Introduction
Liver fibrosis is characterized as reduced liver
regeneration and liver dysfunction, and may lead to portal
hypertension and end-stage liver disease (ESLD) (1). Currently, liver transplantation (LT)
is the only option that may improve the survival rate of adult and
pediatric patients with ESLD; however, the severe shortage of donor
organs limits the widespread application of LT (2,3).
This problem has been partially alleviated by reduced-size LT
(RSLT), such as living donor LT and split LT (4). However, unavoidable and potentially
serious ischemia/reperfusion injuries that are associated with RSLT
have been reported to impair the regeneration of remnant liver
tissue (5). Therefore, effective
therapeutic strategies aimed at inhibiting hepatocyte
apoptosis/necrosis and stimulating the regeneration would be
beneficial for patients on LT waiting lists, as they would offer
more time and increase the chances of obtaining LT, as well as for
patients with small-for-size syndrome following living or split
LT.
Our increasing knowledge on stem cell biology has
offered new opportunities for stem-cell-based therapies as a
platform in regenerative medicine for liver diseases (6,7).
Mesenchymal stromal cells (MSCs) are considered a promising tool
for cell-based therapy owing to their extensive self-renewal and
multidifferential potency, as well as their ability to stimulate
blood vessel and nerve growth, and particularly their production of
cytokines and growth factors (8,9).
Recently, MSCs derived from bone marrow, adipose tissue or
umbilical cord blood have been successfully applied for the
treatment of ESLD (10–12), and patients with ESLD exhibited
good tolerance and decreased Model for End-stage Liver Disease
scores, as well as improvements in albumin production and liver
function follwing 6-months follow-up. In addition, MSC-based
therapy may be an effective auxiliary therapy for RSLT (13).
Although bone marrow is the most common and
well-characterized source of MSCs, adipose tissue is being
recognized as a promising source suitable for autologous stem-cell
therapy. Adipose tissue is one of the richest sources for MSCs, and
they are easily accessible through less invasive and safe
collection procedures, and they maybe the best choice for
autologous cell transplantation (14). Our previous study demonstrated that
autologous adipose-derived MSCs (ADSCs) transplantation resulted in
enhanced liver regeneration capacity of the remnant liver tissue in
the early phase following repeat partial hepatectomy (15). Although this result was considered
promising and encouraging, the therapeutic efficacy of autologous
MSCs for most clinical applications remains modest, possibly due to
the decreased regenerative potential in aged patients with chronic
diseases (16). To effectively
understand the properties of ADSCs affected by age and disease,
autologous clinical applications are required. To the best of our
knowledge, human infantile ADSCs have not yet been compared with
adult and elderly cell populations with benign ESLD. Accordingly,
the aim of the present study was to investigate how the age of
patients with benign ESLD affected the biological and functional
characteristics of ADSCs, which is crucial to increasing the
potential effectiveness of autologous cell therapy.
Materials and methods
Patients
A total of 30 male patients who underwent
living-donor LT for benign ESLD, between January and December 2014,
in Tianjin First Central Hospital (Tianjin, China), were enrolled
in the present study. During the LT procedure, samples of abdominal
subcutaneous adipose tissue were obtained from three distinct age
groups: i) Infant, <1 year old (n=10); ii) adult, 20–40 years
old (n=10); and iii) elderly, >50 years old (n=10). Samples were
immediately transported to the Department of Clinical Laboratory
Medicine, Tianjin First Central Hospital.
The present study was authorized by the Ministry of
Health (Beijing, China). Following approval by the Medical Ethics
Committee of Tianjin First Center Hospital, all patients signed a
written, informed consent, in accordance with the Institutional
Review Board guidelines for the protection of human patients. In
cases involving infants, informed consent was obtained from their
parents or legal guardians.
Preparation and characterization of
ADSCs
Isolation and culture of ADSCs was performed as
previously described (17).
Briefly, isolated adipose tissues were minced and digested with
type I collagenase (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
for 30 min at 37°C with shaking. The mixture was washed,
centrifuged at 300 × g for 5 min at 37°C and resuspended in
Dulbecco's modified Eagle's medium/Ham's F12 (DMEM/F12; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), and sieved
through a Falcon mesh cell strainer (70 µm; BD Biosciences,
Franklin Lakes, NJ, USA) to eliminate the undigested fragments.
Following centrifugation at 300 × g for 5 min at 37°C, ADSCs were
resuspended and cultured in DMEM/F12 containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C, 5%
CO2 and 95% humidity. Cells were grown to 80%
confluence, and adherent cells were detached with trypsin/EDTA
(Sigma-Aldrich; Merck KGaA) and used in subsequent experiments at
the third passage. Cultured ADSCs were blocked in 10% human AB
serum (Sigma-Aldrich; Merck KGaA), then incubated with fluorescein
isothiocyanate-conjugated CD45 (cat no. 555482), CD73 (cat no.
561254), CD90 (cat no. 55595), CD34 (cat no. 555821) and CD105 (cat
no. 562408), all obtained from BD Biosciences, for 40 min at 4°C at
a dilution of 1:50. After adding 2 ml PBS, cells were centrifuged
at 150 × g for 5 min at 8°C. Then data were acquired using a flow
cytometer (FACSCalibur), and data were analyzed using the CellQuest
6.0 software (both from BD Biosciences) (18). The differentiation capacity of
ADSCs into adipogenic and osteogenic lineages was analyzed as
previously described (18). The
ADSCs were seeded in DMEM/F12 at 2×104
cells/cm2 in six-well tissue culture plates. When the
cells reached 100% confluency, DMEM/F12 was subsequently replaced
with specific inducer medium. Adipogenic inducer medium was
DMEM/F12 containing 1 µmol/l dexamethasone, 0.5 mmol/l
3-isobutyl-1-methylxanthine, 5 mg/l insulin and 100 µmol/l
indomethacin (all Sigma-Aldrich; Merck KGaA). Osteogenic inducer
medium was DMEM/F12 containing 100 nmol/l dexamethasone, 10 mmol/l
β-sodium glycerophosphate and 50 µg/ml vitamin C (all
Sigma-Aldrich; Merck KGaA). Following a 21-day induction period,
adipocytes and osteoblasts were identified by Oil Red O staining
(Sigma-Aldrich; Merck KGaA) and Alizarin red S staining (Genmed
Gene Pharmaceutical Technology, Co., Ltd., Shanghai, China) at room
temperature for 10 min, respectively. Cells were visualized under
an inverted Olympus IX71 microscope at ×200 magnification and
photomicrographs were recorded using UIS2 software (both from
Olympus Corporation, Tokyo, Japan).
Cell proliferation assay
Cell proliferation was monitored with the highly
sensitive xCELLigence Real-Time Cell Analyzer (RTCA; ACEA
Biosciences, Inc., San Diego, CA, USA), which is able to measure
the status of cell growth in real time. Briefly, ADSCs were
incubated in 60 mm culture plates for 24 h, and 5,000 cells in 100
µl of culture medium were subsequently seeded into each
sensor-containing well of an E-Plate 16 microtiter plate (Roche
Diagnostics, Indianapolis, IN, USA), which was locked into the RTCA
device and incubated at 37°C with 5% CO2 for 72 h. While
seeded cells were propagated in the incubator, impedance values
were converted into cell index (CI) value corresponding to each
well. CI value is defined as relative change in measured electrical
impedance to represent cellular proliferation status. CI was read
automatically every 15 min for 72 h. All experiments were carried
out in triplicate. Data were analyzed using xCELLigence software,
version 1.2 (RTCA; ACEA Biosciences, Inc.).
