Introduction
Androgens are anabolic hormones that, in addition to
their androgenic activity, are involved in the regulation of
metabolic function, energy utilization, carbohydrate metabolism,
fat metabolism and nitrogen retention in several extragenital
tissues (1). High endogenous
concentrations of testosterone induce increases in muscle size and
strength (2) and confer
physiological and psychological advantages in sports (3). Therefore, androgenic-anabolic
steroids (AAS) are widely abused by athletes (4). However, the side effects to organs
and systems that result from androgenic-anabolic steroid abuse
should be addressed. These systems include the cardiovascular
system, the central nervous system and the male and female
reproductive systems (5). Certain
androgenic herbs may be able to mimic the endocrine signaling
process in the body and increase serum androgen levels, such as
Tribulus terrestris (TT) (6–9).
As a well-known traditional Chinese medicine, TT
extracts have been used in China and India for the treatment of
multiple disorders due to its range of properties, which include
aphrodisiac effects (9,10), protective effects against
ischemia/reperfusion injury, anticancer and antihypertensive
properties (10). TT extracts,
which are primary saponins, may be used by athletes and
bodybuilders who are concerned about increasing muscle mass,
strength and improving performance, even if the underlying
biological pathways remain to be determined. The primary effect of
TT is claimed to be an increase in anabolic and androgenic function
through the activation of endogenous testosterone production,
providing a natural, safe and legal method to increase testosterone
levels with no side effects (6,9,11,12).
However, several research groups have previously reported that TT
extracts have no significant effect on the performance and plasma
levels of testosterone in intact and castrated male rats (13), in rugby athletes (14), men undergoing resistance training
(15,16) and untrained females (17). However, a previous study
demonstrated that TT extracts promoted the performance of rats
undergoing high intensity exercise for 5 weeks, by preventing a
heavy training-induced decrease in serum testosterone (18). The aim of the present study was to
further determine the underlying mechanisms.
Testosterone exerts its biological functions
primarily via the androgen receptor (AR), which is a member of the
ligand-dependent transcription factor superfamily (19). Upon binding to androgens, cytosolic
AR translocates into the nucleus and binds to the promotor/enhancer
regions of specific DNA sequences known as the androgen response
elements. It then regulates target gene transcription by recruiting
coactivators or corepressors. Exercise is involved in regulating
testosterone secretion along with AR expression and activity. In
general, testosterone levels are increased following exercise, in
particular resistance training in men; however, testosterone levels
are reduced in response to high intensity training and overtraining
(3,20,21).
As for AR, exercise increases AR binding capacity in a skeletal
muscle fiber type-dependent manner. Endurance training leads to a
significant increase in AR binding capacity in the soleus (composed
of predominantly slow twitch muscle fibers), whereas resistance
training elicits a significant increase in the extensor
digitorumlongus (composed of predominantly fast twitch muscle
fibers). Exercise also increases AR protein expression levels. Lee
et al (22) demonstrated
that AR protein concentration increased by 106 and 279% in rat
plantaris muscle following 7 and 21 days of overload exercise,
respectively, and AR mRNA expression levels increased by 430%
following 7 days of exercise. Exercise-induced upregulation of AR
content was also observed in female rats (23) and aging men (24). However, it has also been reported
that AR protein levels in the vastus lateralis decreased (20,25)
or remained unchanged (26)
following resistance exercise.
Insulin-like growth factor-1 (IGF-1), a peptide
hormone, is involved in promoting muscle hypertrophy, muscle repair
and alleviating muscle damage. The primary effect of IGF-1 is
mediated by binding to the IGF-1 receptor (IGF-1R), a
widely-expressed cell surface heterotetramer that is similar to the
insulin receptor. IGF-1 reduced age-associated wasting of skeletal
muscle (27). Resistance training,
which is the most useful treatment for the loss of muscle mass and
strength in aging people, was determined to upregulate IGF-1 and
IGF-1R expression levels (28).
IGF-1R is involved in mediating physiological cardiomyocyte
hypertrophy, as evidenced by the prevention of the hypertrophic
response to swim training in cardiomyocyte-specific IGF-1R knockout
mice (29). Androgen response
elements are located within the promoter regions of IGF-1 and
IGF-1R and testosterone administration in humans and rodents
increases circulating and skeletal muscle IGF-1 levels.
Testosterone deficiency is also associated with reduced levels of
IGF-1 in humans. Therefore, IGF-1 and IGF-1R are the target genes
of AR. In addition, the enhancing effect of testosterone on the
proliferation of primary human skeletal muscle cells in
vitro was blocked by small interfering RNA targeting human
IGF-1R (30), indicating the
existence of cross-talk between androgens and IGF-1R. Androgens are
known to cross-talk with multiple signal transduction pathways,
including the growth hormone/IGF-1 axis (31), the phosphatidylinositol
3-kinase/protein kinase B pathway (32), the Wnt/b-catenin signaling pathway
(33) and Notch signaling
(34). However, the mechanisms by
which circulating IGF-1 and muscular IGF-1R mediate the effects of
testosterone on the muscle remain to be fully understood. The aim
of the present study was to detect the expression of AR and
IGF-1/IGF-1R following TT extract-induced testosterone increases in
rats undergoing high intensity exercise, and its relationship with
the promoted performance.
