Introduction
Cardiac hypertrophy and fibrosis are two important
pathological alterations that occur during the progressive
development of heart failure (1,2).
Cardiac fibrosis refers to the excessive accumulation of
extracellular matrix (ECM) and fibroblast deposition in the heart
(3). Cardiac fibrosis is important
in pathological cardiac remodeling and correlates with the
occurrence of several fatal heart diseases, including heart
failure, severe arrhythmia and sudden cardiac death (3,4). It
has previously been demonstrated that isoprenaline (ISO) is
important in cardiac fibrosis via increasing and/or activating
several effector molecules, including reactive oxygen species
(ROS), p38 mitogen-activated protein kinase (p38MAPK) and
extracellular signal-regulated kinase (ERK) (3,5).
Astragaloside IV (AsIV) is one of the major active
ingredients of the plant Astragalus membranaceus, which is
referred to as HuangQi in Chinese. As an important Qi-invigorating
medicinal herb, A. membranaceus is extensively used in
traditional Chinese medicine for the treatment of cardiovascular
diseases, hepatitis, and kidney and skin diseases (2,6,7).
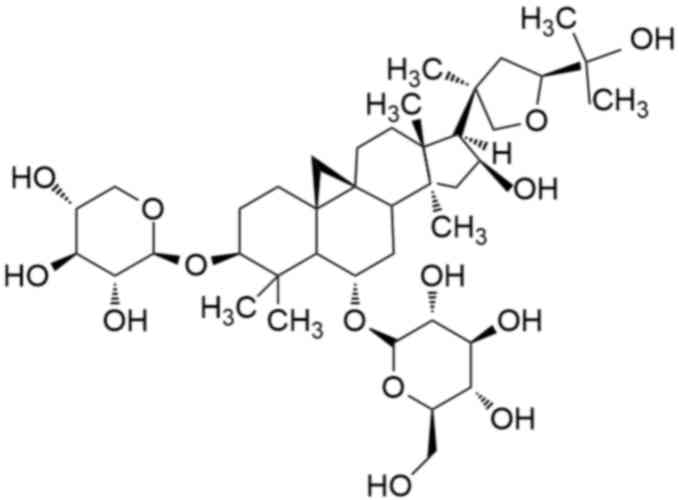
Chemically, AsIV is a cycloartane triterpene saponin with a
well-defined molecular formula, the chemical structure of which is
presented in Fig. 1. It has
previously been demonstrated that AsIV exhibits cardioprotective
effects via its antioxidative and anti-inflammatory properties
(8,9). In addition, AsIV has been reported to
improve cardiac function via the inhibition of cardiomyocyte
hypertrophy and apoptosis (6,8).
However, the function of AsIV in cardiac fibrosis remains to be
fully elucidated, with only one previous study, to the best of our
knowledge, currently available reporting its anti-fibrotic effect
in coxsackievirus-induced cardiomyopathy (10). Therefore, the present study aimed
to examine whether AsIV possesses antifibrotic activity under ISO
stimulation and aimed to elucidate the associated underlying
molecular mechanism.
Materials and methods
Animals
A total of 80 Sprague Dawley rat pups (age, 1–3
days; weight, 7±2 g, 40 male, 40 female), provided by the Animal
Center of Jinzhou Medical University, were used for the current
study. Rat pups, together with their mothers, were maintained at
standard conditions with temperature at 25±1°C, and relative
humidity at 70±10%. Animals were given free access to food and
water and maintained under a 12/12 h light/dark cycle. The
experiments were performed in accordance with the U.S. National
Institute of Health guidelines for the Use of Laboratory Animals
and ethical approval was granted by the Jinzhou Medical University
Committee on Animal Care.
Cardiac fibroblast (CF) culture
The rats were anesthetized by ether inhalation and
euthanized by cervical dislocation. Primary cultures of CFs were
prepared as previously described, following the method for culture
of cardiomyocytes (2), with the
exception that pre-attached CFs rather than cardiomyocytes were
collected for use. CFs were identified by their specific marker
vimentin. Dispersed CFs were cultured in Dulbecco's modified
Eagle's medium (DMEM; Corning Incorporated, Corning, NY, USA)
supplemented with 10% fetal bovine serum (GE Healthcare Life
Sciences, Logan, UT, USA) in a humidified atmosphere of 5%
CO2, at 37°C.
CF proliferation assay
CFs were digested with 0.25% trypsin and ethylene
diamine tetraacetic acid, then cultured in 96-well plates in the
presence and absence of 100 µM AsIV (>98% purity; Nanjing
Jingzhu Bio-Technology Co., Ltd., Nanjing, China) or the specific
inhibitor of JNK, SP600125 (10 µΜ, Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany) for 30 min prior to treatment with 10 µM ISO
(Sigma-Aldrich; Merck KGaA). Following incubation for 24 h in a
humidified atmosphere of 5% CO2, at 37°C, the
supernatant was removed, and 100 µl DMEM containing 10 µl Cell
Counting Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was added to each well for a further 4 h at 37°C. The
optical density was measured at a wavelength of 450 nm using an
automated ELISA plate reader (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
ELISA
The secretion of collagen type I by CFs was measured
using a commercial ELISA kit (catalog no. CSB-E 12732r; Cusabio
Biotech Co., Ltd., Wuhan, China) according to the manufacturer's
protocol.
