Introduction
Hepatocellular carcinoma (HCC) is a common lethal
malignancy that is highly vascularized and is the third most common
cause of cancer-associated mortality worldwide (1). Angiogenesis is important for tumor
progression and metastasis; it is a complex process regulated by a
variety of factors, among which vascular endothelial growth factor
(VEGF) appears to serve a dominant role (2). Previous studies have indicated that
VEGF is overexpressed in HCC and is closely associated with tumor
angiogenesis (3,4). Therefore, targeting the VEGF/VEGF
receptor (VEGFR) signaling pathway has attracted attention in
anti-angiogenesis therapy research.
The soluble form of VEGFR-1 (also known as soluble
fms-like tyrosine kinase-1, sFlt-1), which is an alternatively
spliced variant, has been hypothesized to be a potent antagonist of
VEGF. Full-length VEGFR-1 is a glycoprotein with seven
extracellular immunoglobulin (Ig)-like domains, a transmembrane
domain and an intracellular tyrosine kinase binding domain
(5,6). The spliced sFlt-1 comprises only the
first six extracellular Ig-like domains (7). Although VEGFR-1 has an increased
affinity for VEGF compared with VEGFR-2, the tyrosine kinase
activity of VEGFR-2 is more effective by a factor of ten (8). VEGFR-2 is considered to be the
primary receptor involved in angiogenesis. Despite having the same
affinity as VEGFR-1 for VEGF, sFlt-1 does not initiate signal
transduction, owing to the absence of the transmembrane domain and
the intracellular tyrosine kinase domain. sFlt-1 exerts
anti-angiogenesis effects in two ways: i) Sequestering free
ligands; and ii) forming inactive heterodimers with VEGFR-2
(9). A variety of studies have
demonstrated that the administration of sFlt-1 may suppress tumor
growth and metastasis through anti-angiogenesis mechanisms
(10–13). However, gene therapy remains
limited, as therapeutic molecules that are encoded by the target
gene may not effectively localize to tumor sites following systemic
injection.
Mesenchymal stem cells (MSCs) are immune privileged
pluripotent cells (14) that are
easy to isolate and culture in vitro. In addition to their
multilineage differentiation potential, MSCs have been observed to
migrate to the site of wounds and tumors (15), including glioma (16), hepatoma and lung tumors (17). These features make MSCs an ideal
delivery vehicle for gene therapy, and MSCs engineered to carry
therapeutic genes have been demonstrated to serve an antitumor role
in certain tumor models (18–22).
The present study evaluated the anti-angiogenic
effects of lentiviral transfected MSCs engineered to secrete sFlt-1
(LV-sFlt-1-MSCs) via co-culturing of these cells with human
umbilical vein endothelial cells (HUVECs) in a three-dimensional
co-culture system. To investigate the antitumor effects of sFlt-1
and the tropism of MSCs for the tumor site, LV-sFlt-1-MSCs were
produced by lentiviral transduction and were implanted into mice as
part of a pre-established HCC subcutaneous mouse model. The results
of the present study suggested that MSCs may serve as delivery
vehicles for gene therapy in HCC.
Materials and methods
Cell culture
The HCC cell line SMMC-7721 was purchased from
Shanghai Institute of Biochemistry and Cell Biology, Chinese
Academy of Science (Shanghai, China). BALB/C mouse bone
marrow-derived MSCs were obtained from Cyagen Biosciences (Santa
Clara, CA, USA). SMMC-7721 cells were maintained in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). MSCs were incubated in DMEM/Ham's F12
nutrient mixture (HyClone; GE Healthcare Life Sciences, Logan, UT,
USA). All cell cultures were supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml
streptomycin and 100 U/ml penicillin (Gibco; Thermo Fisher
Scientific, Inc.), and incubated at 37°C in a 5% CO2
atmosphere. HUVECs (ScienCell Research Laboratories, Inc.,
Carlsbad, CA, USA) were incubated in endothelial growth medium
(PromoCell GmbH, Heidelberg, Germany) supplemented with 5% FBS.
