Introduction
Alzheimer's disease (AD) is one of the most common
chronic neurodegenerative disorders. The pathology of AD is
associated with the formation of amyloid-β (Aβ) plaques and the
hyperphosphorylation of tau proteins, which may lead to the
formation of intracellular neurofibrillary tangles (1). Postoperative cognition dysfunction
(POCD) may occur partly due to Aβ accumulation and tau
hyperphosphorylation induced by volatile anesthetics (2).
Insulin-like growth factor (IGF)-1 protects neurons
against the toxic effects of Aβ (3). Circulating IGF-1 may traverse the
blood-brain barrier (4) to
increase Aβ clearance from the brain and reduce tau phosphorylation
via inhibiting glycogen synthase kinase 3β (GSK3β) (5–7).
Previous population-based studies involving elderly individuals
have revealed that low circulating IGF-1 levels contribute to
cognitive decline (8,9). A previous study demonstrated that
circulating IGF-1 levels were reduced in patients anesthetized with
sevoflurane, which negatively correlated with POCD (10).
Halogenated inhalational anesthetics, including
halothane, enflurane, isoflurane, methoxyflurane, desflurane and
sevoflurane enter and primarily leave the body through the
respiratory system. However, due to the lipophilic properties of
the drugs, they may be absorbed and require cellular metabolism for
detoxification and excretion (11,12).
This process occurs primarily in the liver; therefore, volatile
anesthetics may disproportionately affect hepatic function
following surgery (13).
The aim of the present study was to investigate the
effect of sevoflurane exposure on the production of IGF-1 in the
liver, which is the primary site of IGF-1 production (14,15).
Sevoflurane was selected for investigation in the current study, as
it is currently the most frequently used inhalational
anesthetic.
Materials and methods
Cell line and reagents
The BRL rat hepatocyte cell line was obtained from
the Institute of Biomedical Sciences, Fudan University (Shanghai,
China). BRL hepatocytes were cultured in Dulbecco's modified
Eagle's medium (HyClone; GE Healthcare Bio-Sciences, Logan, UT,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and antibiotic-antimycotic
mixture containing 100 U/ml penicillin and 100 mg/ml streptomycin
(Cellgro; Corning, Inc., Corning, NY, USA). Cells were maintained
at 5% CO2 and 37°C and subcultured to a density of
1×105/ml every 2 days. All experiments were performed
using cells in the logarithmic phase of growth. Sevoflurane was
purchased from Shanghai Hengrui Pharmaceutical Co., Ltd. (Shanghai,
China) ThemicroRNA-98 inhibitor and inhibitor control were
purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Animals
A total of10 C57BL/6J mice in each group (age, 18
months; weight, 23.9–40.1 g; female: Male, 1:1) were provided by
the Laboratory Animal Center of Shanghai Tenth People's Hospital
(Shanghai, China). The housing and treatment of the animals were in
accordance with institutional guidelines and were approved by the
Institutional Animal Care and Use Committee of Shanghai Ninth
People's Hospital (Shanghai, China): Room temperature 22–26°C, 12-h
light dark cycle with free access to food and water. Estimates of
sample size were based on previous reports (16,17).
A sample size of 10 should enable the detection of >15%
difference with 80% power and 5% significance level, with an
assumed standard deviation of 12.5% (18). Therefore, the present study used a
sample size of 10 mice.
Treatment of BRL hepatocytes and adult
mice with sevoflurane
The minimal alveolar concentration (MAC), which
leads 50% patients to anesthesia, was used to assess the efficacy
of inhalational anesthetics. Previous studies have employed
concentrations of between 1.5 and 4.1%, with an exposure time of
between 2 and 6 h (16,18–22).
In the present study, hepatocytes and mice were treated with 1MAC
or 2MAC sevoflurane (1 MAC=1.5% sevoflurane in adult mice) for 4 h
or 8 h prior to quantification of IGF-1 expression level.
Cultured hepatocytes were treated with sevoflurane
as previously described (2).