Measurement of intracellular ROS
Dihydroethidium (DHE; Molecular Probes; Thermo
Fisher Scientific, Inc.) was used to determine intracellular ROS
levels in cultured CFs. Briefly, CFs were incubated with 10 µM DHE
for 30 min at 37°C in the dark. Cells were then washed 3 times with
phosphate-buffered saline (PBS) to remove unincorporated dye. CFs
loaded with DHE were then treated with 100 µM AsIV for 30 min,
followed by treatment with 10 µM ISO for an additional 30 min. ROS
levels were subsequently analyzed using an inverted fluorescence
microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Western blotting
Primary cultures of cardiac fibroblasts were
pretreated with 100 µM AsIV or 10 mM N-acetylcysteine (NAC,
Sigma-Aldrich; Merck KGaA) for 30 min, then treated with 10 µM ISO
for a further 24 h in a humidified atmosphere of 5% CO2
at 37°C. Cells were washed with cold PBS and harvested with freshly
prepared lysis buffer [150 mM NaCl, 50 mM Tris-HCl, pH 8.0, 0.1%
sodium dodecyl sulfate, 1% Triton X-100 and 1% proteinase
inhibitors (Sigma-Aldrich, Merck KGaA)]. Following quantification
of total protein by Lowry's method, equal amounts of protein (50
µg) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel
electrolysis and blotted onto a polyvinylidene fluoride membrane.
The membrane was blocked with 1% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) for 1 h and then incubated overnight at
4°C with the primary rabbit anti-rat polyclonal antibodies against
ERK (1:1,000; catalog no. sc-292838; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), phosphorylated (p)-ERK (1:1,000; catalog
no. sc-101760; Santa Cruz Biotechnology, Inc.), p38MAPK, (1:800;
catalog no. 21683; Signalway Antibody LLC, College Park, ML, USA),
p-p38MAPK (1:800; catalog no. 11581; Signalway Antibody LLC), c-Jun
