Introduction
Thyroid cancer is one of the more common endocrine
malignancies with a rapidly rising incidence in recent years
(1–3). Histologically it consists mainly of
anaplastic thyroid cancer (ATC), papillary thyroid cancer (PTC),
and follicular thyroid cancer (FTC) (4). Almost all thyroid cancers are derived
from follicular cells that comprise the simple unicellular
epithelium of normal thyroid. Follicular thyroid cell-derived
tumours include PTC and FTC, poorly differentiated thyroid cancer
(PDTC) and undifferentiated ATC, whereas parafollicular C
cell-derived medullary thyroid cancer accounts only for a small
proportion (2 to 3%) of cases (5).
PTC and FTC are classified as differentiated thyroid cancers (DTCs)
which may be cured with surgery or radioiodine treatment. The
10-year survival rate for PTC and FTC is >90%, while PDTC and
undifferentiated ATC have poor prognoses (6). Approximately 5 to 23% of DTC patients
develop distant metastases, which are the main cause of mortality
(7). The appropriate extent of
surgery for thyroid cancer is controversial: Certain researchers
recommend partial, and others total, thyroidectomy; others advocate
prophylactic central cervical lymph node dissection, while only
rarely do researchers suggest lymphadenectomy (8–10).
Although radioactive iodine is effective, the appropriate use and
dosage remain controversial (11,12).
Recently, molecular analysis of thyroid cancer has been usually
applied for diagnostic purposes, involving preoperative fine-needle
biopsy specimens, as well as to define targetable pathways altered
in the disease to guide the clinical trials of drug therapy
(13,14).
Hormones promote cell proliferation and augment
random genomic mutation, thus increasing the opportunity for
tumorigenicity (15). In fact,
there is an obvious gender disparity in the occurrence of DTC, with
females having three times the incidence of DTC than males
(16,17). Previously, researchers have
demonstrated that thyroid cancer cells express higher levels of
estrogen receptors (ERs) and progesterone receptors (PRs) compared
with normal cells (18). Two ERs,
ERα and ERβ, have different biological functions (19). In thyroid cancer cells, ERα
expression is enhanced; however, ERβ expression is low or absent
(11). An ERα agonist has been
revealed to enhance the proliferation of thyroid cancer cells,
while an ERβ agonist did not (16,20).
Therefore, an ERα-mediated signalling pathway may be critical for
the proliferation of thyroid cells. The lemur tyrosine kinases
(LMTKs) belong to a family of transmembrane serine/threonine
tyrosine kinases, and have been shown to be localized in
cytoplasmic membrane vesicles and involved in endosomal trafficking
(21). Accumulating evidence
indicates an important role for LMTK3 in various types of cancer.
RNA interference screening has identified LMTK3 as a potential
therapeutic target in colon cancer and leukemia cells (22,23).
In breast cancer, LMTK3 isoform knockdown repressed ERα activity,
while an LMTK1/2 isoform knockdown did not. Additionally, LMTK3 was
identified to interact with ERα in vivo and phosphorylation
of ERα by LMTK3 was revealed to protect ERα from proteosomal
degradation (24).
Similarly to other cancers, thyroid cancer
initiation and progression is mediated through the accumulation of
multiple genetic and epigenetic alterations of critical molecules
and signalling pathways (25).
Identification of the altered molecular makers is crucial for the
diagnosis and treatment of thyroid cancer. LMTK3 has been
recognized as a potential biomarker or a prognostic marker for
various malignancies, including breast cancer, gastric cancer and
colorectal cancer (26–28). However, the clinical significance
of LMTK3 and its association with thyroid cancer has yet to be
identified. In the present study, LMTK3 expression in thyroid
cancer was examined and its associated clinical significance was
explored.
Materials and methods
Cell culture
The human thyroid carcinoma cell line SW579 was
purchased from the American Type Culture Collection (American Type
Culture Collection, Manassas, VA, USA). SW579 was cultured in
RPMI-1640 (Gibco Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (FBS; HyClone™,
Logan, UT, USA). Cells were kept at 37°C in a humidified incubator
containing 5% CO2.
Patients and serum
The serum specimens were obtained from patients at
the Fourth Hospital of Harbin Medical University (Harbin,
Heilongjiang, China) who had not undergone surgery. All serum
specimens were derived from 106 thyroid carcinoma patients (26 male
and 80 female; age range: 25 to 72 years; average age: 48.26±14.67
years) and 52 benign thyroid tumor patients. Patients who had
undergone any form of pre-operative chemotherapy and/or radiation
therapy were excluded. None of the patients enrolled in this study
suffered from any other type of cancer. The clinical and
pathological features are presented in Table I. A total of 52 benign thyroid
tumor patients and 50 healthy volunteers were enrolled. A serum
separator tube was used to isolate serum. Blood samples were
allowed to clot for 2 h at room temperature before centrifugation
for 15 min at 1,000 × g. Thereafter, serum was collected and
immediately placed at −80°C to avoid protein or mRNA degradation.
All procedures were approved by the ethics committee of the Fourth
Hospital of Harbin Medical University (Heilongjiang Province,
China).
 | Table I.Clinical and histopathological
characteristics in patients with thyroid cancer. |
Table I.
Clinical and histopathological
characteristics in patients with thyroid cancer.
