Introduction
Acute myocardial infarction (MI) remains a primary
cause of death among all types of cardiovascular disease. Early
reperfusion via fibrinolysis or angioplasty is the predominant
therapy to attenuate myocardial damage (1). Despite declining mortality rates in
recent years, the prevalence of acute MI is increasing in the
elderly population (2). Post-MI
ischemia/reperfusion contributes to myocardial damage and
pathological remodeling. Therefore, attenuating the extent of
myocardial injury may be a useful strategy for the management of
patients post-MI.
Myocardial ischemia/reperfusion may activate various
molecular and cellular pathways that protect cardiomyocytes from
ischemic injury (3). Among these
signaling pathways, the Notch signaling pathway has been
demonstrated to be involved in the process of cardiovascular
disease. Notch proteins have been identified with at least five
Notch ligands (Jagged1 and 2, and Delta1, 2 and 3) and 4 Notch
receptors (Notch 1–4) (4). The
Notch signaling pathway may be involved in infarct healing and
cardiac repair (5,6), and activation of Notch signaling may
have a protective effect following cardiac injury (7–9). In
addition, Notch signaling may activate the hypoxia-inducible
factor-1α (HIF-1α) gene via the Hes 1/signal transducers and
activators of the transcription 3 pathway (10). HIF-1 is a major regulator of the
hypoxic response following MI (11) and serves a key role in regulating
oxygen homeostasis. Activation of HIF-1α by reduced oxygen or
increased oxidative stress exerts a cardioprotective effect in the
myocardium (12).
Astragaloside, the primary active constituent of
Astragalus membranaceus (Huangqi), is widely used for the
treatment of myocardial ischemic diseases in China (13–18).
A previous study conducted by the authors (19) demonstrated that Astragaloside may
attenuate post-MI myocardial ischemia in rats by promoting
angiogenesis and upregulating vascular endothelial growth factor
(VEGF) protein expression. However, the underlying mechanisms by
which Astragaloside protects against myocardial injury in rats
post-infarction remain to be fully elucidated. Little is understood
about the effect of Astragaloside on HIF-1α and the Notch1/Jagged1
signaling pathway. The purpose of the present study was to
investigate the mechanisms underlying the cardioprotective effect
of Astragaloside against myocardial injury in a post-infarction rat
model, and the potential involvement of HIF-1α and Notch1/Jagged1
signaling.
Materials and methods
Materials
Astragaloside was purchased from Chengdu Mansite
Biological Technology Co., Ltd. (Chengdu, China). Polyclonal
anti-HIF-1α (cat. no. #4426) and monoclonal anti-Notch1 (cat. no.
#3608) primary antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA), and monoclonal anti-Jagged1
(cat. no. ab124524) and anti-GAPDH (cat. no. ab181602) antibodies
were purchased from Abcam (Cambridge, UK). A biotinylated
horseradish peroxidase (HRP) -conjugated secondary antibody (cat.
no. A0216) was purchased from Beyotime Institute of Biotechnology
(Shanghai, China). TRIzol® reagent was obtained from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Reverse
transcription (RT), bicinchoninic acid protein (BCA) assay and
SYBR®-Green Real-Time Polymerase Chain Reaction (PCR)
kits were purchased from Thermo Fisher Scientific, Inc.
Radioimmunoprecipitation assay (RIPA) lysis buffer was obtained
from JRDUN Biotechnology (Shanghai, China).
Animals and experimental protocol
Male Wistar rats (mean weight, 250±30 g; age, 2 to 3
months; n=45) were supplied by the Changchun Yisi Experimental
Animal Technology Co. (Changchun, China). They were housed at
22±2°C with 40–50% humidity, a 12 h/12 h light/dark cycle and free
access to food and water. The study protocol was approved by the
Ethical Committee of the Harbin Medical University (Harbin, China)
and performed in accordance with the guidelines of Laboratory
Animals published by the US National Institutes of Health.
Rats were randomly divided into sham control or
acute MI groups. Animals were subjected to coronary artery ligation
to induce acute MI as described previously (19). Briefly, rats were anesthetized with
an intraperitoneal injection of 40 mg/kg 1% sodium pentobarbital
(Merck KGaA, Darmstadt, Germany). Following tracheal intubation,
all rats received positive pressure aerobic ventilation with a
small animal ventilator (55–705B; Harvard University, Cambridge,
MA, USA), and the chest was opened at the left fourth intercostal
space. The heart was exteriorized following incision of the
pericardium. A 6–0 silk suture was used to ligate the left anterior
descending branch ~3–5 mm distal to the aortic root. Successful
generation of the acute MI model was verified by regional cyanosis
of the infarct areas. Sham control animals were subjected to an
identical surgical procedure without coronary artery ligation.