N-terminal kinase (JNK; 1:800; catalog no. 21241; Signalway
Antibody LLC) and p-JNK, (1:800; catalog no. sc-135642; Santa Cruz
Biotechnology, Inc.). Following 2 h incubation at room temperature
with the polyclonal horseradish peroxidase-conjugated secondary
goat anti-rabbit antibody (1:2,500; catalog no. sc-2004; Santa Cruz
Biotechnology, Inc.), the bands were visualized by an enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.). Optical
density for each band was assessed using Quantity One software,
version 4.6.9 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean, from at least three independent experiments. One-way
analysis of variance followed by Fisher's least significant
difference test was used to compare groups by use of SPSS version
17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
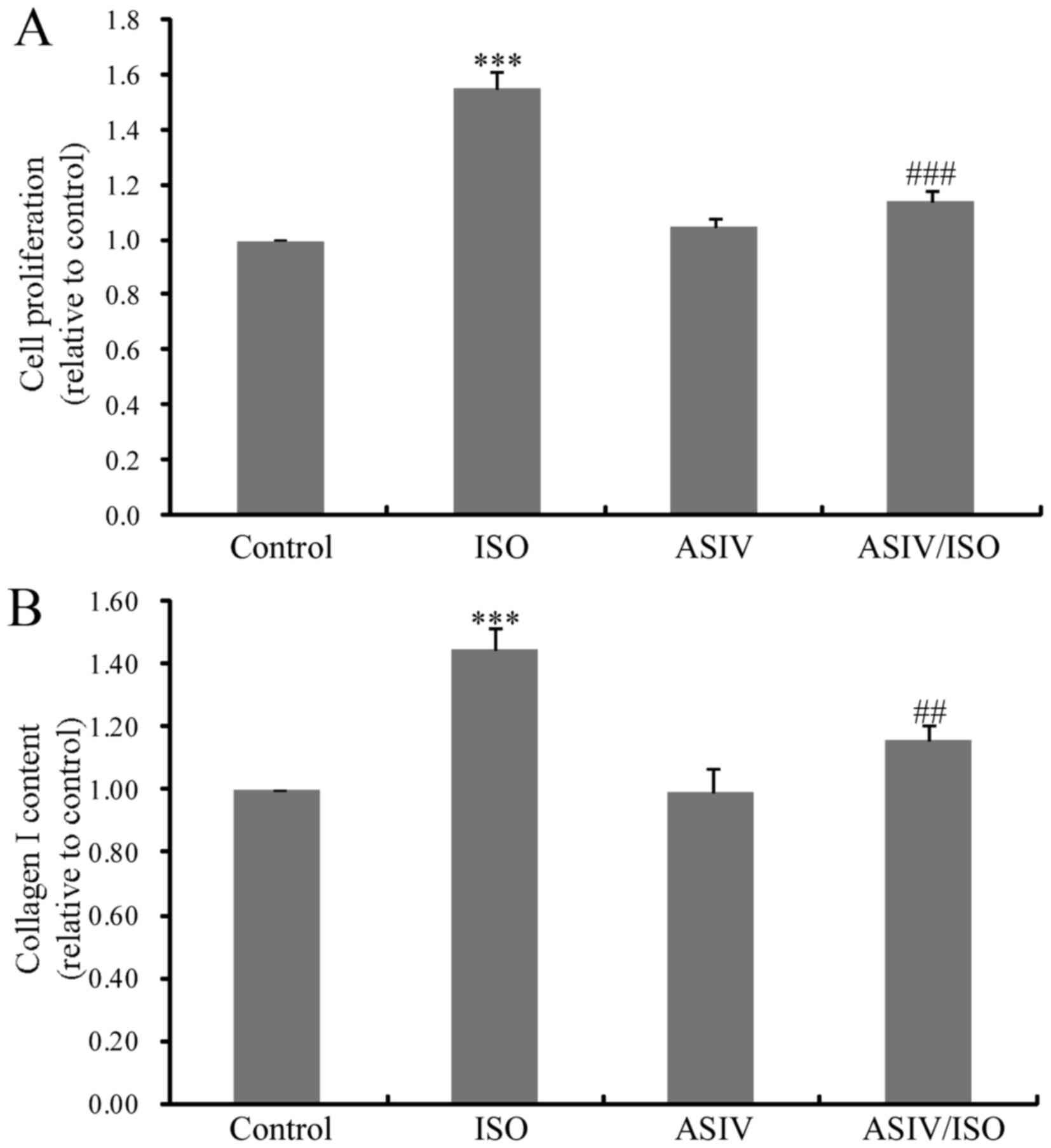
AsIV attenuates ISO-induced CF
proliferation and type I collagen synthesis
To evaluate the effects of AsIV on ISO-induced
cardiac fibrosis, CF proliferation and type I collagen levels were
examined. As presented in Fig. 2A,
CF proliferation was significantly increased upon ISO stimulation
for 24 h. Treatment with AsIV (100 µM) inhibited ISO-triggered CF
proliferation. Increased collagen synthesis in ISO-treated cultured
CFs was also significantly decreased with application of AsIV
(Fig. 2B).
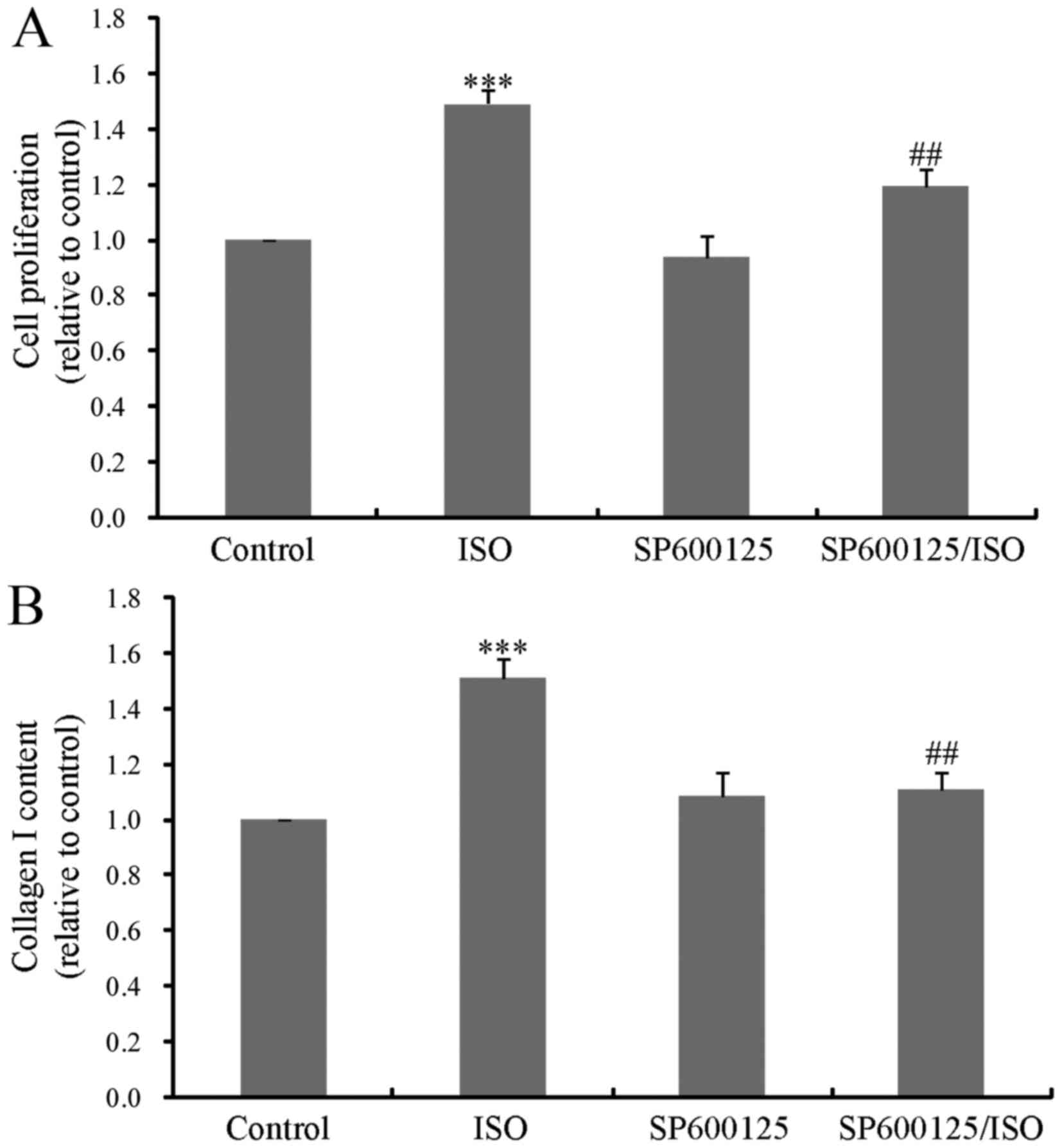
JNK mediates ISO-induced CF
proliferation and type I collagen synthesis
The MAPK signaling pathway is composed of ERK,
p38MAPK and JNK. Previous studies have demonstrated that ERK and
p38MAPK are required for β-adrenergic activation of CF
proliferation (3,5,11).
However, it remains to be elucidated whether JNK is involved in
this process. To investigate this, CF proliferation and type I
