Introduction
Myocardial infarction (MI), additionally known as a
heart attack, is a primary cause of disability and death worldwide
(1). Chest pain or discomfort is
the most common symptom, and this disease may be recognized by
certain clinical features and imaging, or may be defined by
pathology (2). In 2013, there were
~8.6 million cases of MI worldwide, and ~1 million individuals are
affected by MI in the United States annually (3,4).
Every 6th man and every 7th woman in Europe will succumb as a
result of MI (5,6). In addition, diabetes, high blood
pressure, smoking, excessive alcohol intake, poor diet and lack of
exercise are risk factors of MI (7,8).
Therefore, the need for the development of novel and effective
therapeutic strategies for the treatment of MI is imperative.
MI may induce alterations of left ventricular
architecture, and aging is a primary risk factor that results in
heart alterations and cardiovascular disease (9–11).
Additionally, short telomeres have been reported to be risk
predictors for age-associated disease, including heart disease
(12). A previous study revealed
that telomerase activation may elongate telomeres and delay ageing
and associated diseases, including MI (13). Certain other studies suggested that
telomerase expression may stimulate cardiomyocyte proliferation and
contribute to functional heart recovery following MI (14,15).
Weischer et al (16)
demonstrated that short telomere length is associated with a modest
increased risk of MI by studying 19,838 individuals for up to 19
years. In addition, one study indicated that telomerase has
beneficial effects on heart function (17). High expression levels of telomerase
via telomerase reverse transcriptase production may reduce the
magnitude of heart attacks (18).
Harrington et al (19)
suggested that telomerase limits the damage resulting from heart
attacks. Therefore, telomerase may serve important roles in MI.
However, the underlying molecular mechanism of telomerase
activation on MI remains to be fully understood.
In the present study, the array data of GSE62973 was
downloaded and differentially expressed genes (DEGs) were analyzed
in samples of mice that were injected with an adeno-associated
virus that expressed telomerase, an adeno associated-virus with an
empty expression cassette, or no virus, prior to induction of
myocardial infarction. In addition, functional enrichment analysis
was performed. A protein-protein interaction (PPI) network was
generated and significant modules were analyzed. Subsequently,
genes associated with telomerase were identified. The present study
aimed to identify key genes and pathways associated with MI
following telomerase activation, and investigate the possible
underlying molecular mechanism of this process.
Materials and methods
Microarray data
The array data of GSE62973 deposited by Bär et
al (13) was downloaded from
the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) database. An
adeno-associated virus that expressed telomerase or an empty
expression cassette were used to infect mice in this data, and the
MI model was subsequently established. The model was used to study
the effect of telomerase activation in disease. A total of 4
myocardial infarction samples that were treated with an
adeno-associated virus that expressed telomerase
(infarct+telomerase), 4 myocardial infarction samples treated with
an adeno-associated virus with an empty expression cassette
(infarct) and 3 myocardial infarction samples that were not
infected with viruses (control) were included in the present study.
The raw data was downloaded for subsequent analysis, which were
based on the platform of GPL10787 (Agilent-028005 SurePrint G3
Mouse GE 8×60K Microarray).
Data pre-processing
The raw data was pre-processed using an Agilent
signal-channel chip provided by the Linear Models for Microarray
and RNA-Seq Data (Limma; http://www.bioconductor.org/packages/release/bioc/html/limma.html)
in the R package (20). Background
correction, normalized expression intensity and condensed
microarray data were included in the pre-processing protocol.
Subsequently, combined with an annotation file of the platform, the
probe identity was transformed to a gene symbol, and probes without
corresponding gene symbols were eliminated. If a number of probes
mapped to one gene symbol, then the mean value was set as final
expression value of this gene.
DEGs analysis
The DEGs were analyzed in infarct vs. control,
infarct + telomerase vs. control and infarct + telomerase vs.
infarct by using the Limma package. The P-values of DEGs from the
Limma package were calculated by Student's unpaired t-test
(21). |log2FC|≥0.5 and P<0.05
were used as cut-off criteria, and were considered to indicate a
statistically significant difference.
Functional enrichment analysis
Gene ontology (GO) is used for gene annotation, and
molecular function (MF), biological process (BP) and cellular
component (CC) were included in this tool (22). Kyoto Encyclopedia of Genes and
Genomes (KEGG) may be used to place associated gene sets into their
pathways (23). Database for
Annotation, Visualization and Integrated Discovery (DAVID;
https://david.ncifcrf.gov/) is an
integrated data-mining environment and is used for gene list
analysis (24).
GO annotation and KEGG pathway enrichment analysis
were performed for upregulated and downregulated genes by DAVID.
Gene counts ≥2 and P<0.05 were set to determine significant
enrichment.
PPI network analysis
The Search Tool for the Retrieval of Interacting
Genes (25) database may be used
to predict interactions between proteins. Neighbourhood, gene
fusion, co-occurrence, co-expression experiments, databases and
textmining were the source of the prediction method of this
database. The input gene sets were DEGs in 3 comparison groups, and
the species was mouse. PPI score=0.4, and protein nodes that
interacted with each other were DEGs. PPI networks were generated
using Cytoscape software version 3.4.0 (National Institutes of
Health, Bethesda, MD, USA) (26).
Key nodes in the PPI network were obtained by
calculating the degree values of nodes. Degree values represented
the number of other nodes that interacted with the node. The
greater the degree values, the more likely that the nodes were key
nodes in the network.
