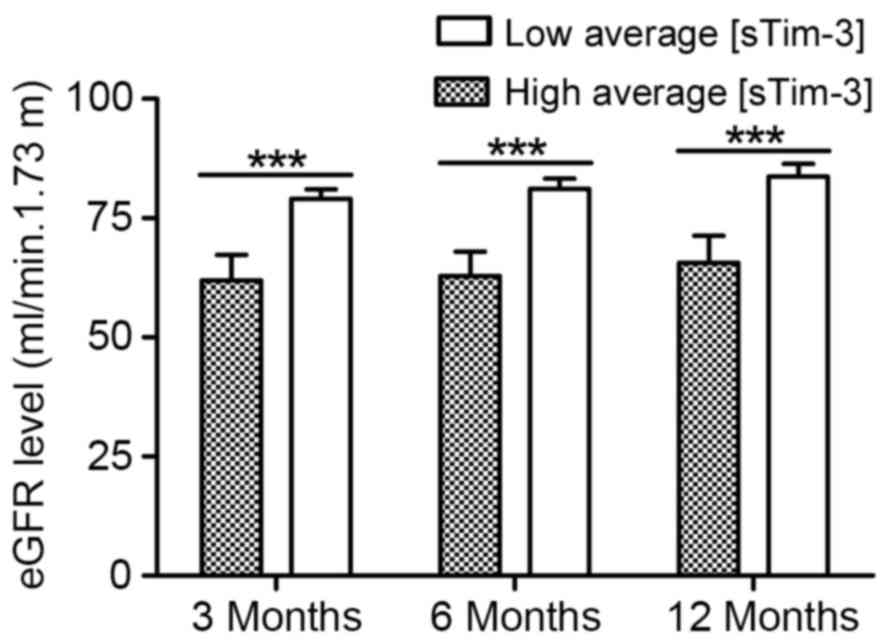
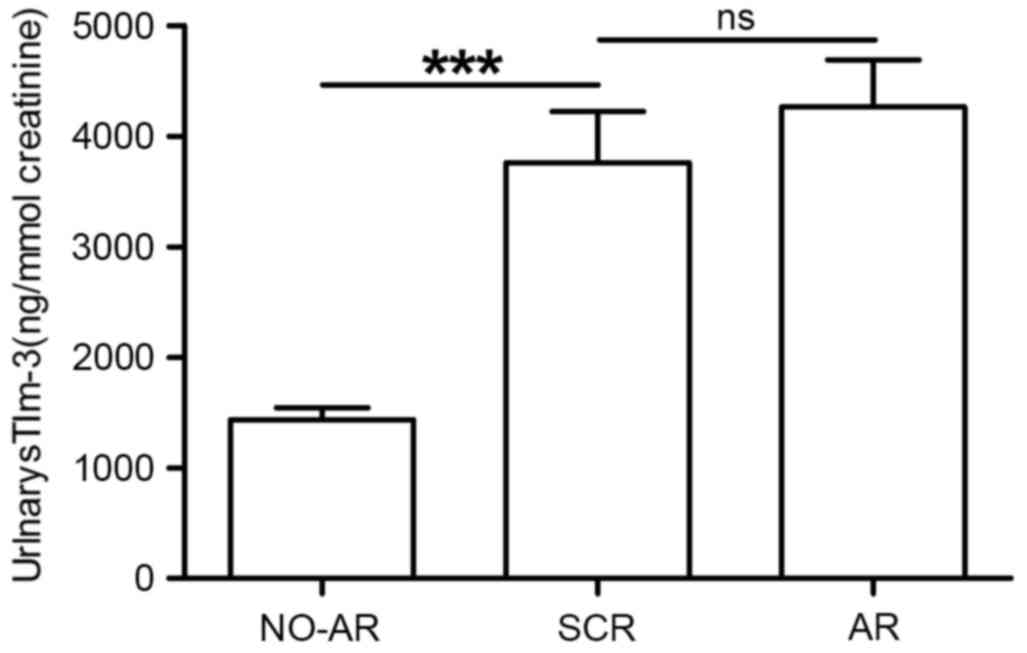
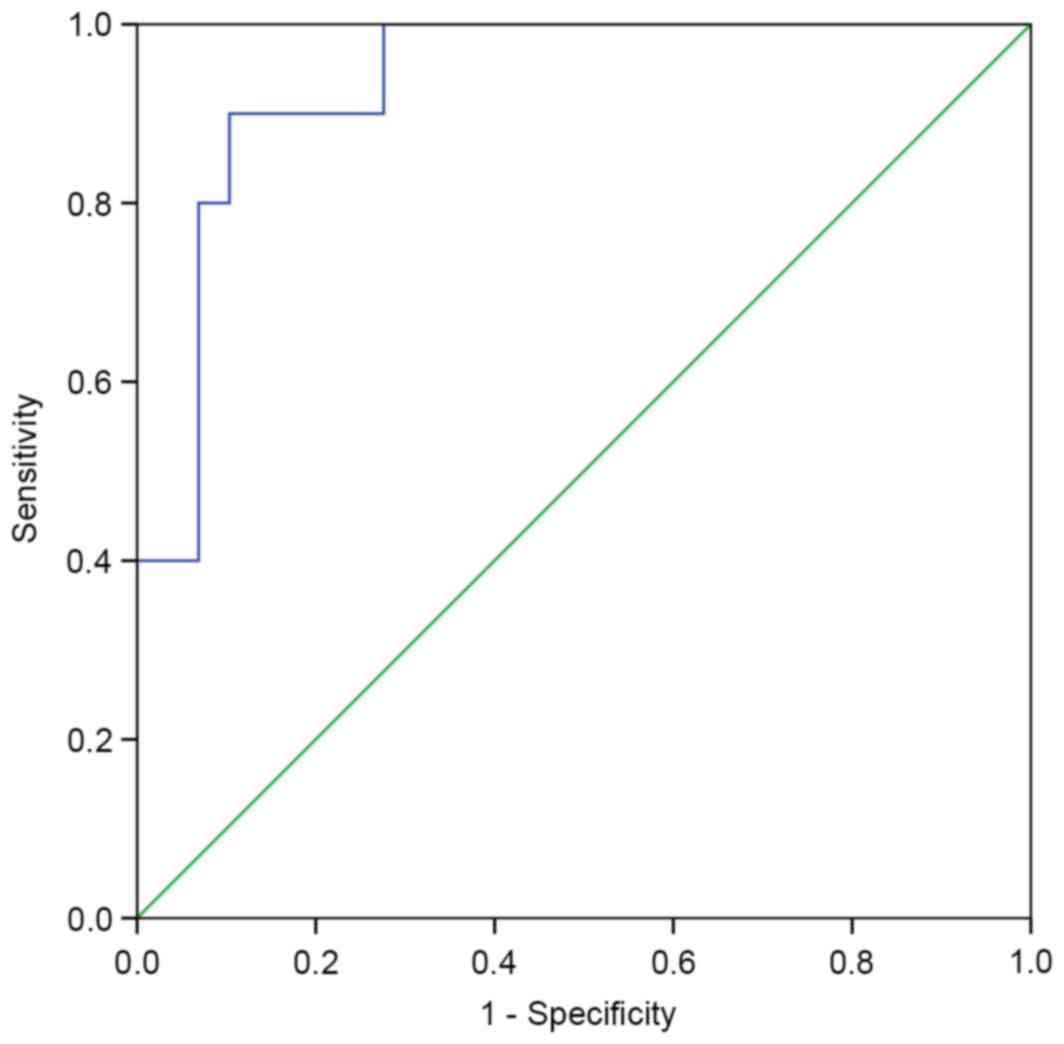
|
1
|
Curtis JJ: End-stage renal disease
patients: Referral for transplantation. J Am Soc Nephrol. 9:(12
Suppl). S137–S140. 1998.PubMed/NCBI
|
|
2
|
Karam VH, Gasquet I, Delvart V, Hiesse C,
Dorent R, Danet C, Samuel D, Charpentier B, Gandjbakhch I, Bismuth
H and Castaing D: Quality of life in adult survivors beyond 10
years after liver, kidney and heart transplantation.
Transplantation. 76:1699–1704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hariharan S, Johnson CP, Bresnahan BA,
Taranto SE, McIntosh MJ and Stablein D: Improved graft survival
after renal transplantation in the United States, 1988 to 1996. N
Engl J Med. 342:605–612. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meier-Kriesche HU, Schold JD, Srinivas TR
and Kaplan B: Lack of improvement in renal allograft survival
despite a marked decrease in acute rejection rates over the most
recent era. Am J Transplant. 4:378–383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beckingham IJ, Nicholson ML and Bell PR:
Analysis of factors associated with complications following renal
transplant needle core biopsy. Br Urol. 73:13–15. 1994. View Article : Google Scholar
|
|
6
|
Thongboonkerd V, Mcleish KR, Arthur JM and
Klein JB: Proteomic analysis of normal human urinary proteins
isolated by acetone precipitation or ultracentrifugation. Kidney
Int. 62:1461–1469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pieper R, Gatlin CL, Mcgrath AM, Makusky
AJ, Mondal M, Seonarain M, Field E, Schatz CR, Estock MA, Ahmed N,
et al: Characterization of the human urinary proteome: A method for
high-resolution display of urinary proteins on two-dimensional
electrophoresis gels with a yield of nearly 1400 distinct protein
spots. Proteomics. 4:1159–1174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hauser IA, Spiegler S, Kiss E, Gauer S,
Sichler O, Scheuermann EH, Ackermann H, Pfeilschifter JM, Geiger H,
Gröne HJ and Radeke HH: Prediction of acute renal allograft
rejection by urinary monokine induced by IFN-gamma (MIG). J Am Soc
Nephrol. 16:1849–1858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muthukumar T, Dadhania D, Ding R,
Snopkowski C, Naqvi R, Lee JB, Hartono C, Li B, Sharma VK, Seshan
SV, et al: Messenger RNA for FOXP3 in the urine of renal-allograft
recipients. N Engl J Med. 353:2342–2351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Riordan E, Orlova TN, Mei JJ, Butt K,