collagen synthesis were measured following 24 h treatment with ISO
in the absence or presence of the JNK inhibitor, SP600125. As
presented in Fig. 3A and B,
ISO-induced CF proliferation and collagen synthesis were
significantly decreased by pretreatment with SP600125, indicative
of a key role of JNK in ISO-induced cardiac fibrosis.
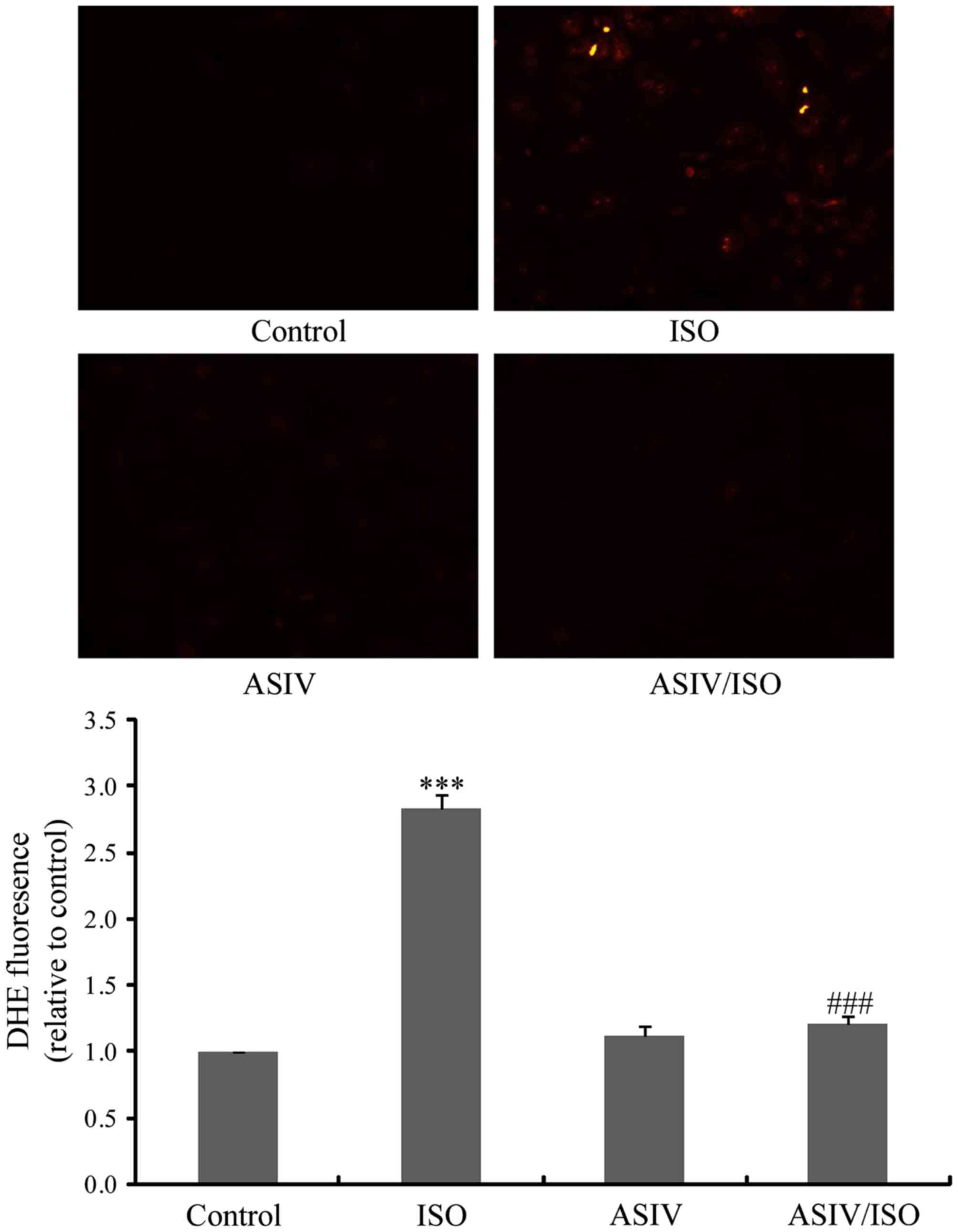
AsIV decreases ISO-induced ROS
generation in CFs
AsIV is a potent antioxidant (12,13).
Furthermore, ROS is important in ISO-induced cardiac fibrosis
(3,14). The present study therefore examined
the effects of AsIV on ROS production in CFs. As presented in
Fig. 4, ISO-induced ROS production
was effectively decreased by pretreatment with 100 µM AsIV.
AsIV and NAC attenuate ISO-induced
phosphorylation of MAPK family members
In view of the important role of MAPK molecules in
ISO-induced cardiac fibrosis, the present study investigated the
effects of AsIV on MAPK activation. As presented in Fig. 5, ISO activated the three MAPKs,
ERK, p38MAPK and JNK. Conversely, AsIV inhibited the activation of
these three MAPKs. In addition, NAC, which is a typical ROS
scavenger, exhibited similar inhibitory effects to those
demonstrated by AsIV on MAPK activation.
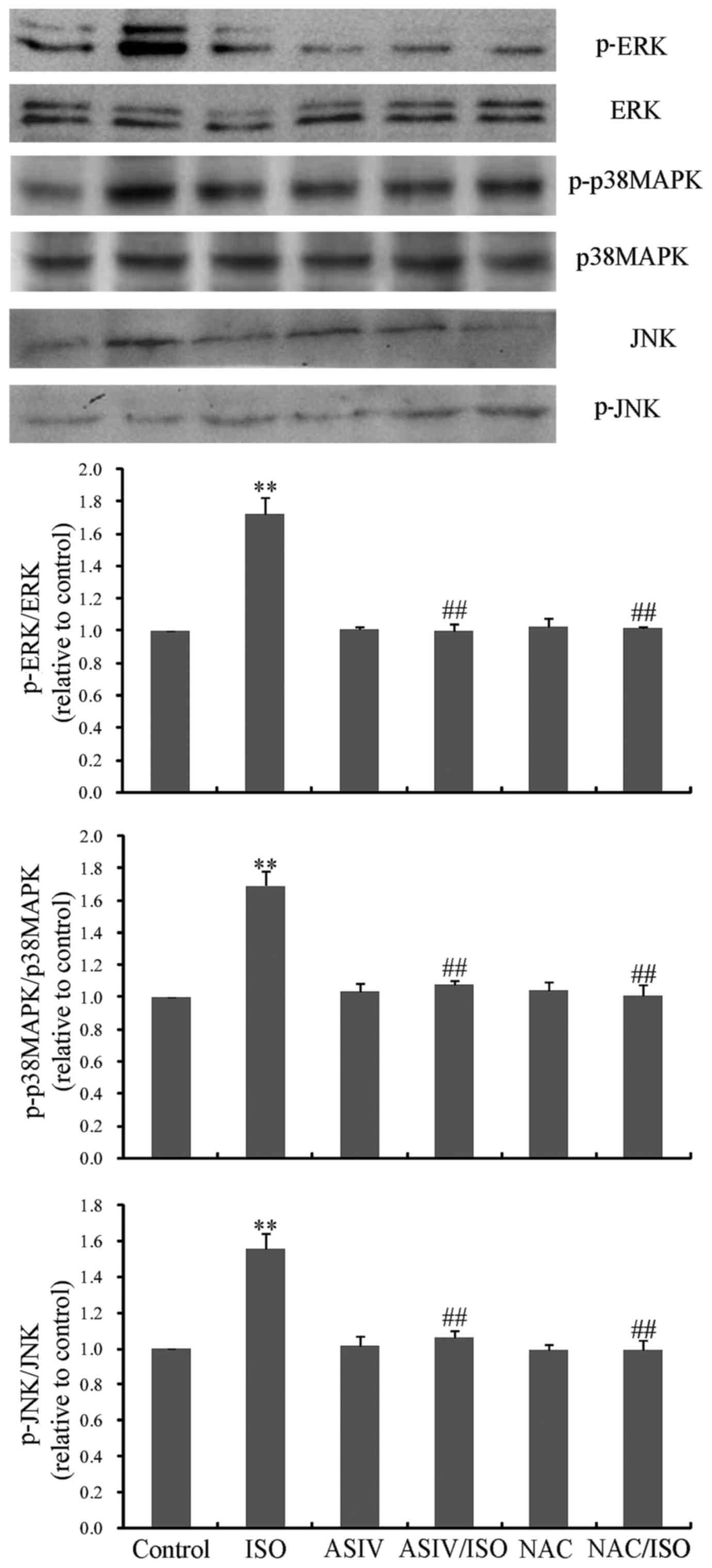 | Figure 5.Effects of AsIV and NAC on ISO-induced
activation of the MAPK family. Activation was measured by analyzing
the expression levels of phosphorylated proteins, as detected by
western blotting. A total of 100 µM AsIV was added to 10 µM
ISO-induced CFs to measure activation of ERK, p38MAPK and JNK MAPK
signaling pathways. This was repeated with the addition of 10 mM
NAC to 10 µM ISO-induced CFs. Data are expressed as the mean ±
standard error of the mean from three independent experiments.