Cell migration assay
Cell migration was monitored in real-time using the
RTCA system (ACEA Biosciences, Inc.) and expressed as the cell
migration index (CMI). The RTCA platform is able to measure cell
migration status in real time. Following overnight starvation of
1×105 cells in DMEM/F12 medium with 0.2% FBS,
1×104 cells in 100 µl serum-free medium were seeded in
the upper chamber of a CIM-Plate 16 (Roche Diagnostics), and the
lower chamber was filled with 160 µl DMEM/F12 medium containing 20%
FBS. The CIM plate was placed in the RTCA device and incubated for
72 h at 37°C in a 5% CO2 atmosphere. A reading of the
impedance on the underside was taken every 10 min and reported as a
CII, which was derived from the relative change in electrical
impedance set against the baseline reading that was taken with no
cells and only DMEM/F12 medium. All experiments were performed in
triplicate. Data were analyzed using xCELLigence software.
Determination of ADSCs antiapoptotic
ability
Apoptotic cell death was evaluated using Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptotic
Detection kit (BD Biosciences) and flow cytometry, according to the
manufacturer's protocol. To compare the antiapoptotic ability of
the three ADSC groups, passage 3 cells were trypsinized, adjusted
to a concentration of 3×105 cells/ml and seeded into
6-well plates. ADSCs were grown to 80% confluence, dexamethasone
(1×10−6 mol/l) was added and incubated at 37°C with 5%
CO2 and 95% humidity for 48 h. Following incubation,
cells were collected by trypsinization, and centrifuged at 150 × g
for 5 min at 8°C, and suspended in binding buffer (BD Biosciences).
Annexin V-FITC/PI was added and the cells were incubated in the
dark at room temperature for 15 min prior to analyzing using a flow
cytometer (FACSCalibur), and data were analyzed using the CellQuest
6.0 software (both from BD Biosciences).
Reverse transcription-quantitative
reverse transcription polymerase chain reaction (RT-qPCR)
mRNA expression levels of the osteogenic-specific
gene osteopontin (OPN) and the adipogenic-specific gene peroxisome
proliferator-activated receptor-γ (PPAR-γ) were detected by
RT-qPCR. Briefly, total RNA was extracted from 1×106
ADSCs using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse transcribed into cDNA using PrimeScript RT
Reagent kit (Takara Biotechnology Co., Ltd., Dalian, China),
according to the respective manufacturer's protocols. RNA purity
was verified by an optical density (OD)260/OD280 nm absorption
ratio >1.9. qPCR was performed using a LightCycler 480 system
(Roche Diagnostics, Basel, Switzerland) and SYBR Premix Ex Taq
(Takara Biotechnology Co., Ltd.). Sequences of the primers used
were as follows: PPAR-γ forward, 5′-AGTCCTCACAGCTGTTTGCCAAGC-3′ and
reverse, 5′-GAGCGGGTGAAGACTCATGTCvTGTC-3′; OPN forward,
5′-AAACGCCGACCAAGGTACAG-3′ and reverse,
5′-ATGCCTAGGAGGCAAAAGCAA-3′; GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-
3′. The PCR cycling conditions were a hot-start step of 95°C for 10
min, followed by a 40-cycle program including a denaturation phase
at 95°C for 30 sec, annealing at 60°C for 45 sec and extension for
30 sec at 72°C. Data were analyzed using the LightCycler 480
software version 1.5 (Roche Diagnostics). Relative gene expression
analysis was calculated relative to the housekeeping gene GAPDH
(19). The assay was replicated in
3 independent experiments.
Co-culture of ADSCs and peripheral
blood mononuclear cells (PBMCs)
PBMCs were isolated from heparinized peripheral
blood samples of the healthy volunteers using Ficoll-Hypaque
(Sigma-Aldrich; Merck KGaA) density gradient centrifugation at 400
× g for 25 min at room temperature, after receiving informed
consent. The immunosuppressive capacity of ADSCs was tested in a
co-culture of ADSCs with PBMCs as follows. A total of
1.0×106 cells were mitotically inactivated with
mitomycin C (50 µg/ml; Sigma-Aldrich; Merck KGaA) for 30 min at
37°C in serum-free medium. ADSCs were subsequently washed 3 times
with PBS and seeded into a 96-well (5.0×103 cells/well)
or 6-well (5.0×104 cells/well) flat-bottomed plate
(Nalge Nunc International; Thermo Fisher Scientific, Inc.) for
proliferation or for flow cytometry, respectively, in triplicate.
Following overnight adherence at 37°C, PBMCs (5.0×104
cells/well in the 96-well plates; 5.0×105 cells/well in
the 6-well plates) were seeded onto the cultured ADSCs at a ratio
of 1:10 (ADSC:PBMSC), stimulated with phytohemagglutinin (PHA; 5
µg/ml; Sigma-Aldrich; Merck KGaA) and interleukin 2 (IL-2; 5 ng/ml;
R&D Systems, Minneapolis, MN, USA) to activate T-cell
proliferation, and cultured at 37°C in a humidified atmosphere of
5% CO2. PBMCs cultured alone with PHA and IL-2 served as
a control. Following culture for 3 days, PBMCs were collected via
pipetting for T-cell proliferation assay and flow cytometric
analysis. Supernatants from the cell cultures were collected and
stored at −80°C for subsequent analysis of cytokine production.
Suppression of T-cell
proliferation
MTT colorimetry was used to measure T-cell
proliferation. Briefly, following removal of the PBMCs to a new
96-well plate, 5 mg/ml MTT solution (Sigma-Aldrich; Merck KGaA) was
added to each well and the plate was incubated for 4 h at 37°C.
Subsequent to the incubation, 100 µl dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA) was added to terminate the reactions,
and the plates were placed on a shaker (150 rpm) for 10 min to
fully dissolve the crystals. The absorbance value at 570 nm was
measured using an automatic BioTek Synergy 2 Multi-Mode microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA), with each
sample assayed in triplicate. Data were analyzed using the Gen5
version 3.03 data analysis software (BioTek Instruments, Inc.). The
proliferation inhibitory rate was calculated according to the
formula: Inhibition rate (%) = [1- (average absorbance for
experimental group/average absorbance for control group)] ×100.
Flow cytometric assessment of
CD4+ regulatory T cells (Tregs)
For examination of
CD4+/CD25+/forkhead box P3
(FoxP3)+Tregs, monoclonal antibodies specific to
CD4-FITC (cat no. 555346) and CD25-allophycocyanin (cat no. 555434)
surface staining at a dilution of 1:50 was conducted in co-cultured
and harvested PBMCs. Following the incubation for 20 min in the
dark at 37°C, the cells were washed with PBS, and
FoxP3-phycoerythrin (cat no. 560046) (all from BD Biosciences)
intracellular staining was conducted and incubation for 30 min in
the dark at 37°C followed, as previously described (20). Data were acquired using a flow
cytometer (FACSCalibur flow cytometer; Becton-Dickinson) and data
were analyzed using the CellQuest 6.0 software (BD
Biosciences).
