Introduction
The aryl hydrocarbon receptor (AhR) is a cytosolic
receptor and ligand-activated transcriptional factor. In the
absence of a ligand, cytoplasmic AhR is present in a primarily
dormant state in an inactive complex that contains two 90-kDa
heat-shock proteins (HSP90), hepatitis B virus X-associated
protein, p23 and the AhR inhibitory protein (1) It has been hypothesized that HSP90
maintains AhR in a conformation that is capable of high-affinity
ligand binding to prevents its nuclear translocation (2). AhR ligands consist primarily of
polycyclic aromatic hydrocarbons (PAHs) (3). Upon binding a ligand, AhR is
activated. AhR is able to dissociate from HSP90, translocate into
the nucleus and form a heterodimer with the closely associated
nuclear protein aryl hydrocarbon receptor nuclear translocator
(ARNT) (4). The AhR-ARNT
heterodimer creates a specific DNA recognition sequence, GCGTG,
within a responsive element, known as the xenobiotic responsive
element (XRE). XREs are located in the promoter regions of a number
of receptor-regulated genes, including CYP1A1, CYP1A2, CYP1B, and
CYP2E1 (1). CYP1A1 is of
particular interest because it is the most active of the group of
cytochrome P450s that metabolize ligands of AhR, PAHs, into
reactive species.
Cardiac CYP1A1 transcription was significantly
induced by treatment with AhR ligands, including
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), benzo(a)pyrene
(BaP), 3,3,4,4,5-pentachlorobiphenyl (PCB 126), β-naphthoflavone
(bNF) and 3-methylcholanthrene, in a variety of mammalian species
(5,6). Previous studies investigating the
expression of constitutive CYP1A1 mRNA have demonstrated that TCDD
and BaP are the most potent inducers of CYP1A1 expression (3). It has been suggested that CYP1A1 mRNA
can be superinduced by treatment with TCDD, which is the most
potent AhR ligand described (7).
TCDD is a pervasive environmental contaminant that induces hepatic
and myocardial oxidative stress (8,9).
CYP1A1 belongs to the cytochrome P450 family (family
1, subfamily A, polypeptide 1). CYP1A1 was initially thought to be
localized only to the endoplasmic reticulum, until a mitochondrial
form of CYP1A1 was characterized in the livers of rats that were
pre-treated with bNF and, more recently, on inner mitochondrial
membranes (10). CYP1A1 has been
demonstrated to produce elevated levels of reactive oxygen species
(ROS) in fish and rodent mitochondria and microsomes when
challenged with the PAH compounds 3,3′,4,4′-tetrachlorobiphenyl
(11) and PCB 126 (12). It has been previously indicated
that the CYP1A1 metabolism serves a major role in detoxifying
foreign chemicals and metabolic activation, which leads to
oxidative damage (3). A previous
study established a link between CYP1A1 and oxidative stress
(8). However, little is currently
understood regarding the mechanisms underlying mitochondrial
oxidative stress in human cardiomyocytes.
In the present study, it was investigated whether
the AhR-induced overexpression of CYP1A1 causes mitochondrial
oxidative stress-related effects in the human cardiac-derived AC16
cell line. The present study may aid to determine the involvement
of AhR and its downstream gene product, CYP1A1, in the mechanisms
underlying dioxin-induced subacute levels of mitochondrial
oxidative stress.
Materials and methods
Chemicals
The reagent, TCDD, was purchased from AccuStandard,
Inc. (New Haven, CT, USA). A pre-calculated volume of TCDD (10 µl)
was first dissolved in dimethyl sulfoxide (DMSO; 300.5 µl). A
concentration of 100 nM TCDD stock liquid of TCDD was obtained. The
appropriate stock solutions of TCDD were added directly to the
culture media to achieve a final concentration of 0.1, 1, 5 and 10
nM of TCDD. The concentration of DMSO was not allowed to exceed
0.05% (v/v). Dulbecco's modified Eagle's medium (DMEM)/F-12 was
purchased from Beijing Aipoo HuaMei Biotechnology Co., Ltd.
(Beijing, China). Fetal bovine serum (FBS) was purchased from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). A High-Capacity
cDNA Reverse Transcription kit and SYBR-Green Super Mix were
purchased from Takara Biotechnology Co., Ltd. (Dalian, China). The
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) primers were synthesized by AuGCT DNA-SNY Biotechnology,
Inc. (Beijing, China). Rabbit anti-human CYP1A1 (catalog no.
sc-39397)/AhR (catalog no. sc-0816R) polyclonal antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Cell cultures and treatments
The AC16 human myocardial cell line (Land Biology,
Inc., Guangzhou, China) was cultured in standard DMEM that was
supplemented with 10% FBS and 1% penicillin-streptomycin (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA) in 25 cm3
plastic culture flasks. The cells were grown in the culture flasks
at 37°C in a 5% CO2 humidified incubator. The
appropriate stock solutions of TCDD were added directly to the
culture media to achieve a final concentration of 0.1 nM or 10 nM
of TCDD. To measure the effects of TCDD on cell viability and
cardiomyocyte mitochondrial membrane oxidative injury, cells were
grown at a density of 7.5×104 cells/well in 96-well
tissue culture plates. For the analyses used to determine mRNA and
protein levels and the intracellular production of oxidants, the
cardiomyocytes were seeded into 6-well plates at a density of
1.5×106 cells/well. To measure mitochondrial activity,
the cells were cultured in 24-well tissue culture plates at a
density of 1.5×105 cells/well.
The effect of TCDD on cell
viability
The cytotoxic effects of TCDD on AC16 myocardial
cell viability were determined by measuring the capacity of the
reducing enzymes present in viable cells to MTT to formazan
crystals. Briefly, cardiomyocytes were treated with various
concentrations of TCDD (0, 0.1, 1, 5 or 10 nM) for 24 h. A volume
of 20 µl MTT solution (5 mg/ml in PBS) was then added, and the
cells were incubated for 4 h at 37°C. The supernatants were then
aspirated, and 150 µl DMSO was added to each well. The plates were
shaken for 10 min to thoroughly dissolve the formazan crystals. The
absorbance at 570 nm was measured for each well using a microplate
reader (Infinite™ M2000; Tecan, Männedorf, Switzerland).
