Introduction
Autologous fat grafting is a promising surgical
technique for soft tissue augmentation, reconstruction and
rejuvenation. Fat tissue is abundant, readily available and
relatively low cost, and can be harvested with minimal trauma to
the donor site. Fat grafts have been extensively used in numerous
fields, including the treatment of Romberg's disease, depressed
scars, wrinkles, senile atrophy, pitting acne, breast augmentation,
and hand and face rejuvenation (1). Despite these uses, transplanted fat
often has a low survival rate, and adipose tissue can be quickly
resorbed and replaced with fibrous tissue and oil cysts (2–4). Fat
graft retention rates have been reported to range between 10 and
90% (5,6). Several theories have been suggested
to explain this phenomenon (7,8). At
present, the generally accepted explanation for the decreasing fat
volume is insufficient blood supply following transplantation
(8–10). Therapeutic approaches that
stimulate angiogenesis, including co-transplanting fat with
proangiogenic growth factors, endothelial progenitor cells and
mesenchymal stem cells (MSCs) from various sources, have
significantly improved fat graft retention (11–16).
Such co-transplanted cells likely promote neovascularization via
the paracrine action of proangiogenic factors, a mechanism that has
also been observed during the treatment of other ischemic diseases
(17–19).
In addition to cell-based therapies for the
treatment of ischemic diseases, interest in the vesicles released
by these cultured cells has garnered interest. Extracellular
vesicles (EVs), which are released by almost all cells, are
normally comprised of three types: Microvesicles, exosomes and
apoptotic bodies (20).
Furthermore, EVs have been detected in various body fluids,
including blood, malignant pleural effusion, urine, saliva and
cerebrospinal fluid (21).
Previously, EVs were considered inert cellular debris, the
consequence of cell damage or the result of dynamic plasma membrane
turnover. However, EVs have more recently been reported to serve an
important role in intercellular communication (22). The structure of EVs consists of a
lipid bilayer membrane, similar to the cellular membrane, which
envelops host-specific proteins, mRNAs and microRNAs. Numerous
studies have investigated the role of EVs in tissue repair and
regeneration (18,22,23).
In addition, animal studies have demonstrated that the systemic
application of EVs derived from cultured bone marrow MSCs
(BMSC-EVs) protects cells during acute kidney injury and
cardiovascular disease (18,23,24).
It has been suggested that BMSC-EVs may protect injured tissues by
inducing angiogenesis (18,24).
Based on the proangiogenic potential of BMSC-EVs,
the present study hypothesized that co-transplantation of fat with
BMSC-EVs during grafting would prevent fat cell death and stimulate
neovascularization, thus helping to prevent graft resorption. To
test this hypothesis, BMSC-EVs were collected from the supernatant
of cultured rat BMSCs using ultra-centrifugation. Subsequently,
human fat tissue, combined with various concentrations of BMSC-EVs,
was subcutaneously injected into nude mice, and the effects were
evaluated 12 weeks post-transplantation.
Materials and methods
Cell culture and isolation of EVs
A total of 80 male Wistar rats (4 weeks old)
weighing 90–110 g were purchased from the Shanghai Chuansha
Experimental Animal Raising Farm (Shanghai, China). The animal
study protocols were approved by the Animal Care and Experiment
Committee of Shanghai Jiao Tong University School of Medicine
(Shanghai, China). They were housed under a temperature of 20–26°C,
a relative humidity of 40–70% and a day/night cycle of 12/12 h,
with free access to food and water. BMSCs were cultured as
previously described (25).
Briefly, rat bone marrow cells were extracted from the femurs of
male Wistar rats. To remove the majority of the non-adherent blood
cells, primary culture of bone marrow cells was performed by
seeding cells at 1.6×104 cells/cm2 in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Hyclone; GE Healthcare Life Science, Logan, UT,
USA) and 0.2% penicillin/streptomycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C. The medium was changed every 3 days.
After 6–7 days of culture the primary adherent cells (P0) were
harvested using trypsin/EDTA (0.25% w/v trypsin and 0.02% EDTA;
Invitrogen; Thermo Fisher Scientific, Inc.), and were subcultured
at 1.6×104 cells/cm2 in a 10-cm diameter
tissue culture dish in 10 ml DMEM containing 10% FBS. The cells
were passaged in the same manner every 3 days. Passage 4 cells were
evaluated for typical MSC characteristics, including the cell
surface marker expression profile and multi-lineage differentiation
potential (data not shown), and were used for the collection of
EVs. Once the cells reached 70% confluence, they were cultured in
serum-free media under hypoxic conditions (94% N2, 5%
CO2 and 1% O2) for 48 h. The culture supernatants were
collected and centrifuged at 2,000 × g for 20 min at 4°C to remove
any large particles of debris. After an additional centrifugation
at 100,000 × g for 1 h at 4°C, the pellet was washed twice in PBS
(26). The collected BMSC-EVs were
then resuspended in 50 µl PBS and stored at −80°C for use in the
subsequent experiments. The amount of BMSC-EVs was determined by
measuring the total protein content using the Bradford method
(Sigma-Aldrich; Merck KGaA).
Scanning electron microscopy
The EVs were suspension-fixed in 4% paraformaldehyde
dissolved in PBS for 12 h at 4°C. Subsequently, the EVs were
dehydrated through a graded series of ethanol/water (50–100%, v/v)
solutions, and then dried at the critical point. The samples were
gold-sputtered and examined under a Hitachi S-4800 field emission
scanning electron microscope (Hitachi, Tokyo, Japan) at an
acceleration voltage of 10 kV.
Characterization of the BMSC-EVs
The size distribution of the EVs was measured using
a particle size analyzer (Nicomp 380 ZLS; Particle Sizing Systems,
Port Richey, FL, USA) (27). The
samples were illuminated by red laser light (15 mW, 635 nm), and a
digital camera captured the scattered light. The EVs were
automatically tracked and sized, based on Brownian motion and their
diffusion coefficient. The results are displayed as representative
size distribution profiles.
A phenotypic profile of the EVs was determined using
bead-based flow cytometry. Briefly, the BMSC-EVs were bound to
aldehyde/sulfate latex beads (4 mm; Molecular Probes; Thermo Fisher
Scientific, Inc.) suspended in 2-(N-morpholino) ethane sulfonic
acid (MES) buffer (0.025 M MES, 0.154 M NaCl; pH 6.0) overnight at
4°C with gentle agitation. The reaction was terminated by
incubation for 30 min with 200 mM glycine at room temperature, to
saturate any remaining free binding sites on the beads. After two
washes in PBS containing 4% FBS, the BMSC-EVs-coated beads were
stained with the following antibodies: Fluorescein isothiocyanate
(FITC)-or phycoerythrin (PE)-conjugated anti-cluster of
differentiation CD81 (catalog no. 559519, 1:400), CD63 (catalog no.
551458, 1:200), CD29 (catalog no. 555005, 1:200), CD90 (catalog no.
554894, 1:200), CD45 (catalog no. 559135, 1:200), CD31 (catalog no.
555026, 1:200) purchased from BD Biosciences (San Diego, CA, USA),
with or without the following secondary antibodies: PE
streptavidin; catalog no. 554061; 1:200 and goat anti-mouse 488;
catalog no. A11001; 1:200, at 4°C overnight. Beads without BMSC-EVs
were prepared to determine the forward and side scatter signals of
the beads. All data were collected on an Epics Altra Hypersort™
system (Beckman Coulter, Inc., Brea, CA, USA) and were analyzed
with FlowJo Software 7.6.1 (FlowJo, LLC, Ashland, OR, USA).