| Characteristic | Whole (n=106) | Male (n=26) | Female (n=80) | Male vs. female
(P-value) |
|---|
| Age (years) | 48.67±2.26 |
|
| 0.019a |
| ≥50 | 37 (34.9) | 14 (53.8) | 23 (28.8) |
|
|
<50 | 69 (65.1) | 12 (46.2) | 57 (71.2) |
|
| Tumor size (mean ±
SEM) | 9.5±0.9 | 10.2±0.6 | 9.1±1.3 | 0.465 |
| Degree of
differentiation | 4/34/64 | 1/8/15 | 3/26/49 | 0.997 |
|
(poor/intermediate/good) | (3.9/33.3/62.7%) | (4.2/33.3/62.5%) | (3.8/33.3/62.8%) |
|
| Capsule
invasion | 62 (58.5%) | 13 (0.5%) | 49 (61.5%) | 0.315 |
| Vascular
invasion | 26 (24.5%) | 3 (2.9%) | 21 (26.9%) | 0.076 |
| Stages |
|
|
| 0.727 |
|
I+II | 89 (83.9%) | 22 (84.6%) | 67 (83.8%) |
|
|
III+IV | 17 (16.1 %) | 4 (15.4%) | 13 (16.2%) |
|
| Pathological
types |
|
|
| 0.141 |
|
Papillary cancer | 68 (64.1%) | 17 (65.4%) | 51 (63.8%) |
|
|
Follicular cancer | 23 (21.7%) | 6 (23%) | 17 (21.2%) |
|
|
Medullary cancer | 12 (11.3%) | 3 (11.5%) | 9 (11.3%) |
|
|
Undifferentiated Cancer | 3 (2.8%) | 2(7.6%) | 1 (1.3%) |
|
ELISA assay for LMTK3
The level of LMTK3 was measured using a human LMTK3
ELISA kit (MyBioSource, Inc., San Diego, CA, USA) according to the
manufacturer's protocol. Briefly, whole blood samples (100 µl) were
added to high-binding polystyrene plates coated with capture
monoclonal antibody for LMTK3. Immobilized antigen was detected
with diluted biotinylated secondary antibody (dilution, 1:100),
followed by horseradish peroxidase-conjugated streptavidin. For
calibration, recombinant LMTK3 protein and two control standards
were performed in parallel with the tested samples on each
plate.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections 4
µm thick were chosen for immunohistochemical staining. Anti-LMTK3
human monoclonal antibody was purchased from Abcam (Cambridge, UK;
cat. no. ab137260; dilution, 1:1,000). The tissue sections were
dewaxed in xylene and then hydrated in a series of graded alcohols.
Specimens were heated in 10 mM sodium citrate buffer (pH 6.0) and
subsequently EDTA (pH 8.0), prepared for LMTK3, at 100°C for 5 min
to expose the antigens. The specimens were then washed with PBS (pH
7.4) and incubated with 3% H2O2 at 37°C for
15 min to eliminate endogenous peroxidase activity, and 5% bovine
serum albumin for 30 min to reduce non-specific binding. The slides
were kept overnight at 4°C with primary antibodies (LMTK3 antibody
with a dilution of 1:200). Following washing, the specimens were
incubated with peroxidase-labeled polymer conjugated to goat
anti-human LMTK3 (dilution, 1:4,000; cat. no. A0201; Beyotime
Institute of Biotechnology, Haimen, China) in Tris-HCl buffer at
room temperature for 30 min. Signals were visualized with
diaminobenzidine and the slides were counterstained with
hematoxylin. For negative controls, the primary antibody was
substituted with PBS.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
First, the cells were incubated with antibiotic-free
medium for 24 h prior to transfection. For the LMTK3 knockdown, the
cells were transfected with siRNA against LMTK3 using Lipofectamine
2000 (Invitrogen: Thermo Fisher Scientific, Inc.). Transfection
complexes were added to the medium at final oligonucleotide
concentration of 50 nM. Total RNA samples from human tissues were
isolated using Trizol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturer's protocol. Total RNA
(1 µg) was reverse-transcribed using High-Capacity cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) to obtain complementary DNA (cDNA). The SYBR Green PCR Master
Mix kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) was
applied in RT-qPCR to quantify the level of LMTK3, with GAPDH as an
internal control. The RT-qPCR was performed on a 7500 FAST
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) for 40 cycles. The primers were designed as follows, The
primers for GAPDH: Sense primer: 5′-AAGAAGGTGGTGAAGCAGGC-3′,
antisense primer: 5′-TCCACCACCCAGTTGCTGTA-3′. The primers for
LMTK3: sense primer: 5′- TCGGCTTCAAGGAATTTGAGA-3′, antisense
primer: 5′-GGGTGGTCATGTCTGAGTGTGA-3′.
Small interfering RNA (siRNA) and
transfection
The target sequence GGAAUUUGAGAACCCUGAATT, for mouse
LMTK3 was purchased from Shanghai GenePharma Co., Ltd. (Suzhou,
China). As a control, LMTK3 siRNA negative control (NC) was also
used: UUCUCCGAACGUGUCACGUTT. The cells were transfected with siRNAs
using Lipofectamine reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol and kept for a
further 48 h prior to being used in the subsequent experiments.
Cell proliferation assay
SW579 cells were seeded into 96-well plates and
treated with saline, LMTK3 siRNA or NC. The serum-free medium was
removed and the cells were cultured with regular culture medium for
a further 48 h. To monitor cell survival, SW579 cells were
incubated for 4 h with 0.5 mg/ml MTT (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany), and dissolved in 150 µl
dimethylsulfoxide (DMSO; Sigma-Aldrich; Merck Millipore).
Absorbance was recorded at 490 nm using an Easy Reader 340 AT (SLT
Labinstruments GmbH, Crailsheim, Germany). Results are presented as
the percentage of survival, taking the control as 100% survival.
Experiments were repeated six times.
Terminal dUTP nick end labeling
(TUNEL) assay
Apoptotic SW579 cells in different groups were
detected using a TUNEL assay as previously described (29). Air-dried slides were fixed with 4%
paraformaldehyde for 30 min at room temperature, washed three times
with PBS, and then permeabilized with 1% Triton X-100 for 4 min at
4°C. Subsequently, each slide was removed to a terminal
deoxynucleotidyl transferase (TdT) -labeled nucleotide mix and kept
at 37°C for 60 min in the dark. Slides were rinsed twice with PBS
and then counterstained with 10 mg/ml 4,6-diamidino-2-phenylindole
for 5 min at 37°C.
Cell cycle analysis
The effect of LMTK3 siRNA on cell cycle distribution
was measured by flow cytometric analysis of the DNA content of cell
nuclei following staining with propidium iodide (PI; Sigma-Aldrich;
Merck Millipore). SW579 cells were seeded into 60-mm flasks, and
allowed to attach overnight. The cells were rinsed with PBS and
fixed in 75% ethanol overnight at 4°C. The cells were then treated
with 80 mg/ml RNaseA (Sigma-Aldrich; Merck Millipore) and 50 mg/ml
PI for 30 min, and analyzed using a Coulter Epics XL Flow Cytometer
(Beckman Coulter, Miami, FL, USA).