CO2, O2 and sevoflurane levels were
continuously monitored. O2 (21%), 5% CO2 and
1MAC or 2MAC sevoflurane were delivered using an anesthesia machine
(Avance CS2; GE Healthcare Life Sciences, Chalfont, UK) to BRL
hepatocytes cultured in 6-well plates in a sealed plastic box at
37°C. Cells were seeded at a density of 1×106 cells/well
and cultured in 1 ml cell culture media. Control BRL hepatocytes
were exposed to 5% CO2 plus 21% O2. The
effect of microRNA-98 on sevoflurane-mediated regulation of IGF-1
inhibition was investigated using 100 nM microRNA-98 inhibitor
according to the manufacturer's protocol (RiboBio Co., Ltd.). Cells
were treated with the same concentration of the inhibitor or
inhibitor control 48 h prior to sevoflurane exposure.
The anesthesia of adult mice with sevoflurane was
performed as previously described (23). Briefly, the adult mice were exposed
to 1MAC or 2MAC anesthetics and 100% oxygen delivered by an
anesthesia machine in individual, environmentally controlled
chambers.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Mice were sacrificed by decapitation immediately
following sevoflurane anesthesia. The liver was removed rapidly and
frozen in liquid nitrogen for subsequent processing. Total RNA from
treated cells (2×106) or liver samples was isolated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) as previously
described (24). A total of 1 µg
RNA was reverse transcribed. cDNA was synthesized using random
hexamer primers (Invitrogen; Thermo Fisher Scientific, Inc.) and
the SuperScript II reverse transcriptase enzyme (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Sequences are as follows: IGF-1-F, CTG GAC CAG AGA CCC TTT GC;
IGF-1-R, GGA CGG GGA CTT CTG AGT CTT; GAP DH-F, AGG TCG GTG TGA ACG
GAT TTG; GAP DH-R, TGT AGA CCA TGT AGT TGA GGT CA. The thermal
cycling parameters are as follows: 95°C for 30 sec, 95°C for 5 sec
for 40 cycles, 60°C for 30–34 sec. microRNAs were prepared using a
microRNA extraction kit (Tiangen Biotech Co., Ltd., Beijing,
China). The expression of microRNA-98 was normalized to that of U6
small nuclear RNA. The expression level of IGF-1 was normalized to
that of GAPDH. The data were analyzed using MxPro-Mx3000P software
(version, 4.00; Stratagene; Agilent Technologies, Inc., Santa
Clara, CA, USA) and the 2-ΔΔCq method (25).
Enzyme-linked immunosorbent assay
(ELISA)
IGF-1 levels in the culture medium were quantified
using a rat IGF-1 enzyme immunoassay kit (cat. no.: FRK0032; Assay
Designs, Inc., Ann Arbor, MI, USA) at an absorbance of 450 nm. The
IGF-1 level (ng/ml) was calculated according to the manufacturer's
protocol.
Blood samples were collected from the tail vein of
mice prior to and following exposure to sevoflurane. A total of 500
µl blood was centrifuged at 4,000 × g for 20 min at 4°C, and
the serum samples were stored at −80°C until required for
downstream analysis. Serum IGF-1 concentrations were quantified
using IGF-1 ELISA kit (cat. no. MG-100; R&D Systems Europe,
Ltd., Abingdon, UK) at an absorbance of 450 nm.
Immunoblot analysis
A total of 2×106 BRL hepatocytes and liver tissues
were harvested at the end of each experiment and were placed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) on ice. Homogenates were then
centrifuged at 12,000 × g for 15 min at 4°C, and the protein
concentrations were quantified using a bicinchoninic acid assay kit
(Pierce; Thermo Fisher Scientific, Inc.). A total of 20 µg protein
for each sample were separated using 12% SDS-PAGE gels and
transferred to polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% non-fat
milk for 1.5 hat room temperature, before they were incubated
overnight at 4°C with anti-β-actin (cat. no. ab3280; dilution,
1:1,000; Abcam, Cambridge, UK) and anti-IGF-1 primary antibodies
(cat. no. ab9572; dilution, 1:1,000; Abcam, Cambridge, UK). The
immune complexes were detected by incubating of the membranes in a
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (IgG; cat. no. 33101 ES60; dilution, 1:1,000; Shanghai
Yeasen Biological Technology Co., Ltd., Shanghai, China) for 1 h at
room temperature. Labeled protein bands were detected by enhanced
chemiluminescence (EMD Millipore).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version, 5.0; GraphPad Software, Inc., La Jolla,
CA, USA). Data are expressed as mean ± standard deviation.
Statistical significance was determined using unpaired Student's
t-tests to compare a sevoflurane exposure group with the control
group, or to compare the two groups with different doses of
sevoflurane. P<0.05 was considered to indicate a statistically
significant difference.