Cellular uptake of BMSC-EVs
BMSC-EVs were labeled with Cell Tracker™ CM-Dil
(Thermo Fisher Scientific, Inc.). Briefly, 1 µl cell-labeling
solution was added to 200 µg EVs suspended in 1 ml PBS, and was
incubated for 20 min at 37°C. Subsequently, the mixture was
centrifuged at 100,000 × g for 1 h at 4°C, the supernatants were
removed, and the BMSC-EVs were gently resuspended in PBS. This
washing procedure was repeated 3 times.
Human umbilical vein endothelial cells (HUVECs) were
purchased from ScienCell Research Laboratories (Carlsbad, CA, USA),
and were cultured in DMEM supplemented with 10% FBS and 1%
penicillin-streptomycin at 37°C in a humidified incubator (5%
CO2). Following incubation with CM-Dil-labeled BMSC-EVs
(20 µg/ml) for 2, 4 or 6 h, the cells were washed twice with PBS,
fixed in 4% paraformaldehyde and stained with DAPI. The cells were
then observed under a confocal microscope (Leica TCS SP5; Leica
Microsystems GmbH, Wetzlar Germany). The images were collected and
merged using Image-Pro Plus 6 (Media Cybernetics, Inc., Rockville,
MD, USA).
Cell viability
HUVECs (1.0×103 cells/well) were plated
in 96-well plates (3 wells/group) and treated with 50 or 100 µg/ml
BMSC-EVs for 6 days. At the indicated time-points (1, 2, 3, 4 and 5
days), cell viability was determined using Cell Counting Kit-8
(CCK8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
according to the manufacturer's protocol. The absorbance was
measured at 450 nm using an ELISA reader (Bio-Tek Instruments,
Inc., Winooski, VT, USA). All experiments were repeated ≥3
times.
Tube formation
Matrigel (BD Biosciences) was thawed and pipetted
into a 24-well culture plate, and was incubated at 37°C for 30 min
to allow solidification. HUVECs (5×104) were treated
with 50 or 100 µg/ml BMSC-EVs for 12 h at 37°C. Tube formation was
examined by phase-contrast microscopy (Olympus Corporation, Tokyo,
Japan), and the number of network structures was quantified using
Image-Pro Plus 6 (Media Cybernetics, Inc.). The data were
statistically analyzed using GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA). All experiments were repeated
≥3 times.
Cell migration
Cell migration was assessed by scratch assays.
Briefly, HUVECs were grown to confluence (2×105 cell/ml)
in a 6-well plate and a scratch was made in the cell field using a
1,000 µl pipette tip. The wells were washed with PBS to remove any
debris and incubated with 50 or 100 µg/ml BMSC-EVs for 12 h at
37°C. Bright-field microscopy images were then collected (Olympus
Corporation) and cell migration was assessed using Image-Pro Plus 6
(Media Cybernetics, Inc.). The data were statistically analyzed
using GraphPad Prism 5 software (GraphPad Software, Inc.). All
experiments were repeated ≥3 times.
Isolation and preparation of human fat
tissue
During the period of June 2015 to November 2016,
human liposuction aspirates were obtained from 10 healthy female
donors aged from 28 to 40 who underwent liposuction of the abdomen
at Shanghai 9th People's Hospital (Shanghai, China). Each patient
provided written informed consent. The protocol for this study was
approved by the Ethics Committee of Shanghai Jiao Tong University
School of Medicine. Prior to initiation of the procedure, the
liposuction site was infiltrated with tumescent solution containing
0.08% lidocaine and a 1: 500,000-unit dilution of epinephrine.
Under sterile conditions, the lipid part of the fat aspirate was
rinsed with sterile PBS to remove blood, saline and the local
anesthetic. The adipose portion of the liposuction aspirate was
placed in an upright position to obtain a clear separation of the
fluids and oil. The upper (oil) and lower (blood and infiltration
liquids) layers were removed, and the middle portion containing the
fat particles was immediately transferred to 50 ml centrifuge tubes
and stored at 4°C until further use.
Animal model and study design
Female BALB/c-nu nude mice (age, 6 weeks; weight,
14–17 g) were obtained from the Shanghai Chuansha Experimental
Animal Raising Farm (Shanghai, China). Mice were kept at a
temperature of 20–26°C, a relative humidity of 40–70% and a
day/night cycle of 12/12 h, with food and water ad libitum,
and a total of 27 mice were randomly divided into three groups (n=9
mice/group). In the control (untreated) group, mice were injected
with 1 ml human fat and 200 µl sterile PBS; in the low-dose EV
group, mice were injected with 1 ml human fat and 200 µl sterile
PBS containing 50 µg BMSC-EVs; in the high-dose EV group, mice were
injected with 1 ml human fat and 200 µl sterile PBS containing 100
µg BMSC-EVs.
The scalp of the mouse was chosen as the recipient
site for fat injection due to the absence of subcutaneous fat in
this area. The mice were anesthetized with 0.2 ml 5% chloral
hydrate and a subcutaneous tunnel was created by antegradely
passing a needle from the dorsalis to the scalp. Fat was then
injected using a 14 G needle in the retrograde direction while
pulling the needle out. Following injection, the skin incisions
were closed with #6/0 non-absorbable sutures. A total of 12 weeks
following transplantation, the mice were sacrificed and the grafts
were harvested. Macroscopic inspection revealed that the fat grafts
were surrounded by a thin envelope, making it easy to isolate them
from the host tissue.
Graft volume
The harvested fat tissue grafts were weighed on a
balance and their volumes were determined according to the liquid
overflow method (13). Briefly, a
graduated cylinder was filled with PBS, the fat tissues were
immersed in the cylinder, and the fat volume was determined by the
consequent increase in buffer volume.
Histology and
immunohistochemistry
Following volume determination, the grafts removed
from the scalp were fixed in 4% paraformaldehyde for 12 h, embedded
in paraffin, and cut into sections (size, 8 µm). These sections
were stained with hematoxylin for 15–30 min and eosin for 3–5 min
(H&E stain) for structural analysis and Masson's staining (acid
fuchsin stain for 5–10 min and aniline blue stain for 5 min) for
analysis of the fibrotic area. A total of 5 randomly selected
fields from each sample (n=3 per group) were imaged under an
inverted microscope (Olympus Corporation).
Immunohistochemical staining was performed according
a method previously published (25). In brief, paraffin-embedded sections
(8 mm thick) were incubated with goat anti-CD31 antibody (catalog
no. sc-1506; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C, followed by incubation with a horseradish
peroxidase-conjugated rabbit anti-goat secondary antibody (catalog
no. E0466; Dako; Agilent Technologies, Inc.) at room temperature
for 30 min, and colorization with 3,3′-diaminobenzidine
tetrahydrochloride (Dako; Agilent Technologies, Inc.) at room
temperature for 3 min. A total of 5 randomly selected fields from
each sample (n=3/group) were imaged under a light microscope (Nikon
Eclipse 90i; Nikon Corporation, Tokyo, Japan). The number of
CD31-positive capillaries was calculated using Image-Pro Plus 6
(Media Cybernetics, Inc.), and the data were statistically analyzed
using GraphPad Prism 5 software (GraphPad Software, Inc.) (25).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from the control and low-dose EV groups
were extracted and reverse transcribed to obtain cDNA, as
previously described (25).
RT-qPCR was performed using a Power SYBR-Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) in an Mx3000P
qPCR system thermal cycler (Stratagene; Agilent Technologies, Inc.,
Santa Clara, CA, USA). Cycling conditions were as follows: An
initial predenaturation step at 95°C for 10 min, followed by 40
cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30
sec and extension at 72°C for 45 sec. The primers used for
detection of the following proangiogenic factors: Platelet-derived
growth factor α (PDGF-A), platelet-derived growth factor receptor α
(PDGFR-A), vascular endothelial growth factor A (VEGF-A) and
transforming growth factor β (TGF-β) are listed in Table I. Gene expression was normalized to
GAPDH expression according to the 2−∆∆Cq method
(28). Each sample was tested in
triplicate, and 3 independent experiments were performed.
 | Table I.Primers used in quantitative
polymerase chain reaction analysis. |
Table I.
