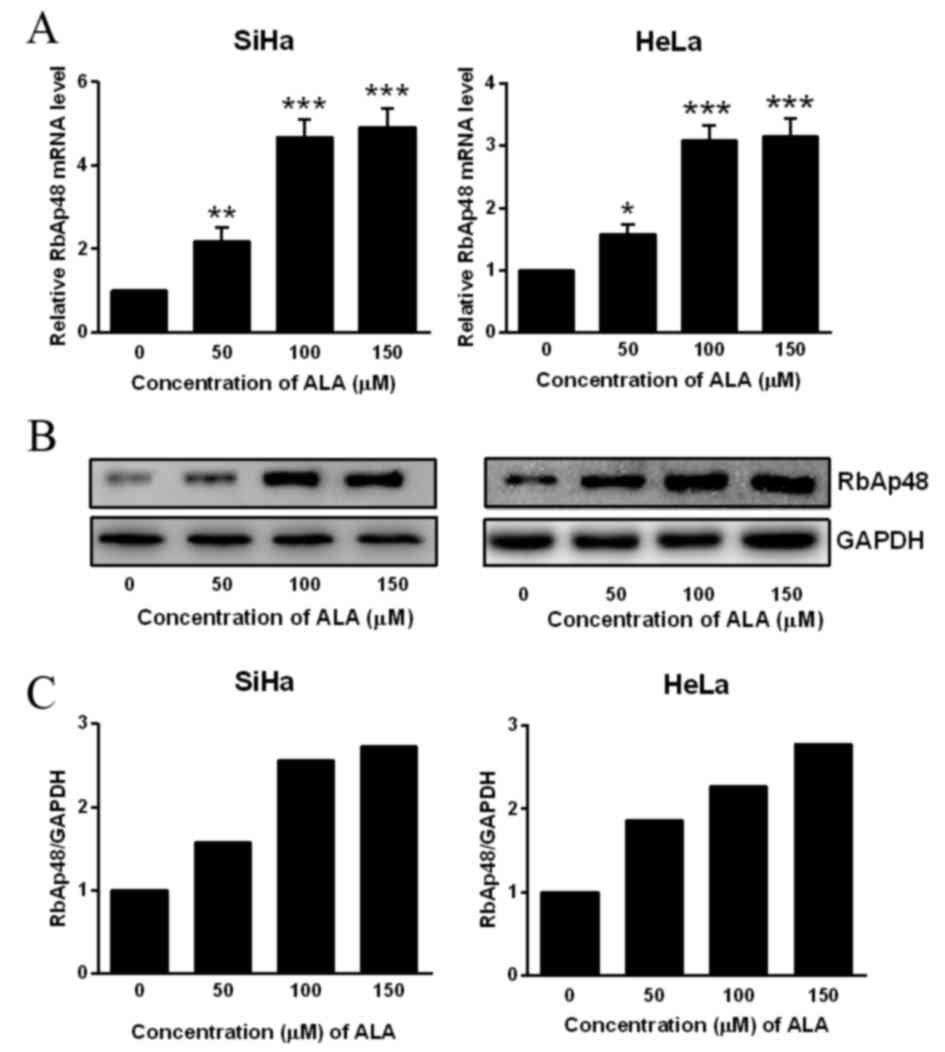
To clarify the function of RbAp48 in ALA-PDT
treatment, the present study initially determined the expression
pattern of RbAp48. mRNA and protein levels of RbAp48 were
significantly upregulated in SiHa and HeLa cells subjected to all
concentrations of ALA-PDT treatment, compared with untreated cells
(P<0.05; Fig. 1). A previous
study verified that RbAp48 controls the transforming activity of
HPV-16 in cervical cancer (14).
Therefore, RbAp48 may be involved in the response of cervical
cancer cells to ALA-PDT treatment.