Materials and methods
Animals, exercise protocol and
administration of TT extracts
A total of 32 male Sprague-Dawley rats (8 weeks old;
210–220 g body weight), obtained from the Animal Center of The
Second Military Medical University (Shanghai, China) were
maintained at a controlled temperature (22–24°C) and humidity
(45–55%) under a 12-h light/dark cycle, with free access to food
and water. The rats were randomly divided into 4 groups, each with
8 rats: i) Control group; ii) TT, TT group; iii) E, high intensity
exercise group; and iv) E+TT, high intensity exercise group plus
TT. The E and E+TT group rats underwent high intensity training for
a period of 5 weeks, as previously described (18). The TT and E+TT group rats were
given TT extracts intragastrically (120 mg/kg) as in a previous
study (18) 30 min prior to
exercise training. Equal amounts of normal saline were administered
to the non-TT treated groups. The animal protocol was approved and
the experiments were supervised by the Ethics Committee of Shanghai
University of Sport (approval no: 2012008).
Composition of TT extracts
TT extracts (saponins >70%) were purchased from
Shanxi Huike Botanical Development Company (Shaanxi, China). The TT
extract powder was dissolved in 70% ethanol and the supernatant was
obtained by ultrasonic extraction and centrifugation at 20,217 × g
for 10 min at 4°C. The composition was determined using ultra-high
performance liquid chromatography-quadrupole-time of flight mass
spectrometry (UHPLC-Q-TOF/MS) revised according to the protocol of
Wu et al (35) by Dr Xin
Dong (College of Pharmacy, The Second Military Medical University,
Shanghai, China).
Hormone assays
In order to avoid the acute effect of exercise on
testosterone and IGF-1 plasma levels, rats were sacrificed 36 h
following the last exercise session. Blood samples were collected
and testosterone (cat no. KGE010) and IGF-1 (cat no. MG100) plasma
levels were determined by ELISA using commercially available kits
from R&D Systems, Inc. (Minneapolis, MN, USA), according to the
manufacturer's protocol.
Relative weight and protein content of
gastrocnemius and soleus
The rat gastrocnemius and soleus muscles (50 mg)
were cut into sections and homogenized on ice with 500 µl
radioimmunoprecipitation assay lysis buffer and 1%
phenylmethylsulfonyl fluoride (ShenergyBiocolor Bioscience and
Technology Co., Ltd., China) in a homogenizer to extract total
protein. The lysates were briefly sonicated on ice, and centrifuged
at 11,963 × g for 10 min at 4°C. Supernatants were collected and
protein concentration was measured using a BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocol.
Western blot analysis
Gastrocnemius and soleus extracts (100 µg) were
resolved by 10% SDS-PAGE and transferred to a nitrocellulose
membrane. The membrane was blocked with 5% non-fat milk at 4°C
overnight. The nitrocellulose membranes were probed overnight at
4°C with the following primary antibodies: Anti-AR (cat no. sc-816;
1:200), anti-IGF-1R (cat no. sc-7952; 1:500), anti-myosin heavy
chain (MHC; cat no. sc-58797; 1:500; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and anti-GAPDH (cat no. SAB-2100894;
1:2,000; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The blots
were washed in TBS containing 0.1% Tween-20 and incubated at room
temperature for 2 h with the following horseradish
peroxidase-conjugated secondary antibodies at a 1:5,000 dilution:
Goat anti-rabbit immunoglobulin (Ig) G (cat no. sc-2004), donkey
anti-goat (cat no. sc-2020) and goat anti-mouse (cat no. sc-2005),
obtained from Santa Cruz Biotechnology, Inc. Following washing with
TBST, the blots were developed using enhanced chemiluminescence
(cat no. WBKLS0100; Merck KGaA) and exposed to X-ray film to
visualize the protein bands. The density of bands was determined
using Tanon Bio-Image software version 1.00 (Tanon Science and
Technology Co., Ltd., Shanghai, China). Experiments were performed
in triplicate.
Statistical analysis
All values were expressed as the mean ± standard
deviation of 3 independent experiments. P<0.05 was considered to
indicate a statistically significant difference. Data were analyzed
using one-way analysis of variance and Fisher's Least Significant
Difference post-hoc test with SPSS software version 20.0 (IBM
Corp., Armonk, NY, USA).
Results
Composition of TT extracts, determined
by UHPLC-Q-TOF/MS
The composition of the TT extracts was detected
using UHPLC-Q-TOF/MS. The most abundant constituents were tigogenin
and diosgenin, which are saponins and accounted for ~60.19 and
11.16% of the total peak area, respectively (Table I). No AAS were present in the TT
extracts.
 | Table I.Ultra-high performance liquid
chromatography-quadrupole-time of flight mass spectrometry analysis
results of Tribulus terrestris extract components. |
Table I.
Ultra-high performance liquid
chromatography-quadrupole-time of flight mass spectrometry analysis
results of Tribulus terrestris extract components.