RNA extraction and RT-qPCR
Following incubation of the AC16 cardiomyocytes with
various concentrations of TCDD (0, 0.1, 1, 5 or 10 nM) for 24 h,
total RNA was extracted using an E.Z.N.A Total RNA kit I (R6834-01;
Omega Bio-Tek, Inc., Norcross, GA, USA) according to the
manufacturer's instructions. The concentration and purity of the
total RNA was evaluated by measuring the 260/280 nm absorbance
ratio using a Thermo Nanodrop 2000 (NanoDrop; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). To obtain cDNA, total RNA
(0.5 µg) was reverse transcribed using a PrimeScript™ RT reagent
kit (DRR037A; Takara Bio, Inc., Otsu, Japan), three times. RT-qPCR
was then performed using a Bio-Rad IQ5 PCR Detection system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) with SYBR Premix Ex
Taq™ II (RR820A; Takara Bio Inc.) according to the manufacturer's
instructions. The following specific primer sequences were used:
CYP1A1 forward, 5′-CCTCCTCAACCTCCTGCTAC-3′ and reverse,
5′-AAGCAAATGGCACAGAYGAC-3′; CYP2C19 forward,
5′-GGTCCTTGTGCTCTGTCTCT-3′ and reverse, 5′-CATATCCATGCAGCACCACC-3′;
GAPDH forward, 5′-TGCACCACCAACTGCTTAGC-3′ and reverse,
5′-GGCATGGACTGTGGTCATGAG-3′.
Protein extraction and western blot
analysis
Cardiomyocytes were pre-treated with various
concentrations of TCDD (0, 0.1, 1, 5 or 10 nM) for 24 h. The cells
were then rinsed with ice-cold PBS, and the cytoplasmic and nuclear
proteins were lysed using radioimmunoprecipitation buffer (HEART
Biological Technology Co., Ltd., Xi'an, China). A total of 25 µg
protein was separated from each treatment group using 12% SDS-PAGE,
and the separated proteins were then electrophoretically
transferred onto polyvinylidene difluoride membranes. The membranes
were blocked in 0.75 g skim milk dissolved in 15 ml tris-buffered
saline with Tween-20 (TBST) and probed with primary polyclonal
rabbit anti-human antibodies against CYP1A1 (catalog no. sc-39397;
1:500), AhR (catalog no. sc-166586; 1:800) and β-actin (catalog no.
sc-130656; 1:400; Santa Cruz Biotechnology, Inc.) at 4°C overnight.
The primary antibodies were then discarded and the membranes were
washed three times with TBST and incubated with the appropriate
horseradish peroxidase-conjugated goat anti-rabbit IgG (catalog no.
A0216; 1:400; Beyotime Institute of Biotechnology, Haimen, China)
for 2 h with shaking at room temperature. The bands were visualized
using enhanced chemiluminescence according to the manufacturer's
instructions(EMD Millipore, Billerica, MA, USA).
Determination of CYP1A1 enzymatic
activity
The activity of 7-ethoxyresorufin O-deacylase (EROD)
was used as a diagnostic marker of CYP1A1 enzymatic activity. The
7-ethoxyresorufin generated resorufin when it was catalyzed by
EROD. The wavelengths used to analyze excitation and emission (λex,
488 nm and λem, 505 nm) were 530 and 590 nm, respectively. The rate
of the change in fluorescence was recorded prior to the experiment
and after the addition of a known quantity of resorufin using a
microplate reader.
Effect of TCDD on intracellular ROS
production
To determine whether TCDD-induced CYP1A1
overexpression resulted in an increase in intra-cellular ROS
production, intracellular oxidant production was analyzed using the
non-fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA;
Beyotime Institute of Biotechnology). Cardiomyocytes were seeded
into 6-well plates at a density of 1.5×106 cells/well
and treated with various concentrations of TCDD. The cells were
incubated with diluted DCFH-DA at 37°C for 20 min and
reverse-blended every 3–5 mins. The cells were then washed three
times with serum-free cell culture medium, and the DCF fluorescence
distribution was detected in 2×107 cells using flow
cytometry.
Detection of living cellular
mitochondrial activity
The mitochondrial activity of cardiomyocytes was
analyzed using the fluorescent probe Mito Tracker RED staining
assay kit (Genmed Scientifics, Inc., Wilmington, DE, USA) according
to the manufacturer's instructions. Mito Tracker RED is a type of
fluorescent dye that contains chlorine methyl thiol groups, which
freely travel through the cell membrane and selectively accumulate
in mitochondria. Cardiomyocytes were seeded in 24-well plates, and
the cell culture fluid was removed when confluence reached 70%. The
medium was then preheated to 37°C (500 µl/well) and added to each
well. Following this, solutions of the liquid Reagents C and D
(20:1; 1 µl/well) were added, and the cells were incubated at 37°C
for 20 min. Finally, fluorescence intensity was analyzed using an
inverted fluorescence microscope.
Fluorometric assays for detecting
mitochondrial membrane damage/oxidation
Cardiolipin is one of the main components in
mitochondrial membranes (13). The
hydrophobic fluorescence dye 10-N-nonyl acrydine orange (NAO) is a
stain that binds specifically to mitochondrial cardiolipin and then
emits fluorescence. The Mitochondria Damage Testing kit (Genmed
Scientifics, Inc.) was used according to the manufacturer's
instructions to directly assess the degree of damage and oxidation
in the mitochondrial membrane. Briefly, the cells were grown in
96-well plates. The culture medium, which contained various
concentrations of TCDD, was removed when the cells covered the
bottom of the well, and the cells were then washed with 100 µl
reagent A. After the solution containing reagent A was thoroughly
drained, the cells were then incubated at 37°C for 20 min using 50
µl/well of reagent B and reagent C (ratio, 1:99). Finally, the
cells were washed with reagent A again. The relative fluorescence
units were measured using a microplate reader (λex 488 nm and λem
505 nm).