Module analysis
Sub-network modules of 3 PPI networks were analyzed
using Clustering with Overlapping Neighborhood Expansion
(ClusterONE) version 1.0, in Cytoscape version 3.4.0 (National
Institutes of Health) (27). The
overlap protein complex may be analyzed and significant sub-network
modules may be screened using ClusterONE software. P<0.0003 was
set to indicate significant modules. Nodes in one module were more
likely to take part in the same biological process. Subsequently,
the KEGG pathways enriched by DEGs in different modules were
analyzed using the DAVID online tool.
Analysis of genes associated with
telomerase
The DEGs in 3 comparison groups were combined, and
then the alterations in expression levels of these DEGs in 3 sample
groups were observed. The present study screened 2 types of genes:
Genes that were upregulated in the control and in the infarct +
telomerase groups compared with in the infarct group (gene
expression decreased in disease and increased following telomerase
treatment), and genes that were downregulated in the control in the
infarct + telomerase groups compared with in the infarct group
(gene expression increased in disease and decreased following
telomerase treatment). Average expression values of genes in the
control, infarct and infarct + telomerase groups were calculated,
and subsequently genes meeting the aforementioned conditions were
screened. Combined with DAVID, KEGG pathways enriched by obtained
genes were analyzed.
Results
DEGs analysis
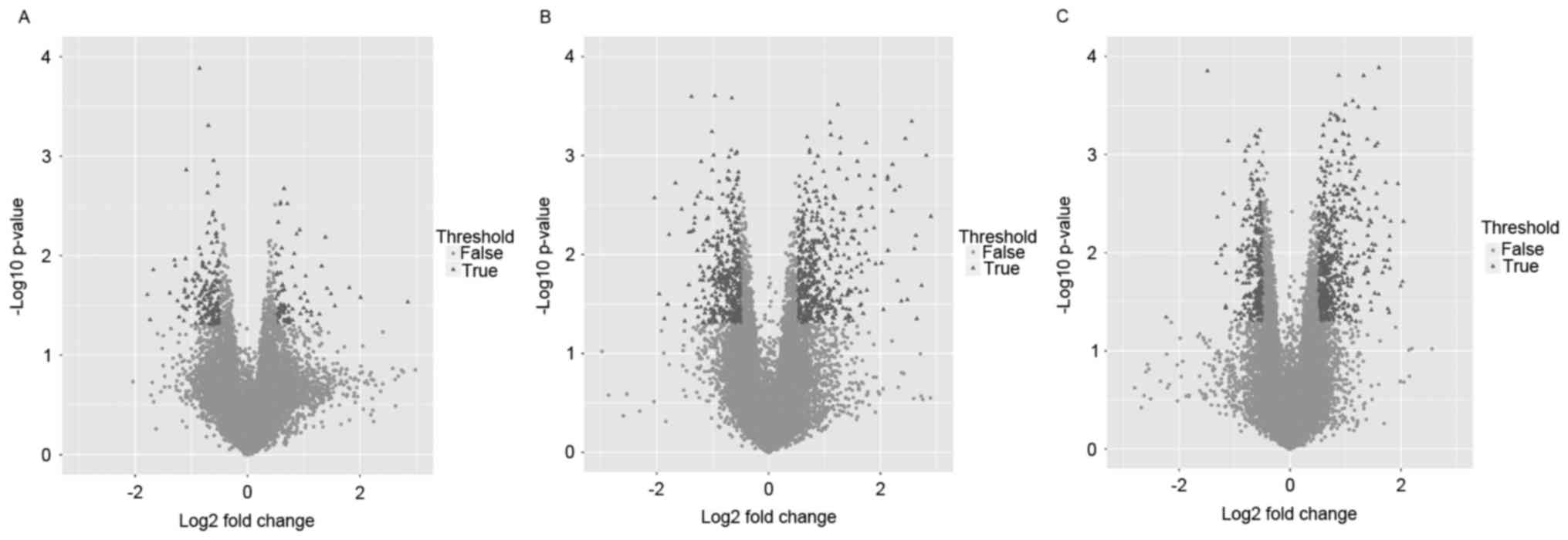
DEGs in 3 comparison groups are presented in
Table I. More DEGs were obtained
in the infarct + telomerase vs. control groups (862 DEGs) and
infarct + telomerase vs. infarct groups (816 DEGs) compared with
the infarct vs. control groups (251 DEGs), and it was suggested
that the expression levels of genes were significantly altered in
infarct + telomerase samples compared with control samples and
infarct samples. The volcano plots of the gene expression
distribution in the 3 groups were demonstrated in Fig. 1.
 | Table I.DEGs in 3 comparison groups. |
Table I.
DEGs in 3 comparison groups.
| Group | Upregulated gene
count | Downregulated gene
count | Total |
|---|
| Infarct vs.
control | 78 | 173 | 251 |
| Infarct +
telomerase vs. control | 467 | 395 | 862 |
| Infarct +
telomerase vs. infarct | 555 | 261 | 816 |
Functional enrichment analysis
GO and KEGG pathway analysis for upregulated and
downregulated DEGs were performed, respectively. Olfactory
transduction was the significantly enriched pathway associated with
downregulated DEGs in the infarct vs. control comparison group
(Table IIA). However, there were
no significantly enriched pathways associated with upregulated DEGs
in this comparison group. Extracellular matrix (ECM)-receptor
interaction and valine, leucine and isoleucine degradation were
significantly enriched pathways by upregulated and downregulated
DEGs in infarct + telomerase vs. control comparison group,
respectively (Table IIB).