Results
Sevoflurane downregulates IGF-1 and
upregulates microRNA-98 in BRL hepatocytes
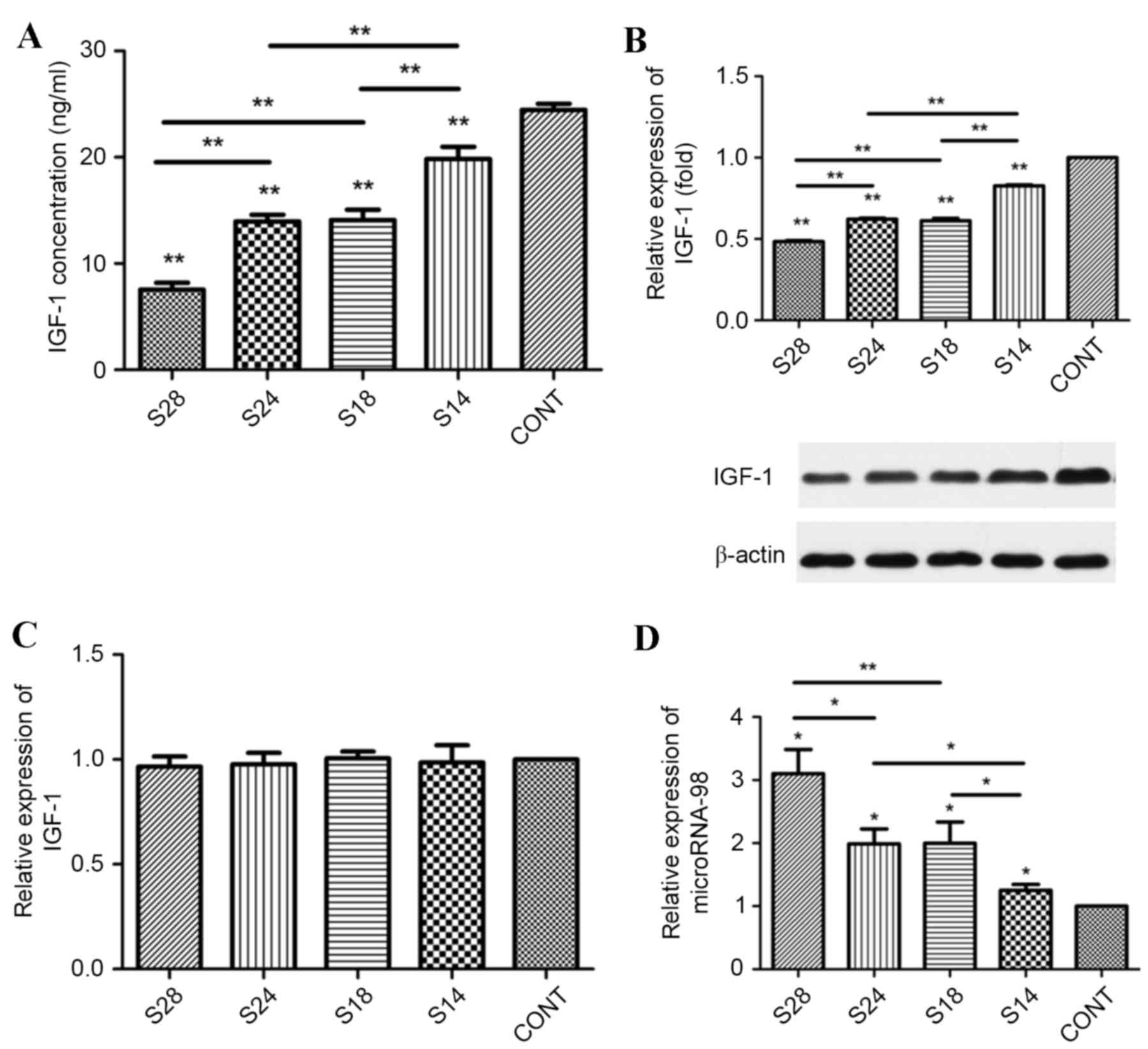
IGF-1 levels were quantified following sevoflurane
exposure in BRL hepatocytes. ELISA analysis revealed that
sevoflurane significantly downregulated IGF-1 protein levels in BRL
hepatocytes in a dose- and time-dependent manner when compared with
untreated controls (Fig. 1A).
