Introduction
Pemphigus is a group of blistering autoimmune
diseases affecting the skin and mucous membranes (1). Although the cause of pemphigus
remains unknown, it is thought that humoral responses to
desmogleins are associated with the development of bullation.
Desmoglein 1 and 3 are the main components of desmosomes, involved
in connecting keratinocytes. Autoantibodies against desmogleins
promote the hydrolysis of plasminogen into plasmin in
keratinocytes, leading to the loss of cell-cell adhesion and the
formation of blisters. It is well known that CD4+ T
cells can regulate humoral responses and may contribute to the
pathogenesis of pemphigus. Pro-inflammatory T cells enhance humoral
responses while regulatory T cells (Tregs) inhibit B-cell
activation and antibody production (1–4).
However, T-cell activation and functional regulation of the humoral
response during the pathogenesis of pemphigus have not been
clarified.
MicroRNAs (miRNAs) are small non-coding RNAs that
regulate growth, development, aging, apoptosis and other important
processes in vivo (5).
Currently, thousands of miRNAs have been identified in humans and
many of them are expressed in immune cells. They are involved in
regulating maturation, differentiation and signal transduction
(6–8). Some miRNAs can regulate the
development and progression of autoimmune diseases by modulating T-
and B-cell function (9,10). However, to the best of our
knowledge, there is no information on how miRNA expression profiles
change in human peripheral blood mononuclear cells (PBMCs) during
the pathogenesis of pemphigus.
To explore the potential role of miRNAs in the
pathogenesis of pemphigus, we characterized the expression profiles
of miRNAs in PBMCs from patients with pemphigus and age- and
gender-matched healthy subjects by microarray analysis.
Furthermore, we validated the high levels of miR-424-5p expression
in PBMCs from patients with pemphigus. The potential gene targets
of miR-424-5p and their possible functional networks were predicted
by bioinformatics.
Patients and methods
Human subjects
Patients with pemphigus diagnosed according to their
clinical, histopathological, and immunological parameters were
recruited at the Nanfang Hospital of Southern Medical University
(Guangzhou, China) (11). They had
new blisters and had not been treated with immunosuppressive drugs.
The exclusion criteria were serious systemic disease, infection,
tumors or any other autoimmune disease. The age- and gender-matched
healthy subjects were recruited from the Physical Examination
Center of the same hospital. Written informed consent was obtained
from individual participants and the experimental protocol was
approved by the Institute Review Board of Nanfang Hospital of
Southern Medical University.
Blood sample collection and
pretreatment
A volume of 5 ml of venous blood was obtained from
the patients and controls, and plasma samples were prepared. PBMCs
were isolated by Ficoll-Hypaque (TBD Science, Tianjin, China)
density gradient centrifugation, lysed in Mix RNAiso blood buffer
(Takara Bio, Inc., Otsu, Japan) and stored at −80°C until use.
Isolation and quality control of
RNA
Total RNA was extracted from individual PBMC samples
and purified using the miRNeasy Mini kit (cat. no. 217004; Qiagen
GmbH, Hilden, Germany), according to the manufacturer's
instructions. The integration of the purified RNA was characterized
in the Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Santa
Clara, CA, USA).
miRNA microarray analysis
The total RNAs were dephosphorylated by phosphatase
and incubated with the Labeling Spike-In kit (Agilent Technologies
Inc.) at 37°C for 30 min. The dephosphorylated RNAs were denatured
by dimethyl sulfoxide (DMSO) and subsequently incubated at 16°C in
a circulating water-bath or cool block for 2 h. The labeled RNAs
were purified with spin columns to remove DMSO in the samples,
dried in vacuum concentrators at 45–55°C for 1 h and dissolved in
nuclease-free water. The dissolved RNAs were mixed with Hyb
Spike-In solution (Agilent Technologies Inc.) to assemble the
hybridization mixture. The mixture was hybridized to the Agilent
Human miRNA array V19.0 (Agilent Technologies Inc.), which covers
2,006 human miRNAs, at 55°C for 20 h. The arrays were washed with
the Gene Expression Wash Buffer kit and subsequently scanned by the
Agilent Microarray Scanner (both from Agilent Technologies Inc.).
Data on miRNA microarray images were extracted by Feature
Extraction software 10.7.1.1 and normalized by Gene Spring software
12.6 (both from Agilent Technologies, Inc.). The similarity between
the samples was analyzed by the principal component analysis (PCA)
and correlation plot.