Cytokine production
Interferon (IFN)-γ and interleukin (IL)-10
concentrations in the supernatants of the T cell proliferation
co-culture aforementioned were measured using a commercially
available IFN-γ (cat no. BMS228) and IL-10 (cat no. BMS215) human
ELISA kits (eBioscience; Thermo Fisher Scientific, Inc.), according
to the manufacturer's instructions.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using one-way analysis of
variance followed by a post-hoc Bonferroni multiple comparison
test. Statistical analyses were performed using SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
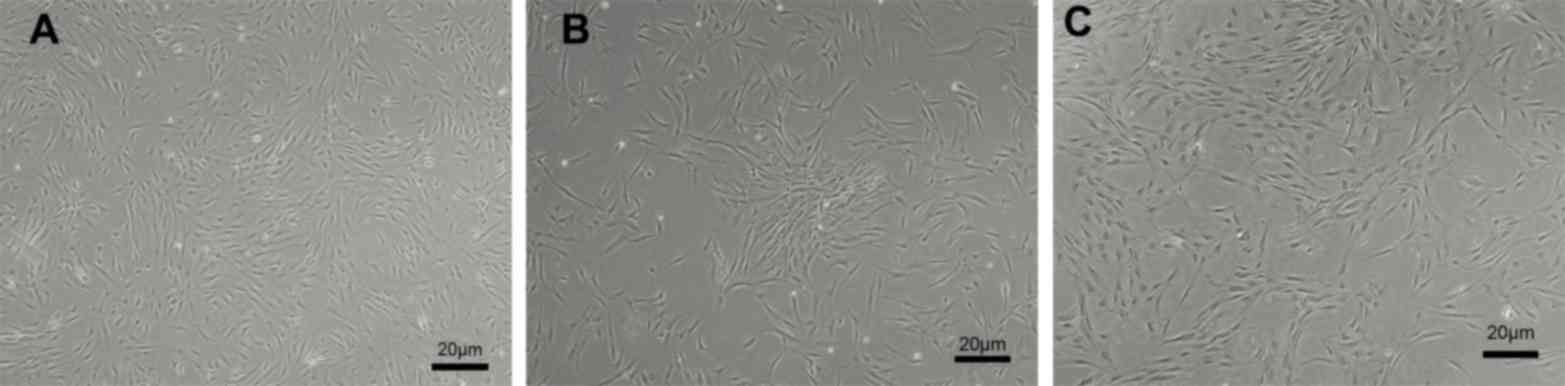
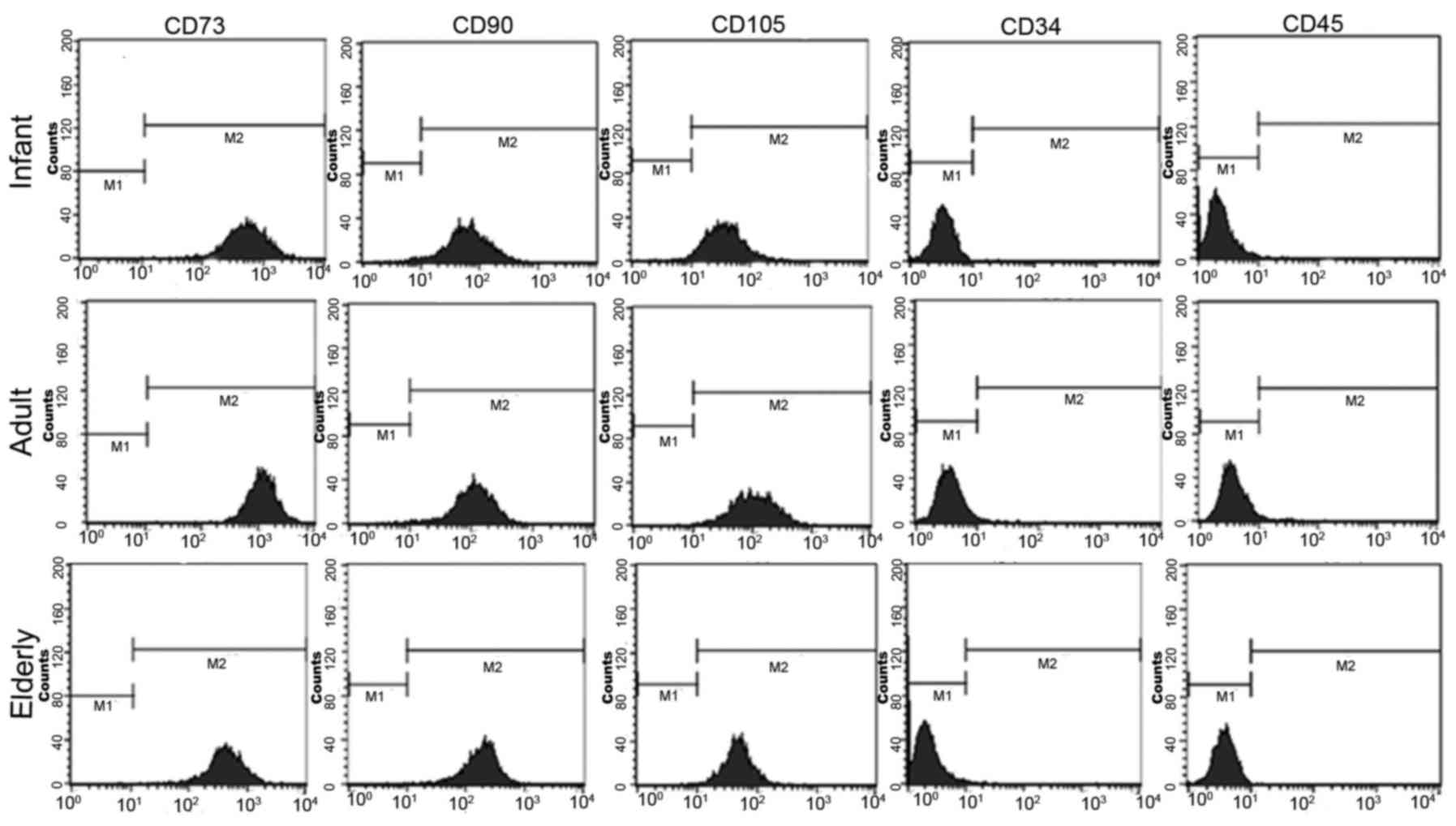
ADSC morphology and immunophenotype is
independent of patient age
ADSCs were successfully isolated from abdominal
subcutaneous adipose tissues from all patients within each of the
three groups of LT recipients (n=10/group), which spanned a wide
range of ages (6 months to 60 years). Homogeneous populations of
ADSCs were cultured to 80% confluence; ADSCs derived from infant,
adult and elderly patients exhibited fibroblast-like morphology and
a whirlpool-like or parallel array (Fig. 1). In addition, ADSCs derived from
all age groups exhibited positive surface antigenicity for CD73,
CD90 and CD105, and exhibited negative surface antigenicity for
CD34 and CD45 (Fig. 2). No notable
differences were identified in relation to patient age.
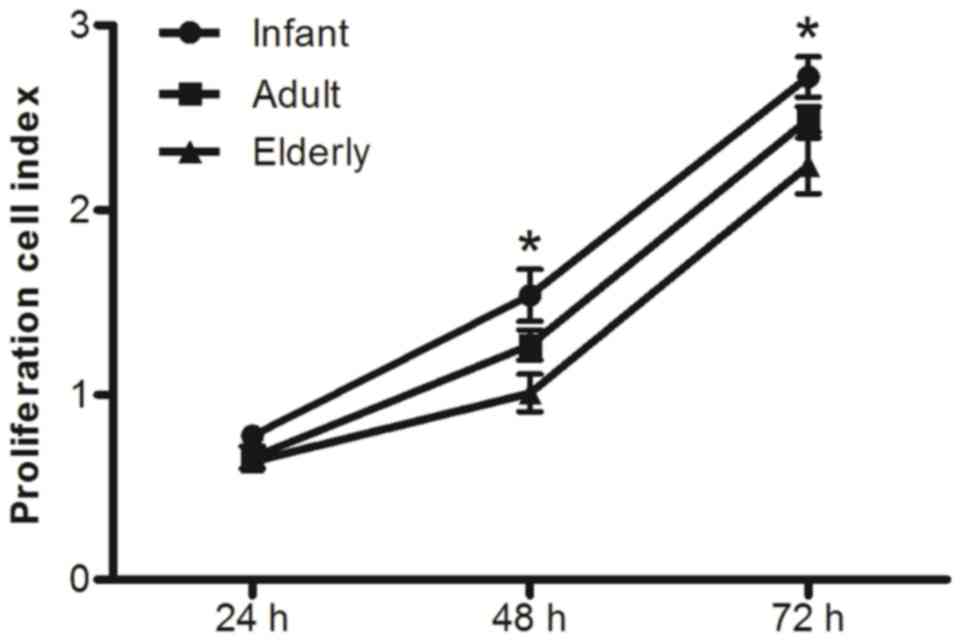
Proliferative potential of ADSCs
The proliferative potential of ADSCs isolated from
adipose tissue of patients with benign ESLD was monitored in real
time for 72 h. Growth curve analysis demonstrated that the
proliferation CI value from infant patients was significantly
increased relative to that observed in cells from both adult and
elderly patients (P<0.05; Fig.