Olfactory transduction and homologous recombination were
significantly enriched pathways by upregulated and downregulated
DEGs in infarct + telomerase vs. infarct comparison group,
respectively (Table IIC).
 | Table II.GO and KEGG analysis for DEGs. |
Table II.
GO and KEGG analysis for DEGs.
|
| Category | Term | Count | P-value |
|---|
| A, infarct vs.
control |
| Upregulated | MF | GO:0019904~protein
domain specific binding | 4 |
1.61×10−2 |
|
|
| GO:0032403~protein
complex binding | 3 |
1.98×10−2 |
|
| CC |
GO:0005576~extracellular region | 13 |
4.93×10−3 |
| Downregulated | BP |
GO:0007186~G-protein coupled receptor
protein signaling pathway | 25 |
7.68×10−4 |
|
|
| GO:0007166~cell
surface receptor linked signal transduction | 30 |
8.89×10−4 |
|
| CC | GO:0016021~integral
to membrane | 55 |
1.47×10−3 |
|
|
|
GO:0031226~intrinsic to plasma
membrane | 12 |
1.50×10−3 |
|
| MF |
GO:0004984~olfactory receptor
activity | 17 |
4.14×10−3 |
|
|
|
GO:0016503~pheromone receptor
activity | 5 |
1.38×10−2 |
|
| PATHWAY | mmu04740:Olfactory
transduction | 12 |
6.80×10−3 |
|
|
| mmu04010:MAPK
signaling pathway | 5 |
4.74×10−2 |
|
| B, Infarct +
telomerase vs. control |
|
| Upregulated | BP | GO:0001568~blood
vessel development | 20 |
2.47×10−6 |
|
|
|
GO:0001944~vasculature development | 20 |
3.53×10−6 |
|
| CC |
GO:0005576~extracellular region | 97 |
1.14×10−16 |
|
|
|
GO:0044421~extracellular region part | 54 |
5.98×10−12 |
|
| MF |
GO:0005201~extracellular matrix structural
constituent | 8 |
2.84×10−6 |
|
|
| GO:0005509~calcium
ion binding | 38 |
3.13×10−5 |
|
| PATHWAY |
mmu04512:ECM-receptor interaction | 14 |
4.50×10−8 |
|
|
| mmu04510:Focal
adhesion | 16 |
5.04×10−5 |
| Downregulated | BP | GO:0006811~ion
transport | 22 |
3.21×10−3 |
|
|
| GO:0009083~branched