Primers used in quantitative
polymerase chain reaction analysis.
| Gene | Primer size
(bp) | Primer
sequences |
|---|
| PDGF-A | 199 |
F:GCAAGACCAGGACGGTCATTTAC |
|
|
|
R:GGCTTCTTCCTGACATACTC |
| PDGFR-A | 193 |
F:CCATGCAGTTGCCTTACGAC |
|
|
|
R:AGAGCCTGCTTTTCACTAGACC |
| VEGF-A | 130 |
F:GGAGATCCTTCGAGGAGCACTT |
|
|
|
R:GGCGATTTAGCAGCAGATATAAGAA |
| TGF-β | 226 |
F:AACCCCCATTGCTGTCCCGTG |
|
|
|
R:GCGCTGAATCGAAAGCCCTGT |
| GAPDH | 235 |
F:GACTTCAACAGCAACTCCCAC |
|
|
|
R:TCCACCACCCTGTTGCTGTA |
Statistical analysis
The data were statistically analyzed using GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). Data
are expressed as the mean ± standard deviation. The significance of
differences between groups was assessed by Student's t-test or
one-way analysis of variance followed by the Newman-Keuls post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
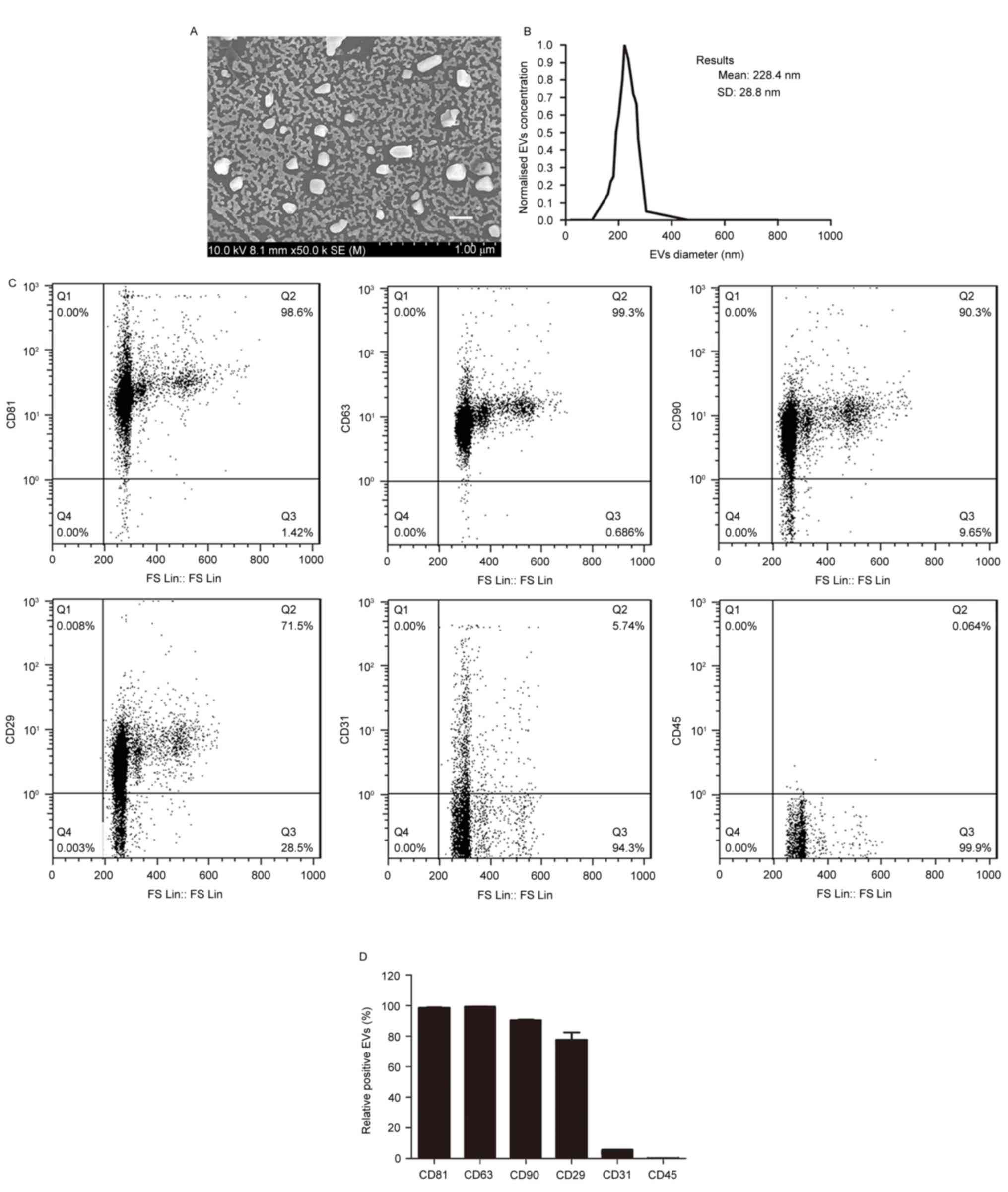
Characterization of BMSC-EVs
To determine whether co-transplantation of fat with
BMSC-EVs improves fat graft retention, EVs were isolated from the
supernatants of rat BMSCs and were characterized. Scanning electron
microscopy demonstrated that the EVs were heterogeneous with a
spheroid-shaped morphology (Fig.
1A). Particle size analysis indicated that the average EV
diameter was 228.4±28.8 nm, with a range between 40 and 300 nm
(Fig. 1B), thus suggesting that
the EVs comprised a mixture of exosomes and microvesicles. Flow
cytometry demonstrated that the EVs expressed high levels of the
following BMSC-positive and EV-positive markers: CD90, CD29, CD81
and CD63, but not of the endothelial and hematopoietic cell
markers: CD31 and CD45 (Fig. 1C and
D). These findings indicated that the EVs were of BMSC
origin.