3). This difference was apparent at 48 h and 72 h, which
indicated a trend toward decreasing proliferative potential with
age. This trend was also apparent when the proliferation CI values
obtained from adult patients were compared with those obtained from
elderly patients; however, no statistical significance was
identified between the CI values of adult and elderly patients.
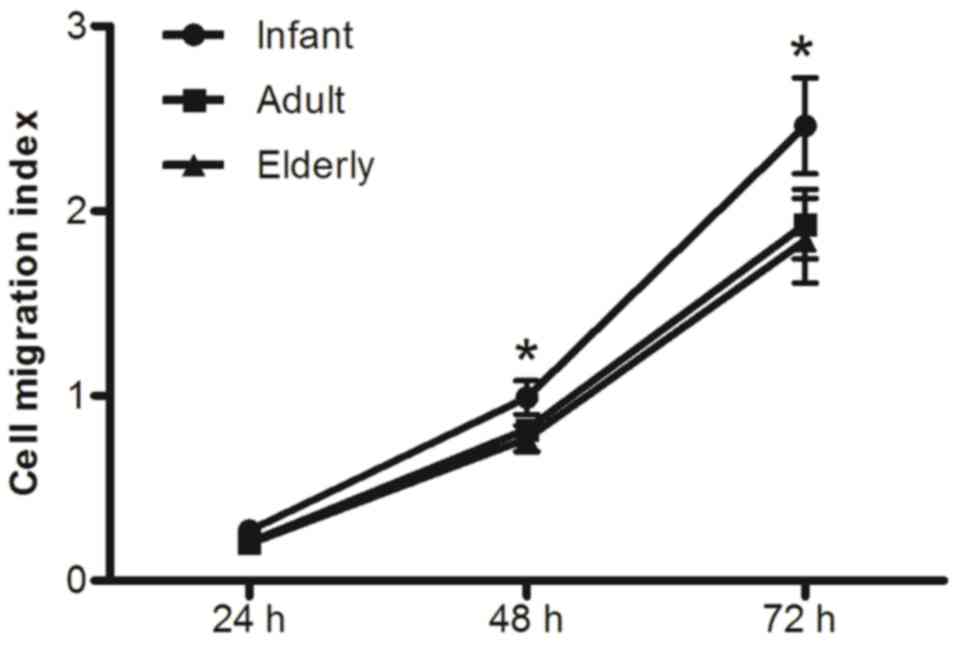
Effects of age on the migration
ability of ADSCs
Migration curve analysis of ADSCs demonstrated that
their CIMI values were significantly increased in infants compared
with adult and elderly patients (P<0.05; Fig. 4). This difference was apparent at
48 h and 72 h, which indicated a trend towards decreasing cell
migratory ability with age. Adult-derived ADSCs exhibited slightly
higher migration capability compared with elderly-derived ADSCs;
however no statistically significant difference was identified
between the two groups (P>0.05).
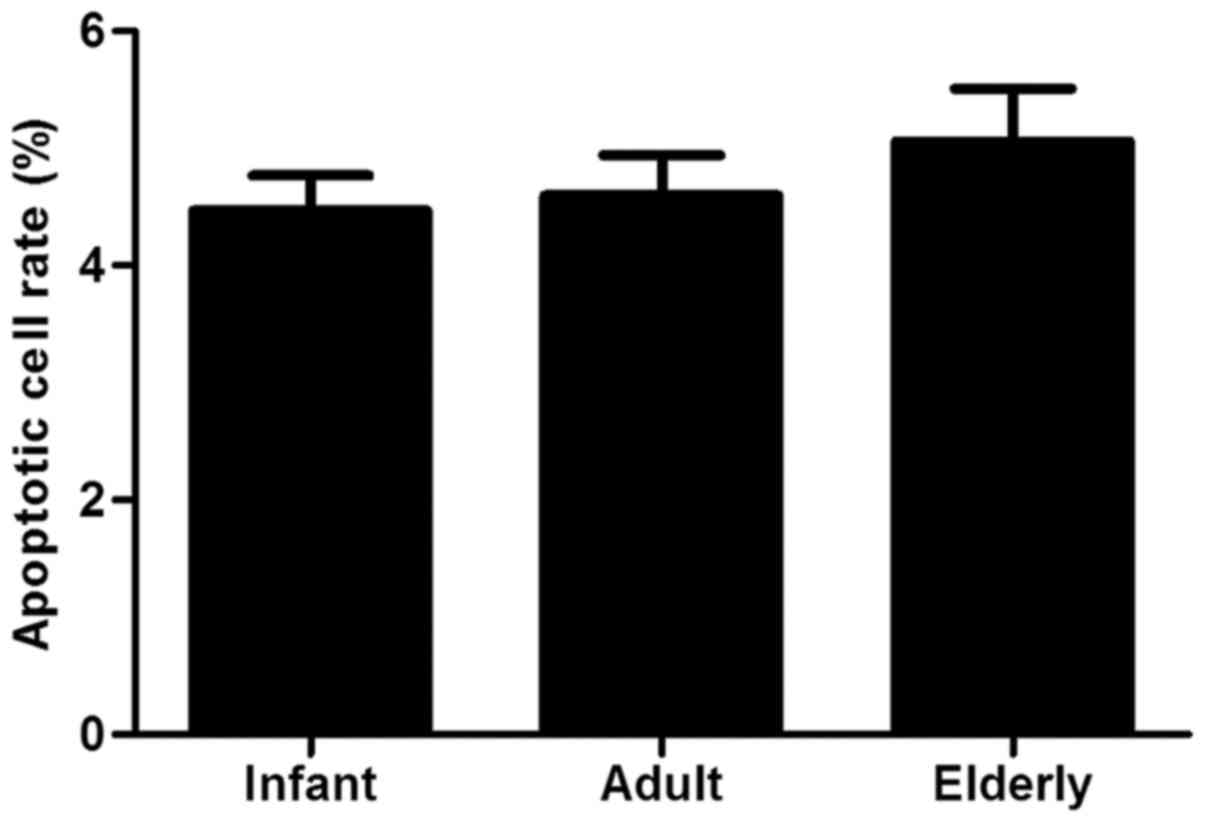
Antiapoptotic ability of ADSCs
Flow cytometric results indicated that no
statistically significant differences were identified among the
three age groups in antiapoptotic capacity induced by high
concentration of dexamethasone (Fig.
5).