| No. | Name | RT (min) | Formula | M±X | Expected m/z | Experimental
m/z | Error (ppm) | Peak area (%) |
|---|
| 1 | Valine |
0.730 |
C5H11NO2 | M+H | 118.0862 | 118.0863 | 0.41 |
0.32 |
| 2 | A | 10.851 |
C9H19NO | M+H | 158.1541 | 158.1539 | −1.21 |
5.56 |
| 3 | B | 11.079 |
C14H24N4 | M+H/M+Na |
249.2079/271.1902 |
249.2074/271.1893 | −1.98/−3.46 |
5.45 |
| 4 | Diosgenin | 12.620 |
C27H42O3 | M+H | 415.3215 | 415.3207 | −3.15 | 11.16 |
| 5 | C | 12.685 |
C31H35N2O6 | M+H/M+Na |
532.2577/554.2393 |
532.2568/554.2387 | −0.98/−1.51 |
3.43 |
| 6 | D | 12.905 |
C23H30O6 | M+H/M+Na |
403.2126/425.1938 |
403.2115/425.1935 | −0.92/−2.76 |
9.11 |
| 7 | Tigogenin | 13.492 |
C27H44O3 | M+H | 417.3369 | 417.3363 | −1.34 | 60.19 |
| 8 | E | 13.842 |
C30H32O8 | M+H/M+Na |
521.2150/543.2009 |
521.2170/543.1989 | −3.81/−3.63 |
4.73 |
| 9 | Rutin |
7.660 |
C27H30O16 | M+H | 611.1608 | 611.1607 | −0.21 |
0.02 |
| 10 |
Kaempferol-3-glucoside |
8.332 |
C21H20O11 | M+H | 449.1071 | 449.1078 | 1.57 |
0.02 |
| 11 | Quercetin |
9.380 |
C15H10O7 | M+H/M-H | 303.0507 | 303.0499 | −2.53 |
0.01 |
TT extracts attenuated high intensity
exercise-induced weight gain in rats
There was no significant difference in weight gain
between the control and TT groups (Table II). A significant reduction in
body weight in the E and E+TT groups was observed from the third
week of training onwards, compared with the control group (week 3,
P<0.05 and P<0.05, respectively; week 4, P<0.01 and
P<0.01, respectively, week 5, P<0.01 and P<0.01,
respectively; Table II).
Treatment with TT extracts significantly attenuated the effect of
high intensity training on weight gain compared with the E group at
week 5 (P<0.05; Table II).
 | Table II.TT extracts enhanced high intensity
exercise-induced weight gain in rats. |
Table II.
TT extracts enhanced high intensity
exercise-induced weight gain in rats.
| Week | Control | E | TT | E+TT |
|---|
| 0 |
240.18±3.69 |
236.23±9.58 |
242.8±14.04 |
228.73±2.05 |
| 1 |
273.8±7.02 |
263.51±11.31 |
276.62±16.27 |
252.73±4.38 |
| 2 |
308.08±11.61 |
295.8±23.02 |
307.34±15.4 |
294.93±5.36 |
| 3 |
351.48±18.25 |
324.66±25.27a |
345.34±15.28 |
324.23±7.61a |
| 4 |
359.25±38.04 |
322.4±25.27b |
380.12±16.45 |
317.23±15.68b |
| 5 |
402.35±30.45 |
310.7±21.23b |
402.06±21.23 |
327.15±15.75b,c |
Effects of TT extract treatment on the
relative weight, protein concentration and protein levels of MHC in
the gastrocnemius and soleus of rats undergoing high intensity
exercise
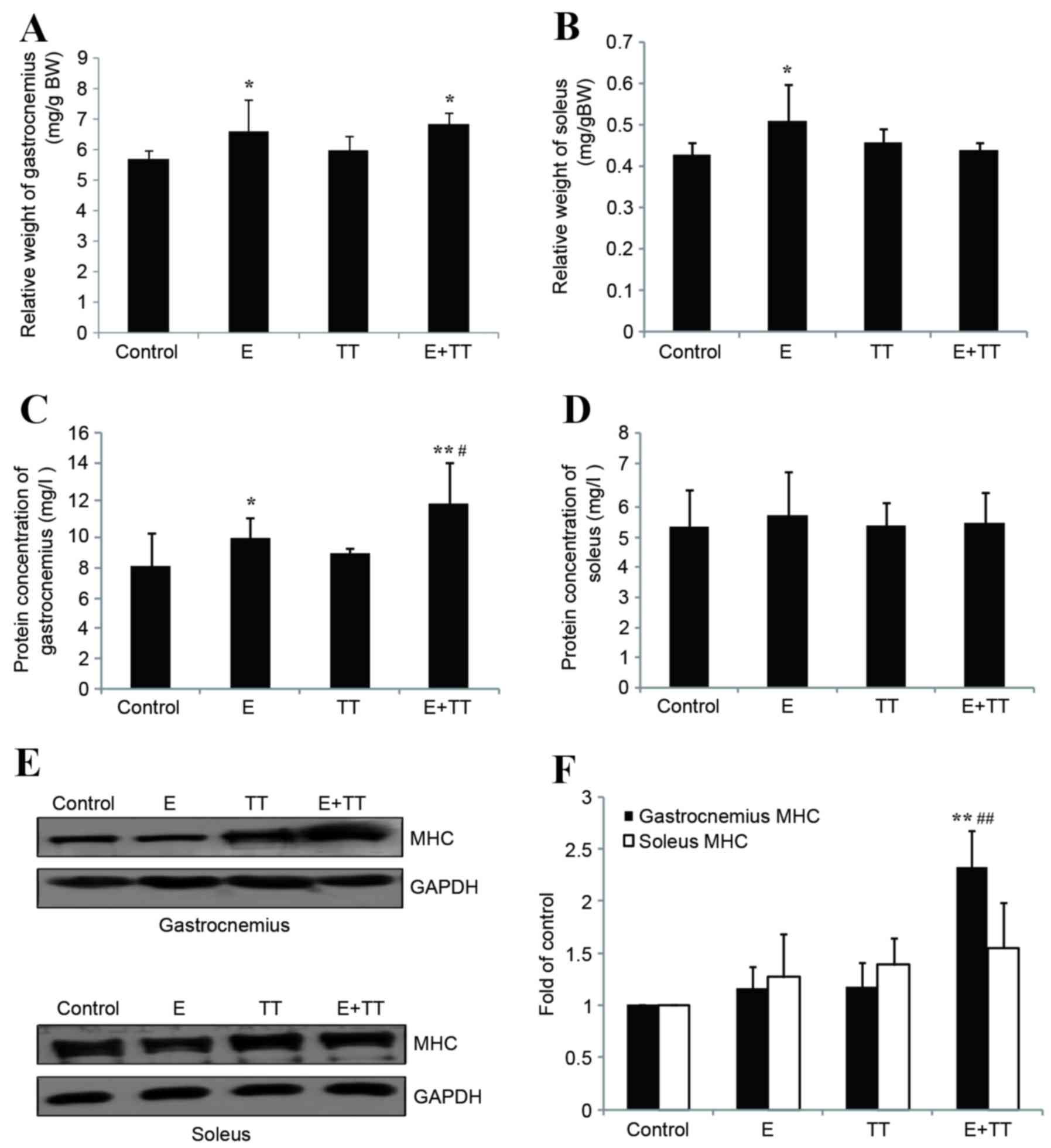
Exercise-induced increases of the relative weight of
the gastrocnemius and soleus and significantly increased relative
weight of the gastrocnemius in the E+TT group compared with the
control (P<0.05; Fig. 1A). The
relative weights of gastrocnemius and soleus did not significantly
differ between the TT group and the control group or in the E+TT
group compared with the E group (Fig.