Inhibition of RbAp48 expression via
siRNA-mediated silencing attenuates reduced viability caused by
ALA-PDT in SiHa and HeLa cells
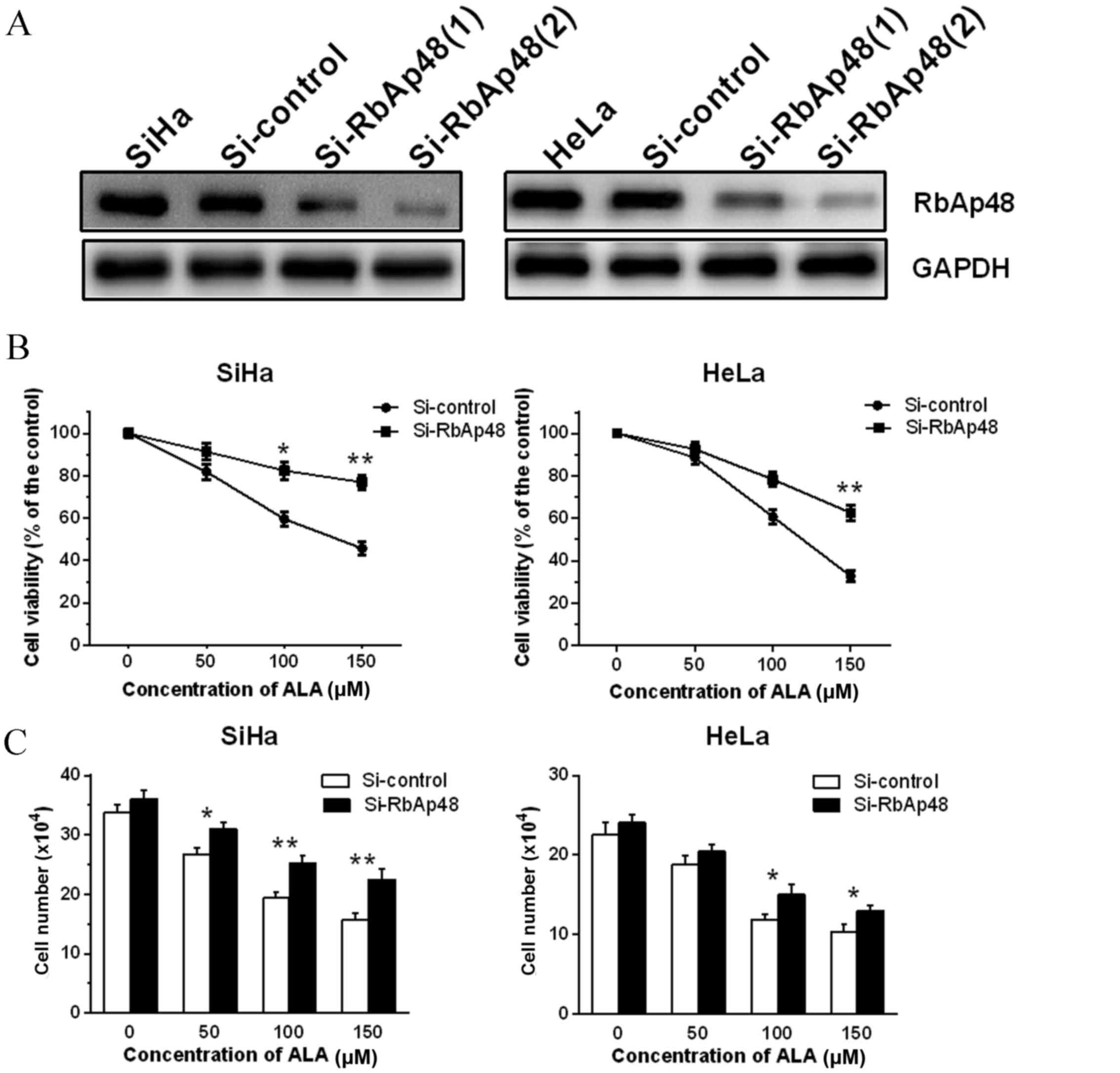
To verify whether altered expression of RbAp48
affects proliferation of PDT-surviving cervical cancer cells, the
current study initially suppressed RbAp48 gene expression in SiHa
and HeLa cells using siRbAp48, and subsequently performed PDT on
cells and evaluated the cell viability. As demonstrated in Fig. 2A, siRbAp48 inhibited protein
expression of RbAp48 compared with pSUPER vector control
(si-control) and parent SiHa or HeLa cells. However, siRbAp48-2 had
a stronger effect. Subsequently, the effect of reduced RbAp48
expression on cellular responses to ALA-PDT in SiHa and HeLa cells
by siRNA knockdown of RbAp48 was investigated. The CCK-8 assay
(Fig. 2B) and cell counting assay
(Fig. 2C) revealed that reduced
RbAp48 impaired ALA-PDT-induced cell growth inhibition in SiHa and
HeLa cervical cancer cell lines compared with si-control cells
(P<0.05).