MSCs and HUVEC were used during the third or fourth passage.
Transfection of MSCs
The lentiviral vectors expressing sFlt-1 (LV-sFlt-1,
9×108 IU/ml) and non-targeting control lentiviral
vectors (LV-NC, 9×108 IU/ml) were constructed by Suzhou
GenePharma Co., Ltd. (Suzhou, China). MSCs (5×105
cells/ml) were transfected at 37°C for 72 h using Lipofectamine
2000 (Thermo Fisher Scientific, Inc.) according to the
manufacturer's guidelines. The suitable multiplicity of infection
was determined to be 50 in the present study. Stably infected MSCs
were obtained by treating the cells with puromycin (2 µg/ml).
Semi-quantitative polymerase chain
reaction (PCR)
LV-sFlt-1-MSCs, LV-NC-MSCs and MSCs were cultured as
aforementioned. Following 72 h, cells (1×106 cells/ml)
were harvested by centrifugation at 1,000 × g at room temperature
for 10 min and the supernatant was stored for ELISA analysis. Total
RNA was isolated from cells using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.), following the manufacturer's instructions.
Analysis was performed using PrimeSTAR HS DNA polymerase (Takara
Biotechnology Co., Ltd., Dalian, China). Thermocycling conditions
were as follows: Initial denaturation at 95°C for 5 min, followed
by 33 cycles of denaturation at 95°C for 30 sec, annealing at 55°C
for 30 sec, extension at 72°C for 30 sec, with a final extension
step at 72°C for 10 min. The primers for sFlt-1 were as follows:
Forward, 5′-GATGGGTTACCTGCGACTG-3′ and reverse,
5′-CCGGGTCTGGAAACGATG-3′. The primers for GAPDH were as follows:
Forward, 5′-AGGGCTGCTTTTAACTCTGGT-3′ and reverse,
5′-TCTCGCTCCTGGAAGATGGTG-3′. PCR products were dissolved on a 12%
agarose gel and visualized following staining with ethidium bromide
(0.5 µg/ml; Thermo Fisher Scientific, Inc.). Densitometric analysis
was performed using ImageJ software version 1.45 (National
Institutes of Health, Bethesda, MD, USA).
Western blot analysis
Cells (1×106 cells/ml) were lysed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) with phenylmethylsulfonyl fluoride (a
protease inhibitor). Protein concentration was determined using a
bicinchoninic acid protein assay. A total of 25 µg total protein
was separated by 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes. The membranes were blocked for 2.5 h with 5%
non-fat milk at room temperature and incubated with monoclonal
rabbit anti-human Flt-1 antibody (1:1,000, cat. no. ab32152; Abcam,
Cambridge, UK) and monoclonal mouse anti-human β-actin antibody
(1:1,000, cat. no. sc-130065; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at 4°C overnight. Subsequently, the membranes were
incubated with the secondary antibody conjugated with horseradish
peroxidase (1:1,000, cat. no. sc-516102; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature. Immunoreactivity was detected
using an enhanced chemiluminescence kit (Beyotime Institute of
Biotechnology), in accordance with the manufacturer's instructions.
β-actin served as an internal control. Blots were semi-quantified
by densitometry using ImageJ software version 1.45.
Tube formation assay
To evaluate the role of sFlt-1 in the tube formation
of HUVECs, LV-sFlt-1-MSCs, LV-NC-MSCs and untransfected MSCs were
co-cultured with HUVECs following the method previously described
by Zeng et al (23). A
15-well µ-slide (ibidi GmbH, Munich, Germany) was coated with
Matrigel (10 µl/well; BD Biosciences, Franklin Lakes, NJ, USA) and
incubated for 30 min at 37°C. Subsequently, HUVECs
(7.5×103 cells/well) were stained with DiI (10 µM, a
cell membrane dye emitting red fluorescence; Beyotime Institute of
Biotechnology) at 37°C for 30 min. HUVECs and LV-sFlt-1-MSCs
(7.5×103 cells/well) were plated in the same well of the
µ-slide at 37°C. Images were digitally captured at 1, 3, 5, 12 and
24 h under a fluorescence microscope (×100 magnification) and
analysis of tube formation was performed using ImageJ software
version 1.45.