Chander PM, Rahman S, Mya M, Hu R, Momin J, Eng EW, et al:
Bioinformatic analysis of the urine proteome of acute allograft
rejection. J Am Soc Nephrol. 15:3240–3248. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng W, Chen J, Jiang Y, Wu J, Shou Z, He
Q, Wang Y, Chen Y and Wang H: Urinary fractalkine is a marker of
acute rejection. Kidney Int. 74:1454–1460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng W, Chen J, Jiang Y, Shou Z, Chen Y
and Wang H: Acute renal allograft rejection is associated with
increased levels of vascular endothelial growth factor in the
urine. Nephrology (Carlton). 13:73–79. 2008.PubMed/NCBI
|
|
13
|
D'elios MM, Josien R, Manghetti M, Amedei
A, de Carli M, Cuturi MC, Blancho G, Buzelin F, del Prete G and
Soulillou JP: Predominant Th1 cell infiltration in acute rejection
episodes of human kidney grafts. Kidney Int. 51:1876–1884. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Monney L, Sabatos CA, Gaglia JL, Ryu A,
Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel
RA, et al: Th-1 specific cell surface protein TIM-3 regulates
macrophage activation and severity of an autoimmune disease.
Nature. 415:536–541. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuchroo VK, Umetsu DT, DeKruyff RH and
Freeman GJ: The TIM gene family: Emerging roles in immunity and
disease. Nat Rev Immunol. 3:454–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sabatos CA, Chakravarti S, Cha E, Schubart
A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ and
Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T
helper type 1 responses and induction of peripheral tolerance. Nat
Immunol. 4:1102–1110. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sánchez-Fueyo A, Tian J, Picarella D,
Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T,
Kuchroo VK, et al: TIM-3 inhibits T helper type 1-mediated auto-
and alloimmune responses and promotes immunological tolerance. Nat
Immunol. 4:1093–1101. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khademi M, Illés Z, Gielen AW, Marta M,
Takazawa N, Baecher-Allan C, Brundin L, Hannerz J, Martin C, Harris
RA, et al: T cell Ig- and mucin-domain-containing molecule-3
(TIM-3) and tim-1 molecules are differentially expressed on human
Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear
cells in multiple screrosis. J Immunol. 172:7169–7176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu JY, Chen JH, Wang YM, He Q and Wu DB:
Improved clinical outcomes in Chinese renal allograft recipients
receiving lower dose immunosuppressants. Transplantation.
78:713–718. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Racusen LC, Solez K, Colvin RB, Bonsib SM,
Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo
AB, et al: The Banff 97 working classification of renal allograft
pathology. Kidney Int. 55:713–723. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Racusen LC, Colvin RB, Solez K, Mihatsch
MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ,
Fishbein MC, et al: Antibody-mediated rejection criteria-an
addition to the Banff 97 classification of renal allograft
rejection. Am J Transplant. 3:708–714. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, He W, Yuan J, Wu K, Zhou H, Zhang
W and Chen ZK: Activation of Tim-3-Galectin-9 pathway improves
survival of fully allogeneic skin grafts. Transpl Immunol.
19:12–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Renesto PG, Ponciano VC, Cenedeze MA,
Saraiva Câmara NO and Pacheco-Silva A: High expression of Tim-3
mRNA in urinary cells from kidney transplant recipients with acute
rejection. Am J Transplant. 7:1661–1665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Escobedo-Villarreal MM, Mercado-Moreira
AB, Muñoz-Espinosa LE, Gamboa-Esparza M, Pérez-Rodríguez E and
Cordero-Pérez P: Urinary protein detection by iTRAQÒ associated
with renal transplant complications and its modification with
therapy. Cir Cir. 83:393–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghys LF, Paepe D, Taffin ER, Vandermeulen
E, Duchateau L, Smets PM, Delanghe J and Daminet S: Serum and
urinary cystatin C in cats with feline immunodeficiency virus
infection and cats with hyperthyroidism. J Feline Med Surg.
18:658–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada T, Arakawa Y, Mii A, Kashiwagi T,
Kaneko T, Utsumi K, Masuda Y, Shimizu A, Iino Y and Katayama Y: A
case of monoclonal immunoglobulin G1-lambda deposition associated
with membranous feature in a patient with hepatitis C viral
infection. Clin Exp Nephrol. 16:468–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mäkelä S, Mustonen J, Ala-Houhala I, Hurme
M, Koivisto AM, Vaheri A and Pasternack A: Urinary excretion of
interleukin-6 correlates with proteinuria in acute Puumala
hantavirus-induced nephritis. Am J Kidney Dis. 43:809–816. 2004.
View Article : Google Scholar : PubMed/NCBI
|