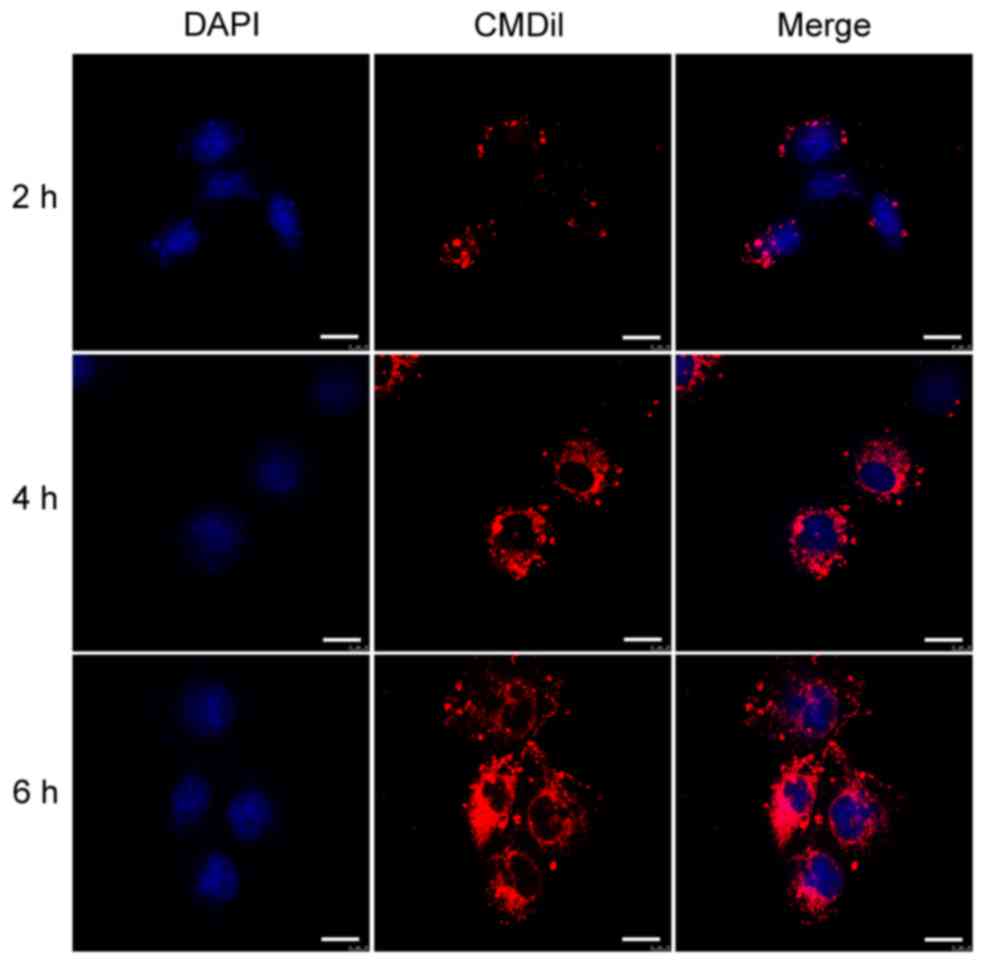
Cellular uptake of BMSC-EVs
In order to determine whether the BMSC-EVs interact
with vascular endothelial cells, HUVECs were incubated with
CM-Dil-labeled BMSC-EVs and observed by confocal microscopy.
Following a 2-h incubation, CM-Dil was observed on the cell
surface. With increasing incubation time, more fluorescent dye
accumulated in the cytoplasm, eventually reaching a maximal signal
at 6 h (Fig. 2). These results
indicated that the EVs were internalized by HUVECs.
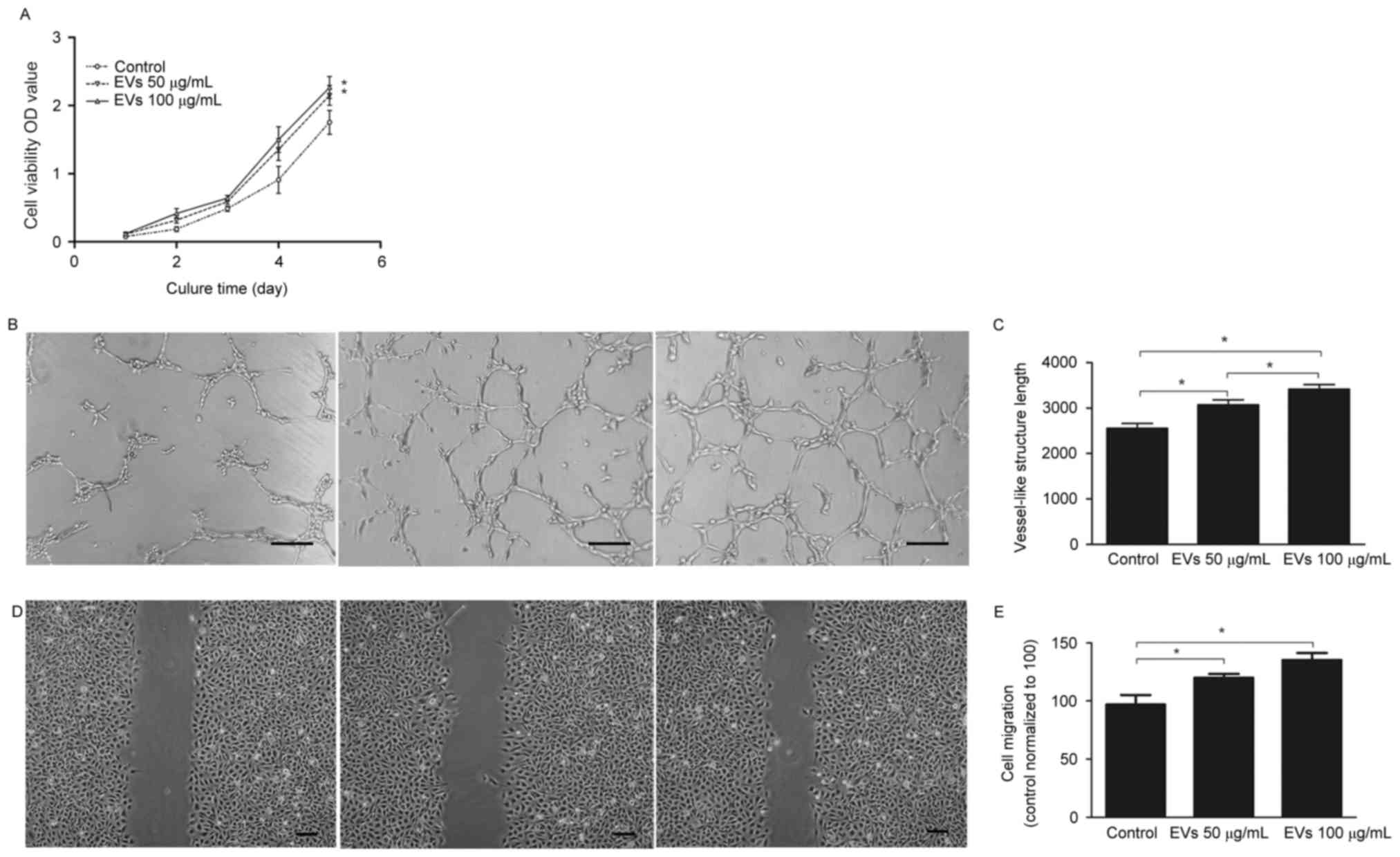
BMSC-EVs promote viability, migration
and tube formation of HUVECs
To determine the effects of BMSC-EVs on endothelial
cells, HUVECs were exposed to 50 or 100 µg/ml BMSC-EVs, and their
cell viability, migration and tube-forming capabilities were
assessed. As presented in Fig. 3A,
100 µg/ml EVs increased cell viability by ~1.3-fold compared with
the control group after 5 days; however, there were no significant
differences between the low (50 µg/ml) and high dose (100 µg/ml)
treatment groups. An in vitro tube formation assay was
conducted to assess the angiogenic effects of BMSC-EVs; the results
demonstrated that more vessel-like structures were observed
following EV treatment, in a dose-dependent manner (Fig. 3B and C). Quantitative and
statistical analysis demonstrated that the high dose EV-treated
HUVECs formed 3,419±97 vessel-like structures per section, whereas
the control cells formed 2,553±107, thus suggesting a ~1.34-fold
increase in vessel-like structures following EV treatment (Fig. 3C). In addition, the cell migration
assay indicated that treatment of HUVECs with high dose BMSC-EVs
resulted in rapid closure of the scratched area, which was
~1.39-fold faster compared with in the non-treated HUVECs; this
effect was dose-dependent (Fig. 3D and
E). These results suggested that BMSC-EVs may promote
endothelial cell viability, migration and tube-forming capabilities
in vitro, indicating that BMSC-EVs may exert
angiogenesis-promoting activity.