A total of 18 rats (35% of rats died) surviving for
24 h were randomly assigned to three groups: MI model, 2.5
mg/kg/day Astragaloside-treated, 10 mg/kg/day Astragaloside-treated
or control (n=6/group). Following surgery, all animals were kept in
an air conditioned-controlled room at 22±2°C with 40–50% humidity
in a 12 h light/dark cycle with free access to water and standard
rat feed. Astragaloside-treated rats were administered with the
designated doses of Astragaloside by intraperitoneal injection for
28 days, whereas rats in the MI model and sham control groups
received equal amounts of saline.
Gross cardiac morphology and
histopathological examination
After 28 days of Astragaloside or saline treatment,
rats were anesthetized with an intraperitoneal injection of 40
mg/kg 1% sodium pentobarbital. The heart was rapidly dissected and
rinsed with saline, following which the left intraventricular
pressure was maintained at 20 mmHg by using water-filled balloon.
The aortic arch, right ventricle, atrium and auricle were removed,
and horizontally sectioned into five 1-mm thick sections. Infracted
areas of the extracted left ventricle were fixed in 4%
paraformaldehyde for 24 h. The fixed tissue specimens were cut into
6-µm sections for hematoxylin-eosin (H&E) staining.
Morphological alterations of the stained tissues were assessed
under a light microscope (CH20BIMF200; Olympus Corporation, Tokyo,
Japan) at ×400 magnification.
HIF-1α, Notch1 and Jagged1 mRNA
expression
Reverse transcription-quantitative PCR (RT-qPCR)
analysis was performed to measure HIF-1α, Notch1 and Jagged1 mRNA
expression. Briefly, total RNA was extracted from 100 mg samples of
ischemic myocardium tissue with TRIzol reagent according to the
manufacturer's protocol. The purity of RNA was measured according
to the A260/A280 ratio
spectrophotometrically. Total RNA (2 µg) from each sample was used
to synthesize cDNA with Moloney Murine Leukemia Virus Reverse
Transcriptase (Thermo Fisher Scientific, Inc.). The following
primer sequences were synthesized by Shanghai Generay Biotech Co.,
Ltd. (Shanghai, China): Forward, 5′-CAGCGATGACACGGAAAC-3′ and
reverse, 5′-AGTGACTCTGGGCTTGAC-3′ for HIF-1α; forward,
5′-CTTCGTGCTCCTGTTCTTTG-3′ and reverse, 5′-GCCTCTGACACTTTGAAACC-3′
for Notch1; and forward, 5′-CACCCGAACTGGACAAAC-3′ and reverse,
5′-AGCCTCAGACTGGGATAC-3′ for Jagged1. These primer pairs resulted
in 210, 105, and 170 bp amplified products, respectively. Rat GAPDH
was used as an internal control with the following primers (182
bp): Forward, 5′-GTCGGTGTGAACGGATTTG-3′ and reverse,
5′-TCCCATTCTCAGCCTTGAC-3′. qPCR reactions were prepared using a
SYBR Real-Time PCR kit. The PCR products were quantified by
detecting the SYBR-Green fluorescence signal intensity and analyzed
by Applied Biosystems Prism 7300 SDS software (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Relative mRNA expression levels of
HIF-1α, Notch1 and Jagged1 were quantified by the 2−∆∆Cq
method, normalized to the levels of GAPDH.
HIF-1α, Notch1 and Jagged1 protein
expression
HIF-1α, Notch1 and Jagged1 protein expression levels
were measured by western blot analysis. Briefly, 20 mg samples of
frozen tissues were powdered and homogenized in 150–250 µl RIPA
lysis buffer. Protein levels were determined by performing a BCA
assay according to the manufacturer's protocol. Prepared protein
samples were separated by 10% SDS-PAGE and transferred onto
nitrocellulose membranes. The membranes were blocked with milk
powder in TBS for 60 min at room temperature, and subsequently
incubated with anti-HIF-1α (1:1,000 dilution), anti-Notch1 (1:1,000
dilution), anti-Jagged1 (1:500 dilution) and anti-GAPDH (1:1500
dilution) primary antibodies. Following overnight incubation at
4°C, the membranes were washed three times with TBST (TBS with 0.1%
Tween-20) and subsequently incubated with HRP-conjugated secondary
antibodies (1:2,000 dilution) in TBS with 0.1% Tween-20 for 30 min
at 37°C. The bands were visualized using Enhanced Chemiluminescence
reagents (EMD Millipore, Billerica, MA, USA), according to the
manufacturer's protocol. The relative protein expression levels of
HIF-1α, Notch1 and Jagged1 were normalized to the intensity of
GAPDH bands from the same sample. All experiments were repeated in
triplicate, and mean values were calculated.
Statistical analysis
Data are presented as mean ± standard deviation.