Reverse transcriptase-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to validate the expression level of
differentially expressed miRNAs in 9 patients and 9 healthy
controls. Total RNAs were isolated from PBMCs and subsequently
reverse transcribed to cDNAs using miScript II RT kit (Qiagen,
Valencia, CA, USA). Briefly, 1 µg total RNA was used as template in
the reaction, and reverse transcribed in a 20-µl reaction mixture
containing 4 µl 5X miScript HiFlex Buffer, 2 µl 10X miScript
Nucleics Mix, 2 µl miScript Reverse Transcriptase Mix and 12 µl
H2O. The thermal profile conditions for reverse
transcription were 37°C for 60 min, 95°C for 5 min and 4°C
indefinitely. The prepared cDNAs were stored at −20°C until
use.
The relative levels of miRNA transcripts normalized
to the internal control (U6) were determined by qPCR using the
miRcute miRNA qPCR Detection kit containing a universal 3′ primer
(SYBR-Green; Tiangen Biotech Co., Ltd., Beijing, China) on a 7900
HT Sequence Detection system (Applied Biosystems, Foster City, CA,
USA) according to the manufacturer's instructions. The 5′ sequences
of primers for U6, 5′-GCTCGCTTCGGCAGCACAT-3′ and for miR-424-5p,
5′-CAGCAGCAATTCATGTTTTAAAA-3′ (Invitrogen Life Technologies). The
reaction mixture contained 5 µl 2X miRcute miRNA Premix, 0.8 µl 50X
ROX Reference Dye, 0.2 µl forward primer, 0.2 µl reverse primer, 1
µl prepared RT products and 2.8 µl H2O. All reactions
were ran in triplicate at 95°C for 2 min, and subjected to 40
cycles at 94°C for 15 sec and 60°C for 1 min. The data were first
normalized to the levels of U6 and analyzed by
2−ΔΔCq.
Statistical analysis
The differences between groups were analyzed using
the Student's t-test. Data from the miRNA microarrays were
considered differentially expressed when there was a P-value of
<0.05 and fold changes were >2-fold or <0.5-fold. The
target genes of differentially expressed miRNA were predicted with
miRanda, miRWalk and TargeScan. The biological processes and
signaling pathways of the differentially expressed miRNAs were
analyzed by the Gene Ontology (GO) analysis and the Kyoto
Encyclopedia of Genes and Genomes (KEGG) analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
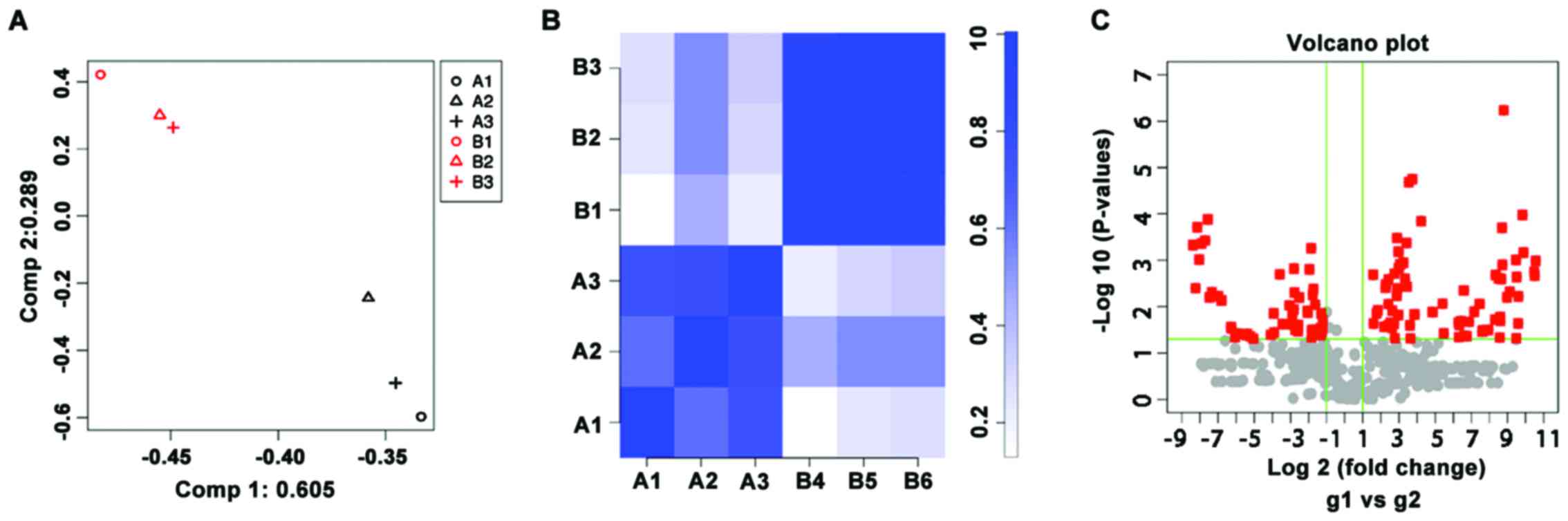
Microarray analysis
To analyze the miRNA expression profiles, 3 patients
with pemphigus and 3 healthy subjects were recruited and RNA from
PBMCs was extracted for microarray analysis. The similarity and
correlation of miRNA expression profiles between the patients and
healthy subjects were analyzed by the PCA and correlation plot
(Fig. 1). There was clear
separation in the miRNA profiles between the 2 groups and the
intra-group miRNA profiles had high correlation coefficients.