Cell migration and invasion
assays
Transwell chambers with a pore size of 8 mm (Corning
Costar, Inc., Corning, NY, USA) were used for cell migration and
invasion assay. Cells were brought to 60% confluency and
transfected with the siRNA for 48 h. For migration assays, cells
were digested with 0.25% trypsin (Beyotime Institute of
Biotechnology), resuspended in serum-free medium and placed in the
upper chamber. As a chemoattractant, the lower chamber contained
10% FBS. Cells were cultured at 37°C in 5% CO2 for 24 h,
and non-migrating cells were removed with a cotton swab. Migrated
cells were washed twice with PBS, fixed in 100% methanol and
stained with hematoxylin. Stained cells were viewed under a
microscope (magnification, ×200), and the number of migrated cells
was counted in five random fields. For invasion assays, the upper
chamber was precoated with Matrigel mixed with serum-free medium
(diluted at 1:3; BD Biosciences, San Jose, CA, USA). Following
solidification of the mixture, 5×105 cells in serum-free
medium were placed into the upper chamber. The lower chamber
contained 10% FBS as a chemoattractant. Cells were cultivated at
37°C in a humidified incubator containing 5% CO2 for 24
h, and non-invading cells were removed with a cotton swab. Invasive
cells were fixed, stained and counted. Stained cells were viewed
under a microscope (magnification, ×200), and the number of
migrated cells was counted in five random fields. Assays were
performed in three independent experiments.
Statistical analysis
All quantitative data are expressed as the mean ±
standard error of the mean and analysed using SPSS software,
version 13.0 (SPSS Inc., Chicago, IL, USA). Two-tailed unpaired
Student's t-test and one-way analysis of variance were used for
statistical evaluation of the data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Histopathological characteristics of
thyroid cancer
Histological diagnoses and tumor features were
derived from 106 thyroid cancer patients (52 with benign tumors and
54 with malignant tumors) and 50 healthy volunteers (Table I). In the whole group (thyroid
cancer patients only, n=106), the mean tumor size was 9.5±0.9 mm
and the average age was 48.67±2.26 years. Male and female subgroup
features were compared.
Serum LMTK3 level was markedly
increased in thyroid cancer patients
It is known that LMTK3 could become a potential
therapeutic target in multiple tumors. Therefore, an ELISA assay
was performed to confirm whether LMTK3 was involved in the
pathological process in thyroid cancer. Serum LMTK3 was derived
from 106 thyroid cancer patients, 52 benign thyroid tumor diseases
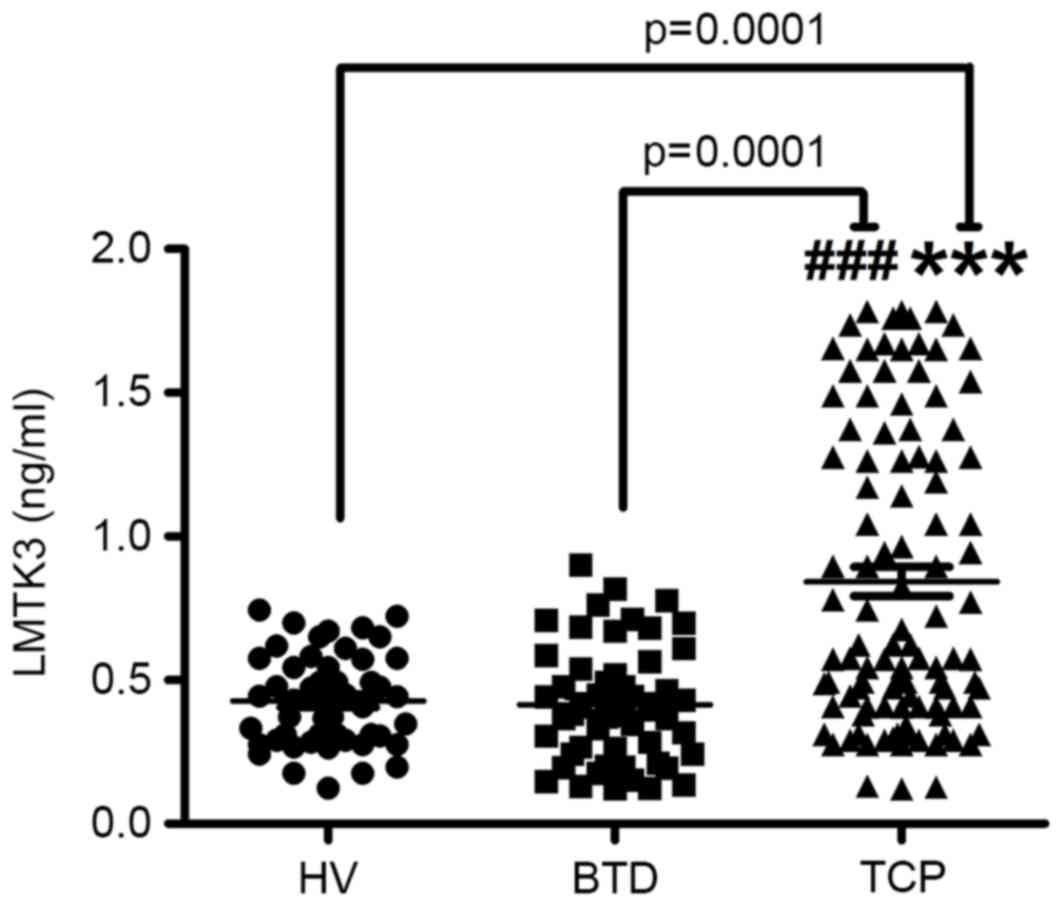
and 50 healthy volunteers, respectively. As presented in Fig. 1, serum expression of LMTK3 was
markedly elevated in patients with thyroid cancer (0.68±0.10 ng/ml)
compared with those with benign thyroid tumors (0.38±0.06 ng/ml,
t=5.708) and in the normal tissues of healthy volunteers (0.41±0.09
ng/ml, t=5.5304). The results further indicated that the LMTK3
level was closely associated with the aggressive stages of thyroid
cancer, whereas no correlations were identified between gender,
age, tumor size and lymph node metastasis, as presented in Table II (P=0.48, P=0.389, P=0.643 and
P=0.752 for gender, age, tumor size and lymph node metastasis,
respectively). In addition, significant differences in the four
pathological types of serum LMTK3 level were identified (Table II).
 | Table II.LMTK3 protein levels and clinical
features in thyroid cancer. |
Table II.