One-way analysis of variance followed by the Student-Newman-Keuls
method was applied for multiple comparisons. All statistical
analyses were performed using SPSS software version 17.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
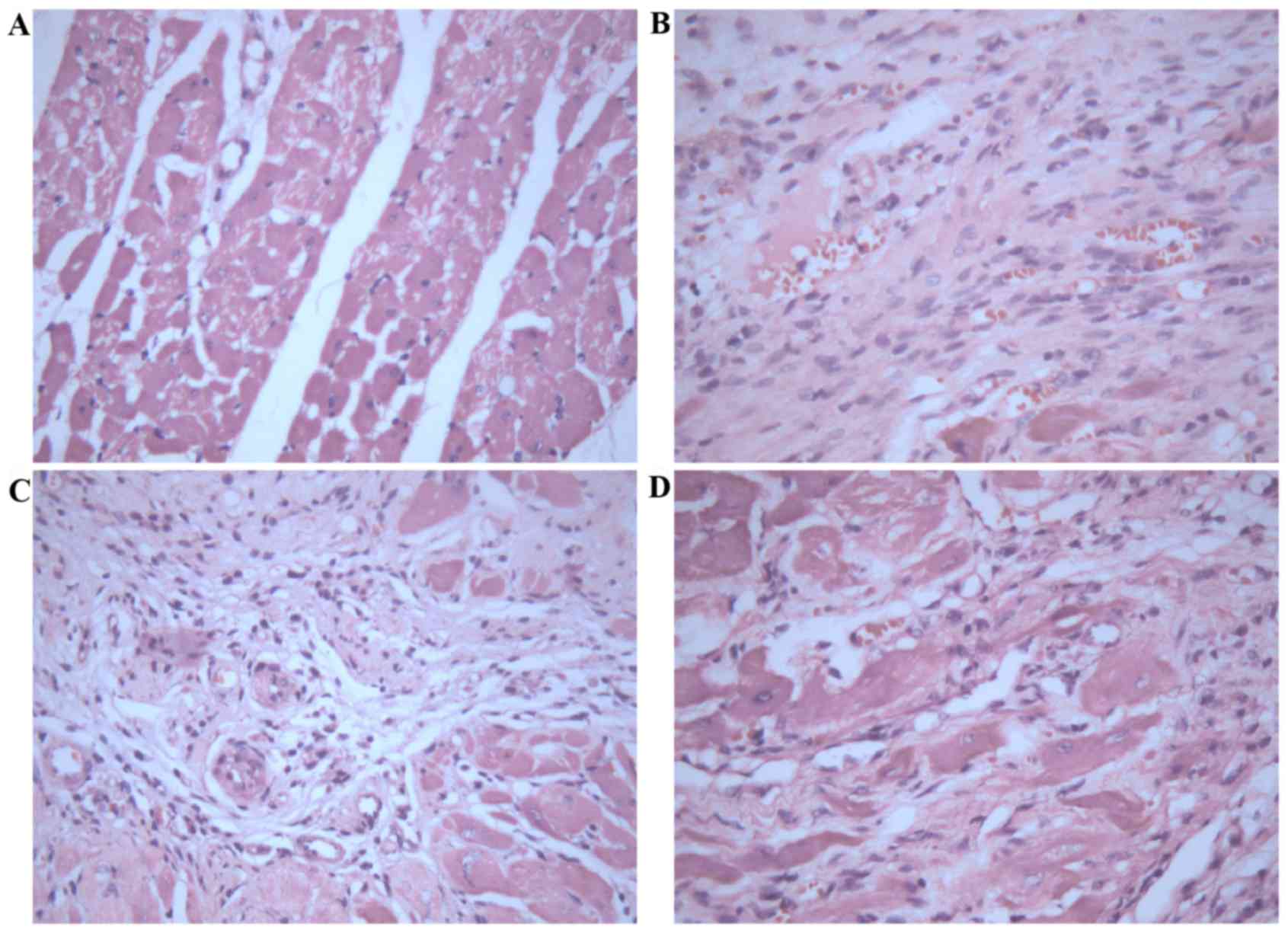
Gross cardiac morphology
Heart structures were healthy in sham control rats
(Fig. 1A), whereas untreated MI
rats exhibited dilation of the heart, pale lesion sites, thinner
ventricular walls and total ventricular wall involvement (Fig. 1B). In contrast, treatment with 2.5
(Fig. 1C) or 10 mg/kg/day
(Fig. 1D) Astragaloside attenuated
the dilation of heart and reduced the infarction size of MI
rats.
Morphological analysis of myocardium
treated with Astragaloside
Histopathological analysis of myocardial tissues was
observed by H&E staining. Myocardial tissues in control rats
demonstrated apparent integrity of the myocardial cell membrane
with no inflammatory cell infiltration (Fig. 2A). Myocardial tissues in infarct
rats exhibited dissolved cardiomyocytes, distorted cardiac muscles,
myocardial necrosis, a large number of fibroblasts or collagen
fibers, and a small amount of inflammatory cell infiltration
(Fig. 2B). In contrast, treatment
with 2.5 (Fig. 2C) or 10 mg/kg/day
(Fig. 2D) Astragaloside attenuated
the above histopathological abnormalities, and the boundary between
the infarct area and non-infarct area was obvious.