Distribution of MSCs in vivo
SMMC-7721 cells (7×106) were stained with
DiI (10 µM) at 37°C for 30 min prior to subcutaneously injecting
the cells into the rear of nude mice. Tumors were allowed to grow
for 2 weeks; subsequently, 5×105 LV-NC-MSCs were
intravenously injected into tumor-bearing mice via the tail vein.
Following 7 days additional growth, the subcutaneous tumor tissues,
livers, lungs, hearts, kidneys and brains were removed and cut into
fresh-frozen (−15°C) sections (5 µm). To visualize the distribution
of MSCs in vivo, the sections were observed under a
fluorescence microscope (×200 magnification).
Animal experiments
Male athymic BALB/C nude mice (age, 4–6 weeks;
weight, 18–22 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China) and maintained under specific
pathogen free conditions at a temperature of 22–25°C, under a 12-h
light/dark cycle with free access to food and water. All
experiments were performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals, and were approved by the Ethics Committee of the Medical
Faculty of the Fujian Medical University (Fuzhou, China).
SMMC-7721 cells (7×106 cells in 0.1 ml
PBS) were subcutaneously implanted into the rear of nude mice. When
the diameter of the tumor nodule reached 0.5 cm, tumor-bearing mice
were randomized into 4 groups (n=10/group): i) PBS (0.1 ml PBS once
a week for 3 weeks); ii) LV-NC-MSCs; iii) LV-sFlt-1; and iv)
LV-sFlt-1-MSCs. LV-NC-MSCs or LV-sFlt-1-MSCs (6×105
cells in 0.1 ml PBS) were intravenously injected via the tail vein
once per week for 3 weeks. LV-sFlt-1 (3×107 TU in 0.1 ml
PBS) was injected once per week for 3 weeks. Tumor size was
measured using a caliper every 5 days. The length (L) and width (W)
of tumors were measured, and tumor volume (V) was calculated using
the following formula: V=L × W2/2. A total of 3 weeks
later, 5 mice in each group were sacrificed. Blood samples were
harvested from mouse hearts (1.5 ml) and plasma was obtained by
centrifugation at room temperature at 1,000 × g for 15 min. Tumor
tissues were removed and minced in PBS. Plasma samples and tumor
tissues were stored at −20°C. The remaining mice were monitored for
survival analysis.
ELISA analysis
Cell culture supernatants (0.1 ml), plasma samples
(0.1 ml) and fresh tumor tissues (0.1 ml) were harvested, and the
concentration of secreted sFlt-1 was detected by ELISA for human
VEGFR1/Flt-1 (cat. no. DVR100B; R&D Systems Inc., Minneapolis,
MN, USA), according to the manufacturer's instructions.
Immunohistochemistry
Tumor tissues were fixed in 4% paraformaldehyde at
room temperature for 24 h and embedded in paraffin. Sections (4 µm)
were cut, dewaxed and dehydrated. Subsequently, sections were
stained with 0.2% hematoxylin for 5 min and 1% eosin for 2 min, and
observed under an optical microscope (×400 magnification).