Statistical analysis
Statistical analysis was performed using SPSS
software (version, 18.0; SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of TCDD on cardiomyocyte
viability
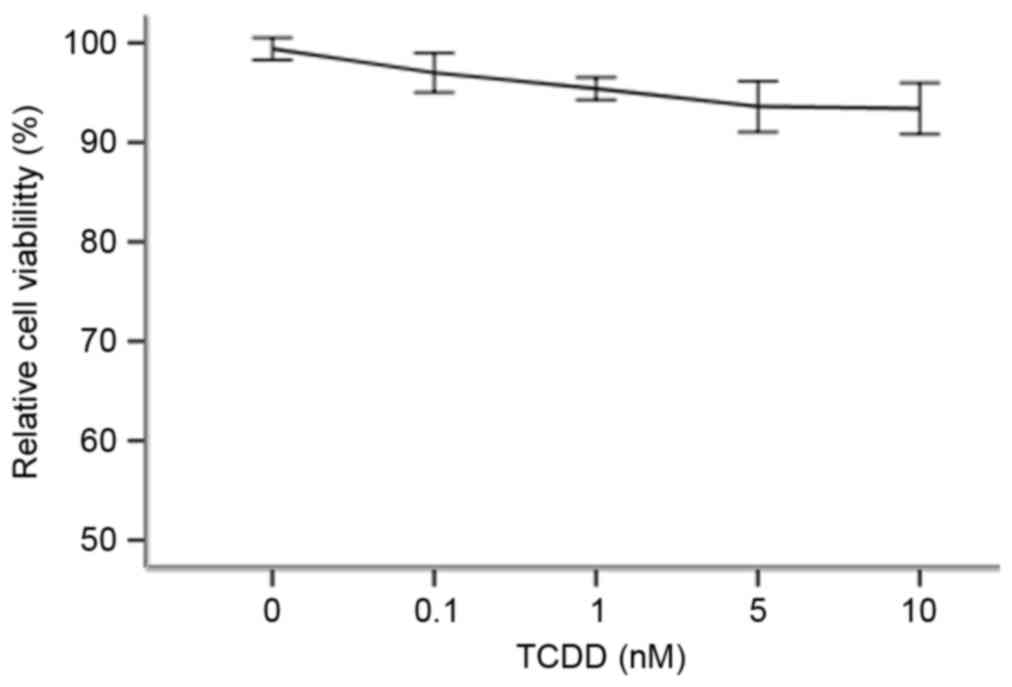
An MTT assay was performed to determine which
optimal concentrations to use in the following studies. As
demonstrated in Fig. 1, when TCDD
was applied at 0.1–10 nM, it demonstrated no significant
cytotoxicity towards cardiomyocytes (P>0.05). The percentage of
surviving cells was calculated for each concentration group by
dividing the difference between the optical density (OD) values of
the intervention group and the blank group by the difference
between the OD values of the negative control group and the blank
group.
The induction of CYP1A1 and AhR gene
expression by TCDD
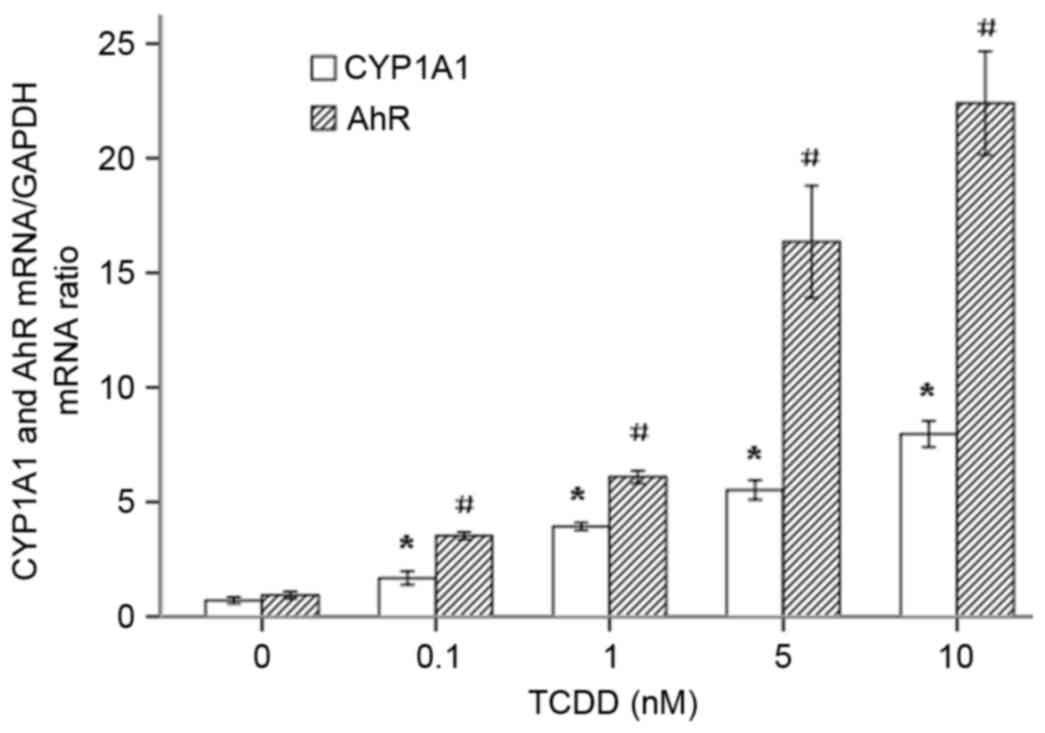
To investigate whether TCDD increases the
transcription of CYP1A1 mRNA by regulating the expression of the
transcription regulator AhR, AC16 cells were treated with
increasing concentrations of TCDD (0.1–10 nM) for 24 h. The ratio
of absorbance at 260/280 of the total RNA of each group was
1.94–2.02 and was maintained at approximately 2.0, as measured
using a Thermo Nanodrop 2000. The RNA samples had sufficient
purity. The expression of the CYP1A1 and AhR mRNAs was measured
using RT-qPCR. As presented in Fig.