1A and B), suggesting that TT extracts do not affect the
relative weight of muscle in sedentary or exercising rats. The
protein content of the gastrocnemius in the E and E+TT group rats
was significantly increased compared with the control group
(P<0.05 and P<0.01, respectively; Fig. 1C), and there was a significantly
greater increase in the E+TT group compared with the E group
(P<0.05; Fig. 1C). Soleus
protein content did not significantly differ between the groups
(Fig. 1D). Similar results were
observed for MHC, with visible increases of gastrocnemius MHC
protein expression levels observed in the TT and E+TT groups
compared with the control group (Fig.
1E). MHC levels were significantly increased in the E+TT group
compared with the control group and the E group (P<0.01 and
P<0.01, respectively; Fig. 1F).
No difference in soleus MHC protein expression level was detected
between the groups (Fig. 1E and
F).
No difference was observed in the relative weight,
protein concentration or the protein levels of MHC in the soleus
following treatment with TT extracts, and TT extract-induced
increases in MHC protein concentration and expression levels were
only observed in the gastrocnemius of the E+TT group rats compared
with the E group. As a result, the gastrocnemius was used for the
following studies as opposed to the soleus.
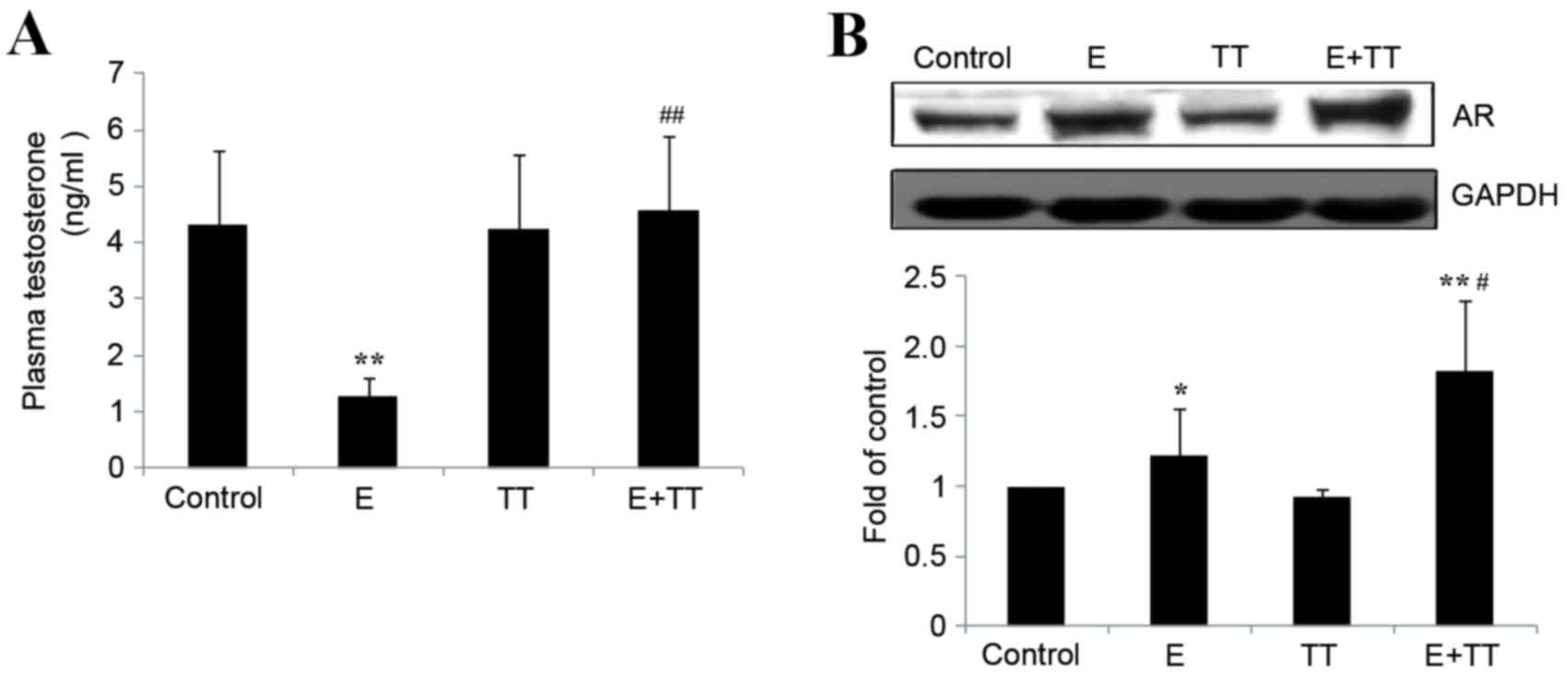
Effects of TT extracts on plasma
testosterone levels and gastrocnemius AR protein levels in rats
undergoing high intensity exercise
Following 5 weeks of high intensity exercise, plasma
testosterone levels in E group were significantly decreased to ~40%
of the control (P<0.01; Fig.