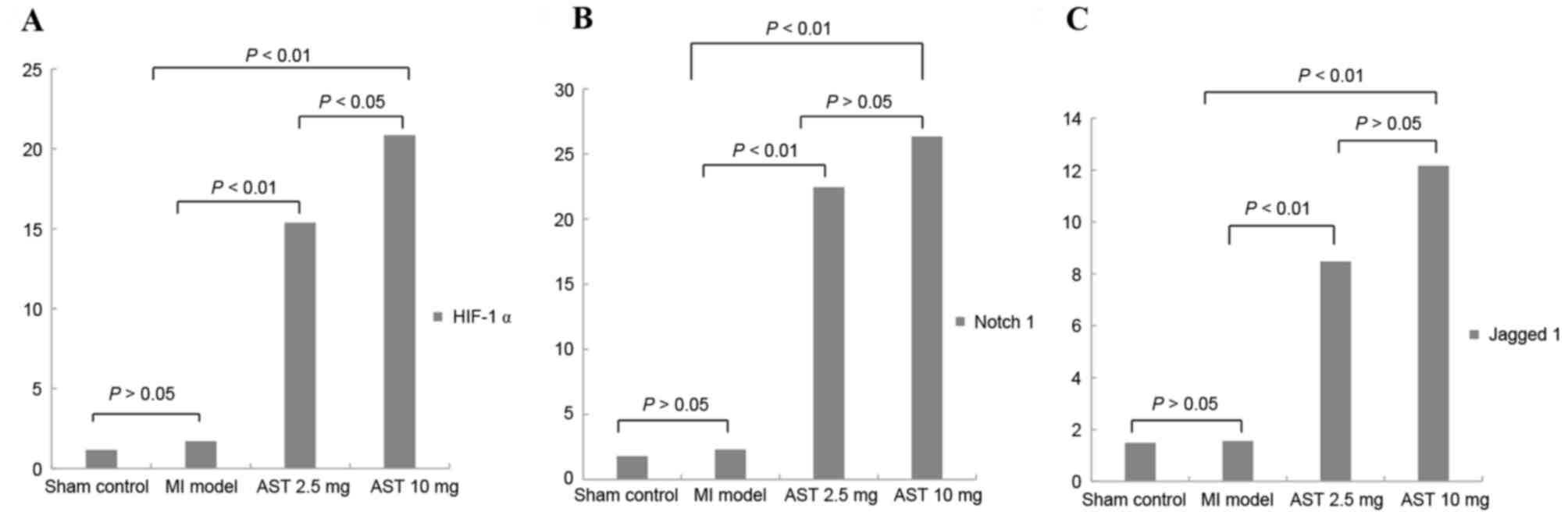
Effects of Astragaloside on HIF-1α,
Notch1, and Jagged1 mRNA expression
As presented in Fig.
3A, no significant differences were observed in HIF-1α mRNA
expression levels in the myocardium of untreated MI rats compared
with sham control rats (1.7006±0.9756 and 1.1495±0.6861,
respectively; P>0.05). Treatment with Astragaloside
significantly increased HIF-1α mRNA expression to 15.3790±4.2757 at
a dose of 2.5 mg/kg/day and 20.8603±6.8249 at 10 mg/kg/day (both
P<0.01). Furthermore, HIF-1α mRNA expression levels were
significantly increased following treatment with 10 mg/kg/day
Astragaloside compared with 2.5 mg/kg/day (P<0.05).
As presented in Fig.
3B, no significant differences were observed in Notch1 mRNA
expression levels in the myocardium of untreated MI rats compared
with sham control rats (2.3227±0.6524 and 1.8110±2.6816,
respectively; P>0.05). Treatment with Astragaloside
significantly increased Notch1 mRNA expression to 22.4513±5.0885 at
2.5 mg/kg/day and 26.3550±10.4480 at 10 mg/kg/day compared with the
expression level in MI model rats (both P<0.01). However, no
significant differences in Notch1 mRNA expression were observed
between the different doses of Astragaloside (P>0.05).
As presented in Fig.
3C, no significant differences were observed in Jagged1 mRNA
levels in the myocardium of untreated MI rats compared with sham
control rats (1.5589±2.0397 and 1.4880±1.5084, respectively;
P>0.05). Treatment with Astragaloside significantly increased
Jagged1 mRNA expression to 8.4783±6.4066 at 2.5 mg/kg/day and
12.1670±2.0818 at 10 mg/kg/day (both P<0.01). However, no
significant differences were observed in Jagged1 mRNA expression
between the different doses of Astragaloside (P>0.05).