Immunohistochemical staining was also performed. Tissue sections
were blocked in 5% goat serum (Gibco; Thermo Fisher Scientific,
Inc.) diluted in PBS for 20 min at room temperature. The primary
antibodies included: Monoclonal rabbit anti-murine cluster of
differentiation (CD)34 (1:50, cat. no. sc-9095; Santa Cruz
Biotechnology, Inc.); monoclonal rabbit anti-human proliferation
marker protein Ki-67 (Ki-67, 1:50, cat. no. sc-15402; Santa Cruz
Biotechnology, Inc.); and polyclonal rabbit anti-human VEGF (1:250,
cat. no. ab46154; Abcam). Sections were incubated with the primary
antibodies at 4°C for 24 h. After washing, the sections were
incubated with a MaxVision™ horseradish peroxidase-labelled polymer
anti-rabbit kit (cat. no. KIT-5005; Fuzhou Maixin Biotech Co.,
Ltd., Fuzhou, China) at room temperature for 15 min. Finally, they
were stained with 3,3′-diaminobenzidince (Fuzhou Maixin Biotech
Co., Ltd.) at room temperature for 5 min. Stained sections were
observed and scored independently by two pathologists. The
proliferation index was determined as the number of [Ki-67-positive
(brown) cells/the number of total cells] × 100, in five random
fields under an optical microscope (×400 magnification).
Microvessel density (MVD)
analysis
MVD was assayed by immunohistochemical staining with
a CD34 antibody under an optical microscope and was evaluated as
previously reported by Weidner et al (24). The sections were visualized under
low magnification (×100) to identify the highest neovascularization
areas (hotspots). Subsequently, these areas were observed at higher
magnification (×200) and the number of microvessels was counted in
5-random fields. The average number of microvessels in five fields
was regarded as the MVD level for the sample.
Statistical analysis
The data are presented as the mean ± standard error
of the mean from at least 3 independent experiments. Survival data
were analyzed using the Kaplan-Meier method, and statistical
significance was determined using the log-rank test. Statistical
significance was evaluated using one-way analysis of variance