2A). This was attenuated by treatment with TT extracts and
significantly increased plasma testosterone levels were observed in
the E+TT group compared with the E group (P<0.01; Fig. 2A) and no significant difference
between the E+TT group and the control (Fig. 2A). Gastrocnemius AR protein levels
were significantly increased in the E and E+TT groups compared with
the control (P<0.05 and P<0.01, respectively; Fig. 2B) and the increase was greater in
the E+TT group compared with the E group (P<0.05; Fig. 2B).
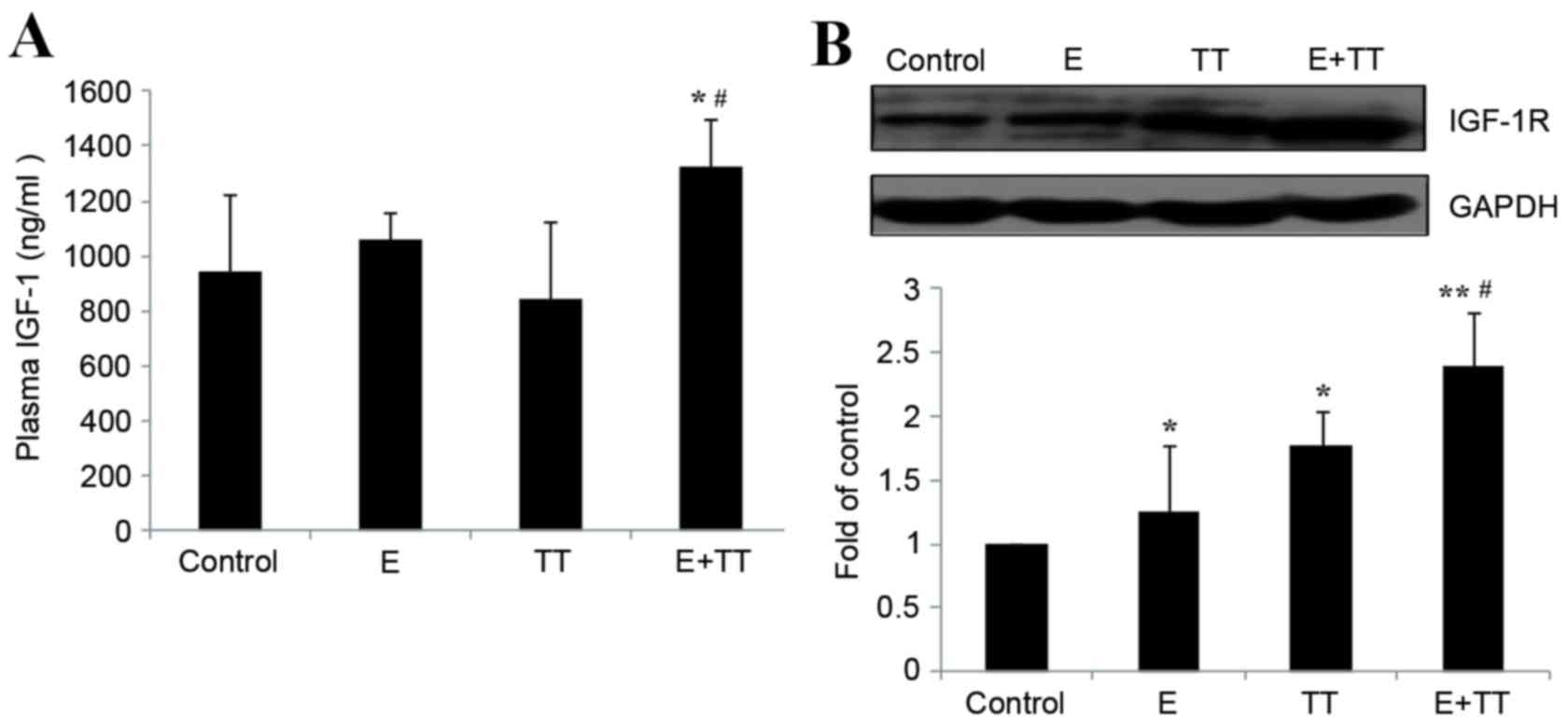
Effects of TT extracts on plasma IGF-1
levels and gastrocnemius IGF-1R protein levels in rats undergoing
high intensity exercise
Following 5 weeks of high intensity exercise, the
plasma levels of IGF-1 in the E group rats remained unaltered
compared with the control group rats (Fig. 3A). TT extracts increased the plasma
levels of IGF-1 in the E+TT group compared with the control group
and the E group (P<0.05 and P<0.05, respectively; Fig. 3A), whereas while no effect was
observed on sedentary rats treated with TT (Fig. 3A). High intensity exercise
significantly increased the protein levels of gastrocnemius IGF-1R
in the E and E+TT groups compared with the control group (P<0.05
and P<0.01, respectively; Fig.