2, TCDD significantly increased the expression of the CYP1A1
and AhR mRNAs in a dose-dependent manner.
The effects of TCDD on CYP1A1 and AhR
protein expression and enzymatic activity
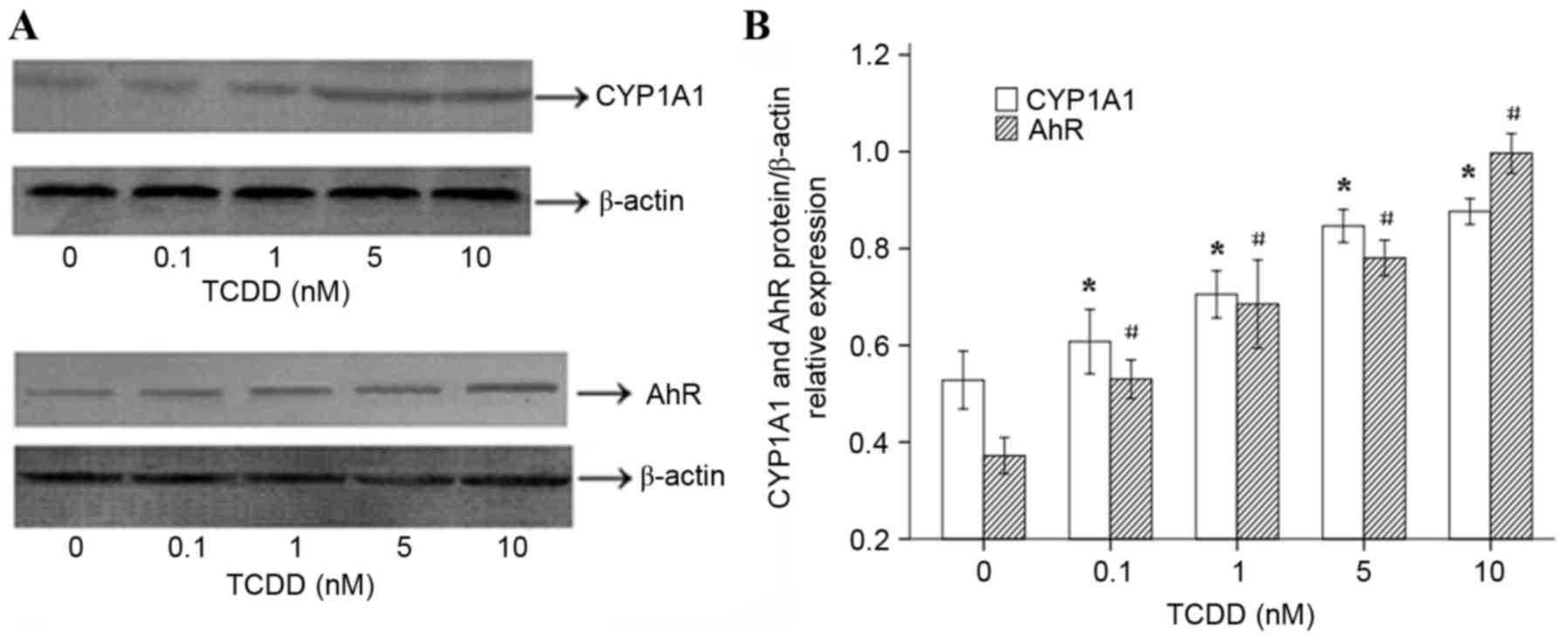
To determine whether the TCDD-induced expression of
the CYP1A1 and AhR mRNAs in AC16 cells is translated into an
increase in the level of functional proteins, western blot analyses
were performed. The results (Fig.
3A) suggested that 24 h of treatment with various
concentrations of TCDD significantly increased the protein
expression levels of CYP1A1 and AhR, which was consistent with the
observed changes in gene expression. ImageJ software (version,
1.43, National Institutes of Health, Bethesda, MD, USA) was used to
calculate and analyze gray values to assess the presence of
comparable amounts of protein in each lane, as presented in
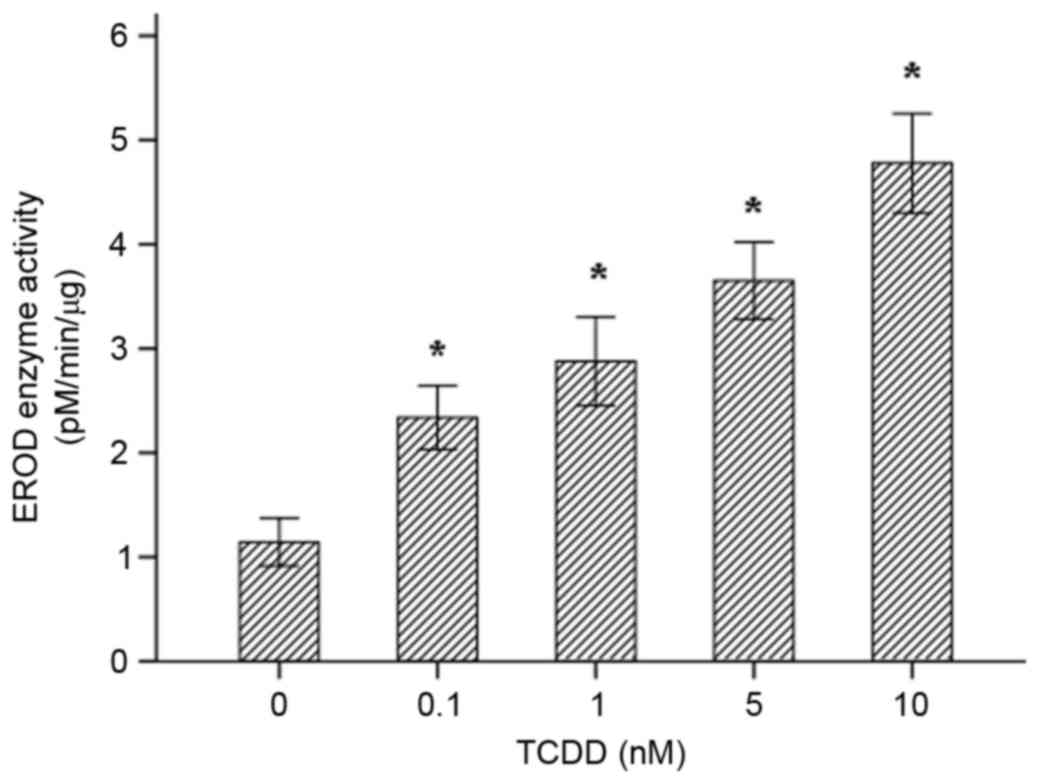
Fig. 3B. The EROD activity
observed in each of the different groups is presented in Fig. 4. AC16 cardiomyocytes that were
incubated for 24 h displayed a distinct concentration-dependent
elevation in CYP1A1 enzymatic activity.