Therefore, the samples in patients were different from healthy
controls according to the biological characteristics, indicating
that the samples were well selected. There were 71 upregulated
(P<0.05, fold change >2) and 53 downregulated (P<0.05,
fold change <0.5) miRNAs on the Agilent Human miRNA array V19.0.
Of these, miR-424-5p was one of the miRNAs that was upregulated by
>1,000-fold in the patients with pemphigus (Table I).
 | Table I.Ten most upregulated or downregulated
miRNAs in PBMC from patients with pemphigus vs. healthy
controls. |
Table I.
Ten most upregulated or downregulated
miRNAs in PBMC from patients with pemphigus vs. healthy
controls.
| miRNAs | Fold change | P-value | miRNA | Fold change | P-value |
|---|
| miR-424-5p | 1507.524 | 0.0010 | miR-595 | 0.007866 | 0.0060 |
| miR-338-3p | 1451.776 | 0.0021 | miR-557 | 0.006176 | 0.0049 |
| miR-340-5p | 1430.471 | 0.0018 | miR-4726-5p | 0.005665 | 0.0062 |
| miR-30e-3p |
942.3967 | 0.0007 | miR-4472 | 0.005267 | 0.0001 |
| miR-145-5p |
908.3626 | 0.0001 | miR-4632-5p | 0.004766 | 0.0004 |
| miR-130b-3p |
781.2204 | 0.0060 | miR-5088 | 0.004145 | 0.0004 |
| miR-199b-5p |
772.3043 | 0.0230 | miR-3648 | 0.003781 | 0.0010 |
| miR-128 |
732.1749 | 0.0023 | miR-4430 | 0.00352 | 0.0002 |
| miR-590-5p |
722.1934 | 0.0479 | miR-4767 | 0.003312 | 0.0040 |
| miR-324-5p |
714.0275 | 0.0010 | miR-1180 | 0.003013 | 0.0005 |
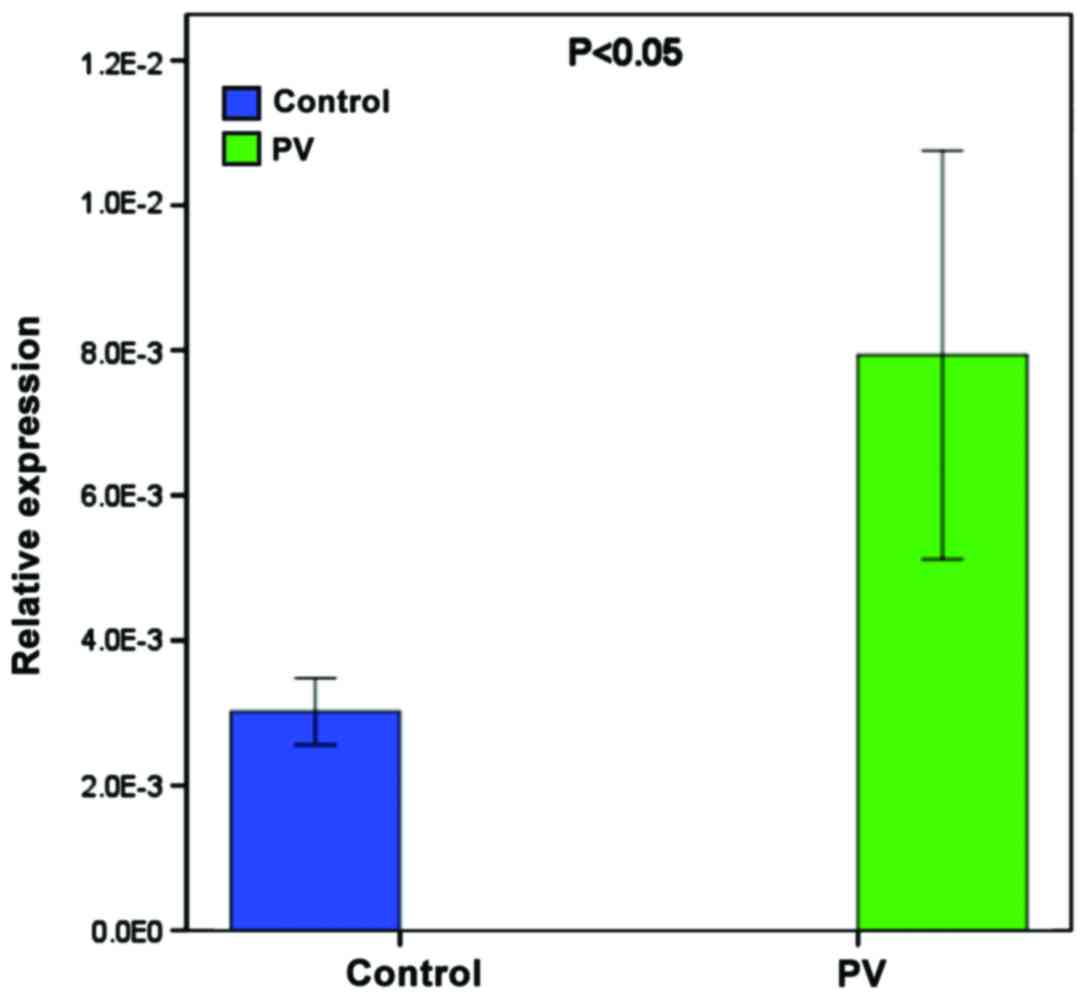
RT-qPCR analysis of miR-424-5p
To validate the higher expression of miR-424-5p,