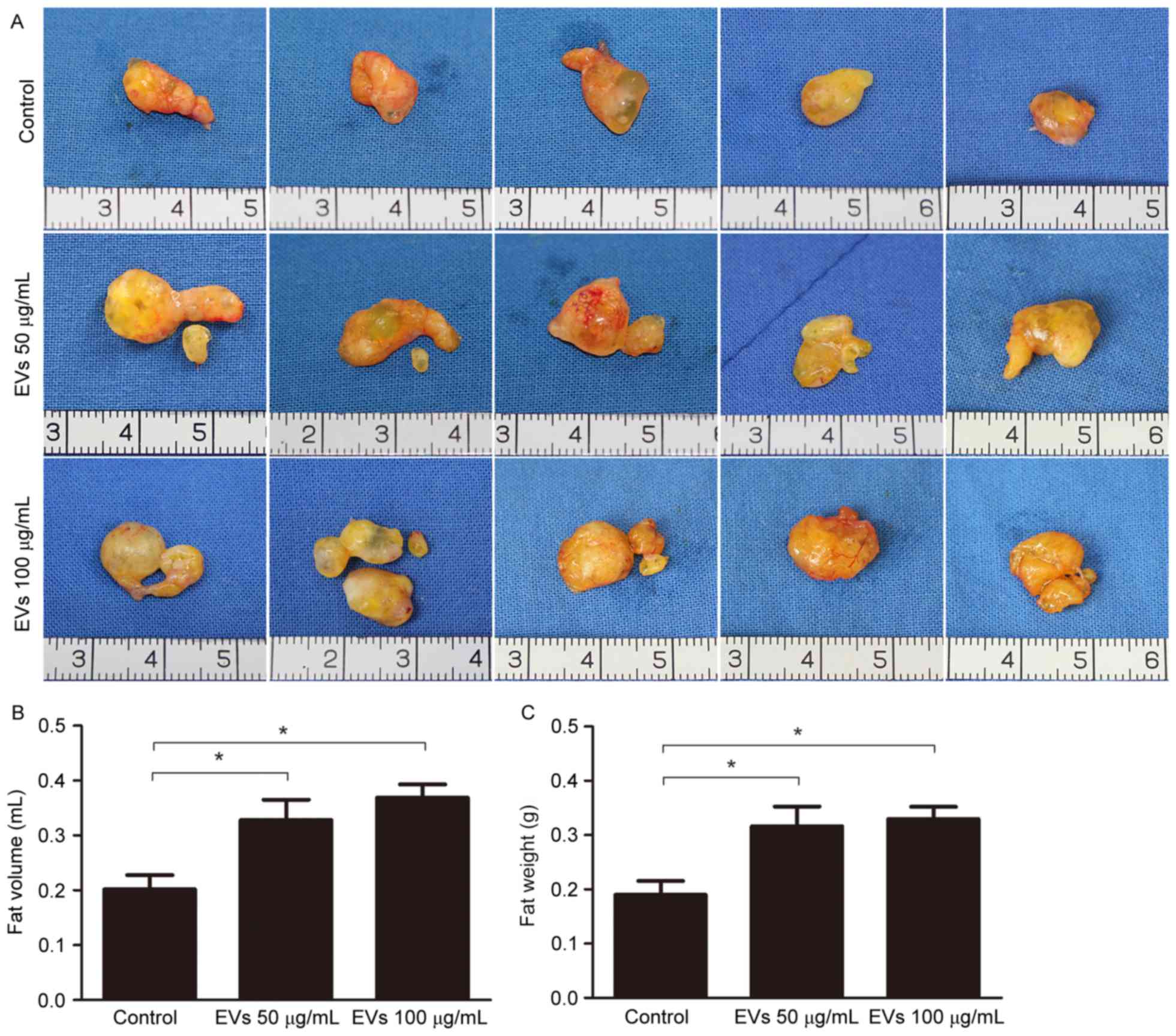
BMSC-EVs improve fat graft
retention
To evaluate the effects of BMSC-EVs on the retention
of transplanted fat grafts in animals, human fat prepared from
liposuction aspirates was co-injected with BMSC-EVs into nude mice.
A total of 12 weeks post-transplantation, all 27 mice in the
control, low-dose EV and high-dose EV groups survived. No
inflammation or abscesses were observed in the surgical areas.
Subsequently, the mice were sacrificed and the fat grafts were
harvested. A total of 5 representative images of grafts from each
group (n=9) are presented in Fig.
4A. The grafts in the EV-treated groups appeared larger than
those in the non-treated group (Fig.
4A). Statistical analysis (n=9/group) demonstrated that average
fat volume in the control, low-dose EV and high-dose EV groups was
0.2018±0.0258, 0.3283±0.0368 and 0.3689±0.0241 ml, respectively,
whereas the average fat weight in these groups was 0.1902±0.0252,
0.3166±0.0363 and 0.3297±0.0228 g, respectively. These results
suggested that EV treatment increased graft retention (Fig. 4B and C).
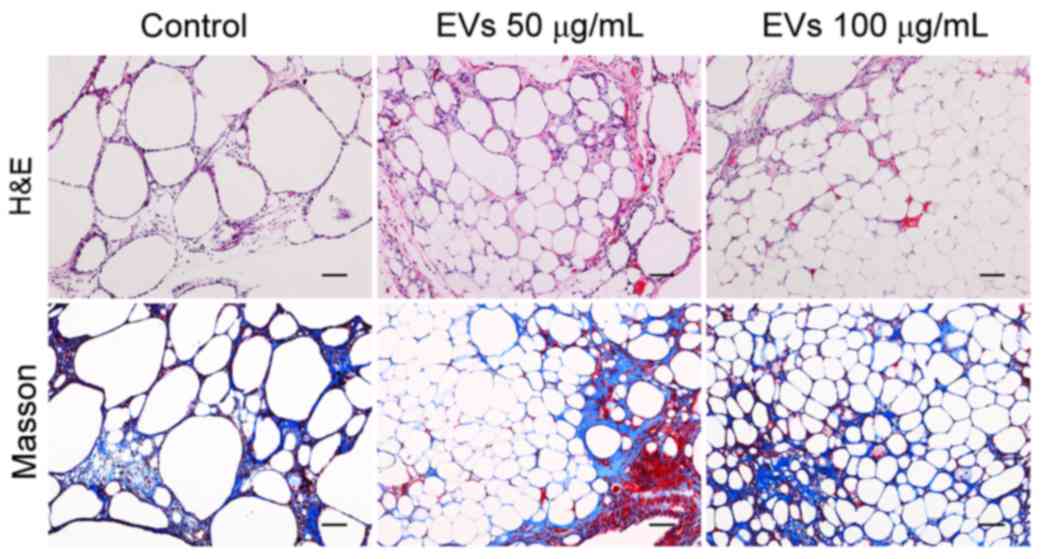
The graft structure was further analyzed by H&E
and Masson staining after sectioning. As presented in Fig. 5, all of the grafts contained intact
adipocytes, fibrous tissue and vacuoles. The grafts from the
EV-treated groups had more intact and nucleated adipocytes with
relatively fewer fibers and vacuoles, whereas more vacuoles were
observed in the control group. No differences were observed between
the low- and high-dose groups. These observations suggested that
BMSC-EVs may enhance adipose tissue regeneration
post-transplantation.