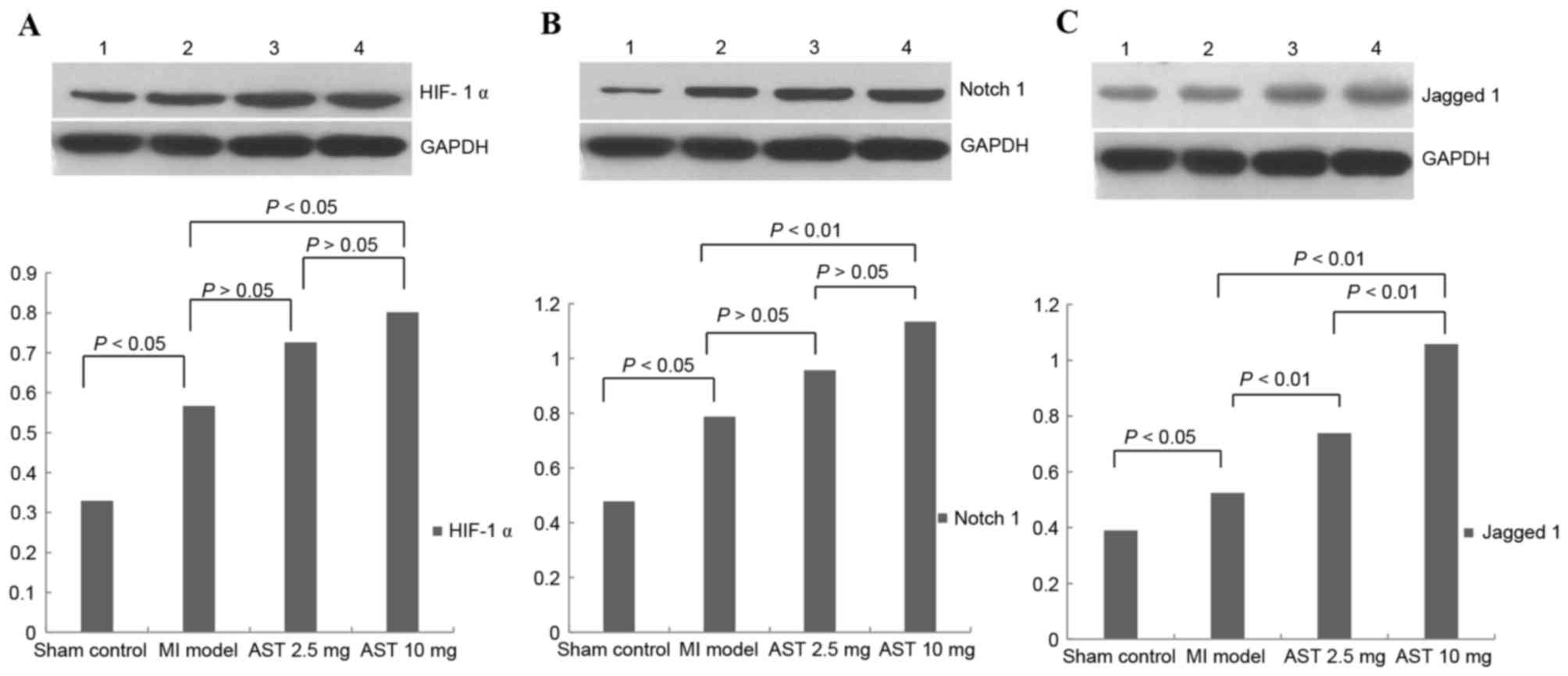
Effects of Astragaloside on HIF-1α,
Notch1 and Jagged1 protein expression
Representative HIF-1α protein bands with a molecular
weight of 120 kDa are presented in Fig. 4A. Myocardial HIF-1α protein levels
were significantly increased in MI model rats compared with sham
control rats (0.5668±0.1855 and 0.3295±0.1553, respectively;
P<0.05). HIF-1α protein expression levels following treatment
with 2.5 and 10 mg/kg/day Astragaloside were 0.7259±0.2112 and
0.8009±0.2167, respectively. Treatment with 10 mg/kg/day
Astragaloside resulted in significantly increased HIF-1α expression
levels compared with MI model rats (P<0.05). HIF-1α protein
expression levels did not differ significantly between rats treated
with different doses of Astragaloside (P>0.05).
Representative Notch1 protein bands with a molecular
weight of 120 kDa are presented in Fig. 4B. Myocardial Notch1 protein
expression levels were significantly increased in MI model rats
compared with sham control rats (0.7873±0.2207 and 0.4781±0.1393,
respectively; P<0.05). Notch1 protein expression levels
increased to 0.9567±0.2099 and 1.1336±0.2014 following treatment
with 2.5 and with 10 mg/kg/day Astragaloside, respectively.
Treatment with 10 mg/kg/day Astragaloside led to significantly
upregulated Notch1 compared with the MI model rats (P<0.01). No
significant differences in Notch1 protein expression levels were
observed between the different doses of Astragaloside
(P>0.05).
Representative Jagged1 protein bands with a
molecular weight of 134 kDa are presented in Fig. 4C. Myocardial Jagged1 protein
expression levels were significantly increased between MI model and
sham control rats (0.5244±0.0915 and 0.3900±0.5689, respectively;
P<0.05). Treatment with 2.5 and 10 mg/kg/day Astragaloside
significantly increased Jagged1 protein expression levels in rats
(0.7380±0.1091 and 1.0580±0.1211, respectively), compared with
untreated MI rats (P<0.01). Furthermore, Jagged1 protein
expression levels were significantly increased following treatment
with 10 mg/kg/day Astragaloside compared with 2.5 mg/kg/day
(P<0.01).