3B) and treatment with TT extracts resulted in a greater
increase than exercise alone (P<0.05; Fig. 3B). Treatment with TT extracts also
increased gastrocnemius IGF-1R protein levels in sedentary rats
compared with the control (P<0.05; Fig. 3B), which suggested that TT extracts
increased gastrocnemius IGF-1R protein expression levels in
sedentary rats and in rats undergoing high intensity exercise.
Discussion
Our previous study demonstrated that TT extracts
promote the performance of rats undergoing 5 weeks of high
intensity exercise, reflected by the extension in the time to
exhaustion from 90 min to ~150 min (a 1.7-fold increase compared
with the E group), which may be associated with the TT-induced
increase of plasma testosterone (18). It of note that commercially
available TT extracts and TT saponins contained in nutritional
supplements recommended for competitive athletes to enhance their
performance may be intentionally contaminated with AAS (36), which may result in inadvertent
doping in competitive sports. In contrast, TT saponins without any
contamination do not lead to positive anti-doping tests (17). To exclude the possibility of AAS
contamination, the chemical components of the TT extracts were
analyzed by UHPLC-Q-TOF/MS, a popular method to identify medicine
components and metabolites (35).
Tigogenin and diosgenin, both saponins, were demonstrated account
for ~71.35% of the total peak area, consistent with the promise of
the manufacturers: >70% TT saponins. In addition, AAS and other
associated hormone precursors were not detected in the extracts.
Together with the results of our previous study (18), the present findings suggested that
TT extracts, in particular TT saponins, are responsible for the
physiological and biological effects which lead to the promotion of
endurance performance of rats undergoing high intensity
exercise.
In the present study, the beneficial effects of TT
extracts on rats undergoing high intensity exercise were
demonstrated by improving weight gain and increasing protein
content, and the MHC protein levels in the gastrocnemius. It has
been demonstrated that exercise promoted contractive muscle
hypertrophy, reflected by the increases of the protein synthesis
and the weight of these muscles, which may contribute to enhanced
exercise performance. MHC constitutes part of the myosin
contractile protein in skeletal muscle. The content of MHC protein
is used to reflect myosin quantity, as MHC is the major determinant
in the speed of contraction of skeletal muscle, contributing to
contractile forces and performance (37,38).
The intensity of the exercise protocol used in the present study
was increased gradually from moderate (week 1–2) to high (week
3–5), resulting in increased protein synthesis and increased
relative weight, but not increased MHC protein levels of the
gastrocnemius (fast twitch fiber), while only increasing of the
relative weight of the soleus (slow twitch fiber). Treatment with
TT extracts resulted in further increases of protein content and
MHC protein levels in the gastrocnemius while no effect was
demonstrated in the soleus, which may explain the increased effect
of TT extracts on the body weight and performance of rats
undergoing high intensity exercise.
Testosterone exerts its biological function by
binding to AR, its cognate receptor. A previous report emphasized
the importance of increased AR levels in exercise-induced muscle
hypertrophy (26). Mitchell et
al (39) demonstrated a
correlation between AR protein content and fiber hypertrophy
despite no statistical significance in adult males undertaking
resistance training. Endurance exercise increased the AR binding
capacity of slow twitch fibers and resistance exercise upregulated
the AR binding capacity of fast twitch fibers (40). The findings of the present study
demonstrated that high intensity exercise led to increased AR
expression in the gastrocnemius (fast twitch fiber), which was
further increased following treatment with TT extracts. These
findings combined with the data that TT extracts induced an
increase in testosterone levels, support the hypothesis that the AR
signaling pathway is involved in the improved performance of rats
undergoing high intensity exercise with administration of TT
extracts. The results of the present study are of particular
physiological significance as to the best of our knowledge, this is
the first report to demonstrate that TT extracts not only increase
testosterone levels but also result in an increase of AR in
skeletal muscles. In addition, TT extracts do not affect plasma
testosterone levels or gastrocnemius AR levels in sedentary rats.
The reason that TT saponins have no effect on testosterone and AR
in non-trained rats but have significant effect on rats undergoing
high intensity training remains to be elucidated.
IGF-1 is associated with muscle mass, conservation
of the musculoskeletal system, metabolic rate and muscle strength
(41,42). The functions of IGF-1 in the blood
and tissues are mediated through binding to tissue IGF-1R (43). IGF-1 is involved in mediating the
effects of testosterone on skeletal muscle progenitor cell growth
and differentiation in vitro through mediation of IGF-1R
(30). However, in MKR mice, which
express a dominant negative form of the IGF-1R in their skeletal
muscle fibers, IGF-1R signaling does not appear to be obligatory
for mediating the anabolic effects of testosterone (30). The results of the present study
demonstrated that, accompanied by a significant increase of
testosterone, a significant increase in plasma IGF-1 levels and a
further increase of the expression of IGF-1R in the gastrocnemius
was induced by TT extracts in rats undergoing high intensity
exercise. This indicates that IGF-1/IGF-1R may be the primary
target of testosterone and one of the major reasons for the
beneficial effects of TT extracts. To the best of our knowledge,
this is the first report to demonstrate that TT extracts activated
the IGF-1/IGF-1R signaling pathway in skeletal muscles.