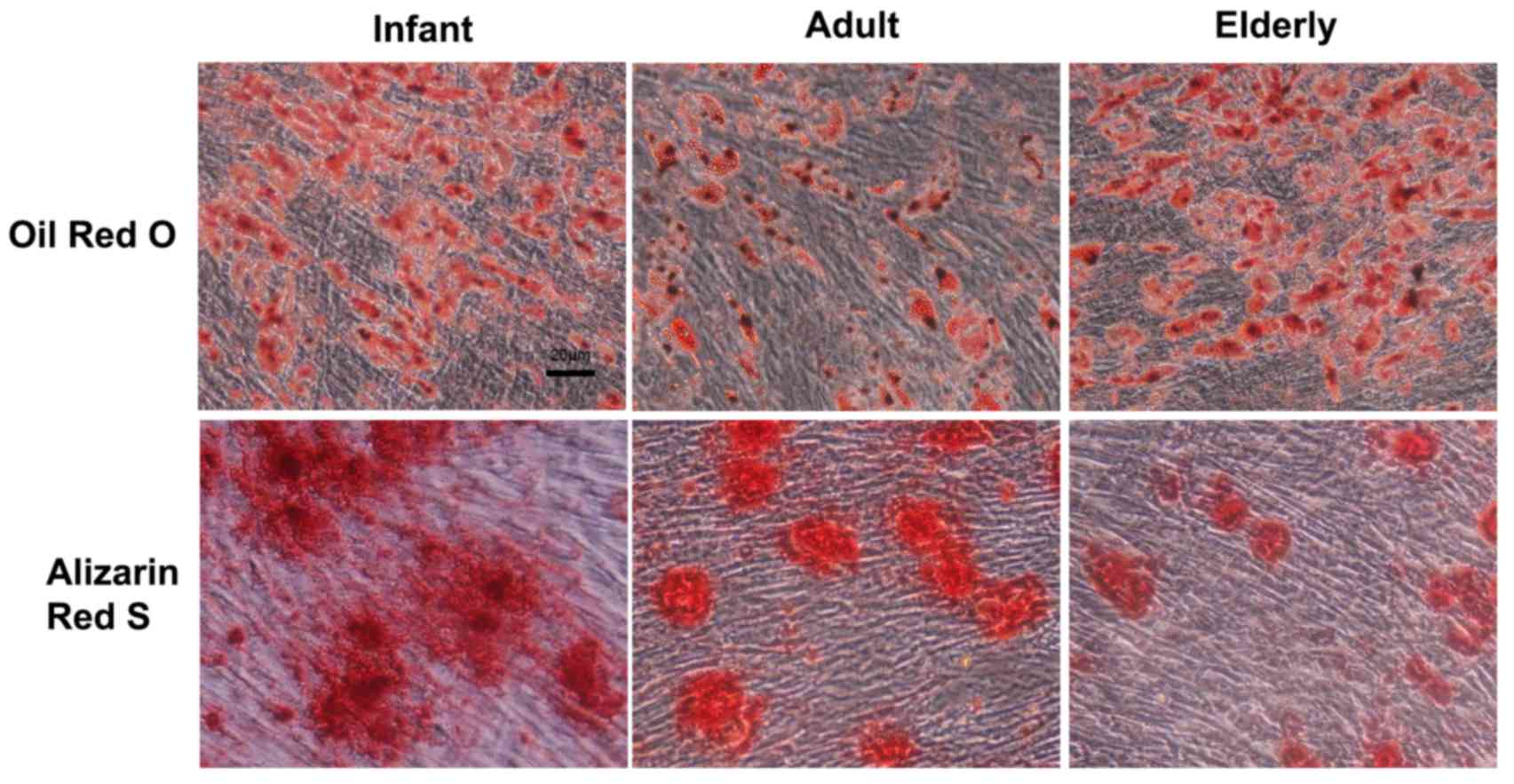
Adipogenic and osteogenic capability
of ADSCs
The adipogenic and osteogenic differentiation
potential of ADSCs derived from three age groups were evaluated by
comparing the amount of lipid-rich vacuoles and calcium deposition
produced in medium only (Fig. 6).
ADSCs were able to efficiently differentiate toward the adipogenic
and osteogenic lineages. Oil Red O staining for
adipocytic-differentiated cells indicated no notable difference in
infant vs. adult or elderly-derived ADSCs. Alizarin Red S staining
for osteogenic-differentiated cells was notably stronger in the
infant-derived ADSCs, which indicated that infant ADSCs produced a
greater amount of calcium deposition compared with cells derived
from adult and elderly patients.
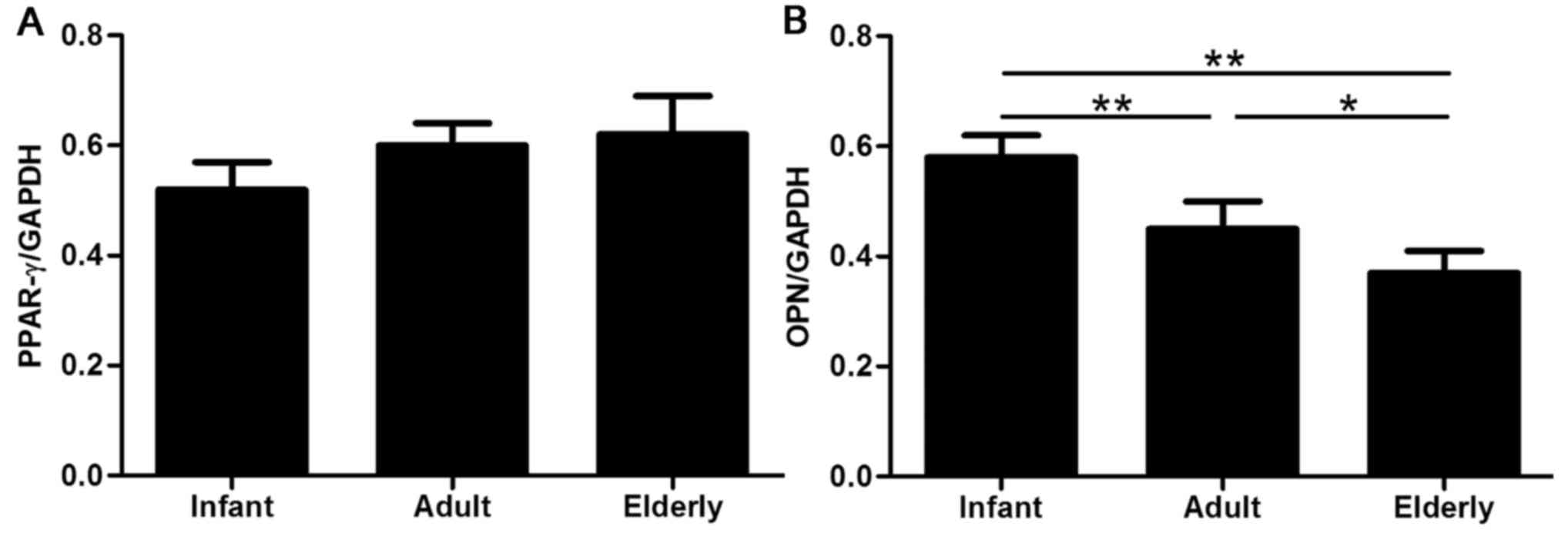
Adipogenic and osteogenic inductions were further
evaluated by RT-qPCR analysis of the lineage-specific genes PPAR-γ
(adipocytes) and OPN (osteocytes). The mRNA expression levels of
PPAR-γ appeared to gradually increase with patient age (Fig. 7A); however, this difference was not
identified as statistically significant, which indicated that age
may not affect adipogenic capacity. Conversely, significant
age-related differences were identified in the OPN mRNA expression
levels of ADSCs isolated from the three age groups, with the
highest level in infant-derived ADSCs (P<0.01) and a notably
increased level in adult-derived ADSCs compared with
elderly-derived ADSCs (P<0.05; Fig.
7B).