ROS generation in cultured
cardiomyocytes stimulated with TCDD
Exposure to TCDD induced a significant increase in
the generation of ROS for concentration of 1, 5 and 10 nM TCDD
(Fig. 5, P<0.05). All values
are presented relative to the control cells (exposed to DMSO
(100%). Treatment with 0.1 nM TCDD did not cause a significant
change in ROS production (P>0.05).
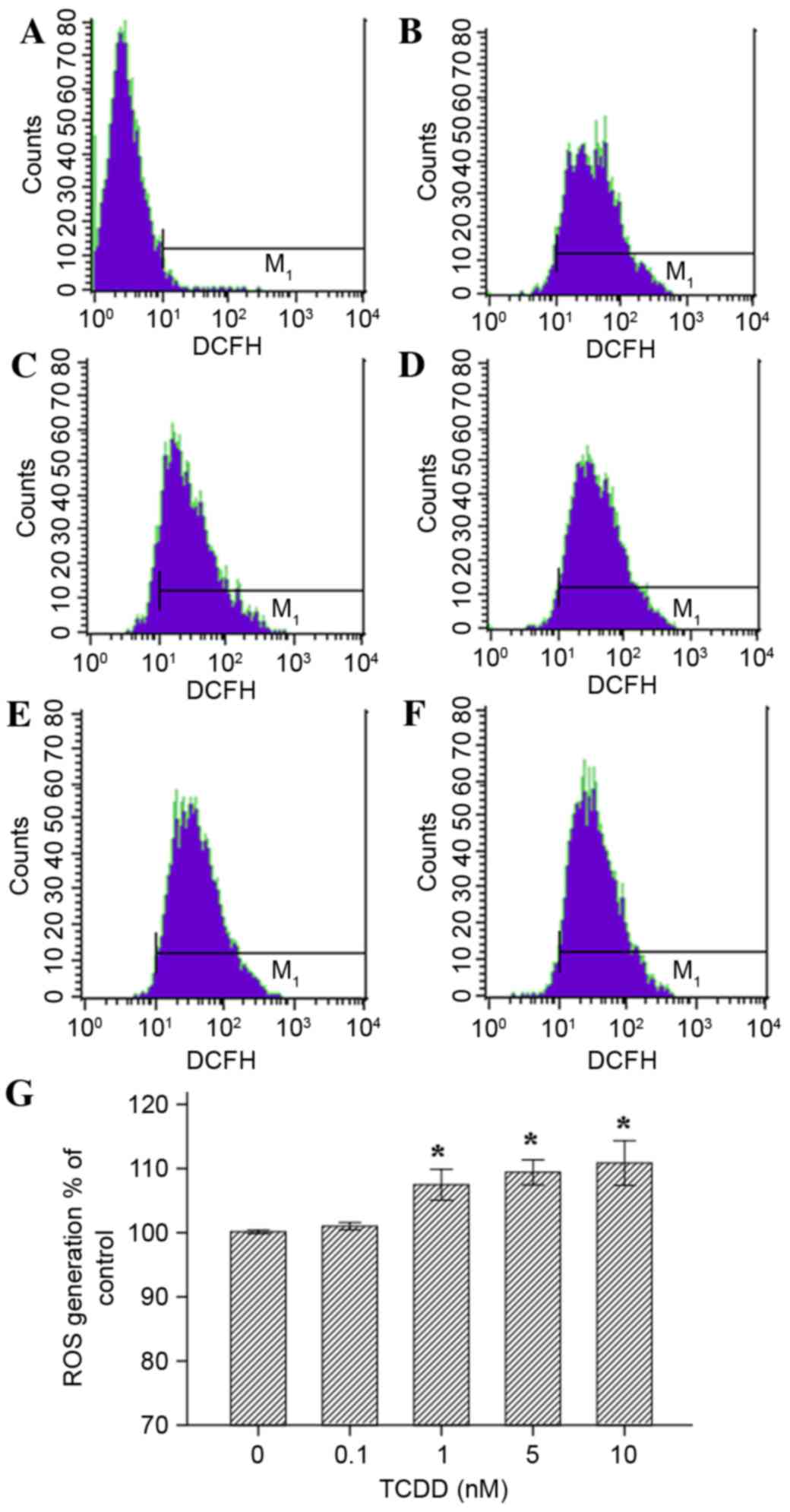 | Figure 5.Effects of TCDD on the generation of
ROS in AC16 cardiomyocytes that were pre-treated with TCDD for 24
h. Intracellular ROS levels were estimated using flow cytometry
with the probe DCFH-DA, which is oxidized to form DCF in the
presence of ROS. The cells were treated as follows: (A) Blank group
(non-probe); (B) control (concentration=0 nM); (C) intervention
group, (0.1 nM); (D) intervention groups (1 nM); (E intervention
group (5 nM); and (F) intervention group (10 nM). (G) A guidance
line has been drawn through the histograms being compared. TCDD,
2,3,7,8-tetrachlorodibenzo-p-dioxin; ROS, reactive oxygen species;
DCFH-DA, 2′,7′-dichlorofluorescein diacetate; DCF,
dichlorofluorescein. |
TCDD reduced mitochondrial
activity
The changes in mitochondrial activity that were
associated with TCDD were assessed qualitatively by staining the
mitochondria with a Mito Tracker RED probe. Mito Tracker RED is the
reduced form of a red fluorescent dye (λex=579 nm and λem=599 nm),
which does not fluoresce on its own. Following incubation with Mito
Tracker RED, the probe penetrates the cell membrane via passive
transport, and the oxidized fluorescence probe inside the cells
selectively gathers on the activated mitochondria. The results
obtained using an inverted fluorescence microscope demonstrated
that TCDD induced changes in mitochondrial activity that resulted
in the loss of red fluorescence (Fig.