chain family amino acid catabolic process | 3 |
6.20×10−3 |
|
| CC | GO:0001673~male
germ cell nucleus | 3 |
3.17×10−2 |
|
|
|
GO:0005739~mitochondrion | 30 |
3.89×10−2 |
|
| MF |
GO:0005244~voltage-gated ion channel
activity | 11 |
6.43×10−4 |
|
|
|
GO:0022832~voltage-gated channel
activity | 11 |
6.43×10−4 |
|
| PATHWAY | mmu00280:Valine,
leucine and isoleucine degradation | 6 |
7.37×10−4 |
|
|
| mmu00380:Tryptophan
metabolism | 5 |
3.50×10−3 |
|
| C, Infarct +
telomerase vs. infarct |
|
| Upregulated | BP |
GO:0007186~G-protein coupled receptor
protein signaling pathway | 68 |
4.03×10−6 |
|
|
| GO:0007166~cell
surface receptor linked signal transduction | 82 |
1.18×10−5 |
|
| CC |
GO:0031224~intrinsic to membrane | 189 |
4.74×10−7 |
|
|
| GO:0016021~integral
to membrane | 179 |
7.71×10−6 |
|
| MF |
GO:0004867~serine-type endopeptidase
inhibitor activity | 14 |
8.09×10−7 |
|
|
|
GO:0030414~peptidase inhibitor
activity | 17 |
8.74×10−7 |
|
| PATHWAY |
mmu00590:Arachidonic acid metabolism | 8 |
1.35×10−3 |
|
|
| mmu04740:Olfactory
transduction | 31 |
7.75×10−3 |
| Downregulated | BP | GO:0042110~T cell
activation | 6 |
3.90×10−3 |
|
|
| GO:0001775~cell
activation | 8 |
6.73×10−3 |
|
| CC |
GO:0005657~replication fork | 3 |
1.68×10−2 |
|
|
|
GO:0005694~chromosome | 8 |
3.81×10−2 |
|
| MF | GO:0003677~DNA
binding | 32 |
3.05×10−4 |
|
|
|
GO:0008190~eukaryotic initiation factor 4E
binding | 2 |
3.65×10−2 |
|
| PATHWAY | mmu03440:Homologous
recombination | 4 |
1.23×10−3 |
|
|
| mmu03430:Mismatch
repair | 3 |
1.31×10−2 |
PPI network analysis
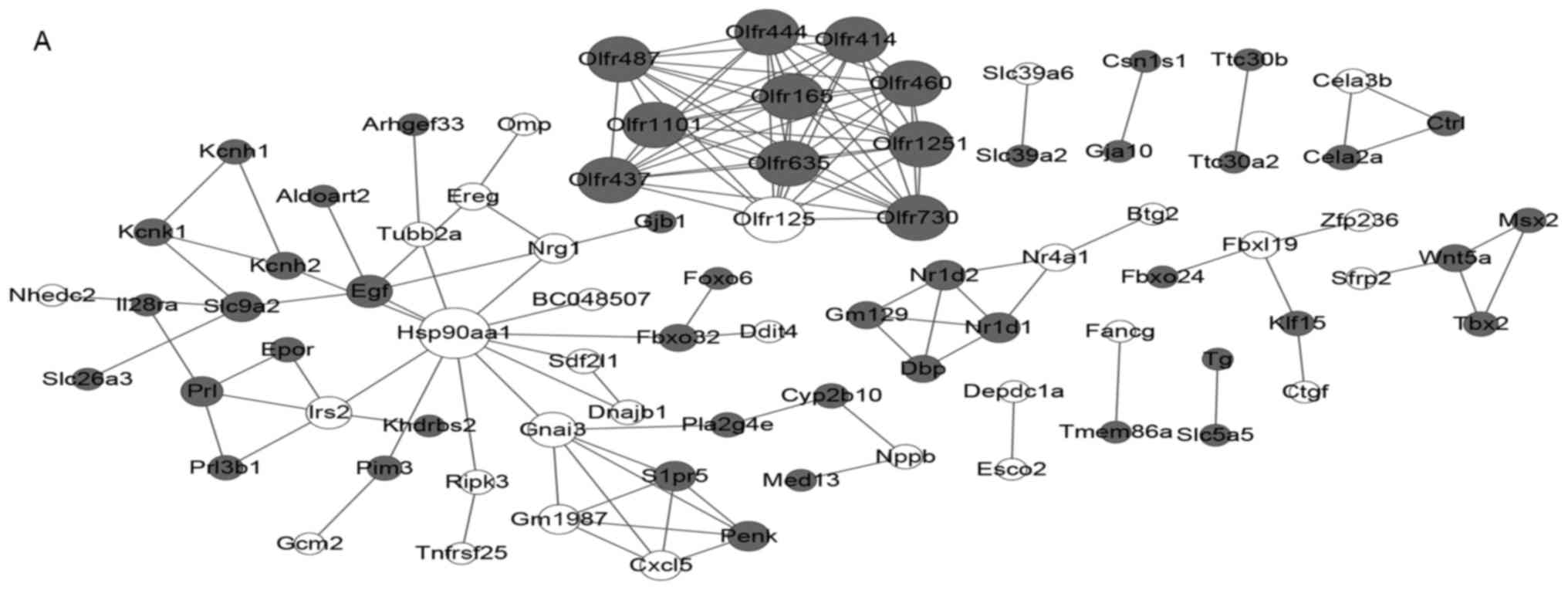
A total of 81 nodes and 133 protein pairs were
included in the PPI network for the infarct vs. control comparison
group (Fig. 2A). A total of 481