**P<0.01 vs. control group; ##P<0.01 vs. ISO
group. ERK, extracellular signal-regulated kinase; p38MAPK, p38
mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; p,
phosphorylated; AsIV, astragaloside IV; CFs, cardiac fibroblasts;
NAC, N-acetylcysteine; ISO, isoprenaline. |
Discussion
The present study demonstrated that AsIV may protect
against ISO-induced CF proliferation and type I collagen synthesis.
The underlying mechanism involved inhibition of ROS production and
the resultant downregulation of MAPK signaling. To the best of our
knowledge, this study is the first to reveal the antifibrotic
effect, and associated mechanism, of AsIV in response to ISO
stimulation.
Cardiac fibrosis is characterized by enhanced CF
proliferation and excessive production and deposition of the ECM,
of which ~85% is composed of type I collagen (15,16).
This consequently results in myocardial stiffness, impaired
diastolic function and cardiac failure (17,18).
Antifibrotic mechanisms are therefore regarded as effective
therapeutic strategies for the treatment of various cardiovascular
diseases. It has previously been demonstrated that AsIV exerts
therapeutic effects on fibrosis in several disorders, including
chronic kidney disease (19),
systemic sclerosis (20), liver
fibrosis (21), and coxsackievirus
B3-induced cardiomyopathy (10).
However, its effect on β-adrenergic receptor-induced cardiac
fibrosis remains to be fully elucidated. The present study
therefore investigated the effects of AsIV on ISO-mediated cardiac
fibrosis and the associated signaling transduction. The results
demonstrated that pretreatment with AsIV significantly inhibited
ISO-induced CF proliferation and type I collagen synthesis, thus
suggesting that AsIV is a promising agent for the prevention and
treatment of cardiac fibrosis under increased sympathetic