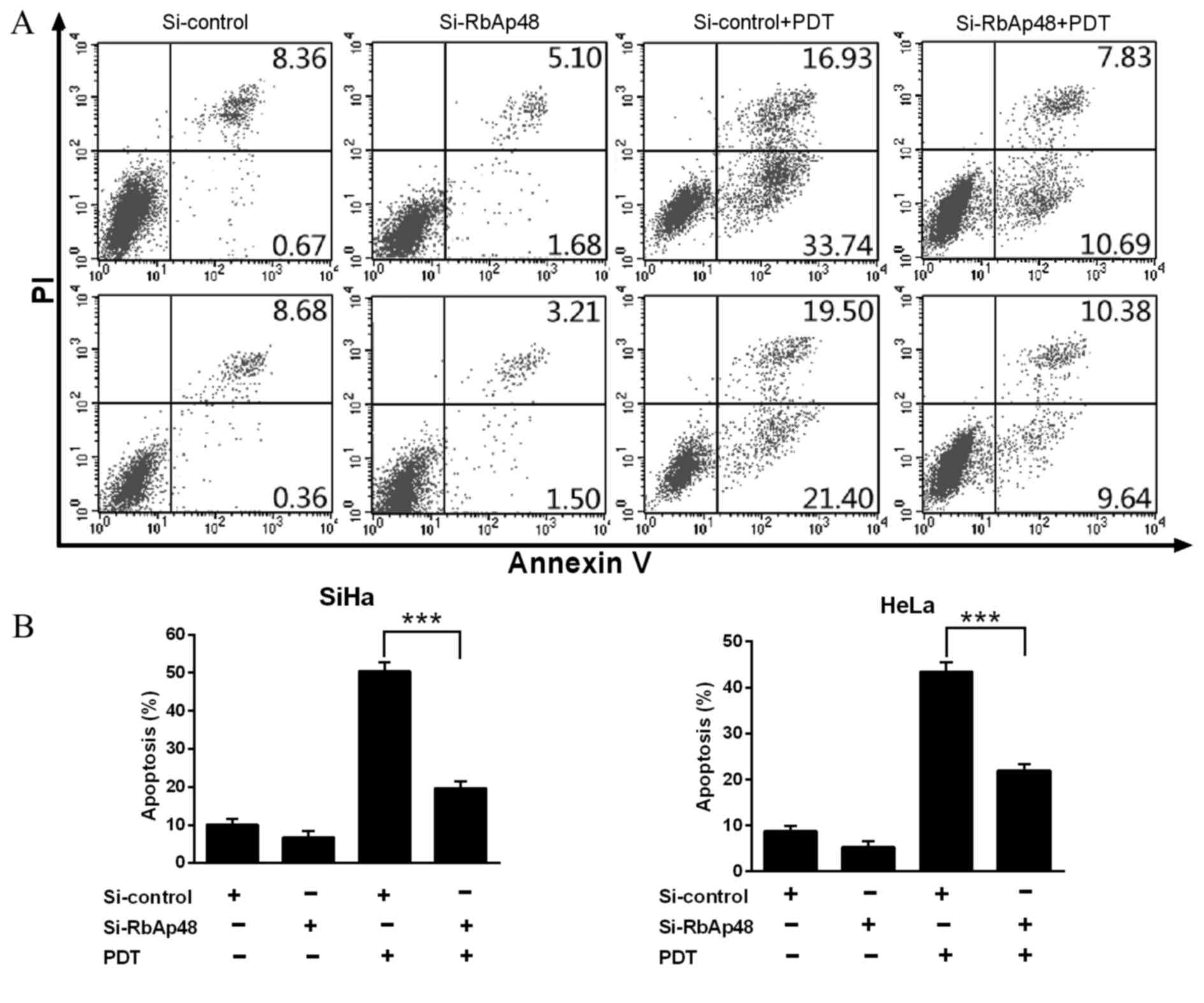
RbAp48 modulates ALA-PDT-induced
apoptosis
The anti-tumor effect of ALA-PDT is predominantly
mediated via apoptosis. Therefore, the current study investigated
whether RbAp48 has a role in PDT-induced apoptosis. Knockdown of
RbAp48 in SiHa and HeLa cells led to a significant reduction in
PDT-induced apoptosis compared with si-control vector groups
(P<0.001 and P<0.001, respectively; Fig. 3).