another 9 patients and age- and gender-matched healthy subjects
were recruited and the levels of miR-424-5p in their PBMCs were
determined by RT-qPCR. The relative levels of miR-424-5p normalized
to U6 in the PBMCs from the patients were significantly higher than
in the healthy subjects (Fig.
2).
Bioinformatics analysis
The target genes of miR-424-5p were predicted using
miRanda, miRWalk and TargeScan. As predicted, an intersection of
3,539 miRNAs were regulated by miR-424-5p. Of these, 2,430 miRNAs
were expressed in humans. GO analysis indicated the functional
categorization of predicted target genes. There were 20 biological
processes (P<0.05, Benjamini <0.05) (12), including intracellular signaling
cascades, phosphate metabolism and regulation of kinase activity
(Table II). There were 242
potential target genes involved in the process of intracellular
signal transduction. Functional annotation of miR-424-5p was
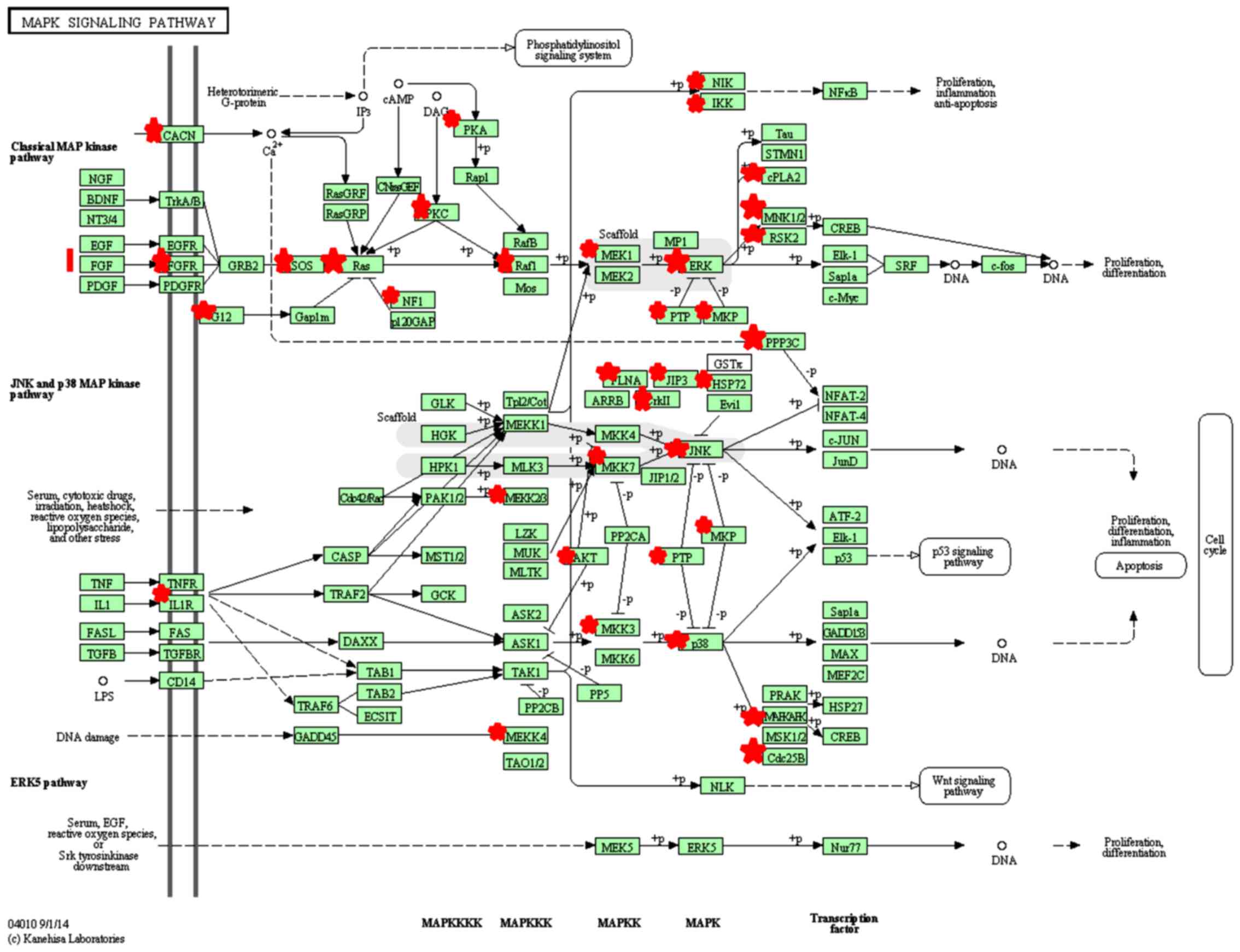
analyzed by KEGG pathway analysis. The target genes were enriched
in 10 signaling pathways (P<0.05, Benjamini <0.05), including
the T-cell receptor signaling pathway and mitogen-activated protein
kinase (MAPK) signaling pathway, suggesting that miR-424-5p may
influence these signaling pathways through target genes (Table III and Fig. 3).
 | Table II.Biological process and functional
annotation of hsa-miR-424-5p analyzed by GO. |
Table II.
Biological process and functional
annotation of hsa-miR-424-5p analyzed by GO.