6).
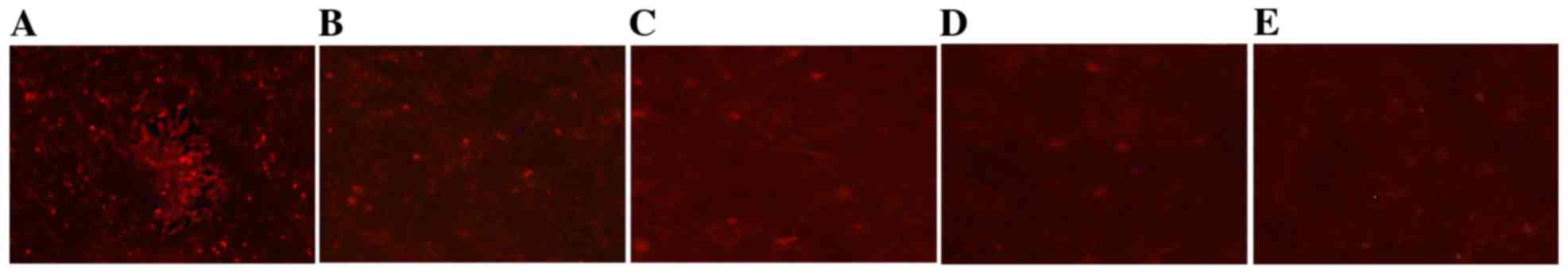 | Figure 6.Effects of TCDD on mitochondrial
activity in AC16 cells. The mitochondria of living cells were
stained using Mito Tracker RED. Digital images of each well were
obtained using inverted fluorescence microscopy at ×40
magnification. (A), (B), (C), (D) and (E) represent 0, 0.1, 1, 5
and 10 nM of TCDD, respectively. TCDD,
2,3,7,8-tetrachlorodibenzo-p-dioxin. |
The effect of TCDD on mitochondrial
mass in cardiomyocytes
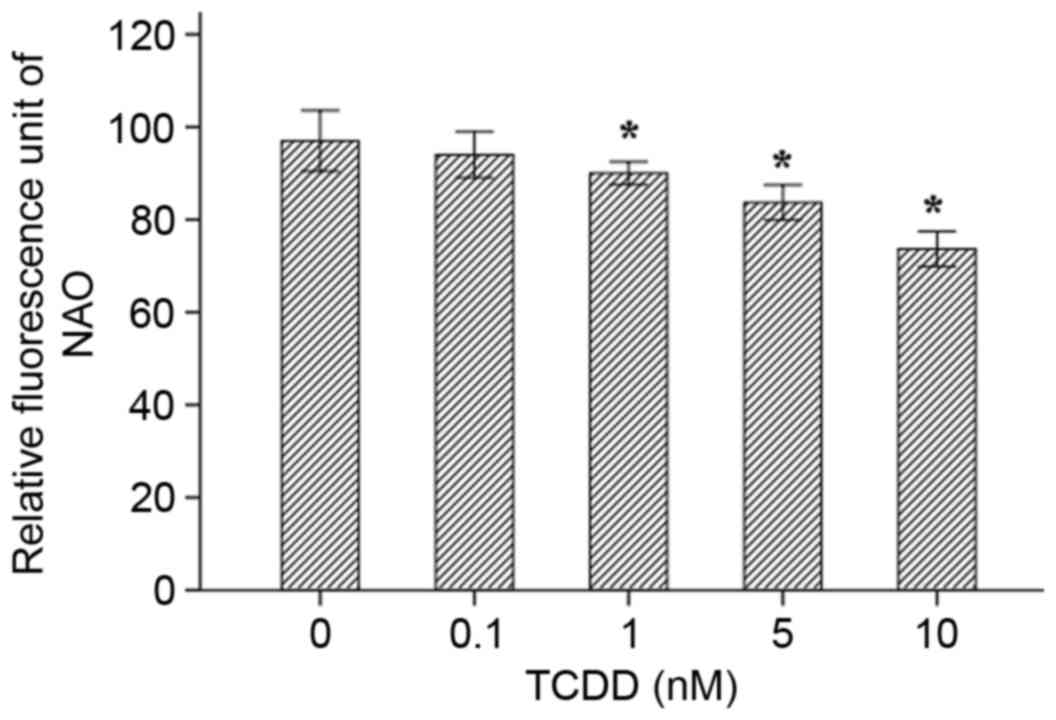
NAO is a fluorescent probe that is widely used to
determine mitochondrial mass in living cells. When the
mitochondrial membrane quality is damage or oxidized, the NAO dye
combines with membrane cardiolipin and is reduced, resulting in a
decrease in fluorescence intensity. The results of the assays
indicated that as TCDD concentration increased, the quality of the
mitochondrial membrane decreased for concentrations of 1, 5 and 10
nM (Fig. 7, P<0.05).