Western blot analysis confirmed these results (Fig. 1B). No significant alterations in in
IGF-1 mRNA levels were observed in sevoflurane-treated BRL
hepatocytes when compared with untreated controls, as determined by
RT-qPCR analysis (Fig. 1C). These
results suggest that sevoflurane may regulate IGF-1 expression in
BRL hepatocytes at a post-transcriptional level. Analysis of
microRNA-98 expression in BRL hepatocytes revealed that sevoflurane
exposure significantly increased microRNA-98 expression in a time-
and dose-dependent manner when compared with untreated controls
(Fig. 1D).
Sevoflurane downregulates circulating
IGF-1 levels in adult mice
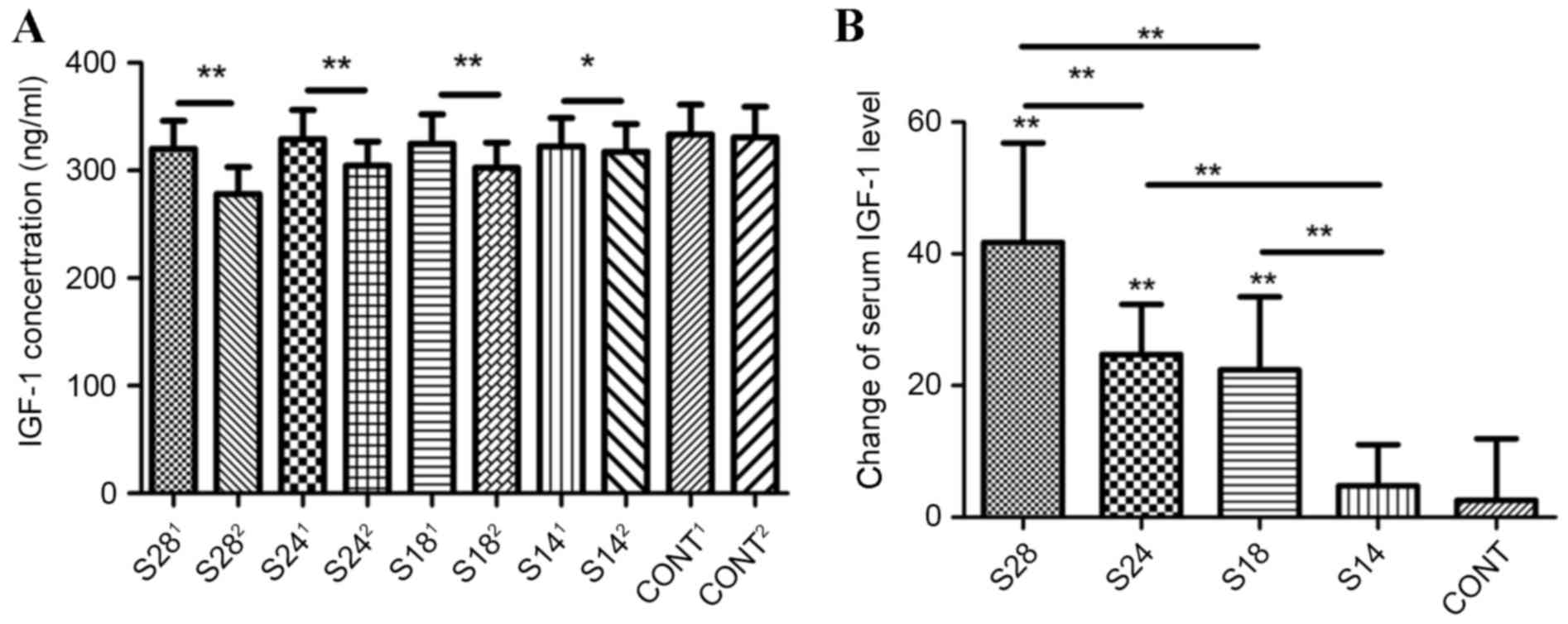
To investigate the effect of sevoflurane on
circulating IGF-1 levels in adult mice, the serum IGF-1
concentration was quantified using ELISA. It was determined that,
administration of sevoflurane significantly reduced serum IGF-1
levels when compared with the same mice prior to treatment
(Fig. 2A). This effect was dose-
and time-dependent (Fig. 2B).