drive.
Oxidative stress is important in the development and
progression of diverse cardiac disorders, including cardiac
fibrosis (14), hypertrophy
(22), apoptosis (23), inflammation (24) and resultant heart failure (25,26).
It has previously been demonstrated that ROS may modulate ECM
remodeling by mediating CF function and stimulating collagen
turnover (26). Antioxidative
mechanisms may prevent numerous adverse cardiovascular events,
including cardiac fibrosis. GKT137831 (14), oleanolic acid (27), 3,3′-diindolymethane (28) and magnolol (29), have been demonstrated to attenuate
cardiac fibrosis via antioxidative bioactivity. The antioxidative
capacity of AsIV has increasingly been identified by previous
studies (12,13). Data from the present study revealed
that application of AsIV effectively suppressed ROS accumulation.
It was therefore concluded that the antifibrotic effects of AsIV
may depend on its antioxidative activity.
One important consequence of oxidative stress is the
phosphorylation of MAPK proteins, including ERK, p38MAPK and JNK.
MAPK activation has been observed in cardiac fibrosis in response
to oxidative stress triggered by angiotensin II (30–33).
In addition, it has been suggested that ISO induces cardiac
fibrosis via activation of p38MAPK and ERK (3,5). To
elucidate the involvement of JNK in ISO-induced cardiac fibrosis,
the effect of SP600125, a selective inhibitor of JNK, was
investigated. The results indicated that SP600125 inhibited CF
proliferation and type I collagen synthesis, suggesting a similarly
indispensible role of JNK in ISO-triggered cardiac fibrosis, as
that observed of ERK and p38MAPK. Furthermore, data from the
present study revealed that the phosphorylation of the three
pro-fibrotic MAPK molecules was abrogated by application of AsIV.