RbAp48 regulates mRNA expression of
tumor suppressors, apoptosis-associated enzymes and oncogenic viral
genes in cervical cancer cells in response to ALA-PDT
To elucidate the molecular mechanism by which RbAp48
functions in the response of cervical cancer cells to ALA-PDT,
RT-qPCR was performed. mRNA expression changes of tumor suppressors
Rb and p53, apoptosis-associated enzyme caspase-3, and HPV
oncogenes, E6 and E7, in cervical cancer cell lines were
investigated. As presented in Fig.
4, silencing RbAp48 significantly reduced mRNA levels of tumor
suppressors p53 (SiHa, P<0.001; HeLa, P<0.001; Fig. 4A) and Rb (SiHa, P<0.01; HeLa,
P<0.05; Fig. 4B) induced by
ALA-PDT treatment in cervical cancer cells compared with the
si-control treatments. Similar results were observed for
apoptosis-associated enzyme caspase-3 (SiHa, P<0.001; HeLa,
P<0.01; Fig. 4C). Notably,
siRNA knockdown of RbAp48 also significantly increased the E6
(SiHa, P<0.01; HeLa, P<0.001; Fig. 4D) and E7 (SiHa, P<0.01; HeLa,
P<0.05; Fig. 4E) levels, that
were reduced by PDT, which have important roles in HPV-induced
cervical cancer. Therefore, RbAp48-mediated sensitivity to ALA-PDT
treatment in cervical cancer cells may be due, at least partially,
to its regulation of the expression of tumor suppressors p53 and
Rb, apoptosis-associated enzyme caspase-3, and the HPV oncogenes,
E6 and E7.
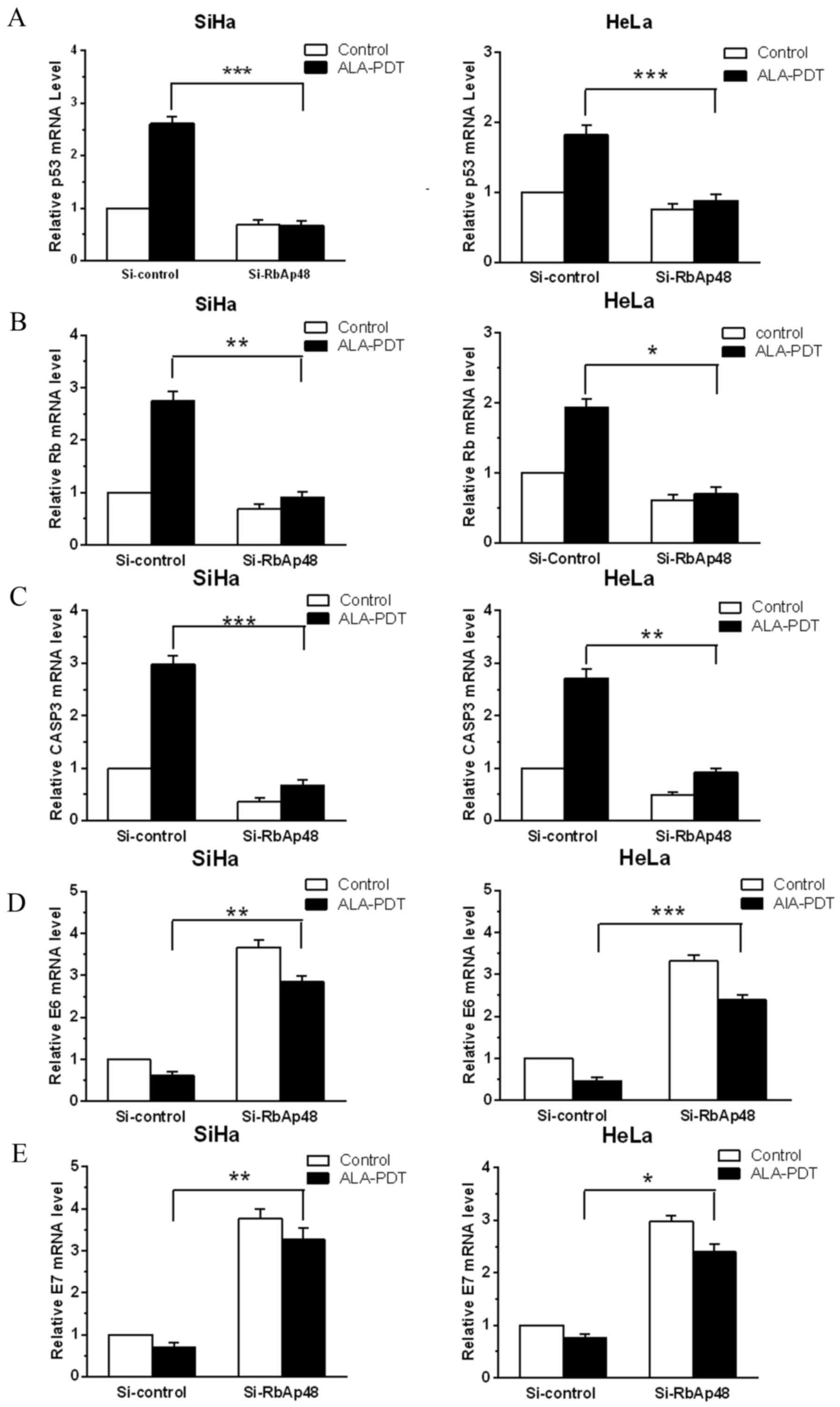 | Figure 4.siRNA knockdown of RbAp48 regulates
mRNA expression of p53, Rb, CASP3, E6 and E7 following ALA-PDT in
cervical cancer cells. mRNA expression of (A) p53, (B) Rb, (C)
CASP3, (D) E6 and (E) E7, in SiHa and HeLa cells with RbAp48
knockdown and/or ALA-PDT were assayed by reverse
transcription-quantitative polymerase chain reaction. Expression of
p53, Rb, CASP3, E6 and E7 in each sample was normalized to that of
β-actin. Normalized values were then calibrated against si-control
transfected values that were arbitrarily set as 1. Quantification
results are presented in the form of bar graphs. Data are presented