Sevoflurane treatment reduces IGF-1
and increases microRNA-98 expression in the liver of adult
mice
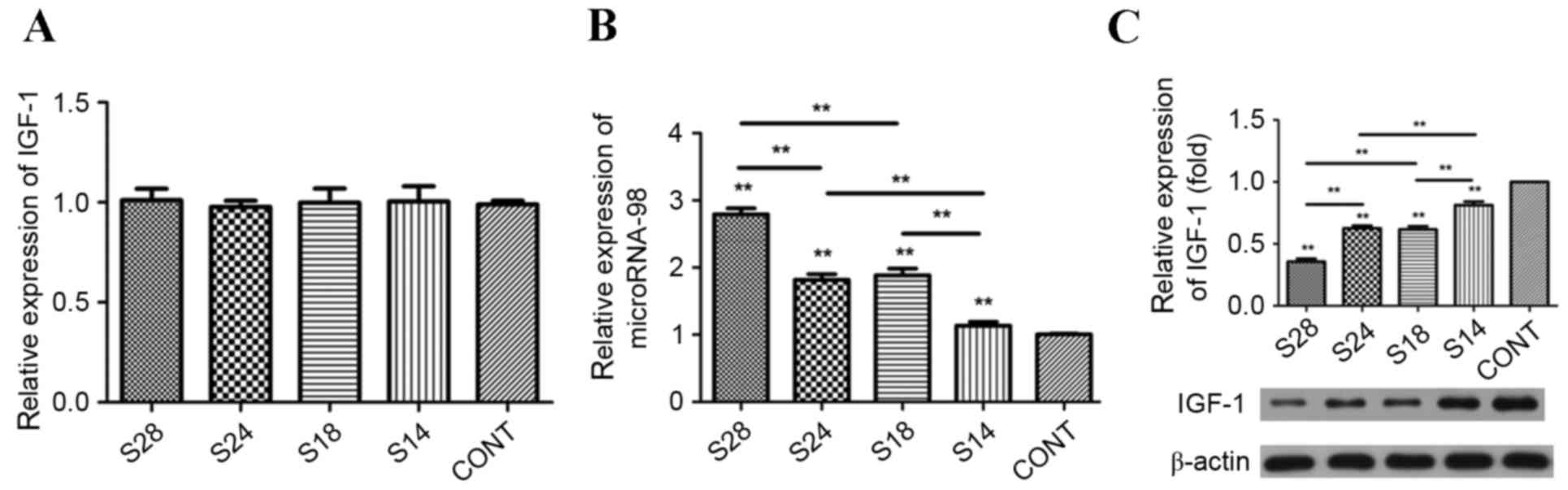
As the majority of serum IGF-1 is produced in the
liver (14,15), the levels of IGF-1 protein in liver
samples were quantified using RT-qPCR and western blot analyses in
the present study. No significant differences in IGF-1 mRNA
expression levels in mouse liver tissues were identified among the
sevoflurane treatment groups (Fig.
3A). However, it was evident that the expression level of
microRNA-98 in the liver of adult mice was significantly increased
in sevoflurane-treated groups in a dose- and time-dependent manner
(P<0.01; Fig. 3B). Similar to
the mRNA expression levels, exposure to sevoflurane significantly
reduced the protein expression levels of IGF-1 in liver tissues
derived from sevoflurane-treated mice when compared with untreated
controls (P<0.01; Fig. 3C).