| GO_ID | Term | Count of genes | P-value | Benjamini |
|---|
| GO:003556 | Intracellular signal
transduction | 242 | 7.9E-10 | 3.3E-6 |
| GO:0051174 | Regulation of
phosphorus metabolic process | 110 | 1.7E-8 | 3.5E-5 |
| GO:0019220 | Regulation of
phosphate metabolic process | 110 | 1.7E-8 | 3.5E-5 |
| GO:0042325 | Regulation of
phosphorylation | 106 | 2.7E-8 | 3.7E-5 |
| GO:0043549 | Regulation of
kinase activity | 85 | 9.4E-8 | 9.7E-5 |
| GO:0045859 | Regulation of
protein kinase activity | 82 | 1.8E-7 | 1.4E-4 |
| GO:0051338 | Regulation of
transferase activity | 85 | 6.1E-7 | 4.2E-4 |
| GO:0006796 | Phosphate metabolic
process | 183 | 7.4E-7 | 4.4E-4 |
| GO:0006793 | Phosphorus
metabolic process | 183 | 7.4E-7 | 4.4E-4 |
| GO:0006468 | Protein amino acid
phosphorylation | 133 | 1.4E-6 | 7.0E-4 |
| GO:0007264 | Small GTPase
mediated signal transduction | 72 | 1.4E-6 | 6.4E-4 |
| GO:0035556 | Protein kinase
cascade | 81 | 6.5E-6 | 2.7E-3 |
| GO:0016055 | Wnt receptor
signaling pathway | 36 | 4.8E-5 | 1.8E-2 |
| GO:0006812 | Cation
transport | 107 | 6.0E-5 | 2.1E-2 |
| GO:0001932 | Regulation of
protein amino acid phosphorylation | 43 | 7.0E-5 | 2.2E-2 |
| GO:0016310 |
Phosphorylation | 145 | 8.1E-5 | 2.4E-2 |
| GO:0033674 | Positive regulation
of kinase activity | 53 | 8.9E-5 | 2.4E-2 |
| GO:0006811 | Ion transport | 139 | 1.2E-4 | 3.1E-2 |
| GO:0000165 | MAPK cascade | 44 | 1.5E-4 | 3.5E-2 |
| GO:0030001 | Metal ion
transport | 91 | 1.5E-4 | 3.4E-2 |
 | Table III.KEGG pathway analysis of
miR-424-5p. |
Table III.