as the mean ± standard deviation from 3 independent experiments.
(*P<0.05; **P<0.01; ***P<0.001 vs. si-control group). p53,
tumor suppressor protein 53; ALA-PDT, 5-aminolevulinic
acid-mediated photodynamic therapy; Si, small interfering RNA;
RbAp48, Retinoblastoma-associated protein 48; Rb, retinoblastoma
protein; ALA, 5-aminolevulinic acid; CASP3, caspase-3. |
Discussion
PDT, which has curative effects and causes
remarkably little scarring, has been clinically approved and has
distinct advantages in its ability to locally treat cervical cancer
and cervical intraepithelial neoplasia (CIN; the precancerous
lesion of cervical cancer) (3,5,8,18).
Various clinical studies have revealed that PDT is of particular
value to women with CIN or early cervical cancer who are interested
in having children later in life (19,20).
Furthermore, PDT, as an ideal adjuvant therapy (4), may be applied prior to or following
chemotherapy or surgery and can be repeated as many times as
necessary without cumulative toxicity (10,21,22).
However, in clinical trials, tumor responses induced by PDT varied
between individual patients. In certain patients, a number of tumor
cells may become resistant to PDT and survive, therefore leading to
relapse and metastasis (3,5,18).
RbAp48, a potential therapeutic target in cervical cancer (14), is a radiation-inducible gene that
has the potential to increase the sensitivity of cervical cancer
cells to radiation (16). In the
present study, mRNA and protein levels of RbAp48 were upregulated
in two cervical cancer cell lines that had been subjected to
ALA-PDT. To further ascertain the function of RbAp48 in PDT-induced
cell destruction, targeted siRNA was used to reduce the expression
of RbAp48 in SiHa and HeLa cells. Subsequently, cells from both
cell lines were subjected to ALA-PDT and viability was examined.
Reduced expression of RbAp48 in cervical cancer cells suppressed
the decrease in cell viability usually induced by ALA-PDT, which
confirmed that RbAp48 is an important contributor to PDT-mediated
cell destruction.