Sevoflurane inhibits IGF-1 translation
by upregulating of microRNA-98 expression
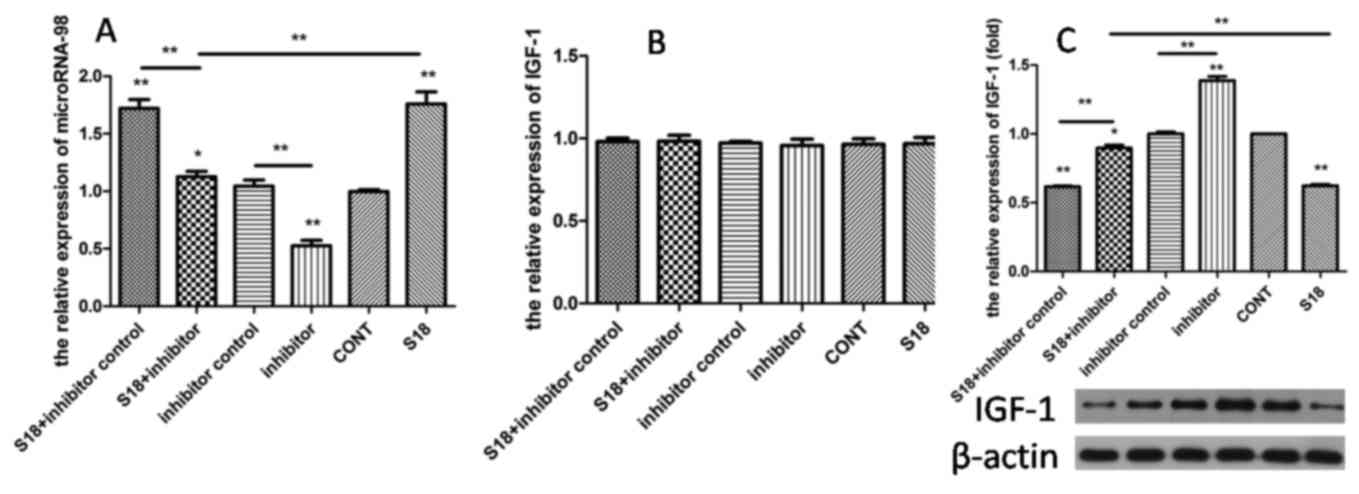
In order to determine whether sevoflurane inhibited
IGF-1 translation by upregulating microRNA-98, BRL hepatocytes were
pretreated with 100 nM microRNA-98 inhibitor and inhibitor control
at48 h prior to exposure to sevoflurane. The microRNA-98 inhibitor
significantly reduced microRNA-98 expression when compared with
those treated with the microRNA-98 inhibitor control (P<0.01;
Fig. 4A). No significant
alterations in IGF-1 mRNA expression levels among all treatment
groups were observed (Fig. 4B).
However, microRNA-98 inhibitor treatment significantly increased
the IGF-1 protein expression levels when compared with the
sevoflurane-only treated group (P<0.01; Fig. 4C).
Discussion
Sevoflurane may affect spatial memory by increasing
the level of Aβ (26) and tau
phosphorylation (22). IGF-1 has
been demonstrated to protect neurons against the toxic effects of
Aβ (3) and reduce tau
phosphorylation by inhibiting GSK3β (5,6). In
addition, low concentrations of circulating IGF-1 may contribute to
cognitive decline (8,9). This suggests that sevoflurane may
impair cognition by reducing the level of circulating IGF-1. To
investigate this further, the present study quantified IGF-1
expression levels in rat BRL hepatocytes and mice following
sevoflurane treatment. Hu et al (27) combined the use of PicTar, miRBase
and TargetScan software programs and determined that one binding
site of microRNA-98 was located within the 3′-untranslated region
(UTR) of IGF-1. Therefore, IGF-1 may be a direct target of
microRNA-98, thereby inhibiting IGF-1 expression at a
post-transcriptional level. As a result, the present study selected
microRNA-98, in order to investigate the mechanisms underlying the
effects of sevoflurane exposure on IGF-1 production. The results of
current study demonstrated that sevoflurane reduced IGF-1 protein
levels, whereas IGF-1 mRNA levels were unaffected. This was
accompanied by an upregulation of microRNA-98 levels. Inhibition of
microRNA-98 reduced the effect of sevoflurane exposure on IGF-1
protein expression levels, which suggests that sevoflurane may
regulate IGF-1 expression at the translational level via
microRNA-98.
microRNAs are non-coding RNAs of ~22 nucleotides,
and are widely expressed in eukaryotic cells. microRNAs regulate
gene expression by repressing translation or by base-pairing with
the 3′-UTR of target mRNAs to induce degradation (28). Sevoflurane may influence
microRNA-98 expression and has been demonstrated to ameliorate
endotoxin-induced acute lung injury by inhibiting the expression of
microRNAs that regulate inflammation (29), increasing the expression of
rno-microRNA-339-3p, rno-microRNA-448 and rno-microRNA-466b-1FNx01
(30). Additionally, the
upregulation of microRNA-101a and microRNA-34b has been
demonstrated to be important for neuroprotection against
sevoflurane (31). Furthermore,
microRNA-1, microRNA-17, microRNA-133 and microRNA-205, associated
with the protein kinase B/GSK/cyclin D1 signaling pathway, were
significantly downregulated by sevoflurane in the liver (32).