KEGG pathway analysis of
miR-424-5p.
| KEGG pathway
term | Count of genes | P-value | Benjamini |
|---|
| Insulin signaling
pathway | 43 | 5.4E-8 | 1.0E-5 |
| Pathways in
cancer | 74 | 4.1E-6 | 3.8E-4 |
| Neurotrophin
signaling pathway | 35 | 2.3E-5 | 8.5E-4 |
| Wnt signaling
pathway | 39 | 6.2E-5 | 1.9E-3 |
| mTOR signaling
pathway | 19 | 8.0E-5 | 2.1E-3 |
| T cell receptor
signaling pathway | 29 | 3.4E-4 | 4.8E-3 |
| GnRH signaling
pathway | 27 | 3.7E-4 | 4.9E-3 |
| Fc epsilon RI
signaling pathway | 23 | 4.1E-4 | 5.1E-3 |
| ErbB signaling
pathway | 24 | 8.3E-4 | 8.6E-3 |
| MAPK signaling
pathway | 52 | 4.8E-3 | 3.8E-2 |
Discussion
The present study examined the miRNA expression
profiles in PBMCs from patients with pemphigus and healthy subjects
by miRNA microarray analysis. We identified 124 differentially
expressed miRNAs, 71 of which were upregulated and 53 of which were
downregulated. Interestingly, we found higher levels of miR-424-5p
expression in PBMCs from patients with pemphigus. We also described
the biological function and regulation network of miR-424-5p, which
may help explore the regulatory role of miR-424-5p in the
pathogenesis of pemphigus.
Previous studies reported that miR-424-5p expression
was dysregulated and correlated with age-related macular
degeneration (13), endometriosis
(14), cancer (15,16)
and Mycobacterium tuberculosis infection (17). The difference is likely due to the
different biological processes regulated by miR-424-5p in different
tissues and organs. However, our data are in disagreement with a
previous study that miR-424-5p expression was significantly
decreased specifically in psoriasis skin. Downregulation of
miR-424-5p expression leads to overexpression of the target genes
MEK1 and cyclin E1, causing the hyper-proliferation of
keratinocytes (18). Furthermore,
miR-424 promotes monocyte differentiation, together with miR-155,
miR-222 and miR-503 (19). The
difference may stem from the different types of cells examined.
We analyzed the potential function of miR-424-5p by
bioinformatics and found many potential target genes in
functionally related groups. GO and KEGG analysis indicated that
the biological function of the potential genes targeted by
miR-424-5p were enriched in intracellular signaling cascades,
phosphate metabolism and regulation of kinase activity. These
potential target genes may regulate a wide range of pathways, such
as the p38 and other MAPK signaling pathways. The MAPK signaling
pathway is dysregulated in the pathogenesis of pemphigus (20). miR-424-5p may regulate the
pathogenesis of pemphigus by targeting the MAPK signaling pathway.
A previous study showed that p38 MAPK is important for regulating
autoimmune responses, cell survival, proliferation, differentiation
and apoptosis (21). Activation of
p38 MAPK was associated with collapse of the cytoskeleton,
disassembly of desmosomes, and keratinocyte apoptosis (22). Pemphigus-specific autoantibodies
can induce p38 MAPK activation in human keratinocytes and mouse
keratinocytes, and inhibition of p38 MAPK activity can prevent
pemphigus-specific, autoantibody-induced cytoskeletal
reorganization, and heat shock protein (HSP) 27 phosphorylation in
human keratinocytes (23).
Furthermore, treatment with p38 inhibitors prevented skin
blistering by inhibiting pemphigus IgG-activated signaling in
epidermal cells in a mouse model of pemphigus induced by adoptive
transfer of pemphigus-specific antibodies (24,25).
Thus, the p38 MAPK signaling pathway is crucial for the
pathogenesis of pemphigus. In addition, HSP27 participates in the
pathogenesis of pemphigus and phosphorylated HSP27 is observed in
skin biopsies from patients with pemphigus, mouse pemphigus models
and cultured keratinocytes. HSP27 phosphorylation has been
considered to be involved in regulation of the cytoskeletal
assembly of actin filaments and keratin intermediate filaments.