nodes and 1,606 protein pairs were included in the PPI network for
infarct + telomerase vs. control comparison group, and
proto-oncogene tyrosine-protein kinase Src (Src) was the hub
node with greatest degree (Fig.
2B). A total of 81 nodes and 133 protein pairs were included in
the PPI network for infarct + telomerase vs. infarct comparison
group, and proto-oncogene tyrosine-protein kinase Fyn (Fyn)
was the hub node with the greatest degree (Fig. 2C). Nodes with greater degree values
in 3 networks are presented in Table
III.
 | Table III.Nodes with greater degree values in 3
networks. |
Table III.
Nodes with greater degree values in 3
networks.
| PPI type | Degree | Node |
|---|
| Infarct vs.
control | 12 | Hsp90aa1 |
|
| 10 |
Olfr1251,Olfr635,Olfr460,Olfr1101,Olfr444,Olfr437,
Olfr125, Olfr165,Olfr414,Olfr487,Olfr730 |
|
| 6 | Gnai3 |
|
| 5 | Irs2,Egf |
| Infarct +
telomerase vs. control | 81 | Src |
|
| 60 | Hsp90aa1 |
|
| 42 | Jund |
|
| 41 | Timp1 |
|
| 38 | Col1a2 |
|
| 37 | Col1a1 |
|
| 35 | Itgb5,Agt |
|
| 34 |
Col3a1,Ptgs2,Edn1 |
|
| 32 | Bgn |
|
| 30 | Tlr2 |
|
| 29 | Serpine1, Fpr2 |
|
| 28 | Sparc, Ccl9 |
|
| 27 | Ccl6 |
|
| 26 | Anxa1,Col5a2 |
| Infarct +
telomerase vs. infarct | 30 | Fyn |
|
| 26 | Agt |
|
| 24 | Slc11a1 |
|
| 21 | Ccl6,Olfr1441 |
|
| 20 |
Olfr10,Olfr578,Olfr1466,Olfr482,Olfr224,Olfr704,Olfr487,Olfr926,Anxa1,Olfr173,Olfr1251,Olfr414,Olfr353,Olfr167,Olfr299,Olfr780,Olfr635,Olfr91,Olfr700,Olfr444,Ccl9,Olfr791 |
|
| 18 | Furin, Cad,
Timp1 |
|
| 17 | Fpr3 |
|
| 15 | Col5a2 |
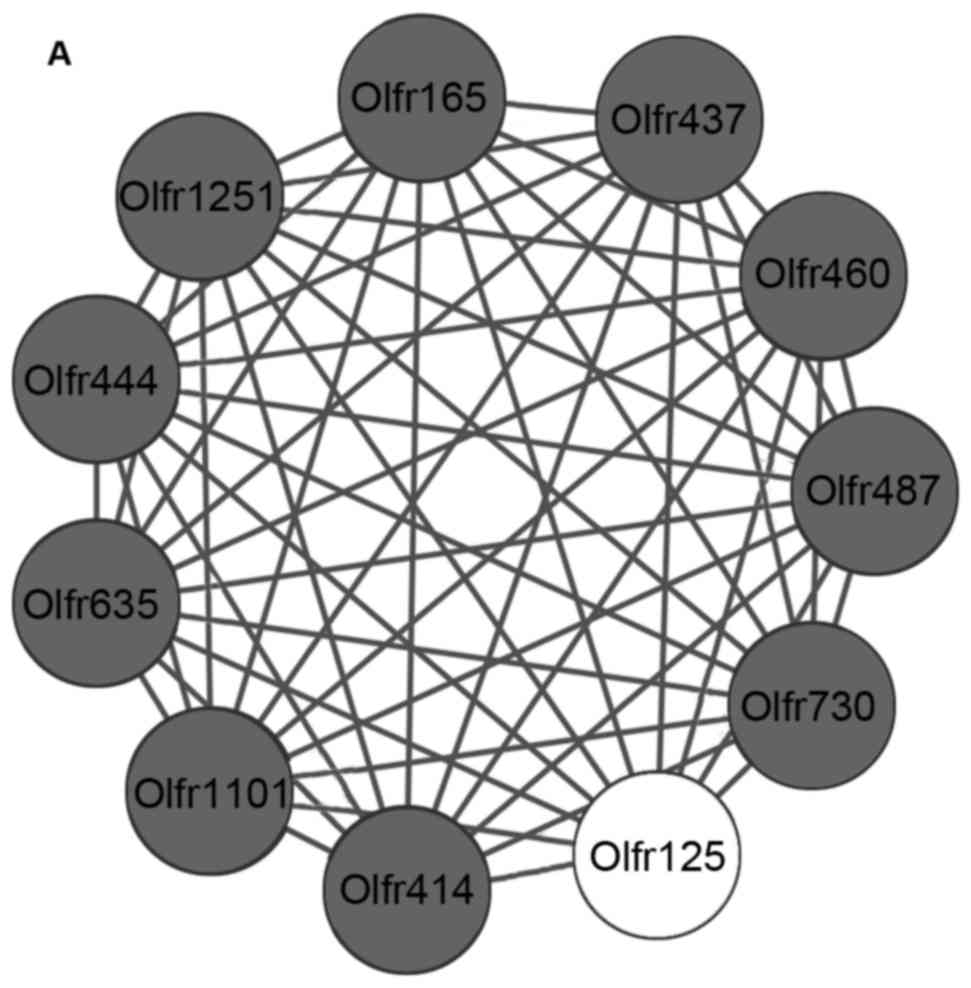
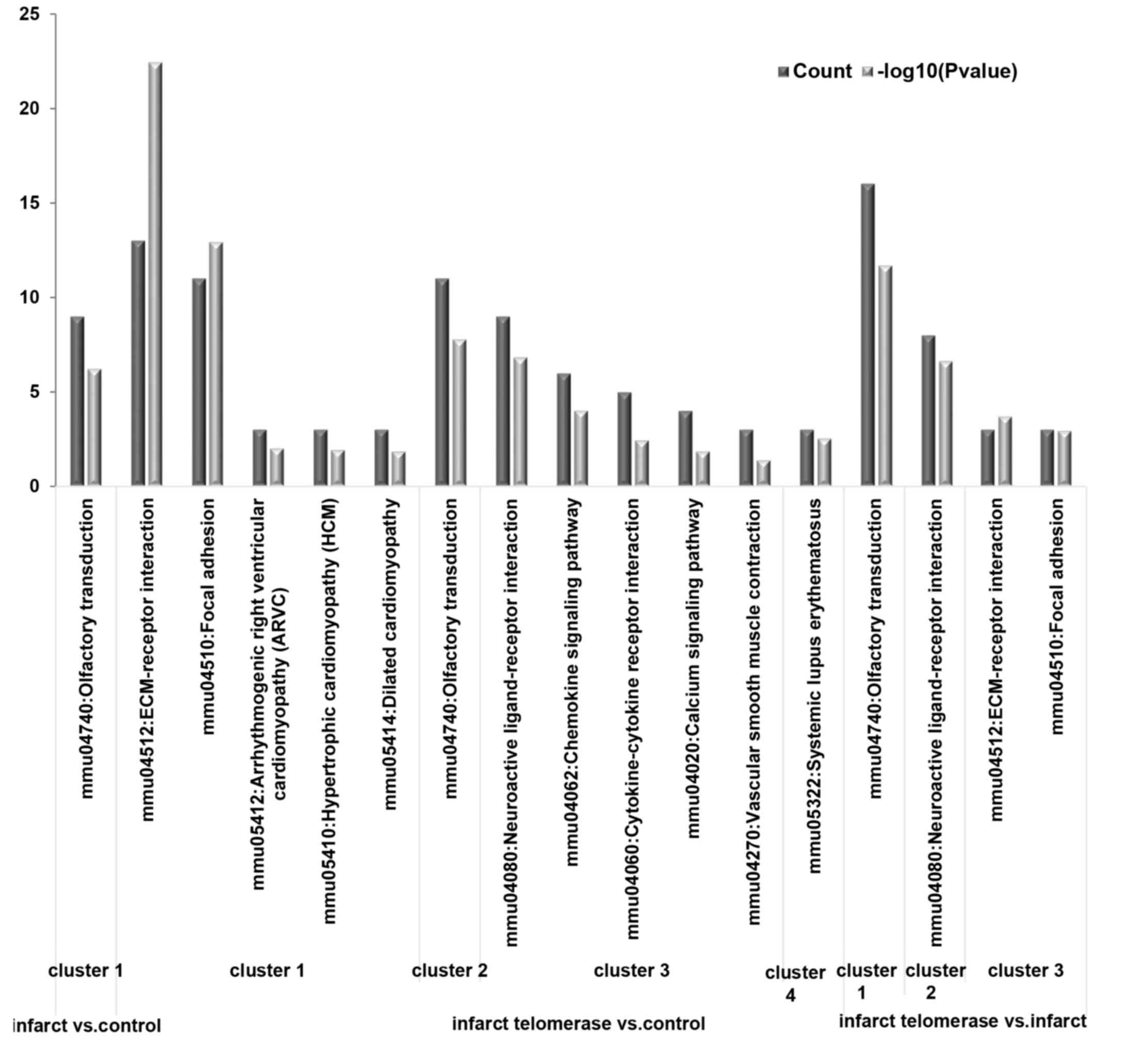
Module analysis
Sub-network modules obtained from 3 PPI networks
were demonstrated in Fig. 3. A
single significant sub-network module was obtained in the infarct
vs. control comparison group (Fig.