The present study determined that sevoflurane
significantly reduced IGF-1 expression and upregulated microRNA-98
expression in the liver, which is in agreement with a previous
study that revealed that IGF-1 was a target of microRNA-98
(27). In addition, the expression
patterns of IGF-1 and microRNA-98 in response to sevoflurane
treatment were consistent with a previous report (27). Inhibition of microRNA-98 reduced
the effect of sevoflurane on IGF-1 protein expression, suggesting
that sevoflurane regulated IGF-1 production by enhancing
microRNA-98 expression.
Acknowledgements
The authors would like to thank Professor Xiaoping
Zhang and Professor Zhongwei Lv (Shanghai Tenth People's Hospital,
Shanghai, China) for providing the instruments used in the present
study. The current study received funding from the Shanghai
Municipal Commission of Health and Family Planning (grant no.
201540104).
References
|
1
|
Iqbal K, Liu F, Gong CX and Grundke-Iqbal
I: Tau in Alzheimer disease and related tauopathies. Curr Alzheimer
Res. 7:656–664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niikura T, Hashimoto Y, Okamoto T, Abe Y,
Yasukawa T, Kawasumi M, Hiraki T, Kita Y, Terashita K, Kouyama K
and Nishimoto I: Insulin-like growth factor I (IGF-I) protects
cells from apoptosis by Alzheimer's V6421 mutant amyloid precursor
protein through IGF-I receptor in an IGF-binding protein-sensitive
manner. J Neurosci. 21:1902–1910. 2001.PubMed/NCBI
|
|
3
|
Reinhardt RR and Bondy CA: Insulin-like
growth factors cross the blood-brain barrier. Endocrinology.
135:1753–1761. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong M and Lee VM: Insulin and
insulin-like growth factor-1 regulate tau phosphorylation in
cultured human neurons. J Biol Chem. 272:19547–19553. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vargas T, Martinez-Garcia A, Antequera D,
Vilella E, Clarimon J, Mateo I, Sanchez-Juan P, Rodriguez-Rodriguez
E, Frank A, Rosich-Estrago M, et al: IGF-I gene variability is
associated with an increased risk for AD. Neurobiol Aging.
32:556.e3–e11. 2011. View Article : Google Scholar
|
|
6
|
Carro E, Trejo JL, Spuch C, Bohl D, Heard
JM and Torres-Aleman I: Blockade of the insulin-like growth factor
I receptor in the choroid plexus originates Alzheimer'slike
neuropathology in rodents: New cues into the human disease?
Neurobiol Aging. 27:1618–1631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen E, Paulsson JF, Blinder P,
Burstyn-Cohen T, Du D, Estepa G, Adame A, Pham HM, Holzenberger M,
Kelly JW, et al: Reduced IGF-1 signaling delays age-associated
proteotoxicity in mice. Cell. 139:1157–1169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuningas M, Mooijaart SP, van Heemst D,
Zwaan BJ, Slagboom PE and Westendorp RG: Genes encoding longevity:
From model organisms to humans. Aging Cell. 7:270–280. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang J, Chen Z, Liang B, Yan J, Zhang Y,
Xu H, Huang Y and Jiang H: The change of circulating insulin like
growth factor binding protein 7 levels may correlate with
postoperative cognitive dysfunction. Neurosci Lett. 588:125–130.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishiyama T, Yokoyama T and Hanaoka K:
Liver function after sevoflurane or isoflurane anaesthesia in
neurosurgical patients. Can J Anaesth. 45:753–756. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malhotra P, Mychaskiw G and Rai A:
Desflurane versus opioid anesthesia for cardiac shunt procedures in
infants with cyantoic congential heart disease. Anesth Pain Med.
3:191–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dabir S, Mohammad-Taheri Z, Parsa T,
Abbasi-Nazari M, Radpay B and Radmand G: Effects of propofol versus
isoflurane on liver function after open thoracotomy. Asian
Cardiovasc Thorac Ann. 23:292–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sjögren K, Liu J, Blad K, Skrtic S, Vidal
O, Wallenius V, LeRoith D, Törnell J, Isaksson OG, Jansson JO and
Ohlsson C: Liver-derived insulin-like growth factor I (IGF-I) is
the principle source of IGF-I in blood but is not required for
postnatal body growth in mice. Proc Natl Acad Sci USA.