Discussion
In the present study, a rat MI model was established
via coronary artery ligation. Histological changes in the infarct
hearts as observed by H&E staining presented dissolved or
disrupted cardiomyocytes, a disorderly myocardial structure and a
large number of fibroblasts or collagen fibers. Results of the
gross study of the hearts revealed increased dilation of the heart,
pale lesion sites, thinner ventricular walls or total ventricular
wall involvement. Treatment with Astragaloside significantly
attenuated these pathological abnormalities. In addition,
Astragaloside treatment increased the mRNA and protein expression
levels of HIF-1α, Notch1 and Jagged1 to certain degrees; however,
these results were dose-dependent. These findings indicated that
the cardioprotective effect of Astragaloside might be partially
mediated by HIF-1α and the Notch1/Jagged1 signaling pathway.
HIF, a nuclear transcriptional regulatory factor,
consists of HIF-1α and HIF-1β. HIF-1α is sensitive to hypoxia and
stabilizes under such conditions. It is widely understood that
hypoxia serves a key role in the process of myocardial ischemia.
Higher HIF-1α expression is observed in the early (20) and late (21) stages of myocardial ischemia. MI
induces upregulation of HIF-1α in ischemic or hypoxic myocardium,
which contributes to cardioprotective effects (20,22,23).
Persisting ischemia at the site of infarction may persist for
numerous days to a later phase. Constitutive overexpression of
HIF-1α attenuates the infarct size and improves cardiac function 4
weeks post-infarction (24). Thus,
HIF-1α has been considered as a therapeutic target for
cardioprotection (25). In the
present study, treatment with 2.5 and 10 mg/kg/day Astragaloside
significantly increased HIF-1α mRNA expression levels compared with
untreated MI rats (P<0.01). HIF-1α protein expression levels
were significantly increased in the 10 mg/kg/day Astragaloside
group than the untreated MI rats (P<0.05). However, obvious
dose-dependent effects of Astragaloside treatment were observed at
the mRNA level (P<0.05).
The Notch signaling pathway is crucial for the
regulation of cellular proliferation, differentiation and
apoptosis. A hypoxic environment in the myocardium may lead to
Notch signaling activation, which attenuates myocardial injury and
improves cardiac function by promoting myocardial regeneration,
protecting ischemic myocardium or inducing angiogenesis (26). Notch1 and Jagged1 are the
predominant forms of Notch signaling expressed in adult myocytes
(27). Furthermore, Notch 1
signaling is activated in proliferating embryonic and immature
cardiomyocytes (8) or in the
border zone of the myocardium following infarction (7). Notch1 signaling additionally inhibits
cardiomyocyte apoptosis in ischemic postconditioning (28). Consistently, myocardial injury may
be attenuated by activation of Notch1 via exogenous administration
of Jagged1 (29). In the current
study, Notch1 and Jagged1 expression in the myocardium was detected
4 weeks after MI. Administration of 2.5 or 10 mg/kg/day
Astragaloside significantly increased Notch1 and Jagged1 mRNA
expression levels compared with MI model rats (both P<0.01).
Compared with MI model rats, treatment with 10 mg/kg/day
Astragaloside significantly increased Notch1 protein expression
(P<0.01). However, significant differences in Jagged1 protein
expression levels were observed following administration with
Astragaloside at either dose (both P<0.01). Furthermore, there
were statistically significant differences in Jagged1 protein
expression levels between the different doses of Astragaloside
(P<0.01). Taken together, the above findings suggested that
upregulation of Notch1/Jagged1 signaling by Astragaloside may be
the underlying mechanism whereby Astragaloside attenuates
myocardial injury following MI.
Numerous studies have investigated the
cardioprotective effects of Astragaloside. A previous study of the
authors demonstrated that Astragaloside improves myocardial
ischemia post-MI by inducing angiogenesis in the ischemic
myocardium of rats (19).
Astragaloside additionally may improve ischemic scope, epicardial
ECG and myocardial enzymes in the acute MI dog heart (13). The cardioprotective effects of
Astragaloside may be linked to the inhibition of calcium overload
in cardiac myocytes (30),
decrease in myocardial apoptosis and transforming growth factor-β
expression (17), increase in
protein expression levels of VEGF (19), or to the antioxidant properties of
Astragaloside (18). Overall,
these data supported the potential cardioprotective effects of
Astragaloside.
There are numerous limitations to the present study.
Infarction repair is a dynamic process, but the time-dependent
changes in HIF-1α, Notch1, and Jagged1 expression were not
investigated. Secondly, although the present study demonstrated the
cardioprotective effects of Astragaloside administration in the
ischemic myocardium, the dose-dependent effect was not obvious. The
dose-dependent effects of Astragaloside on HIF-1α and Jagged1
protein and mRNA expression levels were not consistent in this
study. These inconsistent findings may be associated with
degradation of the extracted protein samples or other upstream
controlling genes that are involved in the regulation of mRNA and
protein expression. The mechanisms underlying the regulation of
HIF-1α, Notch1 and Jagged1 expression require further
investigation.