followed by a post hoc Dunnett's test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
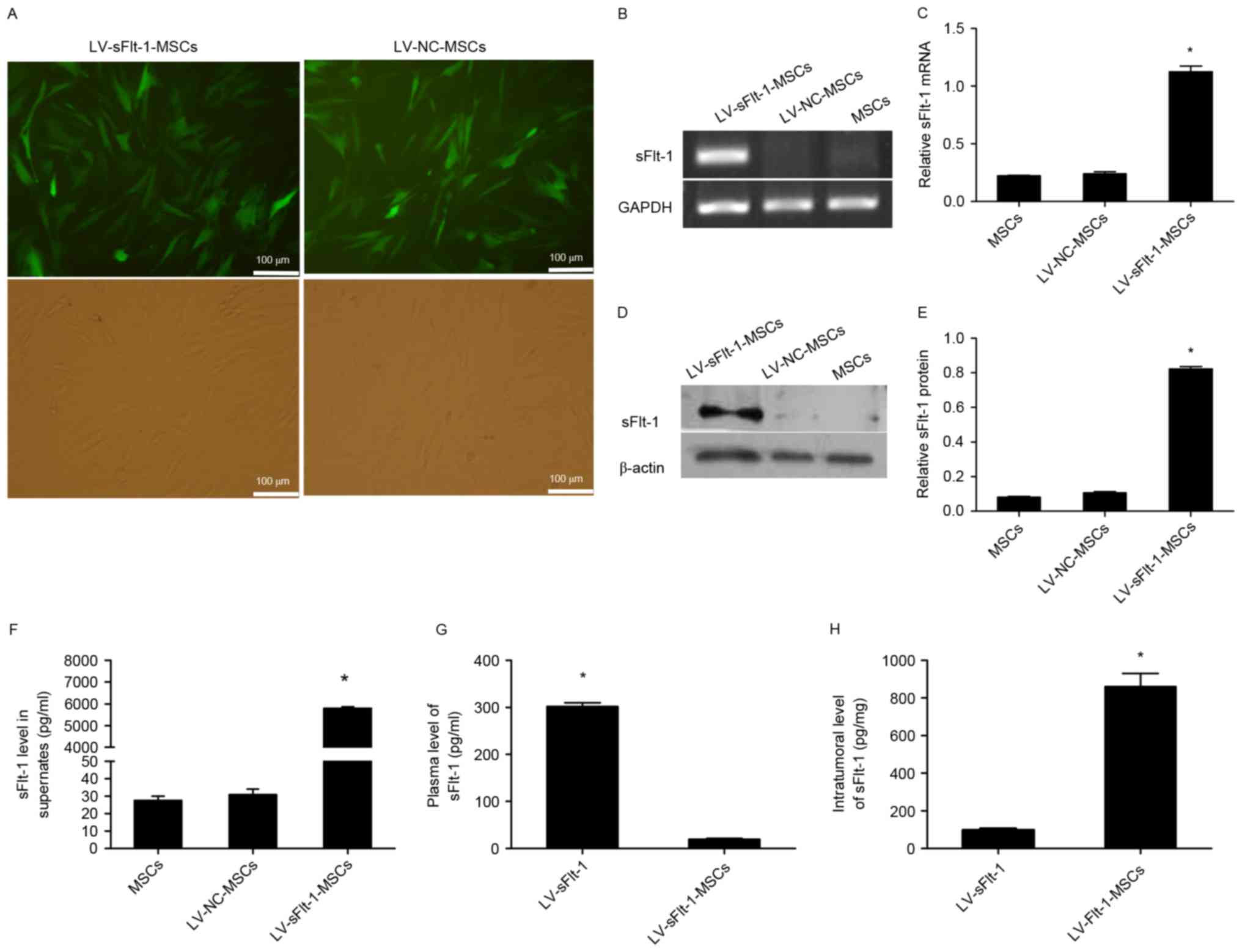
Construction of LV-sFlt-1-MSCs and
expression of sFlt-1 in vitro
MSCs were infected with LV-sFlt-1 or LV-NC, and
stably infected MSCs were obtained by treating the cells with
puromycin (Fig. 1A). To determine
the expression levels of sFlt-1, PCR and western blotting were
performed to analyze lysates from LV-sFlt-1-MSCs, LV-NC-MSCs and
untransfected MSCs. LV-sFlt-1-MSCs significantly overexpressed
sFlt-1 mRNA and protein compared with MSCs and LV-NC-MSCs (Fig. 1B-E). These stably infected MSCs
were used for subsequent experiments.
sFlt-1 levels in cell culture
supernatants, plasma samples and fresh tumor tissues
To determine whether sFlt-1 may be secreted by
lentivirus-infected MSCs, an ELISA for human VEGFR1/Flt-1 was
performed. The secreted sFlt-1 level in the culture supernatants of
LV-sFlt-1-MSCs was 5793.0±73.9 pg/ml, whereas only minimal sFlt-1
was detected in the culture supernatants of LV-NC-MSCs and
untreated MSCs (Fig. 1F).
Human sFlt-1 levels in plasma samples and tumor
tissues were undetectable in control mice treated with PBS or
LV-NC-MSCs (data not shown). The plasma concentration of human
sFlt-1 in the mice treated with LV-sFlt-1 was significantly higher
compared with the concentration in mice treated with LV-sFlt-1-MSCs
(301.7±7.7 pg/ml vs. 19.2±2.2 pg/ml; Fig. 1G). However, the intratumoral level
of human sFlt-1 in the mice treated with LV-sFlt-1-MSCs was
increased compared with the group treated with LV-sFlt-1
(860.7±63.94 pg/mg vs. 14.1±8.79 pg/mg; Fig. 1H).
LV-sFlt-1-MSCs inhibit tube formation
in vitro
To determine the bioactivity of sFlt-1 in
vitro, HUVECs stained with DiI were co-cultured with
LV-sFlt-1-MSCs, LV-NC-MSCs or MSCs in a three-dimensional
co-culture system. The largest number of tubes was observed 5 h
subsequent to co-culturing (Fig. 2A
and B), and the tube-like structures disappeared at 24 h (data
not shown). Tube formation was significantly decreased in the
LV-sFlt-1-MSCs group compared with the LV-NC-MSC or MSC group.