BMSC-EVs promote fat retention through
neovascularization
In the animal model, BMSC-EVs appeared to improve
graft retention by promoting adipose tissue regeneration;
therefore, the present study examined whether BMSC-EVs may
facilitate neovascularization, which is an indispensable process
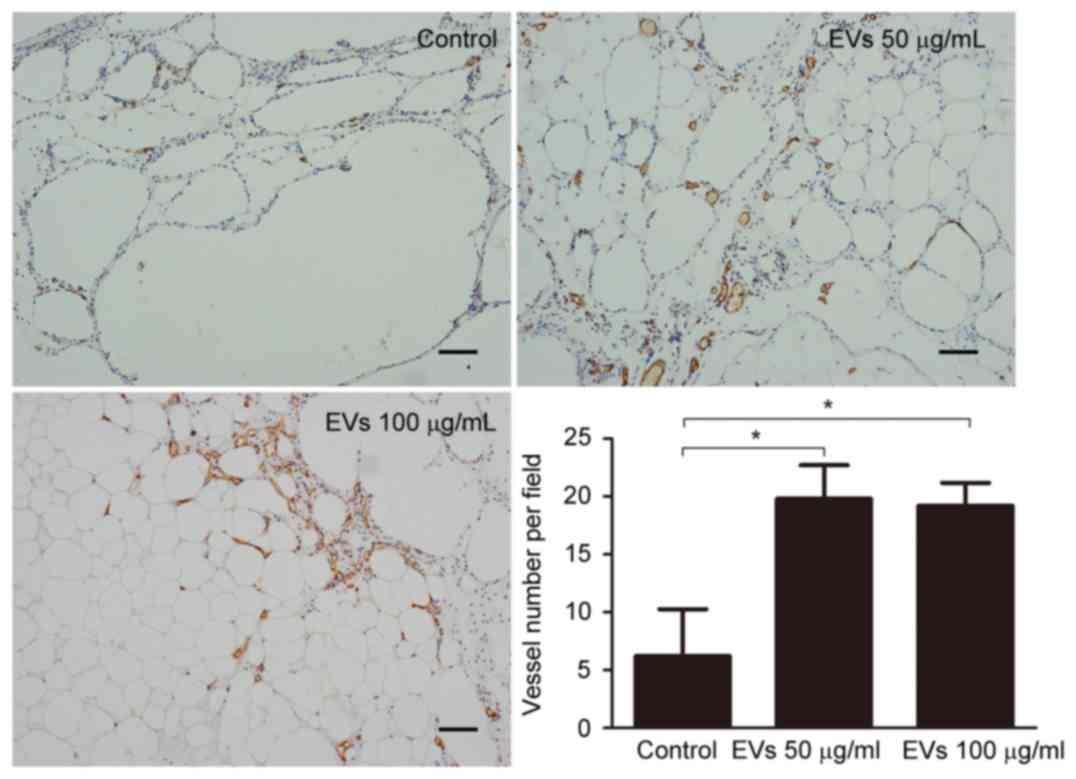
for tissue regeneration. To detect CD31-positive capillaries,
harvested fat tissue grafts were subjected to immunohistochemical
staining with an anti-CD31 antibody. An increased number of
CD31-positive capillaries was observed in the EV-treated groups
compared with in the control group (Fig. 6). Capillary density (vessel number
per field) was calculated from 5 randomly selected fields in each
sample from the control, low-dose EV and high-dose EV groups;
capillary density was 6.20±4.07, 19.80±2.91 and 19.20±1.99,
respectively, indicating that treatment with EVs may promote
neovascularization (Fig. 6).
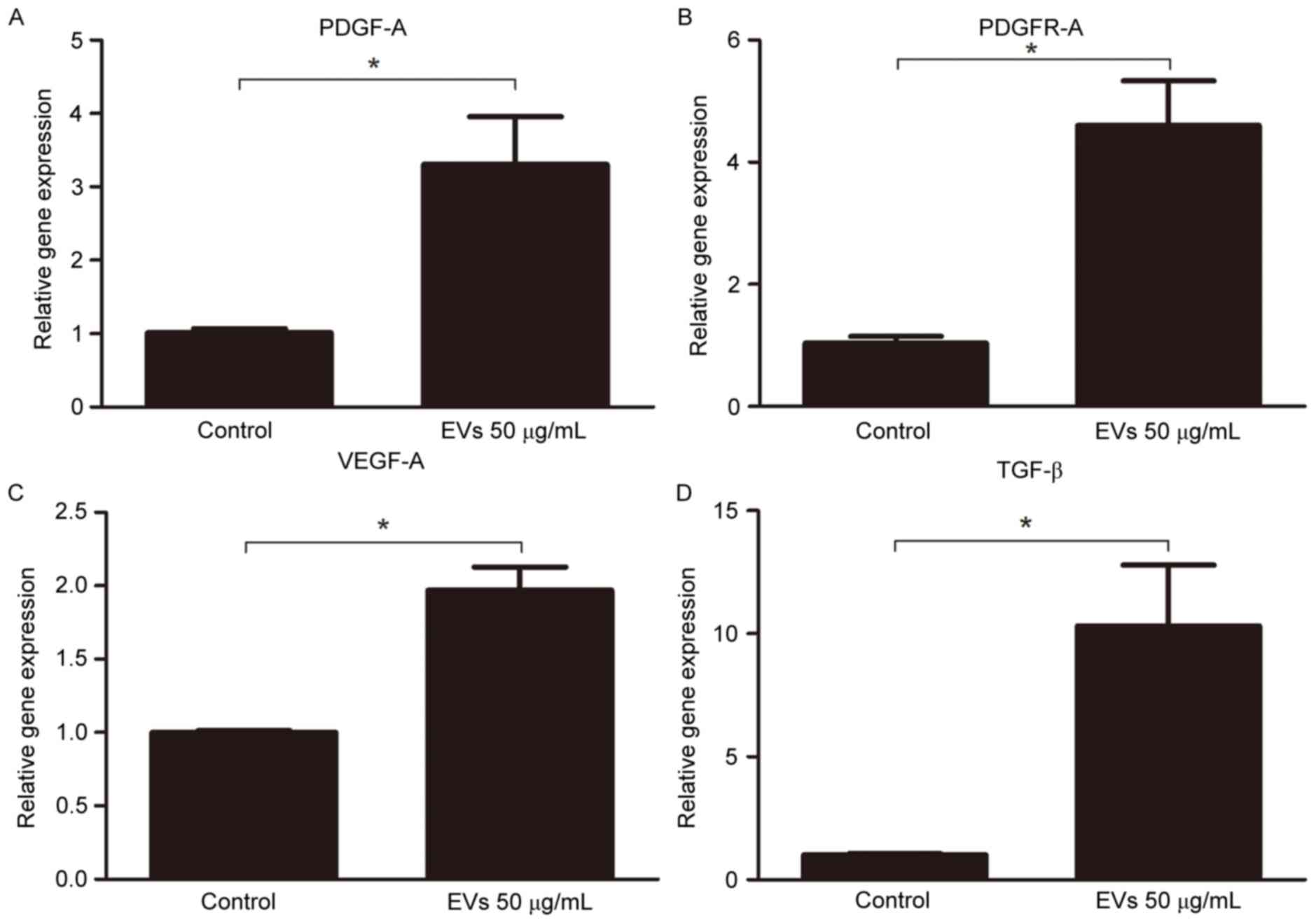
Subsequently, the gene expression levels of proangiogenic factors
were analyzed in the fat grafts by RT-qPCR. The expression levels
of PDGF-A (3.306±0.65-fold), PDGFR-A (4.601±0.73-fold), VEGF-A
(1.970±0.16-fold), and TGF-β (10.310±2.49-fold) were increased in
the 50 µg/ml EV-treated group compared with in the control group
(Fig. 7). These results suggested
that BMSC-EVs may promote neovascularization by stimulating the
secretion of proangiogenic factors.
Discussion
Free fat grafting is an advantageous technique that
may be used as a contouring tool in reconstructive and cosmetic
surgical procedures; however, its use is limited by the
unpredictable and poor long-term retention of the transplanted fat
(4). Although numerous methods for
improving fat graft survival have been investigated, further
improvements are still required.