3A, P=2.750E-6). A total of four significant sub-network
modules were obtained in the infarct + telomerase vs. control
comparison group (Fig. 3B, cluster
1: P=9.860E-8; cluster 2: P=5.186E-7; cluster 3: P=1.388E-6;
cluster 4: P=2.081E-4) and infarct + telomerase vs. infarct
comparison group (Fig. 3C, cluster
1: P<0.0001; cluster 2: P=5.279E-7; cluster 3: P=2.280E-5;
cluster 4: P=4.260E-5). Olfactory receptor gene family associated
genes including olfactory receptor 10 (Or10), olfactory
receptor 444 (Or444) and olfactory receptor 414
(Or414) were significantly enriched in the sub-network
modules of the 3 groups. The KEGG pathway was significantly
enriched by these sub-network modules, as presented in Fig. 4.
Analysis of genes associated with
telomerase
A total of 1,520 DEGs were obtained following
combination and removal of duplications. These genes were
significantly differentially expressed in ≥1 comparison groups.
Expression alterations of 509 genes revealed up-down-up trends in
control, infarct, infarct + telomerase groups. Expression
alterations of 266 genes revealed down-up-down trends in control,
infarct, infarct + telomerase groups. The expression alteration
trends of these genes were in accordance with the control in MI
following addition of telomerase using infarct as a reference, and
suggested that telomerase may affect the expression of these genes.
Signaling pathways significantly enriched by these genes are listed
in Table IV. Olfactory
transduction was a significant pathway enriched by genes associated
with telomerase.
 | Table IV.Pathways significantly enriched by
genes that revealed expression alterations in up-down-up and
down-up-down trends. |
Table IV.
Pathways significantly enriched by
genes that revealed expression alterations in up-down-up and
down-up-down trends.
| Expression
direction | Term | Count | P-value |
|---|
| Up-down-up | mmu04740:Olfactory
transduction | 34 |
8.79×10−5 |
|
|
mmu00590:Arachidonic acid metabolism | 9 |
1.06×10−4 |
|
| mmu00830:Retinol
metabolism | 6 |
7.20×10−3 |
|
| mmu00591:Linoleic
acid metabolism | 5 |
8.97×10−3 |
|
| mmu00980:Metabolism
of xenobiotics by cytochrome P450 | 5 |
3.02×10−2 |
|
| mmu00982:Drug
metabolism | 5 |
4.51×10−2 |
| Down-up-down | mmu03430:Mismatch
repair | 3 |
1.10×10−2 |
Discussion
The present study revealed that Src and
Fyn were the hub nodes of the greatest degrees in the PPI
network for the infarct + telomerase vs. control comparison group
and infarct + telomerase vs. infarct comparison group,
respectively. Olfactory receptor gene family associated genes,
including Or10, Or444 and Or414 were
significantly enriched in the sub-network modules of 3 comparison
groups. In addition, olfactory transduction was a significantly
enriched pathway with downregulated DEGs in the infarct vs. control
comparison group, and was a significantly enriched pathway with
upregulated DEGs in the infarct + telomerase vs. infarct comparison
group. Olfactory transduction was a significant signaling pathway
enriched by genes associated with telomerase.
In the present study, Src was the hub node of
the greatest degree in the PPI network for the infarct + telomerase
vs. control comparison group. Src inhibition may stabilize a
kinase insert domain receptor/cadherin complex and may reduce edema
and tissue injury following MI (28). Cellular-Src blockade lowers
the induction of arrhythmia and improves conduction velocity
following MI (29). Furthermore,
Src protein tyrosine kinases serve preconditioning roles
against MI (30). In addition,
hydrogen sulfide may recruit macrophage migration via the integrin
β1-Src-focal adhesion kinase/protein tyrosine kinase 2-Ras-related
C3 botulinum toxin substrate 1 (Rac1) pathway in MI (31). Therefore, the results of the
present study were in accordance with previous reports (28–31)
and suggested that Src may serve significant roles in MI
following telomerase activation.