In conclusion, the present study demonstrated that
administration of Astragaloside for 28 days was associated with an
improvement in myocardial injury in rats post-infarction. In
addition, Astragaloside treatment increased myocardial expression
levels of HIF-1α, Notch1 and Jagged1. These findings indicated that
the cardioprotective effects of Astragaloside may be mediated
partially via the upregulation of HIF-α and Notch1/Jagged1
signaling, implicating them as therapeutic targets for the
treatment of MI.
Acknowledgements
The present study was supported by the Heilongjiang
Provincial Department of Education (grant no. 12521297).
References
|
1
|
Longacre L Schwartz, Kloner RA, Arai AE,
Baines CP, Bolli R, Braunwald E, Downey J, Gibbons RJ, Gottlieb RA,
Heusch G, et al: New horizons in cardioprotection: Recommendations
from the 2010 National Heart, Lung and Blood Institute Workshop.
Circulation. 124:1172–1179. 2011. View Article : Google Scholar :
|
|
2
|
Forouzanfar MH, Moran AE, Flaxman AD, Roth
G, Mensah GA, Ezzati M, Naghavi M and Murray CJ: Assessing the
global burden of ischemic heart disease, part 2: Analytic methods
and estimates of the global epidemiology of ischemic heart disease
in 2010. Glob Heart. 7:331–342. 2012. View Article : Google Scholar :
|
|
3
|
Zamilpa R and Lindsey ML: Extracellular
matrix turnover and signaling during cardiac remodeling following
MI: Causes and consequences. J Mol Cell Cardiol. 48:558–563. 2010.
View Article : Google Scholar
|
|
4
|
Ishitani T, Hirao T, Suzuki M, Isoda M,
Ishitani S, Harigaya K, Kitagawa M, Matsumoto K and Itoh M:
Nemo-like kinase suppresses Notch signalling by interfering with
formation of the Notch active transcriptional complex. Nat Cell
Biol. 12:278–285. 2010.
|
|
5
|
Gude N and Sussman M: Notch signaling and
cardiac repair. J Mol Cell Cardiol. 52:1226–1232. 2012. View Article : Google Scholar :
|
|
6
|
Li Y, Hiroi Y and Liao JK: Notch signaling
as an important mediator of cardiac repair and regeneration after
myocardial infarction. Trends Cardiovasc Med. 20:228–231. 2010.
View Article : Google Scholar :
|
|
7
|
Gude NA, Emmanuel G, Wu W, Cottage CT,
Fischer K, Quijada P, Muraski JA, Alvarez R, Rubio M, Schaefer E
and Sussman MA: Activation of Notch-mediated protective signaling
in the myocardium. Circ Res. 102:1025–1035. 2008. View Article : Google Scholar :
|
|
8
|
Kratsios P, Catela C, Salimova E, Huth M,
Berno V, Rosenthal N and Mourkioti F: Distinct roles for
cell-autonomous Notch signaling in cardiomyocytes of the embryonic
and adult heart. Circ Res. 106:559–572. 2010. View Article : Google Scholar
|
|
9
|
Li Y, Hiroi Y, Ngoy S, Okamoto R, Noma K,
Wang CY, Wang HW, Zhou Q, Radtke F, Liao R and Liao JK: Notch1 in
bone marrow-derived cells mediates cardiac repair after myocardial
infarction. Circulation. 123:866–876. 2011. View Article : Google Scholar :
|
|
10
|
Lee JH, Suk J, Park J, Kim SB, Kwak SS,
Kim JW, Lee CH, Byun B, Ahn JK and Joe CO: Notch signal activates
hypoxia pathway through HES1-dependent SRC/signal transducers and
activators of transcription 3 pathway. Mol Cancer Res. 7:1663–1671.
2009. View Article : Google Scholar
|
|
11
|
Lee SH, Wolf PL, Escudero R, Deutsch R,
Jamieson SW and Thistlethwaite PA: Early expression of angiogenesis
factors in acute myocardial ischemia and infarction. N Engl J Med.
342:626–633. 2000. View Article : Google Scholar
|
|
12
|
Li X, Zhao H, Wu Y, Zhang S, Zhao X, Zhang
Y, Wang J, Wang J and Liu H: Up-regulation of hypoxia-inducible
factor-1α enhanced the cardioprotective effects of ischemic
postconditioning in hyperlipidemic rats. Acta Biochim Biophys Sin
(Shanghai). 46:112–118. 2014. View Article : Google Scholar
|
|
13
|
Chunli L, Yu C, Wenwei L, Haitao Y, Biao S
and Shijie Y: Effects of Astragalus saponins on ischemic scope,
epicardial ECG, myocardial enzymes in acute myocardial infarcted
dog heart. Baiqiuen Yike Daxue Xuebao. 21:111–113. 1995.(In
Chinese).
|
|
14
|
Zhang Z, Chen LX, Qin LM and B J: Effect
of Astragalus on hemodynamics and oxygen free radical in myocardial
ischemia reperfusion injury in rats. Chin J Info Trad Chin Med.