At present, the exact mechanisms underlying fat
graft survival remain unclear. One generally accepted mechanism
leading to graft loss is a lack of adequate revascularization of
the fat post-transplantation. Karacaoglu et al (9) reported that fat graft survival was
greatest in the supramuscular layer, and indicated that fat grafts
used in relatively more vascular areas underwent lower rates of
resorption. Eto et al (7)
demonstrated that adipocytes died as early as the first day after
ischemia, endothelial cells died second, and finally
adipose-derived stromal cells (ADSCs) died on day 3. Furthermore,
Aygit et al (29) revealed
that revascularization of fat grafts occurred 48 h
post-transplantation, indicating that it is too late for the
survival of adipocytes. Various approaches for accelerating
angiogenesis have been successfully undertaken to enhance fat
survival post-transplantation, including the administration of
basic fibroblast growth factors, interleukin-8 and erythropoietin
(11,30,31);
VEGF-based gene therapy (8,32);
and endothelial cell and MSC therapies (13,16,33).
EVs released by MSCs have recently been reported to
exert proangiogenic effects in numerous ischemic animal models
(18,34). The present study demonstrated that
BMSC-EVs stimulated neovascularization and improved retention of
transplanted fat grafts in a nude mice model. The present results
confirmed that EVs were of BMSC origin, as the cells expressed high
levels of the BMSC-positive markers, CD81, CD63, CD90 and CD29, and
were negative for the endothelial and hematopoietic cell markers,
CD31 and CD45 (Fig. 1C and D).
Confocal microscopy demonstrated that the BMSC-EVs interacted with
endothelial cells in vitro (Fig. 2). Furthermore, the BMSC-EVs
improved HUVEC in vitro cell viability, migration and tube
formation, demonstrating the proangiogenic potential of BMSC-EVs
(Fig. 3). Based on these findings,
the effects of BMSC-EVs on free fat grafts were investigated in an
animal model. The grafts from the EV-treated groups had higher
tissue volume and weight, and improved histology, indicating a
better overall survival than those in the control group at 12 weeks
post-transplantation (Figs. 4 and
5). Immunohistochemical and
RT-qPCR analyses also supported the hypothesis that the observed
improvement in retention of fat graft weight and volume was
attributable to the induction of angiogenesis (Figs. 6 and 7). These observations are also in
agreement with the findings of previous studies, demonstrating a
link between neovascularization and improved fat graft survival
(8,16,35).
In addition to neovascularization, the importance of
adipogenesis for long-term retention of transplanted fat has been
implicated. Previous in vivo studies have demonstrated that
the majority of adipocytes in free grafts die shortly after
transplantation, whereas only ADSCs survive (7,36,37).
In the ‘three zone’ theory suggested by Yoshimura et al
(38), the survival of fat grafts
is largely dependent on adipose tissue regeneration
post-transplantation, and CD34-positive cells are very likely the
seed cells for adipogenic progression. Therefore, recent studies
(14,39) have focused on the therapeutic
effects of adipose-derived cells, including stromal vascular
fraction and ADSCs, on fat grafts. These cells may improve tissue
outcomes by increasing the vascularity and the secretion of growth
factors that improve tissue survival. Yoshimura et al
(40) reported that ADSCs are able
to enhance angiogenesis and improve the survival rate of
non-vascularized grafted fat. In addition, these cells may function
as seed cells for adipogenesis. Compared with these cell-based
therapies, BMSC-EVs serve a proangiogenic role, but do not undergo
adipocyte differentiation. However, the present study demonstrated
that the improvements in fat graft retention achieved by BMSC-EVs
were as good as those from cell-based therapies (16,39),
indicating that revascularization is necessary for free fat
grafting. This finding is in agreement with a recent study, which
suggested that ADSCs are involved in the process of graft
revascularization via paracrine action, but do not undergo
significant adipogenic differentiation (41).
With respect to clinical implications, cross-species
MSCs have been successfully used in various in vivo animal
models. In the nude mice model system used in the present study,
human fat with EVs isolated from rat BMSCs was transplanted into
the mice. In addition, Li et al (42) identified 94 reports of in
vivo cross-species administration of MSCs in various
experimental models. Although the majority of these studies
involved human MSCs, pig, rat or guinea pig MSCs were also
occasionally used. A total of 93.6% of the cases confirmed that the
MSCs were successfully engrafted and functioned well in the
different species, with only 6.4% of cases reporting failure. In
the present study, rat BMSC-EVs were internalized by HUVECs and
promoted their viability, migration and tube-forming capabilities
in vitro. In the nude mice experiments, the results
indicated that rat BMSC-EVs improved the retention of human fat
grafts, which suggests that BMSC-EVs function across species
barriers, in accordance with a previous report that rat MSCs
prolonged xenogenic skin graft survival in mice (43).
In conclusion, the present study is the first, to
the best of our knowledge, to demonstrate that co-transplantation
of fat grafts with BMSC-EVs improves the long-term retention and
transplanted fat quality in a mouse model. In addition, the present
findings suggested that this beneficial effect is likely mediated
by the proangiogenic effects of BMSC-EVs. These results suggested
that effective employment of BMSC-EVs may have potential as a
therapeutic option in fat transplantation. However, the detailed
mechanisms underlying the function of BMSC-EVs remain to be
investigated.
Acknowledgements
The present study was supported by the Major State
Basic Research Development Program of China (grant no.
2011CB964704) and the National Natural Science Foundation of China
(grant nos. 81271714 and 31170944).
References
|
1
|
Atik B, Oztürk G, Erdoğan E and Tan O:
Comparison of techniques for long-term storage of fat grafts: An
experimental study. Plast Reconstr Surg. 118:1533–1537. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong Z, Peng Z, Chang Q, Zhan W, Zeng Z,
Zhang S and Lu F: The angiogenic and adipogenic modes of adipose
tissue after free fat grafting. Plast Reconstr Surg. 135:556e–567e.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizoguchi T, Kijima Y, Hirata M, Kaneko K,
Arima H, Nakajo A, Higashi M, Tabata K, Koriyama C, Arigami T, et
al: Histological findings of an autologous dermal fat graft
implanted onto the pectoralis major muscle of a rat model. Breast
Cancer. 22:578–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu M, Zhou Z, Chen Y, Schreiber R, Ransom
JT, Fraser JK, Hedrick MH, Pinkernell K and Kuo HC: Supplementation
of fat grafts with adipose-derived regenerative cells improves
long-term graft retention. Ann Plast Surg. 64:222–228. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wetterau M, Szpalski C, Hazen A and Warren
SM: Autologous fat grafting and facial reconstruction. J Craniofac
Surg. 23:315–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herold C, Ueberreiter K, Busche MN and
Vogt PM: Autologous fat transplantation: Volumetric tools for
estimation of volume survival. A systematic review. Aesthetic Plast
Surg. 37:380–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eto H, Kato H, Suga H, Aoi N, Doi K, Kuno
S and Yoshimura K: The fate of adipocytes after nonvascularized fat
grafting: Evidence of early death and replacement of adipocytes.
Plast Reconstr Surg. 129:1081–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu F, Li J, Gao J, Ogawa R, Ou C, Yang B
and Fu B: Improvement of the survival of human autologous fat
transplantation by using VEGF-transfected adipose-derived stem
cells. Plast Reconstr Surg. 124:1437–1446. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karacaoglu E, Kizilkaya E, Cermik H and
Zienowicz R: The role of recipient sites in fat-graft survival:
Experimental study. Ann Plast Surg. 55:63–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamaguchi M, Matsumoto F, Bujo H,
Shibasaki M, Takahashi K, Yoshimoto S, Ichinose M and Saito Y:
Revascularization determines volume retention and gene expression
by fat grafts in mice. Exp Biol Med (Maywood). 230:742–748. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamed S, Egozi D, Kruchevsky D, Teot L,
Gilhar A and Ullmann Y: Erythropoietin improves the survival of fat
tissue after its transplantation in nude mice. PLoS One.