In addition, Fyn was the hub node of the
greatest degree in the PPI network for the infarct + telomerase vs.
infarct comparison group in the present study. Activation of
nuclear factor erythroid 2-related factor 2 via the Rac1/glycogen
synthase kinase-3β/Fyn signaling pathway may prevent angiotensin
II-induced cardiomyopathy (32),
and inhibition of nephrin activation by c-maf inducing protein via
the C-Src tyrosine kinase-CREB-binding protein-Fyn axis, serves a
key role in angiotensin II-induced podocyte damage (33). Pre-treatment with angiotensin II
may limit MI in isolated rabbit hearts (34). Additional angiotensin II receptor
blocker treatment has minimal impact on the development of coronary
atherosclerosis in patients with acute MI compared with an
angiotensin-converting enzyme inhibitor alone (35). Although there may be little direct
research on the roles of Fyn in MI in previous studies, it
may be hypothesized from the results of the present study that
Fyn serves an important role in MI following telomerase
activation.
In addition, in the present study, the olfactory
receptor gene family associated genes, including Or10, Or444
and Or414, were significantly enriched in the sub-network
modules of 3 comparison groups. A previous study revealed that the
olfactory receptor 10J5 gene was expressed in the human aorta,
coronary artery and umbilical vein endothelial cells, and served
functional roles in angiogenesis (36). Drutel et al (37) suggested that olfactory receptor
genes may serve roles in cardiac progression. Certain studies have
suggested that olfactory receptors are involved in olfactory signal
recognition and muscle regeneration (38,39).
In addition, reduced blood flow to a part of the heart, due to
thrombosis, etc., results in damage to the heart muscle, and
subsequently, MI occurs (2).
Therefore, the olfactory receptor gene family associated genes may
indirectly serve important roles in heart disease, including
MI.
In the present study, olfactory transduction was a
significantly enriched pathway by downregulated DEGs in the infarct
vs. control comparison group, and was a significantly enriched
pathway by upregulated DEGs in the infarct + telomerase vs. infarct
comparison group, and olfactory transduction was the significant
pathway enriched by genes associated with telomerase. Li et
al (40) demonstrated that
olfactory transduction was a significant pathway enriched with
dysregulated genes in coronary artery disease. Additionally,
olfactory receptor gene family associated genes may serve important
roles in heart disease, including MI. Therefore, it may be
hypothesized that the olfactory transduction pathway may be
involved in the development of MI.
In conclusion, Src, Fyn and olfactory
receptor gene family associated genes serve significant roles in
MI. These genes and their associated signaling pathways were
differentially expressed in MI following telomerase activation in
the present study. Therefore, telomerase activation may serve
important roles in MI partly via Src, Fyn and
olfactory receptor gene family associated genes. However, a
limitation of the present study is that as of yet, there is no
experimental verification to conclude this, and further experiments
are required in the future to verify these findings.
References
|
1
|
Thygesen K, Alpert JS, White HD, et al:
Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of
Myocardial Infarction: Universal definition of myocardial
infarction. J Am Coll Cardiol. 50:2173–2195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thygesen K, Alpert JS, Jaffe AS, Simoons
ML, Chaitman BR and White HD: JointESC/ACCF/AHA/WHF Task Force for
Universal Definition of Myocardial Infarction, Authors/Task Force
Members Chairpersons. Thygesen K, Alpert JS, et al: Third universal
definition of myocardial infarction. J Am Coll Cardiol.
60:1581–1598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vos T, Barber RM, Bell B, Bertozzi-Villa
A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhardt L,
Dicker D, et al: Global, regional, and national incidence,
prevalence, and years lived with disability for 301 acute and
chronic diseases and injuries in 188 countries, 1990–2013: A
systematic analysis for the global burden of disease study 2013.
Lancet. 386:743–800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
http://www.nhlbi.nih.gov/health/health-topics/topics/heartattack/
|
|
5
|
Task Force on the management of ST-segment
elevation acute myocardial infarction of the European Society of
Cardiology (ESC), . Steg PG, James SK, Atar D, Badano LP,
Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq
G, et al: ESC guidelines for the management of acute myocardial
infarction in patients presenting with ST-segment elevation. Eur
Heart J. 33:2569–2619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doyle F, McGee H, Conroy R, Conradi HJ,
Meijer A, Steeds R, Sato H, Stewart DE, Parakh K, Carney R, et al:
Systematic review and individual patient data meta-analysis of sex
differences in depression and prognosis in persons with myocardial
infarction: A MINDMAPS study. Psychosom Med. 77:419–428. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mehta PK, Wei J and Wenger NK: Ischemic
heart disease in women: A focus on risk factors. Trends Cardiovasc
Med. 25:140–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
World Health Organization (WHΟ): Global
atlas on cardiovascular disease prevention and control. WHO;
Geneva: 2011
|
|
9
|
Dominguez LJ, Galioto A, Ferlisi A, Pineo
A, Putignano E, Belvedere M, Costanza G and Barbagallo M: Ageing,
lifestyle modifications, and cardiovascular disease in developing
countries. J Nutr Health Aging. 10:143–149. 2006.PubMed/NCBI
|
|
10
|
Liew CC and Dzau VJ: Molecular genetics
and genomics of heart failure. Nat Rev Genet. 5:811–825. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cohn JN, Ferrari R and Sharpe N: Cardiac
remodeling-concepts and clinical implications: A consensus paper
from an international forum on cardiac remodeling. Behalf of an
international forum on cardiac remodeling. J Am Coll Cardiol.