Cancer is defined as abnormal cell growth
characterized by increased cell proliferation and decreased
apoptosis. With exposure to PDT, cells initiate complex responses,
including the formation of highly reactive singlet oxygen or other
free radicals like hydrogen peroxides or superoxide anions, which
can kill tumor cells directly by apoptosis and/or necrosis
(10,23). The finding that PDT may cause an
apoptotic response in target cells without complete disruption of
the tissue has provided an explanation for the ideal
characteristics required of any ablative technology for focally
treating early cervical cancer. Certain studies have illustrated
that apoptosis is critical for the therapeutic efficacy of PDT
(24,25) and reduced expression of RbAp48
inhibited apoptosis in exocrine gland cells (26). The current study investigated the
effect of reduced RbAp48 on apoptosis, induced by PDT, which was
assessed by flow cytometric analysis using the Annexin
V-fluorescein isothiocyanate/propidium iodide double staining
method. As presented in Fig. 3,
reduced expression of RbAp48 in cervical cancer cells decreased
PDT-induced apoptosis. It is therefore possible that PDT-mediated
induction of RbAp48, at least partially, contributes to subsequent
apoptosis of treated cells in addition to its inhibitory effect in
malignant transformation, and its ability to enhance the
radiosensitivity of cervical cancer cells. However, detailed
studies are required to strengthen this hypothesis.
It is clear that p53 is associated with several
types of human cancers and has great significance in cancer
therapy. Studies have demonstrated that p53 has an important role
in ALA-PDT-induced apoptosis in the HepG2 human liver cancer cell
line (27) and the sensitivity of
mutated p53 HT29 human colon adenocarcinoma cells to PDT can be
increased by introducing the wild-type p53 gene (28). Rb is capable of suppressing cancer
growth, partly by binding and inhibiting the activity of the E2F
family of transcription factors (29–31).
A previous study demonstrated that PDT increased the amount of
hypo-phosphorylated Rb protein and decreased the amount of
hyper-phosphorylated Rb protein in A431 cells, and suggested that
Rb-E2F was involved in PDT-induced cell cycle arrest and apoptosis
(32). The current study reported
that reduced RbAp48, combined with ALA-PDT, reduced mRNA levels of
p53 and Rb in cervical cancer cells, compared with PDT treatment
alone. Caspases are critical mediators of apoptosis (33). Reports have indicated that
caspase-3 is a death protease that is frequently activated during
PDT-induced apoptosis, and is part of the mitochondria-initiated
cytochrome c-mediated caspase-dependent pathway (34,35).
The present study demonstrated that RbAp48 altered the expression
of caspase-3 in cervical cancer cells in response to ALA-PDT.
It is well established that E6 and E7 are major
viral oncogenes that promote host cell proliferation and decreased
cell apoptosis, and increase genomic instability to facilitate the
migration of HPV-infected cells (36). E6 and E7 proteins form complexes
with p53 and Rb, respectively, which leads to dysregulation of cell
cycle control mechanisms and the release of transcription factor
E2F, which then activates the expression of cell cycle-associated
genes. Viral oncogenes E6 and E7 are essential for HPV-induced
cervical carcinogenesis and support the viral life cycle (37). As RbAp48 controls the transforming
activity of HPV-16 in cervical carcinogenesis by affecting mRNA
levels of E6 and E7, the present study conducted RT-qPCR to
investigate the effect of altered RbAp48 on E6 and E7 in cervical
cancer cells that had been subjected to PDT. The results indicate
that ALA-PDT effectively decreased mRNA levels of E6 and E7.
Silencing RbAp48 increased mRNA levels of E6 and E7 in cervical
cancer cells compared with cervical cancer cells subjected to
ALA-PDT alone. It is therefore possible that the regulation of p53
and Rb, caspase-3, and E6 and E7, by RbAp48 contributes, at least
in part, to the cell destruction caused by PDT.
In conclusion, the results of the present study
indicated that RbAp48 is involved in the anti-proliferative effects
and apoptosis induced by PDT in cervical cancer cells. As earlier
studies demonstrated that reduced expression of RbAp48 is specific
to cervical cancer, and considering the important role RbAp48 has
in regulating cervical carcinogenesis, measuring RbAp48 expression
may have the potential to predict the response that individual
cervical cancers will have to ALA-PDT. However, a detailed study to
investigate the involvement of RbAp48 in PDT-mediated cell
destruction, and the association with the expression of RbAp48 and
PDT-mediated therapeutic efficacy in cervical cancer and its
precancerous form, CIN, are required to valiadate these
findings.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81572559) and the
Foundation for Outstanding Young Scientist of Shandong Province
(grant no. BS2010YY056).