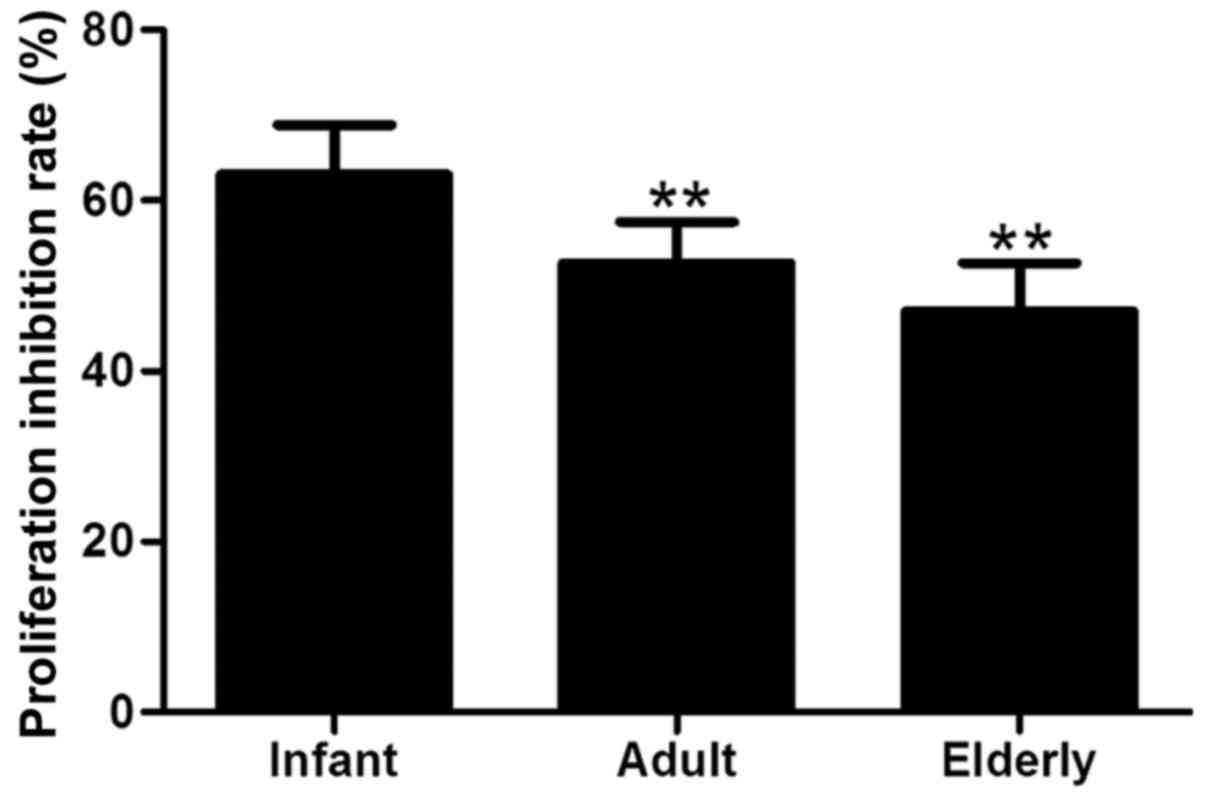
Suppression of T-cell
proliferation
The effects of biological aging on ADSC-mediated
suppression of T-cell proliferation was examined following
co-culture with PBMCs. ADSCs were demonstrated to strongly inhibit
the proliferation of T cells in response to PHA and IL-2
stimulation. The adult- and elderly-derived ADSCs were
significantly less efficient at suppressing of T-cell proliferation
compared with infant-derived ADSCs (P<0.01; Fig. 8), indicating an age-associated
decline in the immunomodulatory capacity ADSCs.
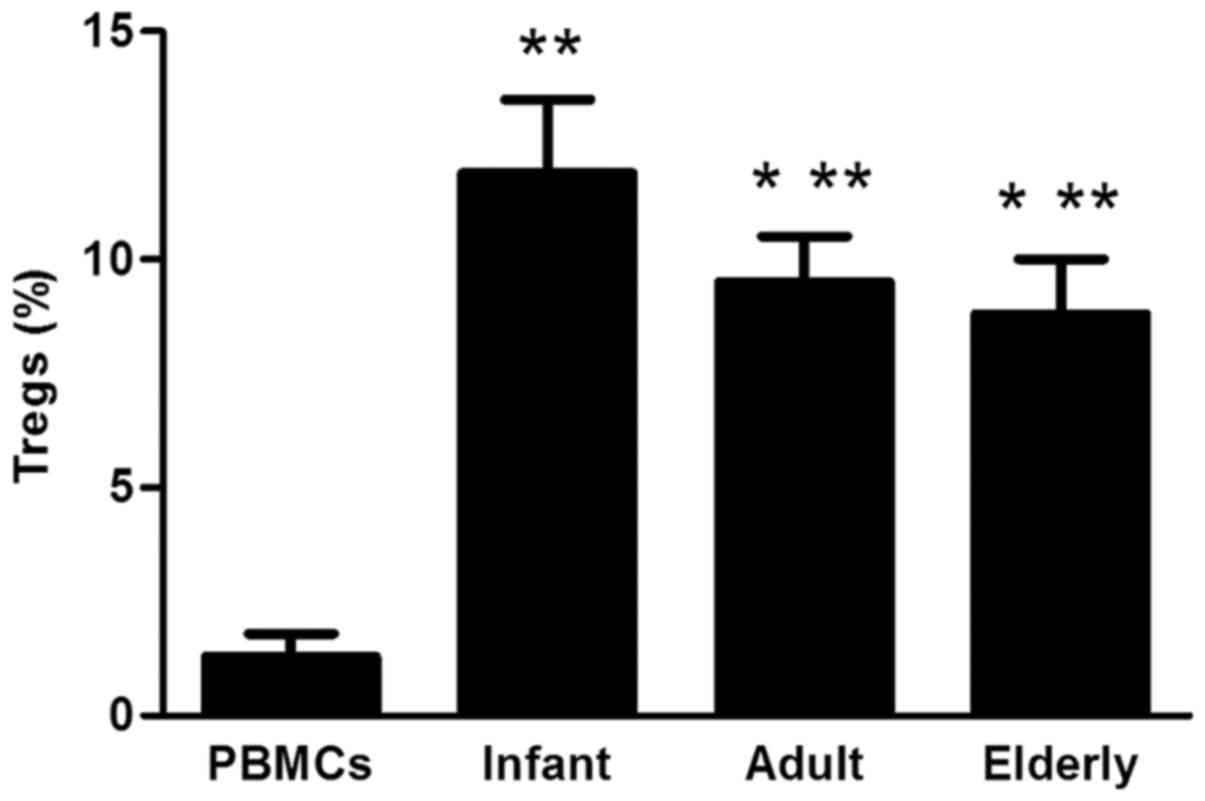
CD4+/CD25+/FoxP3+Treg
analysis
ADSCs from each of the three groups were revealed to
significantly increase the proportion of
CD4+/CD25+/FoxP3+Tregs when
co-cultured with PBMCs, compared with PBMCs alone (P<0.01;
Fig. 9). The proportion of
CD4+/CD25+/FoxP3+Tregs was
significantly lower in adult- and elderly-derived ADSCs compared
with infant-derived ADSCs (P<0.05).
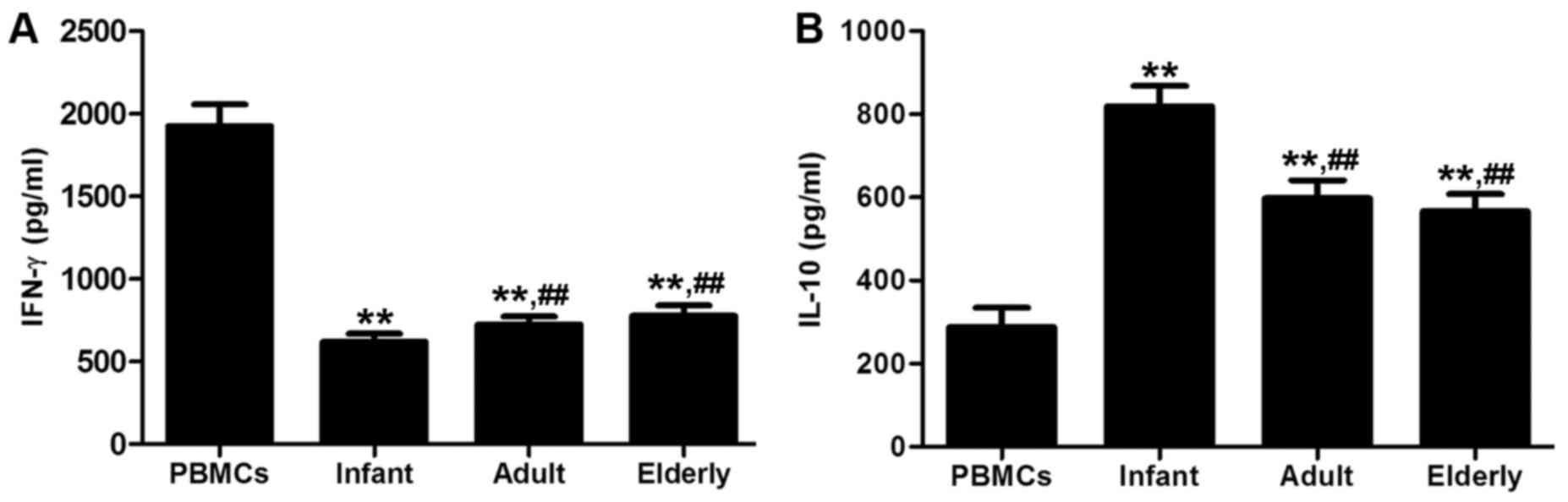
Cytokine production
The concentrations of proinflammatory cytokine IFN-γ
were significantly decreased in the supernatants of all ADSC groups
co-cultured with PBMCs, compared with the concentration in PBMCs
alone (P<0.01; Fig. 10A).