In addition, the inhibitory effects of AsIV on MAPK signaling were
mimicked by the selective ROS inhibitor, NAC; thus, indicating that
AsIV inhibits MAPK signaling in a ROS-dependent manner.
In conclusion, the present study demonstrated that
AsIV effectively inhibited ISO-induced CF proliferation and type I
collagen synthesis via attenuation of ROS-mediated MAPK signaling
activation. These findings may aid to further elucidate the
protective role and underlying molecular mechanism of AsIV in the
cardiovascular system.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81374008), the
Natural Science Foundation of Liaoning Province (grant no.
2015020360) and the President Foundation, Aohongboze Foundation of
Liaoning Medical University (grant no. XZJJ20140111). The authors
would like to thank Dr Guannan Wang (Jinzhou Medical University,
Jinzhou, China) for his assistance in drawing the chemical
structure of AsIV.
References
|
1
|
Yu LM and Xu Y: Epigenetic regulation in
cardiac fibrosis. World J Cardiol. 7:784–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai H, Jia G, Liu X, Liu Z and Wang H:
Astragalus polysaccharide inhibits isoprenaline-induced cardiac
hypertrophy via suppressing Ca2+-mediated calcineurin/NFATc3 and
CaMKII signaling cascades. Environ Toxicol Pharmacol. 38:263–271.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu H, Tian A, Wu J, Yang C, Xing R, Jia P,
Yang L, Zhang Y, Zheng X and Li Z: Danshensu inhibits β-adrenergic
receptors-mediated cardiac fibrosis by ROS/p38 MAPK axis. Biol
Pharm Bull. 37:961–967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morita N, Mandel WJ, Kobayashi Y and
Karagueuzian HS: Cardiac fibrosis as a determinant of ventricular
tachyarrhythmias. J Arrhythm. 30:389–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim J, Eckhart AD, Eguchi S and Koch WJ:
Beta-adrenergic receptor-mediated DNA synthesis in cardiac
fibroblasts is dependent on transactivation of the epidermal growth
factor receptor and subsequent activation of extracellular
signal-regulated kinases. J Biol Chem. 277:32116–32123. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Wang HX, Zhang YJ, Yang YH, Lu ML,
Zhang J, Li ST, Zhang SP and Li G: Astragaloside IV attenuates
inflammatory cytokines by inhibiting TLR4/NF-кB signaling pathway
in isoproterenol-induced myocardial hypertrophy. J Ethnopharmacol.
150:1062–1070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang X, Cao X, Huang Y, Chen J, Yao X,
Zhao M, Liu Y, Meng J, Li P, Li Z, et al: Effects of treatment with
Astragalus Membranaceus on function of rat leydig cells. BMC
Complement Altern Med. 15:2612015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mei M, Tang F, Lu M, He X, Wang H, Hou X,
Hu J, Xu C and Han R: Astragaloside IV attenuates apoptosis of
hypertrophic cardiomyocyte through inhibiting oxidative stress and
calpain-1 activation. Environ Toxicol Pharmacol. 40:764–773. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao P, Wang Y, Zeng S, Lu J, Jiang TM and
Li YM: Protective effect of astragaloside IV on
lipopolysaccharide-induced cardiac dysfunction via downregulation
of inflammatory signaling in mice. Immunopharmacol Immunotoxicol.
37:428–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen P, Xie Y, Shen E, Li GG, Yu Y, Zhang
CB, Yang Y, Zou Y, Ge J, Chen R and Chen H: Astragaloside IV
attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in
coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol.
658:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu N, Xing R, Yang C, Tian A, Lv Z, Sun
N, Gao X, Zhang Y and Li Z: HIP-55/DBNL-dependent regulation of
adrenergic receptor mediates the ERK1/2 proliferative pathway. Mol
Biosyst. 10:1932–1939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Y, Li S, Wu H, Bian Z, Xu J, Gu C, Chen
X and Yang D: Beneficial effects of astragaloside IV against
angiotensin II-induced mitochondrial dysfunction in rat vascular
smooth muscle cells. Int J Mol Med. 36:1223–1232. 2015.PubMed/NCBI
|
|
13
|
Wang SG, Xu Y, Xie H, Wang W and Chen XH:
Astragaloside IV prevents lipopolysaccharide-induced injury in H9C2
cardiomyocytes. Chin J Nat Med. 13:127–132. 2015.PubMed/NCBI
|
|
14
|
Somanna NK, Valente AJ, Krenz M, Fay WP,
Delafontaine P and Chandrasekar B: The Nox1/4 dual inhibitor
GKT137831 or Nox4 knockdown Inhibits Angiotensin-II-Induced adult
mouse cardiac fibroblast proliferation and migration. AT1
physically associates with Nox4. J Cell Physiol. 231:1130–1141.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porter KE and Turner NA: Cardiac
fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther.