35:569–582. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boonekamp JJ, Simons MJ, Hemerik L and
Verhulst S: Telomere length behaves as biomarker of somatic
redundancy rather than biological age. Aging Cell. 12:330–332.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bär C, de Bernardes Jesus B, Serrano R,
Tejera A, Ayuso E, Jimenez V, Formentini I, Bobadilla M, Mizrahi J,
de Martino A, et al: Telomerase expression confers cardioprotection
in the adult mouse heart after acute myocardial infarction. Nat
Commun. 5:58632014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soonpaa MH, Koh GY, Klug MG and Field LJ:
Formation of nascent intercalated disks between grafted fetal
cardiomyocytes and host myocardium. Science. 264:98–101. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Senyo SE, Steinhauser ML, Pizzimenti CL,
Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP and Lee
RT: Mammalian heart renewal by pre-existing cardiomyocytes. Nature.
493:433–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weischer M, Bojesen SE, Cawthon RM,
Freiberg JJ, Tybjærg-Hansen A and Nordestgaard BG: Short telomere
length, myocardial infarction, ischemic heart disease, and early
death. Arterioscler Thromb Vasc Biol. 32:822–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buechner N, Drose S, Jakob S, Brandt U,
Altschmied J and Haendeler J: Mitochondrial TERT enhances
mitochondria functions in vivo by protecting mitochondrial DNA
integrity from oxidative damage. Cell Circulation Signaling.
7:(Suppl 1). A572009. View Article : Google Scholar
|
|
18
|
Cohen H: Forced TERT expression in mice.
Scientist. 15:222001.
|
|
19
|
Harrington M: Telomerase limits damage
after heart attack. Lab Anim (NY). 44:462015. View Article : Google Scholar
|
|
20
|
Smyth GK: Limma: Linear models for
microarray data. Bioinformatics and computational biology solutions
using R and Bioconductor Springer. 397–420. 2005. View Article : Google Scholar
|
|
21
|
Berkeley C: Linear models and empirical
Bayes methods for assessing differential expression in microarray
experiments. http://www.bepress.com/sagmb/vol3/iss1/art3.2004
|
|
22
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Altermann E and Klaenhammer TR:
PathwayVoyager: Pathway mapping using the Kyoto encyclopedia of
genes and genomes (KEGG) database. BMC Genomics. 6:602005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
da Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nepusz T, Yu H and Paccanaro A: Detecting
overlapping protein complexes in protein-protein interaction
networks. Nat Methods. 9:471–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weis S, Shintani S, Weber A, Kirchmair R,
Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, et al:
Src blockade stabilizes a Flk/cadherin complex, reducing edema and
tissue injury following myocardial infarction. J Clin Invest.
113:885–894. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rutledge CA, Ng FS, Sulkin MS, Greener ID,
Sergeyenko AM, Liu H, Gemel J, Beyer EC, Sovari AA, Efimov IR and
Dudley SC: c-Src kinase inhibition reduces arrhythmia inducibility
and connexin43 dysregulation after myocardial infarction. J Am Coll
Cardiol. 63:928–934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dawn B, Takano H, Tang XL, Kodani E,
Banerjee S, Rezazadeh A, Qiu Y and Bolli R: Role of Src protein
tyrosine kinases in late preconditioning against myocardial
infarction. Am J Physiol Heart Circ Physiol. 283:H549–H556. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miao L, Xin X, Xin H, Shen X and Zhu YZ:
Hydrogen sulfide recruits macrophage migration by integrin
β1-Src-FAK/Pyk2-Rac pathway in myocardial infarction. Sci Rep.
6:223632016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xin Y, Bai Y, Jiang X, Zhou S, Wang Y,
Wintergerst KA, Tan Y, Cui T and Cai L: Activation of Nrf2 by
Sulforaphane via the AKT/GSK-3β/Fyn pathway prevents angiotensin
ii-induced cardiomyopathy. Circ Res. 117:A802015.
|
|
33
|
Yu L, Lin Q, Feng J, Dong X, Chen W, Liu Q
and Ye J: Inhibition of nephrin activation by c-mip through
Csk-Cbp-Fyn axis plays a critical role in Angiotensin II-induced
podocyte damage. Cell Signal. 25:581–588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Y, Tsuchida A, Cohen MV and Downey JM:
Pretreatment with angiotensin II activates protein kinase C and
limits myocardial infarction in isolated rabbit hearts. J Mol Cell
Cardiol. 27:883–892. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yano H, Hibi K, Nozawa N, Ozaki H, Kusama
I, Ebina T, Kosuge M, Tsukahara K, Okuda J, Morita S, et al:
Effects of valsartan, an angiotensin II receptor blocker, on
coronary atherosclerosis in patients with acute myocardial
infarction who receive an angiotensin-converting enzyme inhibitor.
Circ J. 76:1442–1451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SH, Yoon YC, Lee AS, Kang N, Koo J,
Rhyu MR and Park JH: Expression of human olfactory receptor 10J5 in
heart aorta, coronary artery, and endothelial cells and its
functional role in angiogenesis. Biochem Biophys Res Commun.
460:404–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Drutel G, Arrang JM, Diaz J, Wisnewsky C,
Schwartz K and Schwartz JC: Cloning of OL1, a putative olfactory
receptor and its expression in the developing rat heart. Receptors
Channels. 3:33–40. 1995.PubMed/NCBI
|
|
38
|
Griffin CA, Kafadar KA and Pavlath GK:
MOR23 promotes muscle regeneration and regulates cell adhesion and
migration. Dev Cell. 17:649–661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang N and Koo J: Olfactory receptors in
non-chemosensory tissues. BMB Rep. 45:612–622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li H, Zhong X, Li C, Peng L, Liu W, Ye M
and Tuo B: Analysis of pathways and networks influencing the
differential expression of genes in coronary artery disease. Arch
Biol Sci Belgrade. 66:983–988. 2014. View Article : Google Scholar
|