5:e139862010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong SJ, Lee JH, Hong SM and Park CH:
Enhancing the viability of fat grafts using new transfer medium
containing insulin and beta-fibroblast growth factor in autologous
fat transplantation. J Plast Reconstr Aesthet Surg. 63:1202–1208.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yi C, Pan Y, Zhen Y, Zhang L, Zhang X, Shu
M, Han Y and Guo S: Enhancement of viability of fat grafts in nude
mice by endothelial progenitor cells. Dermatol Surg. 32:1437–1443.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seyhan N, Alhan D, Ural AU, Gunal A,
Avunduk MC and Savaci N: The effect of combined use of
platelet-rich plasma and adipose-derived stem cells on fat graft
survival. Ann Plast Surg. 74:615–620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu M, Dong Z, Gao J, Liao Y, Xue J, Yuan
Y, Liu L, Chang Q and Lu F: Adipocyte regeneration after free fat
transplantation: Promotion by stromal vascular fraction cells. Cell
Transplant. 24:49–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao J, Yi C, Zheng Y, Li L, Qiu X, Xia W,
Su Y, Diao J and Guo S: Enhancement of fat graft survival by bone
marrow-derived mesenchymal stem cell therapy. Plast Reconstr Surg.
132:1149–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
See F, Seki T, Psaltis PJ, Sondermeijer
HP, Gronthos S, Zannettino AC, Govaert KM, Schuster MD, Kurlansky
PA, Kelly DJ, et al: Therapeutic effects of human STRO-3-selected
mesenchymal precursor cells and their soluble factors in
experimental myocardial ischemia. J Cell Mol Med. 15:2117–2129.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bian S, Zhang L, Duan L, Wang X, Min Y and
Yu H: Extracellular vesicles derived from human bone marrow
mesenchymal stem cells promote angiogenesis in a rat myocardial
infarction model. J Mol Med (Berl). 92:387–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tögel F, Weiss K, Yang Y, Hu Z, Zhang P
and Westenfelder C: Vasculotropic, paracrine actions of infused
mesenchymal stem cells are important to the recovery from acute
kidney injury. Am J Physiol Renal Physiol. 292:F1626–F1635. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vader P, Breakefield XO and Wood MJ:
Extracellular vesicles: Emerging targets for cancer therapy. Trends
Mol Med. 20:385–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van der Pol E, Böing AN, Harrison P, Sturk
A and Nieuwland R: Classification, functions, and clinical
relevance of extracellular vesicles. Pharmacol Rev. 64:676–705.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tetta C, Bruno S, Fonsato V, Deregibus MC
and Camussi G: The role of microvesicles in tissue repair.
Organogenesis. 7:105–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reis LA, Borges FT, Simões MJ, Borges AA,
Sinigaglia-Coimbra R and Schor N: Bone marrow-derived mesenchymal
stem cells repaired but did not prevent gentamicin-induced acute
kidney injury through paracrine effects in rats. PLoS One.
7:e440922012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai RC, Chen TS and Lim SK: Mesenchymal
stem cell exosome: A novel stem cell-based therapy for
cardiovascular disease. Regen Med. 6:481–492. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lian J, Lu Y, Xu P, Ai A, Zhou G, Liu W,
Cao Y and Zhang WJ: Prevention of liver fibrosis by intrasplenic
injection of high-density cultured bone marrow cells in a rat
chronic liver injury model. PLoS One. 9:e1036032014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deregibus MC, Cantaluppi V, Calogero R, Lo
Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B and Camussi G:
Endothelial progenitor cell derived microvesicles activate an
angiogenic program in endothelial cells by a horizontal transfer of
mRNA. Blood. 110:2440–2448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Osterman CJ, Lynch JC, Leaf P, Gonda A,
Bennit HR Ferguson, Griffiths D and Wall NR: Curcumin modulates
pancreatic adenocarcinoma cell-derived exosomal function. PLoS One.
10:e01328452015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aygit AC, Sarikaya A, Doganay L, Top H,
Cakir B and Firat MF: The fate of intramuscularly injected fat
autografts: An experimental study in rabbits. Aesthet Plast Surg.
28:334–339. 2004. View Article : Google Scholar
|
|
30
|
Jiang A, Li M, Duan W, Dong Y and Wang Y:
Improvement of the survival of human autologous fat transplantation
by adipose-derived stem-cells-assisted lipotransfer combined with
bFGF. ScientificWorldJournal. 2015:9680572015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shoshani O, Livne E, Armoni M, Shupak A,
Berger J, Ramon Y, Fodor L, Gilhar A, Peled IJ and Ullmann Y: The
effect of interleukin-8 on the viability of injected adipose tissue
in nude mice. Plast Reconstr Surg. 115:853–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yi CG, Xia W, Zhang LX, Zhen Y, Shu MG,
Han Y and Guo SZ: VEGF gene therapy for the survival of
transplanted fat tissue in nude mice. J Plast Reconstr Aesthet
Surg. 60:272–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ko MS, Jung JY, Shin IS, Choi EW, Kim JH,
Kang SK and Ra JC: Effects of expanded human adipose tissue-derived
mesenchymal stem cells on the viability of cryopreserved fat grafts
in the nude mouse. Int J Med Sci. 8:231–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG and
Chopp M: Systemic administration of exosomes released from
mesenchymal stromal cells promote functional recovery and
neurovascular plasticity after stroke in rats. J Cereb Blood Flow
Metab. 33:1711–1715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sezgin B, Ozmen S, Bulam H, Omeroglu S,
Yuksel S, Cayci B and Peker T: Improving fat graft survival through
preconditioning of the recipient site with microneedling. J Plast
Reconstr Aesthet Surg. 67:712–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong Z, Peng Z, Chang Q and Lu F: The
survival condition and immunoregulatory function of adipose stromal
vascular fraction (SVF) in the early stage of nonvascularized
adipose transplantation. PLoS One. 8:e803642013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sunaga A, Sugawara Y, Katsuragi-Tomioka Y
and Kobayashi E: The fate of nonvascularized fat grafts:
Histological and bioluminescent study. Plast Reconstr Surg Glob
Open. 1:e402013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshimura K, Eto H, Kato H, Doi K and Aoi
N: In vivo manipulation of stem cells for adipose tissue
repair/reconstruction. Regen Med. 6:(6 Suppl). S33–S41. 2011.
View Article : Google Scholar
|
|
39
|
Piccinno MS, Veronesi E, Loschi P,
Pignatti M, Murgia A, Grisendi G, Castelli I, Bernabei D, Candini
O, Conte P, et al: Adipose stromal/stem cells assist fat
transplantation reducing necrosis and increasing graft performance.
Apoptosis. 18:1274–1289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoshimura K, Sato K, Aoi N, Kurita M,
Inoue K, Suga H, Eto H, Kato H, Hirohi T and Harii K: Cell-assisted
lipotransfer for facial lipoatrophy: Efficacy of clinical use of
adipose-derived stem cells. Dermatol Surg. 34:1178–1185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Garza RM, Rennert RC, Paik KJ, Atashroo D,
Chung MT, Duscher D, Januszyk M, Gurtner GC, Longaker MT and Wan
DC: Studies in fat grafting: Part IV. Adipose-derived stromal cell
gene expression in cell-assisted lipotransfer. Plast Reconstr Surg.
135:1045–1055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li J, Ezzelarab MB and Cooper DK: Do
mesenchymal stem cells function across species barriers? Relevance
for xenotransplantation. Xenotransplantation. 19:273–285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moadsiri A, Polchert D, Genrich K, Napoles
P, Reina E, Turian J, Smith B and Bartholomew A: Mesenchymal stem
cells enhance xenochimerism in NK-depleted hosts. Surgery.
140:315–321. 2006. View Article : Google Scholar : PubMed/NCBI
|