123:255–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ye Y, Lv X, Wang MH, Zhu J, Chen SQ, Jiang
CY and Fu GS: Alendronate prevents angiotensin II-induced collagen
I production through geranylgeranylation-dependent RhoA/Rho kinase
activation in cardiac fibroblasts. J Pharmacol Sci. 129:205–209.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Segura AM, Frazier OH and Buja LM:
Fibrosis and heart failure. Heart Fail Rev. 19:173–185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tao H, Yang JJ, Shi KH, Deng ZY and Li J:
DNA methylation in cardiac fibrosis: New advances and perspectives.
Toxicology. 323:125–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Che X, Wang Q, Xie Y, Xu W, Shao X, Mou S
and Ni Z: Astragaloside IV suppresses transforming growth factor-β1
induced fibrosis of cultured mouse renal fibroblasts via inhibition
of the MAPK and NF-B signaling pathways. Biochem Biophys Res
Commun. 464:1260–1266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi Q, Mao Y, Yi J, Li D, Zhu K and Cha X:
Anti-fibrotic effects of Astragaloside IV in systemic sclerosis.
Cell Physiol Biochem. 34:2105–2116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Wang X, Han C, Wang X, Xing G, Zhou
L, Li G and Niu Y: Astragaloside IV suppresses collagen production
of activated hepatic stellate cells via oxidative stress-mediated
p38 MAPK pathway. Free Radic Biol Med. 60:168–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang C, Wang F, Zhang Y, Kang Y, Wang H,
Si M, Su L, Xin X, Xue F, Hao F, et al: Celecoxib prevents pressure
overload-induced cardiac hypertrophy and dysfunction by inhibiting
inflammation, apoptosis and oxidative stress. J Cell Mol Med.
20:116–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu B, Zhang J, Liu W, Liu N, Fu X, Kwan H
and Liu S, Liu B, Zhang S, Yu Z and Liu S: Calycosin inhibits
oxidative stress-induced cardiomyocyte apoptosis via activating
estrogen receptor-α/β. Bioorg Med Chem Lett. 26:181–185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kayama Y, Raaz U, Jagger A, Adam M,
Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM and Tsao
PS: Diabetic cardiovascular disease induced by oxidative stress.
Int J Mol Sci. 16:25234–25263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fournier P, Fourcade J, Roncalli J,
Salvayre R, Galinier M and Causse E: Homocysteine in chronic heart
failure. Clin Lab. 61:1137–1145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murdoch CE, Zhang M, Cave AC and Shah AM:
NADPH oxidase-dependent redox signalling in cardiac hypertrophy,
remodelling and failure. Cardiovasc Res. 71:208–215. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao HH, Zhang N, Feng H, Zhang N, Ma ZG,
Yang Z, Yuan Y, Bian ZY and Tang QZ: Oleanolic acid alleviated
pressure overload-induced cardiac remodeling. Mol Cell Biochem.
409:145–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao Z, Hu W, Yin S, Huang Z, Zhu Q, Chen
J, Zang Y, Dong L and Zhang J: 3,3′-Diindolymethane ameliorates
adriamycin-induced cardiac fibrosis via activation of a
BRCA1-dependent anti-oxidant pathway. Pharmacol Res. 70:139–146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liou JY, Chen YL, Loh SH, Chen PY, Hong
CY, Chen JJ, Cheng TH and Liu JC: Magnolol depresses
urotensin-II-induced cell proliferation in rat cardiac fibroblasts.
Clin Exp Pharmacol Physiol. 36:711–716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chan P, Liu JC, Lin LJ, Chen PY, Cheng TH,
Lin JG and Hong HJ: Tanshinone IIA inhibits angiotensin II-induced
cell proliferation in rat cardiac fibroblasts. Am J Chin Med.
39:381–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang W, Chen XF, Huang YJ, Chen QQ, Bao
YJ and Zhu W: 2,3,4′, 5-Tetrahydroxystilbene-2-O-β-D-glucoside
inhibits angiotensin II-induced cardiac fibroblast proliferation
via suppression of the reactive oxygen species-extracellular
signal-regulated kinase 1/2 pathway. Clin Exp Pharmacol Physiol.
39:429–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu M, Zheng Y, Sun HX and Yu DJ:
Inhibitory effects of enalaprilat on rat cardiac fibroblast
proliferation via ROS/P38MAPK/TGF-β1 signaling pathway. Molecules.
17:2738–2751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shyu KG, Wang BW, Chen WJ, Kuan P and Lin
CM: Angiotensin II mediates urotensin II expression by hypoxia in
cultured cardiac fibroblast. Eur J Clin Invest. 42:17–26. 2012.
View Article : Google Scholar : PubMed/NCBI
|