Discussion
Previous studies demonstrated that CYP1A1 was
constitutively expressed and inducible in H9c2 cells and that the
cardiotoxic effects of AhR ligands included structural
malformations, ventricular hypertrophy and abnormal myocyte
contractility in several in vivo models (7,9,14).
In the present study, five different concentrations of the AhR
ligand TCDD were applied to AC16 cardiac cells. The results
demonstrated that this myocardial cell line may constitutively
express the CYP1A1 mRNA and protein, leading to its enzyme
activity. In addition, the process of inducing gene expression of
CYP1A1 was accompanied by a decline in mitochondrial activity and
quality and an increase in mitochondrial ROS.
The AC16 cell line is a commercially available
myogenic cell line that is derived from human heart cells. TCDD is
a congener, which is the most well studied in a family of
halogenated aromatic hydrocarbons known as dioxins (15). Due to their high lipophilicity and
long half-life, dioxins persist in the environment for long periods
of time and accumulate in biological systems. Dioxins additionally
induce a wide range of toxic effects in the heart, including
impairment of cardiomyocyte differentiation and induction of
hypertrophy and heart failure (7,16,17).
Furthermore, AhR ligands have been reported to alter arrangement of
the mitochondria, resulting in twisted and disrupted cristae, in
addition to altering arrangement of sarcomeres (16). AhR ligands can cause the
misalignment or disarrangement of the myofibrillar organization in
cardiomyocytes, which are derived from embryonic stem cells
(16). Mitochondria are important
organelles that are involved in metabolism and apoptosis, and they
serve important roles in cardiomyopathies (18). In the present study, two commonly
used fluorescent dyes were used to analyze mitochondrial activity
and mass in living cells. The results indicated that, following
incubation with TCDD, the red fluorescence intensity in
cardiomyocytes was significantly weaker than the intensity observed
in the control group, indicating that TCDD affects mitochondrial
activity.
The transcriptional regulation of the CYP1A1 gene is
controlled by the soluble intracellular receptor protein, AhR, and
this process serves a major role in cardiovascular diseases, due to
the fact that these two genes are inducible by PAHs. Generally, the
induction of CYP1A1 is considered to be cardiotoxic as it leads to
the generation of ROS (19), DNA
adducts and endogenous arachidonic acid metabolites (1). CYP1A1 is known to metabolize
arachidonic acid into different regioisomers of
hydroxyeicosatetraenoic acids (HETEs) (7). A previous study indicated that HETEs
and possibly other CYP ω-hydroxylation products serve deleterious
roles in cardiac injuries (20).
Furthermore, inhibiting ω-hydroxylase has been reported to reduce
the size of infarcts (21).
Whether this represented a direct effect by AhR ligands on
cardiotoxicity requires further elucidation. To the best of our
knowledge, no previous studies have demonstrated that the AC16
human heart cell line can be induced to express CYP1A1 mRNA,
protein and enzymatic activity. The present experiments, which were
performed in TCDD-treated AC16 cells, indicated that CYP1A1 was
significantly expressed and inducible in these cells. Because AhR
is a transcriptional regulator of CYP1A1, the expression levels of
AhR mRNA and protein were also assessed. As predicted, the results
confirmed that in cardiomyocytes, treatment with TCDD resulted in
markedly higher levels of AhR expression than were observed in the
blank control group. The regulation of CYP1A1 gene expression
involves the activation of a cytosolic transcriptional factor, AhR,
which is the first step in a series of molecular events that
promote the transcription and translation of CYP1A1 (1,22).
In the present study, ROS levels were increased and
the quality of mitochondrial membranes was reduced in TCDD-treated
cardiomyocytes. A previous study (23) demonstrated that an increase in
intracellular ROS involved numerous signaling pathways and
predisposed cells to damage. In addition, ROS can lead to thiol
oxidation and the inhibition of ATP synthesis and translocation of
adenine nucleotides (24). ROS can
also cause the peroxidation of unsaturated fatty acids in membrane
phospholipids, particularly cardiolipin, on the inner mitochondrial
membrane, which leads to the further inhibition of respiratory
chain activity (25). The
initiation of the ROS-dependent opening of mitochondrial
permeability transition pores has been specifically implicated in
cardiomyocyte cell death (26).
Taken together, the observations made in the current study suggest
a previously unsuspected connection between ROS and TCDD-induced
mitochondrial dysfunction.
In conclusion, the present study demonstrates that
AC16 cardiomyocytes respond to TCDD by increasing CYP1A1 activity
and increasing the mRNA and protein expression levels of CYP1A1 and
AhR. The observations support the involvement of the AhR/CYP1A1
signaling pathway in TCDD toxicity in AC16 cells. Due to the fact
that CYP1A1 is involved in xenobiotic metabolism and detoxification
in humans, malfunctions in this enzyme caused by TCDD disrupting
the normal metabolism and detoxification machinery are suggested to
lead to increased susceptibility of the heart to environmental and
other stressors, in addition to increased risk of
cardiomyopathy.
Acknowledgements
The present study was supported by the National
Natural Scientific Foundation of China (grant nos. 81273008 and
30872192).
References
|
1
|
Korashy HM and El-Kadi AO: The role of
aryl hydrocarbon receptor in the pathogenesis of cardiovascular
diseases. Drug Metab Rev. 38:411–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kann S, Huang MY, Estes C, Reichard JF,
Sartor MA, Xia Y and Puga A: Arsenite-induced aryl hydrocarbon
receptor nuclear translocation results in additive induction of
phase I genes and synergistic induction of phase II genes. Mol
Pharmacol. 68:336–346. 2005.PubMed/NCBI
|
|
3
|
Nebert DW, Dalton TP, Okey AB and Gonzalez
FJ: Role of aryl hydrocarbon receptor-mediated induction of the
CYP1 enzymes in environmental toxicity and cancer. J Biol Chem.