LMTK3 protein levels and clinical
features in thyroid cancer.
| Clinical
pathological feature | No. | Mean ± SEM | t-value | P-value |
|---|
| Gender |
|
| −0.713 | 0.48 |
|
Male | 26 | 0.68±0.06 |
|
|
|
Female | 80 | 0.62±0.01 |
|
|
| Age (years) |
|
| −0.866 | 0.389 |
|
≥50 | 37 | 0.67±0.05 |
|
|
|
<50 | 69 | 0.61±0.03 |
|
|
| Tumour size |
|
| −0.465 | 0.643 |
| <2
cm | 54 | 0.61±0.04 |
|
|
| ≥2
cm | 52 | 0.65±0.02 |
|
|
| Lymph node
metastasis |
|
| −0.318 | 0.752 |
|
Negative | 78 | 0.62±0.48 |
|
|
|
Positive | 28 | 0.65±0.35 |
|
|
| Stage |
|
| −4.805 | 0.0001 |
|
I+II | 89 | 0.54±0.09 |
|
|
|
III+IV | 17 | 0.95±0.10 |
|
|
| Patdological
types |
|
|
| 0.0001 |
|
Papillary cancer | 68 | 0.44±0.01 |
|
|
|
Follicular cancer | 23 | 0.96±0.06 |
|
|
|
Medullary cancer | 12 | 0.86±0.03 |
|
|
|
Undifferentiated cancer | 3 | 1.17±0.01 |
|
|
LMTK3 expression in patients with
thyroid cancer
To further ascertain the involvement of LMTK3 in the
development of thyroid cancer, immunohistochemistry, RT-qPCR and
western blotting were employed to measure the expression of LMTK3
in thyroid cancer and benign thyroid tumor tissues.
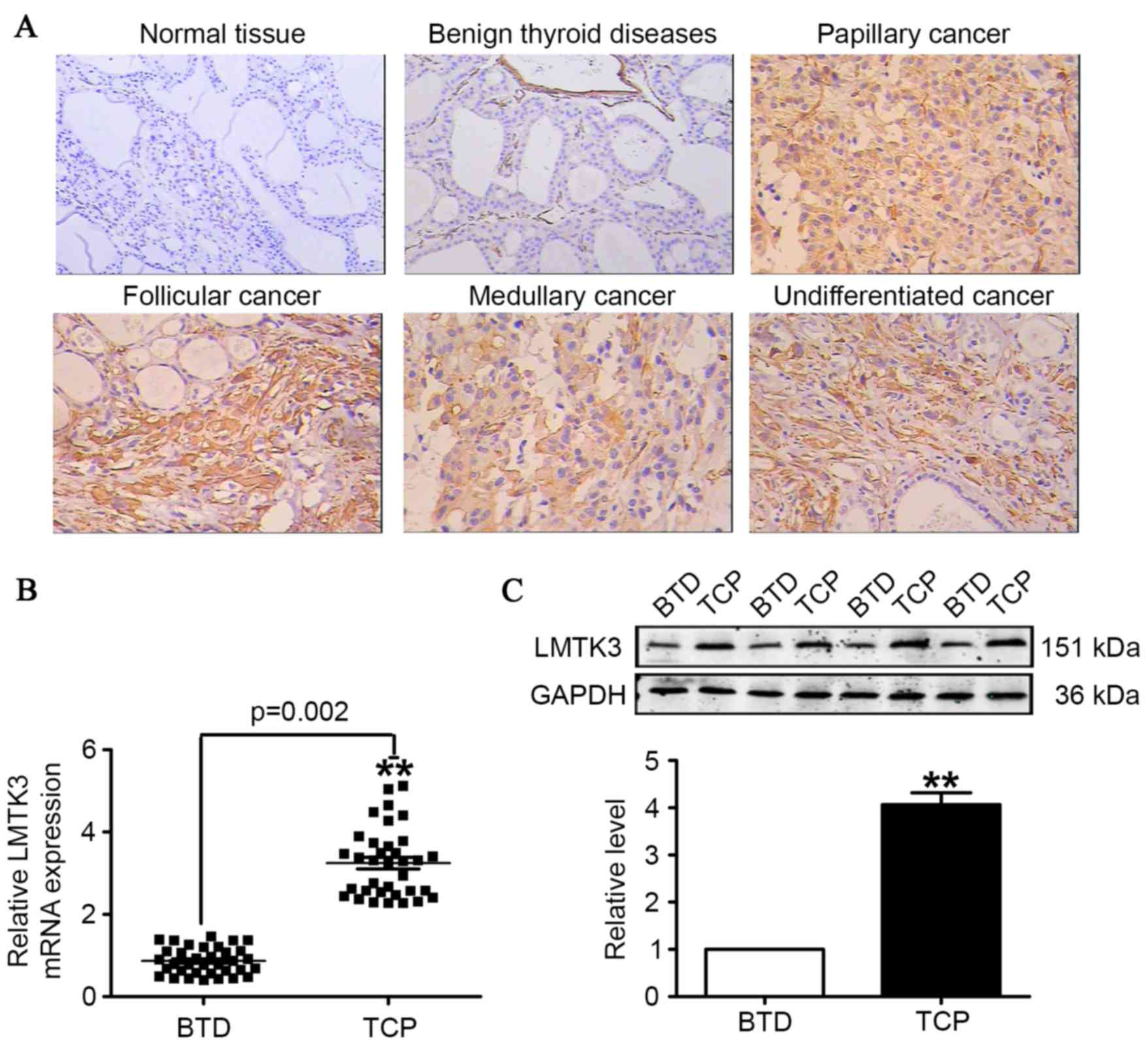
Immunohistochemistry results indicated a stronger expression of
LMTK3 in tissue sections from human thyroid cancer samples, and
LMTK3 was expressed not only in the cell nuclei, but also in the
cytoplasm of tumor cells. By contrast, LMTK3 staining was negative
in corresponding benign thyroid tumor tissues (Fig. 2A). The results demonstrated that
the mRNA level for LMTK3 was almost 4-fold higher than that of the
corresponding benign thyroid tumor disease tissues (Fig. 2B). Additionally, western blotting
results identified that LMTK3 in human thyroid cancer samples was
markedly higher compared with benign thyroid tumor tissues
(Fig. 2C).