The present study remains a descriptive study and
the present conclusion will be fully supported if it is
demonstrated that administration of TT extracts does not increase
the performance of rats undergoing high intensity exercise
following blocking increases of AR and IGF-1R in the
gastrocnemius.
The findings of the present study indicated that the
effect of TT extracts on the performance of rats undergoing high
intensity exercise may be attributed to the induced increases of
circulating testosterone and IGF-1, as well as the increases of AR
and IGF-1R protein expression levels in the gastrocnemius,
resulting in increased muscle weight and myosin content in the
gastrocnemius.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31271274) and the
Key Laboratory of Exercise and Health Sciences, Shanghai University
of Sport, Ministry of Education.
Glossary
Abbreviations
Abbreviations:
|
TT
|
Tribulus terrestris
|
|
AR
|
androgen receptor
|
|
IGF-1
|
insulin growth factor-1
|
|
IGF-1R
|
IGF-1 receptor
|
|
MHC
|
myosin heavy chain
|
|
UHPLC-Q-TOF/MS
|
ultra-high performance liquid
chromatography-quadrupole-time of flight mass spectrometry
|
References
|
1
|
Velders M and Diel P: How sex hormones
promote skeletal muscle regeneration. Sports Med. 43:1089–1100.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schoenfeld BJ: Postexercise hypertrophic
adaptations: A reexamination of the hormone hypothesis and its
applicability to resistance training program design. J Strength
Cond Res. 27:1720–1730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wood RI and Stanton SJ: Testosterone and
sport: Current perspectives. Horm Behav. 61:147–155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Luigi L, Romanelli F, Sgrò P and Lenzi
A: Andrological aspects of physical exercise and sport medicine.
Endocrine. 42:278–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bassil N, Alkaade S and Morley JE: The
benefits and risks of testosterone replacement therapy: A review.
Ther Clin Risk Manag. 5:427–448. 2009.PubMed/NCBI
|
|
6
|
El-Tantawy WH, Temraz A and El-Gindi OD:
Free serum testosterone level in male rats treated with Tribulus
alatus extracts. Int Braz J Urol. 33:554–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gauthaman K and Ganesan AP: The hormonal
effects of Tribulus terrestris and its role in the management of
male erectile dysfunction-anevaluation using primates, rabbit and
rat. Phytomedicine. 15:44–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qureshi A, Naughton DP and Petroczi A: A
systematic review on the herbal extract Tribulus terrestris and the
roots of its putative aphrodisiac and performance enhancing effect.
J Diet Suppl. 11:64–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh S, Nair V and Gupta YK: Evaluation
of the aphrodisiac activity of Tribulus terrestris Linn. In
sexually sluggish male albino rats. J Pharmacol Pharmacother.
3:43–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chhatre S, Nesari T, Somani G, Kanchan D
and Sathaye S: Phytopharmacological overview of Tribulus
terrestris. Pharmacogn Rev. 8:45–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown GA, Vukovich MD, Martini ER, Kohut
ML, Franke WD, Jackson DA and King DS: Effects of
androstenedione-herbal supplementation on serum sex hormone
concentrations in 30- to 59-year-old men. Int J Vitam Nutr Res.
71:293–301. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gauthaman K, Adaikan PG and Prasad RN:
Aphrodisiac properties of Tribulus Terrestris extract
(Protodioscin) in normal and castrated rats. Life Sci.
71:1385–1396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martino-Andrade AJ, Morais RN, Spercoski
KM, Rossi SC, Vechi MF, Golin M, Lombardi NF, Greca CS and
Dalsenter PR: Effects of Tribulus terrestris on endocrine sensitive
organs in male and female Wistar rats. J Ethnopharmacol.
127:165–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rogerson S, Riches CJ, Jennings C,
Weatherby RP, Meir RA and Marshall-Gradisnik SM: The effect of five
weeks of Tribulus terrestris supplementation on muscle strength and
body composition during preseason training in elite rugby league
players. J Strength Cond Res. 21:348–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Antonio J, Uelmen J, Rodriguez R and
Earnest C: The effects of Tribulus terrestris on body composition
and exercise performance in resistance-trained males. Int J Sport
Nutr Exerc Metab. 10:208–215. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown GA, Vukovich MD, Reifenrath TA, Uhl
NL, Parsons KA, Sharp RL and King DS: Effects of anabolic
precursors on serum testosterone concentrations and adaptations to
resistance training in young men. Int J Sport Nutr Exerc Metab.
10:340–359. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saudan C, Baume N, Emery C, Strahm E and
Saugy M: Short term impact of Tribulus terrestris intake on doping
control analysis of endogenous steroids. Forensic Sci Int.
178:e7–e10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin L and Wang X, Cao X and Wang X: The
effects of tribulus terrestris on the time of exhaustion in rats
with high intensity training and its mechanism. J Shanghai
University Sport. 37:73–77. 2013.
|
|
19
|
Matsumoto T, Sakari M, Okada M, Yokoyama
A, Takahashi S, Kouzmenko A and Kato S: The androgen receptor in
health and disease. Annu Rev Physiol. 75:201–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vingren JL, Kraemer WJ, Hatfield DL, Volek
JS, Ratamess NA, Anderson JM, Häkkinen K, Ahtiainen J, Fragala MS,
Thomas GA, et al: Effect of resistance exercise on muscle steroid
receptor protein content in strength-trained men and women.