Dysregulated cytoskeletal arrangements are associated with the loss
of cell-cell adhesion, and it is possible that miR-424-5p regulates
p38 MAPK activation and HSP27 phosphorylation, and blister
formation during the pathogenesis of pemphigus (23,25,26).
We predicted that there were 52 target genes of
miR-424-5p enriched in the MAPK signaling pathway. This indicates
that miR-424-5p may regulate p38 MAPK activation and HSP27
phosphorylation in human PBMCs. miR-424-5p may regulate HSP27
phosphorylation by targeting the upstream MAPKAPK3 and CDC25B.
MAPKAPK3 is a member of the Ser/Thr protein kinase family. This
kinase can be activated by growth factors and stress stimuli, and
is involved in cell responses and gene regulation (27). CDC25B is a member of the CDC25
phosphatase family and is regulated by the p38 MAPK and/or MAPKAP
kinase-2 pathways (28,29). The dysregulated expression of
MAPKAPK3 is associated with the development of various types of
cancer, but its specific role in tumor formation remains to be
determined (30–32). Further investigations are required
focusing on the exact roles of MAPKAPK3 and CDC25B in the
pathogenesis of pemphigus.
In conclusion, we examined the miRNA expression
profile of PBMCs from patients with pemphigus by miRNA microarray
analysis. To the best of our knowledge, we are the first to
identify differentially expressed miRNAs in PBMCs from patients
with pemphigus and demonstrated higher levels of miR-424-5p in
PBMCs from these patients. A bioinformatics approach predicted the
potential target genes, biological functions, and pathways of
miR-424-5p, indicating that miR-424-5p may contribute to the
pathogenesis of pemphigus by regulating the p38 MAPK signaling
pathway. The influence of miR-424-5p on HSP27 phosphorylation by
targeting MAPKAPK3 and CDC25B may also regulate the pathogenesis of
pemphigus.
References
|
1
|
Rizzo C, Fotino M, Zhang Y, Chow S,
Spizuoco A and Sinha AA: Direct characterization of human T cells
in pemphigus vulgaris reveals elevated autoantigen-specific Th2
activity in association with active disease. Clin Exp Dermatol.
30:535–540. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sugiyama H, Matsue H, Nagasaka A, Nakamura
Y, Tsukamoto K, Shibagaki N, Kawamura T, Kitamura R, Ando N and
Shimada S: CD4+CD25high regulatory T cells
are markedly decreased in blood of patients with pemphigus
vulgaris. Dermatology. 214:210–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
von Bubnoff D, Andrès E, Hentges F, Bieber
T, Michel T and Zimmer J: Natural killer cells in atopic and
autoimmune diseases of the skin. J Allergy Clin Immunol. 125:60–68.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu RC, Zhu HQ, Li WP, Zhao XQ, Yuan HJ,
Zheng J and Pan M: The imbalance of Th17 and regulatory T cells in
pemphigus patients. Eur J Dermatol. 23:795–802. 2013.PubMed/NCBI
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. N Engl J Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao C and Rajewsky K: MicroRNA control in
the immune system: basic principles. Cell. 136:26–36. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simpson LJ and Ansel KM: MicroRNA
regulation of lymphocyte tolerance and autoimmunity. J Clin Invest.
125:2242–2249. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heegaard NH, Carlsen AL, Skovgaard K and
Heegaard PM: Circulating extracellular microRNA in systemic
autoimmunity. EXS. 106:171–195. 2015.PubMed/NCBI
|
|
11
|
Amagai M, Tanikawa A, Shimizu T, Hashimoto
T, Ikeda S, Kurosawa M, Niizeki H, Aoyama Y, Iwatsuki K and
Kitajima Y: Committee for Guidelines for the Management of
Pemphigus Disease: Japanese guidelines for the management of
pemphigus. J Dermatol. 41:471–486. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noble WS: How does multiple testing
correction work? Nat Biotechnol. 27:1135–1137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grassmann F, Schoenberger PG, Brandl C,
Schick T, Hasler D, Meister G, Fleckenstein M, Lindner M, Helbig H,
Fauser S, et al: A circulating microRNA profile is associated with
late-stage neovascular age-related macular degeneration. PLoS One.
9:e1074612014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Braza-Boïls A, Salloum-Asfar S,
Marí-Alexandre J, Arroyo AB, González-Conejero R, Barceló-Molina M,
García-Oms J, Vicente V, Estellés A, Gilabert-Estellés J, et al:
Peritoneal fluid modifies the microRNA expression profile in
endometrial and endometriotic cells from women with endometriosis.