LMTK3 knockdown inhibited cell cycle
and retarded proliferation
Flow cytometric analysis and MTT assays were
employed to validate the role of LMTK3 in regulating the cell cycle
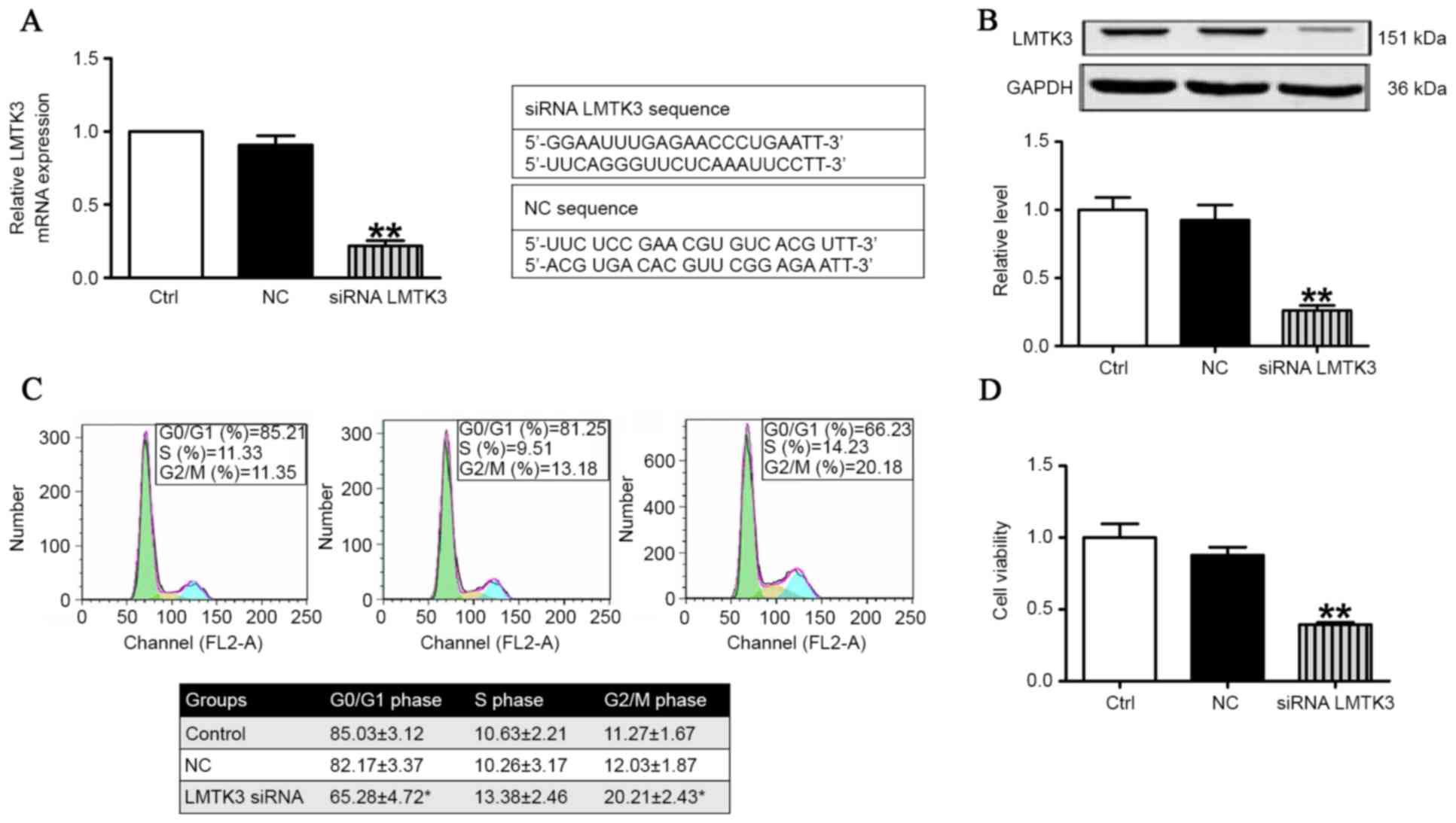
and proliferation in SW579 cells. As presented in Fig. 3A, the mRNA level of LMTK3 was
substantially decreased by treatment with LMTK3 siRNA for 48 h
compared with the negative control (NC). Consistently with Fig. 3A, western blot analysis
demonstrated that the protein expression of LMTK3 was also clearly
reduced in the LMTK3 siRNA-treated group (Fig. 3B). The effect of LMTK3 knockdown on
cell cycle distribution was determined to gain insights into the
mechanism of its anti-proliferative activity. As illustrated in
Fig. 3C, LMTK3 siRNA treatment for
48 h resulted in an accumulation of cells in the G2/M phase that
was accompanied by a reduction in cells with G0/G1 DNA content.
Previously, decreased LMTK3 activity has been demonstrated in
breast cancer, where it is considered to inhibit cell proliferation
(17). Therefore, an MTT assay was
performed to further document the effect of LMTK3 knockdown on
SW579 cell proliferation. The results identified that LMTK3
knockdown clearly inhibited cell proliferation (Fig. 3D), and indicated that LMTK3
silencing is an effective inhibitor of SW579 cell growth.