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, cancer incidence and mortality
worldwide: IARC CancerBase No. 11 [internet]. International Agency
for Research on Cancer Lyon: http://globocan.iarc.frAccessed. December.
2013
|
|
2
|
Li S, Hu T, Lv W, Zhou H, Li X, Yang R,
Jia Y, Huang K, Chen Z, Wang S, et al: Changes in prevalence and
clinical characteristics of cervical cancer in the People's
Republic of China: A study of 10,012 cases from a nationwide
working group. Oncologist. 18:1101–1107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shishkova N, Kuznetsova O and Berezov T:
Photodynamic therapy for gynecological diseases and breast cancer.
Cancer Biol Med. 9:9–17. 2012.PubMed/NCBI
|
|
4
|
Yu CH and Yu CC: Photodynamic therapy with
5-aminolevulinic acid (ALA) impairs tumor initiating and
chemo-resistance property in head and neck cancer-derived cancer
stem cells. PLoS One. 9:e871292014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trushina OI, Novikova EG, Sokolov VV,
Filonenko EV, Chissov VI and Vorozhtsov GN: Photodynamic therapy of
virus-associated precancer and early stages cancer of cervix uteri.
Photodiagnosis Photodyn Ther. 5:256–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi MC, Kim MS, Lee GH, Jung SG, Park H,
Joo WD, Lee C, Lee JH, Hwang YY and Kim SJ: Photodynamic therapy
for premalignant lesions of the vulva and vagina: A long-term
follow-up study. Lasers Surg Med. Jul 14–2015.(Epub ahead of
print). View Article : Google Scholar
|
|
7
|
Saini R and Poh CF: Photodynamic therapy:
A review and its prospective role in the management of oral
potentially malignant disorders. Oral Dis. 19:440–451. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soergel P and Hillemanns P: Photodynamic
therapy for intraepithelial neoplasia of the lower genital tract.
Photodiagnosis Photodyn Ther. 7:10–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dewaele M, Martinet W, Rubio N, Verfaillie
T, de Witte PA, Piette J and Agostinis P: Autophagy pathways
activated in response to PDT contribute to cell resistance against
ROS damage. J Cell Mol Med. 15:1402–1414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Postiglione I, Chiaviello A and Palumbo G:
Enhancing photodynamyc therapy efficacy by combination therapy:
Dated, current and oncoming strategies. Cancers (Basel).
3:2597–2629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demyanenko SV, Uzdensky AB, Sharifulina
SA, Lapteva TO and Polyakova LP: PDT-induced epigenetic changes in
the mouse cerebral cortex: A protein microarray study. Biochim
Biophys Acta. 1840:262–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nicolas E, Morales V, Magnaghi-Jaulin L,
Harel-Bellan A, Richard-Foy H and Trouche D: RbAp48 belongs to the
histone deacetylase complex that associates with the retinoblastoma
protein. J Biol Chem. 275:9797–9804. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Ng HH, Erdjument-Bromage H,
Tempst P, Bird A and Reinberg D: Analysis of the NuRD subunits
reveals a histone deacetylase core complex and a connection with
DNA methylation. Genes Dev. 13:1924–1935. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong L, Yu XP, Bai XH, Zhang WF, Zhang Y,
Zhao WM, Jia JH, Tang W, Zhou YB and Liu CJ: RbAp48 is a critical
mediator controlling the transforming activity of human
papillomavirus type 16 in cervical cancer. J Biol Chem.
282:26381–26391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Torres-Roca JF, Eschrich S, Zhao H, Bloom
G, Sung J, McCarthy S, Cantor AB, Scuto A, Li C, Zhang S, et al:
Prediction of radiation sensitivity using a gene expression
classifier. Cancer Res. 65:7169–7176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng L, Tang W, Wei F, Wang H, Liu J, Lu
Y, Cheng Y, Bai X, Yu X and Zhao W: Radiation-inducible protein
RbAp48 contributes to radiosensitivity of cervical cancer cells.
Gynecol Oncol. 130:601–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soergel P, Loehr-Schulz R, Hillemanns M,
Landwehr S, Makowski L and Hillemanns P: Effects of photodynamic
therapy using topical applied hexylaminolevulinate and
methylaminolevulinate upon the integrity of cervical epithelium.