279:23847–23850. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujii-Kuriyama Y and Mimura J: Molecular
mechanisms of AhR functions in the regulation of cytochrome P450
genes. Biochem Biophys Res Commun. 338:311–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thum T and Borlak J: Testosterone,
cytochrome P450 and cardiac hypertrophy. FASEB J. 16:1537–1549.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Granberg AL, Brunström B and Brandt I:
Cytochrome P450-dependent binding of 7,12-dimethylbenz[a]anthracene
(DMBA) and benzo[a]pyrene (B[a]P) in murine heart, lung and liver
endothelial cells. Arch Toxicol. 74:593–601. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zordoky BN and El-Kadi AO:
2,3,7,8-Tetrachlorodibenzo-p-dioxin and beta-naphthoflavone induce
cellular hypertrophy in H9c2 cells by an aryl hydrocarbon
receptor-dependant mechanism. Toxicol In Vitro. 24:863–871. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Senft AP, Dalton TP, Nebert DW, Genter MB,
Puga A, Hutchinson RJ, Kerzee JK, Uno S and Shertzer HG:
Mitochondrial reactive oxygen production is dependent on the
aromatic hydrocarbon receptor. Free Radic Biol Med. 33:1268–1278.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aboutabl ME and El-Kadi AO: Constitutive
expression and inducibility of CYP1A1 in the H9c2 rat
cardiomyoblast cells. Toxicol In Vitro. 21:1686–1691. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong H, Dalton TP, Miller ML, Chen Y, Uno
S, Shi Z, Shertzer HG, Bansal S, Avadhani NG and Nebert DW:
Knock-in mouse lines expressing either mitochondrial or microsomal
CYP1A1: Differing responses to dietary benzo[a]pyrene as proof of
principle. Mol Pharmacol. 75:555–567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schlezinger JJ, White RD and Stegeman JJ:
Oxidative inactivation of cytochrome P-450 1A (CYP1A) stimulated by
3,3′, 4,4′-tetrachlorobiphenyl: Production of reactive oxygen by
vertebrate CYP1As. Mol Pharmacol. 56:588–597. 1999.PubMed/NCBI
|
|
12
|
Schlezinger JJ and Stegeman JJ: Induction
and suppression of cytochrome P450 1A by
3,3′,4,4′,5-pentachlorobiphenyl and its relationship to oxidative
stress in the marine fish scup (Stenotomus chrysops). Aquat
Toxicol. 52:101–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Claypool SM and Koehler CM: The complexity
of cardiolipin in health and disease. Trends Biochem Sci. 37:32–41.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heid SE, Walker MK and Swanson HI:
Correlation of cardiotoxicity mediated by halogenated aromatic
hydrocarbons to aryl hydrocarbon receptor activation. Toxicol Sci.
61:187–196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Abrew KN, Thomas-Virnig CL, Rasmussen
CA, Bolterstein EA, Schlosser SJ and Allen-Hoffmann BL: TCDD
induces dermal accumulation of keratinocyte-derived matrix
metalloproteinase-10 in an organotypic model of human skin. Toxicol
Appl Pharmacol. 276:171–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neri T, Merico V, Fiordaliso F, Salio M,
Rebuzzini P, Sacchi L, Bellazzi R, Redi CA, Zuccotti M and Garagna
S: The differentiation of cardiomyocytes from mouse embryonic stem
cells is altered by dioxin. Toxicol Lett. 202:226–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zordoky BN and El-Kadi AO: Modulation of
cardiac and hepatic cytochrome P450 enzymes during heart failure.
Curr Drug Metab. 9:122–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao S, Li H, Cai Y, Ye JT, Liu ZP, Lu J,
Huang XY, Feng XJ, Gao H, Chen SR, et al: Mitochondrial binding of
α-enolase stabilizes mitochondrial membrane: Its role in
doxorubicin-induced cardiomyocyte apoptosis. Arch Biochem Biophys.
542:46–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hunter AL, Bai N, Laher I and Granville
DJ: Cytochrome p450 2C inhibition reduces post-ischemic vascular
dysfunction. Vascul Pharmacol. 43:213–219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Granville DJ and Gottlieb RA: Having a
heart attack? Avoid the ‘HETE’! Am J Physiol Heart Circ Physiol.
291:H485–H487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nithipatikom K, Gross ER, Endsley MP,
Moore JM, Isbell MA, Falck JR, Campbell WB and Gross GJ: Inhibition
of cytochrome P450omega-hydroxylase: A novel endogenous
cardioprotective pathway. Circ Res. 95:e65–e71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Denison MS, Fisher JM and Whitlock JP Jr:
Protein-DNA interactions at recognition sites for the dioxin-Ah
receptor complex. J Biol Chem. 264:16478–16482. 1989.PubMed/NCBI
|
|
23
|
Di Lisa F and Bernardi P: Mitochondria and
ischemia-reperfusion injury of the heart: Fixing a hole. Cardiovasc
Res. 70:191–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yue R, Hu H, Yiu KH, Luo T, Zhou Z, Xu L,
Zhang S, Li K and Yu Z: Lycopene protects against
hypoxia/reoxygenation-induced apoptosis by preventing mitochondrial
dysfunction in primary neonatal mouse cardiomyocytes. PLoS One.
7:e507782012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paradies G, Petrosillo G, Pistolese M, Di
Venosa N, Federici A and Ruggiero FM: Decrease in mitochondrial
complex I activity in ischemic/reperfused rat heart: Involvement of
reactive oxygen species and cardiolipin. Circ Res. 94:53–59. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Papanicolaou KN, Ngoh GA, Dabkowski ER,
O'Connell KA, Ribeiro RF Jr, Stanley WC and Walsh K: Cardiomyocyte
deletion of mitofusin-1 leads to mitochondrial fragmentation and
improves tolerance to ROS-induced mitochondrial dysfunction and
cell death. Am J Physiol Heart Circ Physiol. 302:H167–H179. 2012.
View Article : Google Scholar : PubMed/NCBI
|