LMTK3 knockdown suppresses the
migration and invasion of SW579 cells
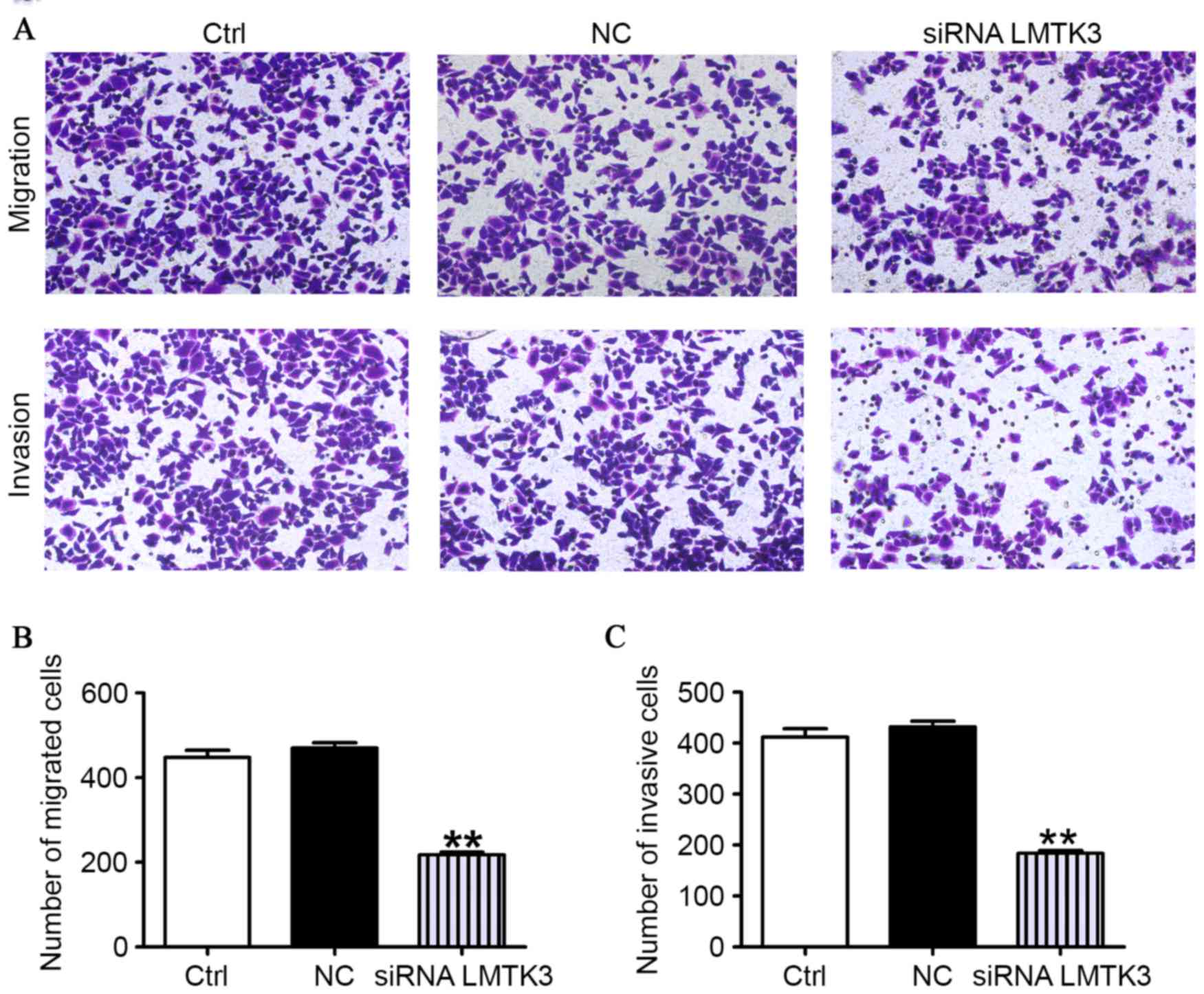
As presented in Fig.
4A, the migratory capability of SW579 cells transfected with
the LMTK3 siRNA was clearly reduced compared with the control group
(Ctrl). However, the cells had approximately similar migration
abilities in the NC and the Ctrl groups. To further determine
whether LMTK3 knockdown contributes to mitigate the SW579 cells
invasion, an invasion assay was performed by using 24-well Boyden
chambers coated with Matrigel. As presented in Fig. 4A, the number of SW579 cells was
clearly fewer than that observed for the NC and Ctrl groups. These
data strongly evidently that downregulation of LMTK3 could mediate
a reduction in the migration and invasion of SW579 cells (Fig. 4B).
LMTK3 knockdown promoted SW579 cells
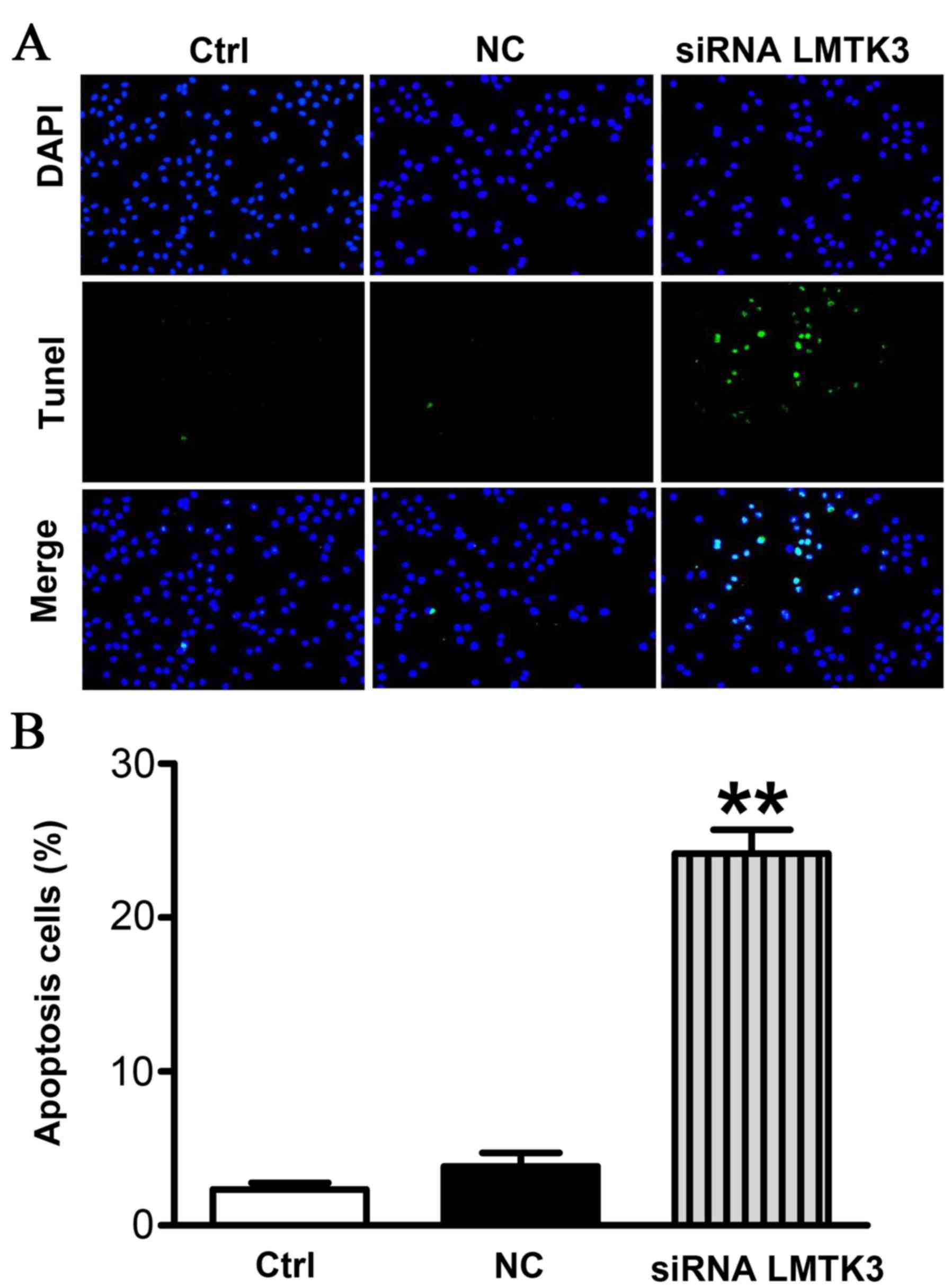
apoptosis
To evaluate the extent of apoptosis in SW579 cells,
apoptotic cells were stained using the TUNEL method. The number of
apoptotic-positive cells was counted in a high-power field
(magnification, ×200). Representative images are presented in
Fig. 5A. A notable increase in the
number of apoptotic-positive SW579 cells was observed in the LMTK3
siRNA treatment group compared with the Ctrl group. However, the NC
group displayed no significant differences (Fig. 5B).
Discussion
LMTK3 has been identified as a potential biomarker
or prognostic factor in numerous types of cancer, including breast,
gastric and colorectal cancer. In all of these, it has been shown
to be increased in cancer cells compared with normal tissues
(1,27,28).
In the present study, a critical association was also revealed
between the serum LMTK3 level and thyroid cancer. It was
identified, to the best of our knowledge for the first time, that
the level of serum and tissue LMTK3 was markedly increased in
patients with thyroid cancer (Figs.
1 and 2). This indicated that
the serum LMTK3 level may be an important diagnostic and prognostic
marker in thyroid cancer. The diagnostic and prognostic
significance of the pre-operative serum LMTK3 level have been
reported in numerous types of cancer. In colorectal cancer
patients, the serum LMTK3 level was reported to be clearly higher
compared with healthy volunteers, suggesting that serum LMTK3 could
be a valuable biomarker for predicting the progression and
prognosis of colorectal cancer (30). Similarly, in non-small cell lung
cancer, the serum LMTK3 level was markedly elevated compared with a
control group (31). Notably, the
present study has identified that the protein and mRNA level of
LMTK3 were significantly increased in patients with thyroid cancer.
Therefore, it may be surmised that LMTK3 is involved in the
pathological progression of thyroid cancer. The present results