Hum Reprod. 30:2292–2302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu K, Hu G, He X, Zhou P, Li J, He B and
Sun W: MicroRNA-424-5p suppresses the expression of SOCS6 in
pancreatic cancer. Pathol Oncol Res. 19:739–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Li T, Guo P, Kang J, Wei Q, Jia
X, Zhao W, Huai W, Qiu Y, Sun L, et al: miR-424-5p reversed
epithelial-mesenchymal transition of anchorage-independent HCC
cells by directly targeting ICAT and suppressed HCC progression.
Sci Rep. 4:62482014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng QL, Liu F, Yang XY, Liu XM, Zhang X,
Zhang C and Zhang ZD: Identification of latent tuberculosis
infection-related microRNAs in human U937 macrophages expressing
Mycobacterium tuberculosis Hsp16.3. BMC Microbiol. 14:372014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jinnin M: Recent progress in studies of
miRNA and skin diseases. J Dermatol. 42:551–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Forrest AR, Kanamori-Katayama M, Tomaru Y,
Lassmann T, Ninomiya N, Takahashi Y, de Hoon MJ, Kubosaki A, Kaiho
A, Suzuki M, et al: Induction of microRNAs, mir-155, mir-222,
mir-424 and mir-503, promotes monocytic differentiation through
combinatorial regulation. Leukemia. 24:460–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Ishii N, Ohata C, Furumura M and
Hashimoto T: Signalling pathways in pemphigus vulgaris. Exp
Dermatol. 23:155–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coulthard LR, White DE, Jones DL,
McDermott MF and Burchill SA: p38(MAPK): stress responses from
molecular mechanisms to therapeutics. Trends Mol Med. 15:369–379.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chernyavsky AI, Arredondo J, Kitajima Y,
Sato-Nagai M and Grando SA: Desmoglein versus non-desmoglein
signaling in pemphigus acantholysis: characterization of novel
signaling pathways downstream of pemphigus vulgaris antigens. J
Biol Chem. 282:13804–13812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild
JJ, Chua MP and Rubenstein DS: Desmosome signaling. Inhibition of
p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton
reorganization. J Biol Chem. 280:23778–23784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HE, Berkowitz P, Jolly PS, Diaz LA,
Chua MP and Rubenstein DS: Biphasic activation of p38MAPK suggests
that apoptosis is a downstream event in pemphigus acantholysis. J
Biol Chem. 284:12524–12532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berkowitz P, Hu P, Warren S, Liu Z, Diaz
LA and Rubenstein DS: p38MAPK inhibition prevents disease in
pemphigus vulgaris mice. Proc Natl Acad Sci USA. 103:pp.
12855–12860. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berkowitz P, Diaz LA, Hall RP and
Rubenstein DS: Induction of p38MAPK and HSP27 phosphorylation in
pemphigus patient skin. J Invest Dermatol. 128:738–740. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meunier I, Lenaers G, Bocquet B, Baudoin
C, Piro-Megy C, Cubizolle A, Quilès M, Jean-Charles A, Cohen SY,
Merle H, et al: A dominant mutation in MAPKAPK3, an actor of p38
signaling pathway, causes a new retinal dystrophy involving Bruch's
membrane and retinal pigment epithelium. Hum Mol Genet. 25:916–926.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lemaire M, Ducommun B and Nebreda AR:
UV-induced downregulation of the CDC25B protein in human cells.
FEBS Lett. 584:1199–1204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lemaire M, Froment C, Boutros R, Mondesert
O, Nebreda AR, Monsarrat B and Ducommun B: CDC25B phosphorylation
by p38 and MK-2. Cell Cycle. 5:1649–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song GQ and Zhao Y: MicroRNA-211, a direct
negative regulator of CDC25B expression, inhibits triple-negative
breast cancer cells' growth and migration. Tumour Biol.
36:5001–5009. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kar S, Wang M, Ham SW and Carr BI:
Fluorinated Cpd 5, a pure arylating K-vitamin derivative, inhibits
human hepatoma cell growth by inhibiting Cdc25 and activating MAPK.
Biochem Pharmacol. 72:1217–1227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang M, Zhu XY, Wang L and Lin Y:
Expression and signi-ficance of CDC25B, PED/PEA-15 in esophageal
carcinoma. Cancer Biother Radiopharm. 30:139–145. 2015. View Article : Google Scholar : PubMed/NCBI
|