7:7–8. 2000.(In Chinese).
|
|
15
|
Feng L, Meng D, Chen XJ, Yang D and Zhang
JN: Protective action of Astragalosides on myocardial injury
induced by isoproterenol in rats. Chin Pharm J. 1313–1316. 2006.(In
Chinese).
|
|
16
|
Wang N, Zhu K, Yue H, et al: Protective
effects of Astragalus saponins on cardiomyocytes injury induced by
oxidative stress. J Hebei Trad Chin Med Pharmacol. 22:8–9. 2007.(In
Chinese).
|
|
17
|
Jia H, Wweiping J, Jiajie L and Li WZ:
Protective effect of astragalosides on myocardial damage in
diabetic mice. Acta Universitatis Medicinalis Anhui. 290–294.
2012.
|
|
18
|
Han D, Zhang YW, Liu M, et al: The
hemodynamic and antioxidant effects of Astragaloside on ventricular
remodeling rats. Chin J Lab Diag. 17:1956–1959. 2013.(In
Chinese).
|
|
19
|
Cao BD, Jiang W, Wang HD, Wang LY, Yu JM
and Zhang XB: The effect of Astragalus on VEGF in ischemic
myocardium of rats. Chin J Crit Care Med. 1033–1035. 2014.
|
|
20
|
Blanco P, ampín J, García Rivero SA,
Cepeda XL Otero, Vázquez Boquete A, Vila J Forteza and Fonseca R
Hinojal: Immunohistochemical expression of HIF-1alpha in response
to early myocardial ischemia. J Forensic Sci. 51:120–124. 2006.
View Article : Google Scholar
|
|
21
|
Parisi Q, Biondi-Zoccai GG, Abbate A,
Santini D, Vasaturo F, Scarpa S, Bussani R, Leone AM, Petrolini A,
Silvestri F, et al: Hypoxia inducible factor-1 expression mediates
myocardial response to ischemia late after acute myocardial
infarction. Int J Cardiol. 99:337–339. 2005. View Article : Google Scholar
|
|
22
|
Adluri RS, Thirunavukkarasu M, Dunna NR,
Zhan L, Oriowo B, Takeda K, Sanchez JA, Otani H, Maulik G, Fong GH
and Maulik N: Disruption of hypoxia-inducible transcription
factor-prolyl hydroxylase domain-1 (PHD-1-/-) attenuates ex vivo
myocardial ischemia/reperfusion injury through hypoxia-inducible
factor-1α transcription factor and its target genes in mice.
Antioxid Redox Signal. 15:1789–1797. 2011. View Article : Google Scholar :
|
|
23
|
Poynter JA, Manukyan MC, Wang Y, Brewster
BD, Herrmann JL, Weil BR, Abarbanell AM and Meldrum DR: Systemic
pretreatment with dimethyloxalylglycine increases myocardial
HIF-1alpha and VEGF production and improves functional recovery
after acute ischemia/reperfusion. Surgery. 150:278–283. 2011.
View Article : Google Scholar
|
|
24
|
Kido M, Du L, Sullivan CC, Li X, Deutsch
R, Jamieson SW and Thistlethwaite PA: Hypoxia-inducible factor
1-alpha reduces infarction and attenuates progression of cardiac
dysfunction after myocardial infarction in the mouse. J Am Coll
Cardiol. 46:2116–2124. 2005. View Article : Google Scholar
|
|
25
|
Ong SG and Hausenloy DJ: Hypoxia-inducible
factor as a therapeutic target for cardioprotection. Pharmacol
Ther. 136:69–81. 2012. View Article : Google Scholar
|
|
26
|
Zhou XL and Liu JC: Role of Notch
signaling in the mammalian heart. Braz J Med Biol Res. 47:1–10.
2014. View Article : Google Scholar
|
|
27
|
Croquelois A, Domenighetti AA, Nemir M,
Lepore M, Rosenblatt-Velin N, Radtke F and Pedrazzini T: Control of
the adaptive response of the heart to stress via the Notch1
receptor pathway. J Exp Med. 205:3173–3185. 2008. View Article : Google Scholar :
|
|
28
|
Yu B and Song B: Notch 1 signalling
inhibits cardiomyocyte apoptosis in ischaemic postconditioning.
Heart Lung Circ. 23:152–158. 2014. View Article : Google Scholar
|
|
29
|
Pei H, Yu Q, Xue Q, Guo Y, Sun L, Hong Z,
Han H, Gao E, Qu Y and Tao L: Notch1 cardioprotection in myocardial
ischemia/reperfusion involves reduction of oxidative/nitrative
stress. Basic Res Cardiol. 108:3732013. View Article : Google Scholar
|
|
30
|
Meng D, Chen XJ, Yang D, Bian YY, Li P and
Zhang JN: Effects of Astragaloside on the cultivation of calcium
overload in rat cardiac myocytes. Chin J Clin Rehab. 8:462–463.
2004.
|