Steroids. 74:1033–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Safarinejad MR, Azma K and Kolahi AA: The
effects of intensive, long-term treadmill running on reproductive
hormones, hypothalamus-pituitary-testis axis, and semen quality: A
randomized controlled study. J Endocrinol. 200:259–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee WJ, Thompson RW, McClung JM and Carson
JA: Regulation of androgen receptor expression at the onset of
functional overload in rat plantaris muscle. Am J Physiol Regul
Integr Comp Physiol. 285:R1076–R1085. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aizawa K, Iemitsu M, Maeda S, Otsuki T,
Sato K, Ushida T, Mesaki N and Akimoto T: Acute exercise activates
local bioactive androgen metabolism in skeletal muscle. Steroids.
75:219–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hulmi JJ, Ahtiainen JP, Selänne H, Volek
JS, Häkkinen K, Kovanen V and Mero AA: Androgen receptors and
testosterone in men-effects of protein ingestion, resistance
exercise and fiber type. J Steroid Biochem Mol Biol. 110:130–137.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ratamess NA, Kraemer WJ, Volek JS, Maresh
CM, Vanheest JL, Sharman MJ, Rubin MR, French DN, Vescovi JD,
Silvestre R, et al: Androgen receptor content following heavy
resistance exercise in men. J Steroid Biochem Mol Biol. 93:35–42.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahtiainen JP, Hulmi JJ, Kraemer WJ, Lehti
M, Nyman K, Selänne H, Alen M, Pakarinen A, Komulainen J, Kovanen
V, et al: Heavy resistance exercise training and skeletal muscle
androgen receptor expression in younger and older men. Steroids.
76:183–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McMahon CD, Chai R, Radley-Crabb HG,
Watson T, Matthews KG, Sheard PW, Soffe Z, Grounds MD and
Shavlakadze T: Lifelong exercise and locally produced insulin-like
growth factor-1 (IGF-1) have a modest influence on reducing
age-related muscle wasting in mice. Scand J Med Sci Sports.
24:e423–e435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo L, Lu AM, Wang Y, Hong A, Chen Y, Hu
J, Li X and Qin ZH: Chronic resistance training activates autophagy
and reduces apoptosis of muscle cells by modulating IGF-1 and its
receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp
Gerontol. 48:427–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim J, Wende AR, Sena S, Theobald HA, Soto
J, Sloan C, Wayment BE, Litwin SE, Holzenberger M, LeRoith D and
Abel ED: Insulin-like growth factor I receptor signaling is
required for exercise-induced cardiac hypertrophy. Mol Endocrinol.
22:2531–2543. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Serra C, Bhasin S, Tangherlini F, Barton
ER, Ganno M, Zhang A, Shansky J, Vandenburgh HH, Travison TG,
Jasuja R and Morris C: The role of GH and IGF-I in mediating
anabolic effects of testosterone on androgen-responsive muscle.
Endocrinology. 152:193–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Knapczyk-Stwora K, Grzesiak M, Duda M,
Koziorowski M and Slomczynska M: Effect of flutamide on
folliculogenesis in the fetal porcine ovary-regulation by Kit
ligand/c-Kit and IGF1/IGF1R systems. Anim Reprod Sci. 142:160–167.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SH, Johnson D, Luong R and Sun Z:
Crosstalking between androgen and PI3K/AKT signaling pathways in
prostate cancer cells. J Biol Chem. 290:2759–2768. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kretzschmar K, Cottle DL, Schweiger PJ and
Watt FM: The androgen receptor antagonizes Wnt/β-Catenin signaling
in epidermal stem cells. J Invest Dermatol. 135:2753–2763. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tarulli GA, Butler LM, Tilley WD and
Hickey TE: Bringing androgens up a NOTCH in breast cancer. Endocr
Relat Cancer. 21:T183–T202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu JL, Leung EL, Zhou H, Liu L and Li N:
Metabolite analysis of toosendanin by an ultra-high performance
liquid chromatography-quadrupole-time of flight mass spectrometry
technique. Molecules. 18:12144–12153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cavalcanti Gde A, Leal FD, Garrido BC,
Padilha MC and de Aquino Neto FR: Detection of designer steroid
methylstenbolone in ‘nutritional supplement’ using gas
chromatography and tandem mass spectrometry: Elucidation of its
urinary metabolites. Steroids. 78:228–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JH and Thompson LV: Non-weight
bearing-induced muscle weakness: The role of myosin quantity and
quality in MHC type II fibers. Am J Physiol Cell Physiol.
307:C190–C194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schilling BK, Fry AC, Chiu LZ and Weiss
LW: Myosin heavy chain isoform expression and in vivo isometric
performance: A regression model. J Strength Cond Res. 19:270–275.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitchell CJ, Churchward-Venne TA, Bellamy
L, Parise G, Baker SK and Phillips SM: Muscular and systemic
correlates of resistance training-induced muscle hypertrophy. PLoS
One. 8:e786362013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deschenes MR, Maresh CM, Armstrong LE,
Covault J, Kraemer WJ and Crivello JF: Endurance and resistance
exercise induce muscle fiber type specific responses in androgen
binding capacity. J Steroid Biochem Mol Biol. 50:175–179. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Frystyk J: Exercise and the growth
hormone-insulin-like growth factor axis. Med Sci Sports Exerc.
42:58–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gibney J, Healy ML and Sönksen PH: The
growth hormone/insulin-like growth factor-I axis in exercise and
sport. Endocr Rev. 28:603–624. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Philippou A, Halapas A, Maridaki M and
Koutsilieris M: Type I insulin-like growth factor receptor
signaling in skeletal muscle regeneration and hypertrophy. J
Musculoskelet Neuronal Interact. 7:208–218. 2007.PubMed/NCBI
|