Lasers Surg Med. 42:624–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi MC, Jung SG, Park H, Lee SY, Lee C,
Hwang YY and Kim SJ: Photodynamic therapy for management of
cervical intraepithelial neoplasia II and III in young patients and
obstetric outcomes. Lasers Surg Med. 45:564–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahn TG, Lee BR, Kim JK, Choi BC and Han
SJ: Successful full term pregnancy and delivery after concurrent
chemo-photodynamic therapy (CCPDT) for the uterine cervical cancer
staged 1B1 and 1B2: Preserving fertility in young women. Gynecol
Oncol Case Rep. 2:54–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jerjes W, Upile T, Betz CS, El Maaytah M,
Abbas S, Wright A and Hopper C: The application of photodynamic
therapy in the head and neck. Dent Update. 34:478–480, 483-484,
486. 2007.PubMed/NCBI
|
|
22
|
Hopper C: Photodynamic therapy: A clinical
reality in the treatment of cancer. Lancet Oncol. 1:212–219. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Douillard S, Rozec B, Bigot E, Aillet L
and Patrice T: Secondary reactive oxygen species production after
PDT during pulmonary tumor growth in sera of nude mice.
Photodiagnosis Photodyn Ther. 10:62–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gupta S, Dwarakanath BS, Muralidhar K and
Jain V: Role of apoptosis in photodynamic sensitivity of human
tumour cell lines. Indian J Exp Biol. 41:33–40. 2003.PubMed/NCBI
|
|
25
|
Ke MS, Xue LY, Feyes DK, Azizuddin K,
Baron ED, McCormick TS, Mukhtar H, Panneerselvam A, Schluchter MD,
Cooper KD, et al: Apoptosis mechanisms related to the increased
sensitivity of Jurkat T-cells vs A431 epidermoid cells to
photodynamic therapy with the phthalocyanine Pc 4. Photochem
Photobiol. 84:407–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishimaru N, Arakaki R, Omotehara F, Yamada
K, Mishima K, Saito I and Hayashi Y: Novel role for RbAp48 in
tissue-specific, estrogen deficiency-dependent apoptosis in the
exocrine glands. Mol Cell Biol. 26:2924–2935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yow CM, Wong CK, Huang Z and Ho RJ: Study
of the efficacy and mechanism of ALA-mediated photodynamic therapy
on human hepatocellular carcinoma cell. Liver Int. 27:201–208.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mikes J, Koval' J, Jendzelovský R, Sacková
V, Uhrinová I, Kello M, Kuliková L and Fedorocko P: The role of p53
in the efficiency of photodynamic therapy with hypericin and
subsequent long-term survival of colon cancer cells. Photochem
Photobiol Sci. 8:1558–1567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weintraub SJ, Chow KN, Luo RX, Zhang SH,
He S and Dean DC: Mechanism of active transcriptional repression by
the retinoblastoma protein. Nature. 375:812–815. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goodrich DW: The retinoblastoma
tumor-suppressor gene, the exception that proves the rule.
Oncogene. 25:5233–5243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nevins JR: The Rb/E2F pathway and cancer.
Hum Mol Genet. 10:699–703. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahmad N, Gupta S and Mukhtar H:
Involvement of retinoblastoma (Rb) and E2F transcription factors
during photodynamic therapy of human epidermoid carcinoma cells
A431. Oncogene. 18:1891–1896. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Furre IE, Møller MT, Shahzidi S, Nesland
JM and Peng Q: Involvement of both caspase-dependent and
-independent pathways in apoptotic induction by
hexaminolevulinate-mediated photodynamic therapy in human lymphoma
cells. Apoptosis. 11:2031–2042. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fukuhara H, Inoue K, Kurabayashi A,
Furihata M, Fujita H, Utsumi K, Sasaki J and Shuin T: The
inhibition of ferrochelatase enhances 5-aminolevulinic acid-based
photodynamic action for prostate cancer. Photodiagnosis Photodyn
Ther. 10:399–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Münger K, Phelps WC, Bubb V, Howley PM and
Schlegel R: The E6 and E7 genes of the human papillomavirus type 16
together are necessary and sufficient for transformation of primary
human keratinocytes. J Virol. 63:4417–4421. 1989.PubMed/NCBI
|
|
37
|
Govan VA: Strategies for human
papillomavirus therapeutic vaccines and other therapies based on
the E6 and E7 oncogenes. Ann N Y Acad Sci. 1056:328–343. 2005.
View Article : Google Scholar : PubMed/NCBI
|