Conversely, the concentrations of anti-inflammatory cytokine IL-10
were significantly increased in the supernatants of all ADSC groups
co-cultured with PBMCs compared with those in the culture of PBMCs
alone (P<0.01; Fig. 10B).
Adult- and elderly-derived ADSCs significantly increased the
production of IFN-γ and significantly decreased the production of
IL-10 compared with infant-derived ADSCs (P<0.05).
Discussion
A previous clinical trial involving the
transendocardial administration of MSCs in patients with benign
ESLD demonstrated that this technique was feasible, safe and
offered potential therapeutic benefits (21). However, cellular therapeutic
products such as this may be affected by aging and cell senescence,
which are known to influence the properties and function of MSCs
(22). Previous studies have
reported adipose tissue to be an attractive autologous source of
adult stem cells owing to its abundance and surgical accessibility
(23,24). The present study comprised a
comprehensive analysis of age-associated ADSC characteristics using
patients with benign ESLD, which represented a cohort of potential
candidates for autologous cell therapy, and specifically elaborated
on the properties of infant-derived ADSCs. The data demonstrated
that increased age may be associated with reduced proliferative,
migratory, differentiation and immunosuppressive capabilities and
infant-derived ADSCs may have biological advantages for clinical
application compared with to adult-or elderly-derived ADSCs.
The present study compared cell morphologies and
immunophenotypes of ADSCs from the three patient sources. Notably,
ADSCs derived from infant, adult and elderly patients exhibited
typical MSC morphology and phenotype that was independent of
patient age. That is, regardless of patient age, analysis of cell
surface markers on ADSCs revealed the absence of expression of
hematopoietic stem cells markers, such as CD34 and CD45, and an
increased expression of MSC surface markers CD73, CD90 and CD105.
These results were in agreement with a previous report (25), and confirm that ADSCs from all age
groups contained a homogeneous phenotypic population of
fibroblast-like morphology (26).
A crucial factor that may influence clinical
outcomes of cell therapy/transplantation procedures, as well as the
success of tissue engineering, is the proliferative, migratory,
antiapoptotic and differentiation capacities of the cells used
(27). The ability to expand cells
without losing function is key to successfully culturing ADSCs for
therapeutic purposes. In addition, ADSC migration is considered to
serve a pivotal role in MSC-mediated tissue repair (28). The present study used RTCA to
determine cell proliferation and cell migration, similar to a
previous report (29), and
demonstrated an age-related difference in cell proliferation and
migration, both of which were statistically higher in
infant-derived ADSCs compared with adult and elderly patients. This
result suggested that the proliferation and migration of ADSCs
within adipose tissue is affected by advancing age; proliferation
and migration of ADSCs derived from elderly patients were further
decreased, offering preliminary evidence that senescence may reduce
cell proliferation and migration. However, the antiapoptotic
capacity of ADSCs did not change with age (30). In addition, ASDCs derived from all
patients with benign ESLD were able to efficiently differentiate
into adipocytes and osteocytes in the appropriate induction
environment; no significant differences were observed in the
ability of ADSCs to undergo adipogenesis. However, PPAR-γ mRNA
expression analysis suggested that adipogenic capacity may increase
slightly in elderly-derived ADSCs. Conversely, increased calcium
deposition and increased expression of OPN mRNA in infant patients,
and a comparatively lower level of OPN mRNA in elderly patients,
suggested that elderly-derived ADSCs may lose their osteogenic
potential and, in turn, may gain adipogenic potential, which leads
to senile osteoporosis. Notably, previous studies have reported
that adipogenic and osteogenic differentiation potential of ADSCs
were dependent on donor age (31),
whereas other studies indicated that the multilineage
differentiation potential of ADSCs was not affected by age
(32). This disparity may be due
to the choice of age groups, group size, sex, and health or
pathological states of the donors, as well as the isolation and
cultivation conditions (33).
It has also been suggested that MSC therapy may
improve liver function and reduce liver injury in ESLD, as
supported by the notable inhibition of immunocyte proliferation and
migration to the liver, an increased number of circulating Tregs,
the release of soluble immunomodulatory factors and the repair of
damaged target organs (34). To
investigate whether ADSCs derived from patients with benign ESLD
were able to effectively exert immunosuppressive properties,
mitogen-activated T cells are co-cultured in vitro with
ADSCs from the three groups of patients. Results from the present
study indicated that ADSC immunomodulation may occur through a
multistage process involving their ability to suppress T cell
proliferation and convert effector T cells into Tregs, followed by
the downregulated secretion of the proinflammatory cytokine IFN-γ
and an upregulation in anti-inflammatory cytokine IL-10 secretion.
These characteristics appeared to be most effective in the
infant-derived ADSCs, which indicated that there may be an
age-associated decline in ADSC immunoregulatory capacity. To the
best of our knowledge, this is the first study to demonstrate that
infant-derived ADSCs obtained from patients with benign ESLD
substantially enhanced the immunosuppressive ability of ADSCs.
Results from the present and previous studies
suggested that aging may affect the properties of ADSCs and, thus,
may lower the effectiveness of autologous cell therapy. For these
reasons, cell material should be examined prior to use and the
development of effective approaches for the pretreatment or
modification of MSCs from elderly patients may aid in enhancing
their therapeutic potential (35).
Improvements may include the addition of certain growth factors
and/or chemokines, such as platelet-derived growth factor and tumor
necrosis factor α, to MSC culture media, to enhance their
viability, proliferation, migratory properties, which may increase
their therapeutic potential (36,37).
In conclusion, the present results revealed that the
biological and functional characterizations of ADSCs may be
positively correlated with patient age, with infant-derived ADSCs
being the most effective. In addition, the results suggested that
cellular pretreatment strategies may be required to improve the
efficiency of aged cells in autologous therapy.
Acknowledgements
This study was supported by The National Natural
Science Foundation of China (grant no. 81470982), the Tianjin
Municipal Health Bureau Key Project (grant no. 16KG105) and The
Tianjin Research Program of Application Foundation and Advanced
Technology (grant nos. 12ZCZDSY02600 and 13JCYBJC23000).
References
|
1
|
Lindvig KP, Teisner AS, Kjeldsen J, Strøm
T, Toft P, Furhmann V and Krag A: Allocation of patients with liver
cirrhosis and organ failure to intensive care: Systematic review
and a proposal for clinical practice. World J Gastroenterol.
21:8964–8973. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reddy SS and Civan JM: From child-pugh to
model for end-stage liver disease: Deciding who needs a liver
transplant. Med Clin North Am. 100:449–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai YH, Duan WD, Yu Q, Ye S, Xiao NJ,
Zhang DX, Huang ZQ, Yang ZY and Dong JH: Outcomes of liver
transplantation for end-stage biliary disease: A comparative study
with end-stage liver disease. World J Gastroenterol. 21:6296–6303.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gong N and Chen X: Partial liver
transplantation. Front Med. 5:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raut V, Alikhanov R, Belghiti J and Uemoto
S: Review of the surgical approach to prevent small-for-size
syndrome in recipients after left lobe adult LDLT. Surg Today.
44:1189–1196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nicolas CT, Wang Y and Nyberg SL: Cell
therapy in chronic liver disease. Curr Opin Gastroenterol.