96:7088–7092. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yakar S, Liu J, Stannard B, Butler A,
Accili D, Sauer B and LeRoith D: Normal growth and development in
the absence of hepatic insulin-like growth factor I. Proc Natl Acad
Sci USA. 96:7324–7329. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Callaway JK, Jones NC, Royse AG and Royse
CF: Sevoflurane anesthesia does not impair acquisition learning or
memory in the Morris water maze in young adult and aged rats.
Anesthesiology. 117:1091–1101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Callaway JK, Jones NC, Royse AG and Royse
CF: Memory Impairment in Rats after desflurane anesthesia is age
and dose dependent. J Alzheimers Dis. 44:995–1005. 2015.PubMed/NCBI
|
|
17
|
Peng S, Zhang Y, Sun D, Zhang D, Fang Q
and Li G: The effect of sevoflurane anesthesia on cognitive
function and the expression of Insulin-like Growth Factor-1 in CA1
region of hippocampus in old rats. Mol Biol Rep. 38:1195–1199.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu Y, Wu X, Dong Y, Xu Z, Zhang Y and Xie
Z: Anesthetic sevoflurane causes neurotoxicity differently in
neonatal naive and Alzheimer disease transgenic mice.
Anesthesiology. 112:1404–1416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye X, Lian Q, Eckenhoff MF, Eckenhoff R
and Pan JZ: Differential general anesthetic effects on microglial
cytokine expression. PLoS One. 8:e528872013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Gao M, Ma L, Zhang L and Pan N:
Sevoflurane alters the expression of receptors and enzymes involved
in Aβ clearance in rats. Acta Anaesthesiol Scand. 57:903–910. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le Freche H, Brouillette J,
Fernandez-Gomez FJ, Patin P, Caillierez R, Zommer N, Sergeant N,
Buée-Scherrer V, Lebuffe G, Blum D and Buée L: Tau phosphorylation
and sevoflurane anesthesia: An association to postoperative
cognitive impairment. Anesthesiology. 116:779–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie Z, Dong Y, Maeda U, Alfille P, Culley
DJ, Crosby G and Tanzi RE: The common inhalation anesthetic
isoflurane induces apoptosis and increases amyloid beta protein
levels. Anesthesiology. 104:988–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Y, Chen J, Pruckmayr G, Baumgardner
JE, Eckmann DM, Eckenhoff RG and Kelz MB: High throughput modular
chambers for rapid evaluation of anesthetic sensitivity. BMC
Anesthesiol. 6:132006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma Y, Peng J, Liu W, Zhang P, Huang L, Gao
B, Shen T, Zhou Y, Chen H, Chu Z, et al: Proteomics identification
of desmin as a potential oncofetal diagnostic and prognostic
biomarker in colorectal Cancer. Mol Cell Proteomics. 8:1878–1890.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong Y, Zhang G, Zhang B, Moir RD, Xia W,
Marcantonio ER, Culley DJ, Crosby G, Tanzi RE and Xie Z: The common
inhalational anesthetic sevoflurane induces apoptosis and increases
beta-amyloid protein levels. Arch Neurol. 66:620–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu YK, Wang X, Li L, Du YH, Ye HT and Li
CY: MicroRNA-98 induces an Alzheimer's disease-like disturbance by
targeting insulin-like growth factor 1. Neurosci Bull. 29:745–751.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McNeill E and VanVactor D: MicroRNAs shape
the neuronal landscape. Neuron. 75:363–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Otsuki T, Ishikawa M, Hori Y, Goto G and
Sakamoto A: Volatile anesthetic sevoflurane ameliorates
endotoxin-induced acute lung injury via microRNA modulation in
rats. Biomed Rep. 3:408–412. 2015.PubMed/NCBI
|
|
30
|
Lu Y, Jian MY, Ouyang YB and Han RQ:
Changes in rat brain microRNA expression profiles following
sevoflurane and propofol anesthesia. Chin Med J (Engl).
128:1510–1515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Li Y, Liu L, Wang Y, Xia Y, Zhang L
and Ji X: Identification of miRNAs Involved in the Protective
effect of sevoflurane preconditioning against hypoxic injury in
PC12 Cells. Cell Mol Neurobiol. 35:1117–1125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morita T, Ishikawa M and Sakamoto A:
Identical microRNAs regulate liver protection during anaesthetic
and ischemic preconditioning in rats: An animal study. PLoS One.
14:e01258662015. View Article : Google Scholar
|