suggested that LMTK3 knockdown could dominantly inhibit
proliferation, invasion and migration of SW579 cells (Figs. 3 and 4). In addition, the results gave a clear
indication that suppression of LMTK3 could promote apoptosis in
SW579 cells (Fig. 5). These
findings not only helped to elucidate details of the mechanism of
LMTK3 in regulating the proliferation, invasion and apoptosis in
thyroid cancer cells, but also advanced the hypothesis that LMTK3
may serve as a novel therapeutic target for patients with thyroid
cancer.
Hormone-related cancers, including breast,
endometrial, ovarian and thyroid cancer, share carcinogenic
mechanisms (15). Zhao et
al (32) indicated that the
exogenous delivery of miRNA to target LMTK3 could inhibit cell
proliferation in the human breast cancer MCF-7 cell line. Recently,
it has been demonstrated that LMTK3 co-localizes with ER in the
nucleus, increasing ER transcription, stability and activity, which
is closely associated with progression and disease outcome in
breast cancer cells (24,27). Notably, in the present study it was
shown that the increased incidence of thyroid cancer is closely
associated with dysregulation of LMTK3 in females (Table I). The results also demonstrated
that the LMTK3 level was positively associated with the disease
stage and pathological type (Table
II). Taking into account the above results and the high level
of ER receptor in thyroid cancer, it may be hypothesized that LMTK3
knockdown reduced proliferation, invasion and migration of thyroid
cancer cells, partly by mediating ER activity. However, the
underlying molecular mechanism governing how LMTK3 mediates ER
activity remains to be explored.
In conclusion, the results of the present study
demonstrated that the serum level of LMTK3 is associated with
thyroid cancer and the disease stage, and thus LMTK3 may be a
useful biomarker for the diagnosis and prognosis of thyroid cancer.
In addition, LMTK3 knockdown could inhibit proliferation, migration
and invasion of thyroid cancer cells. Therefore, LMTK3 may serve as
a novel therapeutic target for patients with thyroid cancer.
However, the exact mechanism of LMTK3 in thyroid cancer cells
requires further investigation.
References
|
1
|
Ito Y, Nikiforov YE, Schlumberger M and
Vigneri R: Increasing incidence of thyroid cancer: Controversies
explored. Nat Rev Endocrinol. 9:178–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kilfoy BA, Zheng T, Holford TR, Han X,
Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, et al:
International patterns and trends in thyroid cancer incidence,
1973–2002. Cancer Causes Control. 20:525–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hundahl SA, Fleming ID, Fremgen AM and
Menck HR: A national cancer data base report on 53,856 cases of
thyroid carcinoma treated in the U.S., 1985–1995 [see commetns].