32:189–194. 2016.PubMed/NCBI
|
|
7
|
Than NN, Tomlinson CL, Haldar D, King AL,
Moore D and Newsome PN: Clinical effectiveness of cell therapies in
patients with chronic liver disease and acute-on-chronic liver
failure: A systematic review protocol. Syst Rev. 5:1002016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Malhotra S, Hu MS, Marshall CD, Leavitt T,
Cheung AT, Gonzalez JG, Kaur H, Lorenz HP and Longaker MT:
Mesenchymal stromal cells as cell-based therapeutics for wound
healing. Stem Cells Int. 2016:41579342016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prockop DJ: Inflammation, fibrosis and
modulation of the process by mesenchymal stem/stromal cells. Matrix
Biol. 51:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK,
Park H, Hwang SG, Kim DJ, Lee BS, Lee SH, et al: Transplantation
with autologous bone marrow-derived mesenchymal stem cells for
alcoholic cirrhosis: Phase 2 trial. Hepatology. 64:2185–2197. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW,
Cho MY, Park HJ, Park SY, Kim BR, Kim JW, et al: Histological
improvement following administration of autologous bone
marrow-derived mesenchymal stem cells for alcoholic cirrhosis: A
pilot study. Liver Int. 34:33–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salama H, Zekri AR, Medhat E, Al Alim SA,
Ahmed OS, Bahnassy AA, Lotfy MM, Ahmed R and Musa S: Peripheral
vein infusion of autologous mesenchymal stem cells in Egyptian
HCV-positive patients with end-stage liver disease. Stem Cell Res
Ther. 5:702014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du Z, Wei C, Yan J, Han B, Zhang M, Peng C
and Liu Y: Mesenchymal stem cells overexpressing C-X-C chemokine
receptor type 4 improve early liver regeneration of small-for-size
liver grafts. Liver Transpl. 19:215–225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maria AT, Maumus M, Le Quellec A,
Jorgensen C, Noël D and Guilpain P: Adipose-derived mesenchymal
stem cells in autoimmune disorders: State of the art and
perspectives for systemic sclerosis. Clin Rev Allergy Immunol.
52:234–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu T, Mu H, Shen Z, Song Z, Chen X and
Wang Y: Autologous adipose tissue-derived mesenchymal stem cells
are involved in rat liver regeneration following repeat partial
hepatectomy. Mol Med Rep. 13:2053–2059. 2016.PubMed/NCBI
|
|
16
|
Mancini O Kizilay, Shum-Tim D, Stochaj U,
Correa JA and Colmegna I: Age, atherosclerosis and type 2 diabetes
reduce human mesenchymal stromal cell-mediated T-cell suppression.
Stem Cell Res Ther. 6:1402015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang YL, Li G, Zou XF, Chen XB, Liu T and
Shen ZY: Effect of autologous adipose-derived stem cells in renal
cold ischemia and reperfusion injury. Transplant Proc.
45:3198–3202. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu T, Zhang Y, Shen Z, Song Z, Zou X,
Chen X, Chen L and Wang Y: Immunomodulatory effects of OX40Ig
gene-modified adipose tissue-derived mesenchymal stem cells in rat
kidney transplantation. Int J Mol Med. 39:144–152. 2017.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu YE, Du ZR, Cai YM, Peng WG, Zheng GZ,
Zheng GL, Wu LB and Li K: Effective expansion of forkhead box
P3+ regulatory T cells via early secreted antigenic
target 6 and antigen 85 complex B from Mycobacterium
tuberculosis. Mol Med Rep. 11:3134–3142. 2015.PubMed/NCBI
|
|
21
|
Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J,
Chen L, Lv S, Li Y, Yu S, et al: Human umbilical cord mesenchymal
stem cells improve liver function and ascites in decompensated
liver cirrhosis patients. J Gastroenterol Hepatol. 27:(Suppl 2).
S112–S120. 2012. View Article : Google Scholar
|
|
22
|
Choudhery MS, Badowski M, Muise A, Pierce
J and Harris DT: Donor age negatively impacts adipose
tissue-derived mesenchymal stem cell expansion and differentiation.
J Transl Med. 12:82014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harasymiak-Krzyżanowska I, Niedojadło A,
Karwat J, Kotuła L, Gil-Kulik P, Sawiuk M and Kocki J: Adipose
tissue-derived stem cells show considerable promise for
regenerative medicine applications. Cell Mol Biol Lett. 18:479–493.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schneider S, Unger M, van Griensven M and
Balmayor ER: Adipose-derived mesenchymal stem cells from
liposuction and resected fat are feasible sources for regenerative
medicine. Eur J Med Res. 22:172017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Efimenko A, Dzhoyashvili N, Kalinina N,
Kochegura T, Akchurin R, Tkachuk V and Parfyonova Y:
Adipose-derived mesenchymal stromal cells from aged patients with
coronary artery disease keep mesenchymal stromal cell properties
but exhibit characteristics of aging and have impaired angiogenic
potential. Stem Cells Transl Med. 3:32–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu W, Niklason L and Steinbacher DM: The
effect of age on human adipose-derived stem cells. Plast Reconstr
Surg. 131:27–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yarygin KN, Lupatov AY and Kholodenko IV:
Cell-based therapies of liver diseases: Age-related challenges.
Clin Interv Aging. 10:1909–1924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai A, Qiu R, Li L, Zheng D, Dong Y, Yu D,
Huang Y, Rao S, Zhou Y and Mai W: Atorvastatin treatment of rats
with ischemia-reperfusion injury improves
adipose-derivedmesenchymal stem cell migration and survival via the
SDF-1α/CXCR-4 axis. PLoS One. 8:e791002013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lai SL, Cheah SC, Wong PF, Noor SM and
Mustafa MR: In vitro and in vivo anti-angiogenic activities of
Panduratin A. PLoS One. 7:e381032012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ong WK and Sugii S: Adipose-derived stem
cells: Fatty potentials for therapy. Int J Biochem Cell Biol.
45:1083–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kornicka K, Marycz K, Tomaszewski KA,
Marędziak M and Śmieszek A: The effect of age on osteogenic and
adipogenic differentiation potential of human adipose derived
stromal stem cells (hASCs) and the impact of stress factors in the
course of the differentiation process. Oxid Med Cell Longev.
2015:3091692015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Beane OS, Fonseca VC, Cooper LL, Koren G
and Darling EM: Impact of aging on the regenerative properties of
bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal
cells. PLoS One. 9:e1159632014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sethe S, Scutt A and Stolzing A: Aging of
mesenchymal stem cells. Ageing Res Rev. 5:91–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Owen A and Newsome PN: Mesenchymal stromal
cell therapy in liver disease: Opportunities and lessons to be
learnt? Am J Physiol Gastrointest Liver Physiol. 309:G791–G800.
2015.PubMed/NCBI
|
|
35
|
Petrou P, Gothelf Y, Argov Z, Gotkine M,
Levy YS, Kassis I, Vaknin-Dembinsky A, Ben-Hur T, Offen D, Abramsky
O, et al: Safety and clinical effects of mesenchymal stem cells
secreting neurotrophic factor transplantation in patients with
amyotrophic lateral sclerosis: Results of phase 1/2 and 2a clinical
trials. JAMA Neurol. 73:337–344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mizuno M, Katano H, Otabe K, Komori K,
Matsumoto Y, Fujii S, Ozeki N, Tsuji K, Koga H, Muneta T, et al:
Platelet-derived growth factor (PDGF)-AA/AB in human serum are
potential indicators of the proliferative capacity of human
synovial mesenchymal stem cells. Stem Cell Res Ther. 6:2432015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kavanagh DP, Suresh S, Newsome PN,
Frampton J and Kalia N: Pretreatment of mesenchymal stem cells
manipulates their vasculoprotective potential while not altering
their homing within the injured gut. Stem Cells. 33:2785–2797.
2015. View Article : Google Scholar : PubMed/NCBI
|