Cancer. 83:2638–2648. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Omur O and Baran Y: An update on molecular
biology of thyroid cancers. Crit Rev Oncol Hematol. 90:233–252.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang IC, Chou FF, Liu RT, Tung SC, Chen
JF, Kuo MC, Hsieh CJ and Wang PW: Long-term outcomes of distant
metastasis from differentiated thyroid carcinoma. Clin Endocrinol
(Oxf). 76:439–447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kebebew E, Weng J, Bauer J, Ranvier G,
Clark OH, Duh QY, Shibru D, Bastian B and Griffin A: The prevalence
and prognostic value of BRAF mutation in thyroid cancer. Ann Surg.
246:466–471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen WT, Ogawa L, Ruan D, Suh I, Duh QY
and Clark OH: Central neck lymph node dissection for papillary
thyroid cancer: The reliability of surgeon judgment in predicting
which patients will benefit. Surgery. 148:398–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baek SK, Jung KY, Kang SM, Kwon SY, Woo
JS, Cho SH and Chung EJ: Clinical risk factors associated with
cervical lymph node recurrence in papillary thyroid carcinoma.
Thyroid. 20:147–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dorn R, Kopp J, Vogt H, Heidenreich P,
Carroll RG and Gulec SA: Dosimetry-guided radioactive iodine
treatment in patients with metastatic differentiated thyroid
cancer: Largest safe dose using a risk-adapted approach. J Nucl
Med. 44:451–456. 2003.PubMed/NCBI
|
|
12
|
Wartofsky L, Sherman SI, Gopal J,
Schlumberger M and Hay ID: The use of radioactive iodine in
patients with papillary and follicular thyroid cancer. J Clin
Endocrinol Metab. 83:4195–4203. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhaijee F and Nikiforov YE: Molecular
analysis of thyroid tumors. Endocr Pathol. 22:126–133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nikiforov YE: Molecular analysis of
thyroid tumors. Mod Pathol. 24 Suppl 2:S34–S43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Henderson BE and Feigelson HS: Hormonal
carcinogenesis. Carcinogenesis. 21:427–433. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen GG, Vlantis AC, Zeng Q and van
Hasselt CA: Regulation of cell growth by estrogen signaling and
potential targets in thyroid cancer. Curr Cancer Drug Targets.
8:367–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rahbari R, Zhang L and Kebebew E: Thyroid
cancer gender disparity. Future Oncol. 6:1771–1779. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kansakar E, Chang YJ, Mehrabi M and Mittal
V: Expression of estrogen receptor, progesterone receptor, and
vascular endothelial growth factor-A in thyroid cancer. Am Surg.
75:785–789. 2009.PubMed/NCBI
|
|
19
|
Lee HR, Kim TH and Choi KC: Functions and
physiological roles of two types of estrogen receptors, ERα and
ERβ, identified by estrogen receptor knockout mouse. Lab Anim Res.
28:71–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng Q, Chen GG, Vlantis AC and van
Hasselt CA: Oestrogen mediates the growth of human thyroid
carcinoma cells via an oestrogen receptor-ERK pathway. Cell Prolif.
40:921–935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inoue T, Kon T, Ohkura R, Yamakawa H,
Ohara O, Yokota J and Sutoh K: BREK/LMTK2 is a myosin VI-binding
protein involved in endosomal membrane trafficking. Genes Cells.
13:483–495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naik S, Dothager RS, Marasa J, Lewis CL
and Piwnica-Worms D: Vascular endothelial growth factor receptor-1
is synthetic lethal to aberrant {beta}-catenin activation in colon
cancer. Clin Cancer Res. 15:7529–7537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tyner JW, Deininger MW, Loriaux MM, Chang
BH, Gotlib JR, Willis SG, Erickson H, Kovacsovics T, O'Hare T,
Heinrich MC and Druker BJ: RNAi screen for rapid therapeutic target
identification in leukemia patients. Proc Natl Acad Sci USA.
106:8695–8700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giamas G, Filipović A, Jacob J, Messier W,
Zhang H, Yang D, Zhang W, Shifa BA, Photiou A, Tralau-Stewart C, et
al: Kinome screening for regulators of the estrogen receptor
identifies LMTK3 as a new therapeutic target in breast cancer. Nat
Med. 17:715–719. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nikiforov YE: Molecular diagnostics of
thyroid tumors. Arch Pathol Lab Med. 135:569–577. 2011.PubMed/NCBI
|
|
26
|
Shi H, Li Q, Ji M, Wu J, Li Z, Zheng X, Xu
B, Chen L, Li X, Lu C, et al: Lemur tyrosine kinase-3 is a
significant prognostic marker for patients with colorectal cancer.
Int J Clin Exp Pathol. 7:1101–1107. 2014.PubMed/NCBI
|
|
27
|
Stebbing J, Filipovic A, Ellis IO, Green
AR, D'Silva TR, Lenz HJ, Coombes RC, Wang T, Lee SC and Giamas G:
LMTK3 expression in breast cancer: Association with tumor phenotype
and clinical outcome. Breast Cancer Res Treat. 132:537–544. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Z, Wu J, Ji M, Shi L, Xu B, Jiang J and
Wu C: Prognostic role of lemur tyrosine kinase 3 in postoperative
gastric cancer. Mol Clin Oncol. 2:756–760. 2014.PubMed/NCBI
|
|
29
|
Fan Y, Chen M, Meng J, Yu L, Tu Y, Wan L,
Fang K and Zhu W: Arsenic trioxide and resveratrol show synergistic
anti-leukemia activity and neutralized cardiotoxicity. PLoS One.
9:e1058902014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi H, Wu J, Ji M, Zhou Q, Li Z, Zheng X,
Xu B, Deng H, Zhao W, Wu C and Jiang J: Serum lemur tyrosine kinase
3 expression in colorectal cancer patients predicts cancer
progression and prognosis. Med Oncol. 30:7542013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Z, Qi X, Zhang X and Yu L: Preoperative
serum LMTK3 as a novel biomarker in non-small cell lung cancer.
Tumour Biol. 35:5007–5011. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao G, Guo J, Li D, Jia C, Yin W, Sun R,
Lv Z and Cong X: MicroRNA-34a suppresses cell proliferation by
targeting LMTK3 in human breast cancer mcf-7 cell line. DNA Cell
Biol. 32:699–707. 2013. View Article : Google Scholar : PubMed/NCBI
|