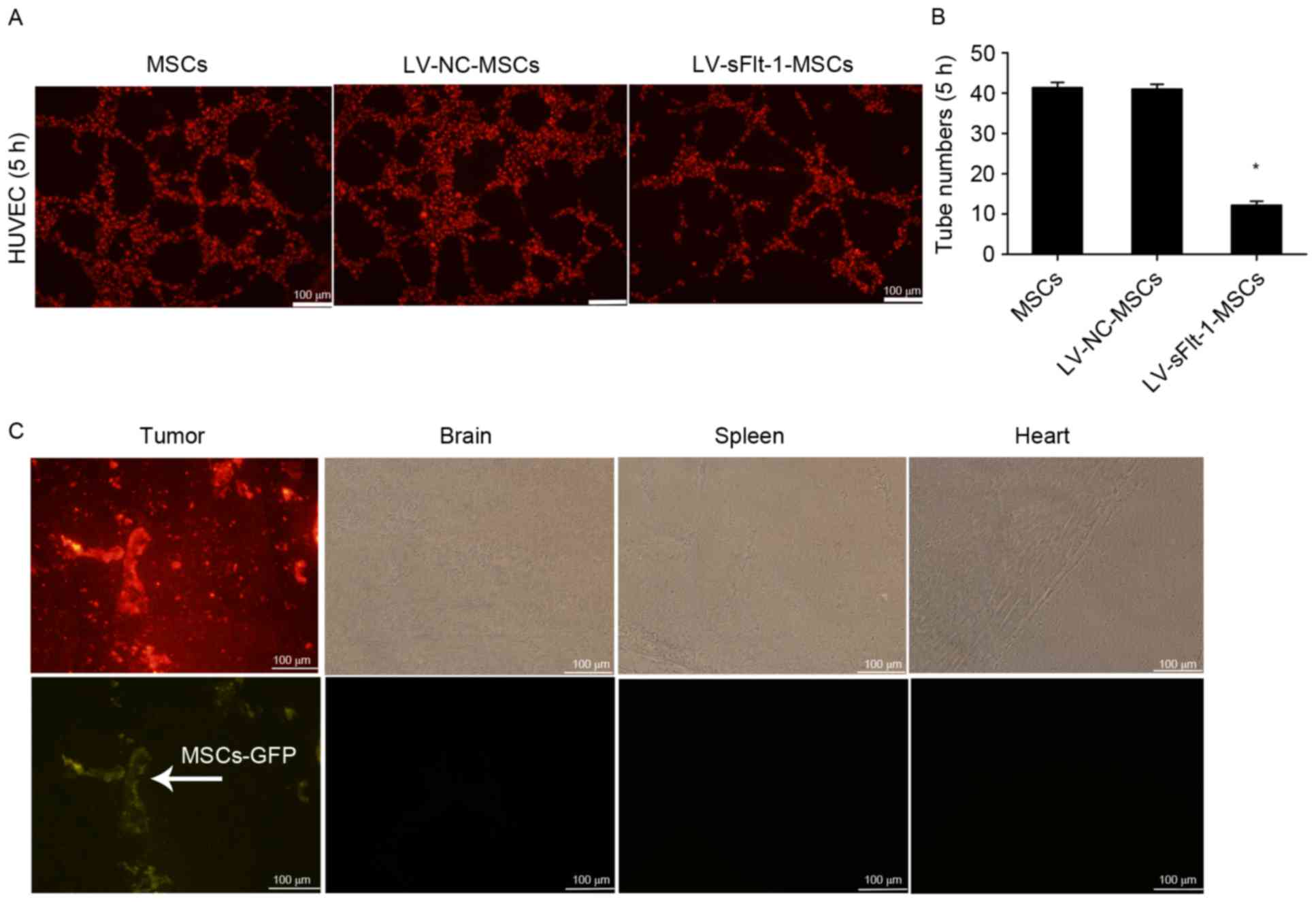 | Figure 2.Effects of sFlt-1 on tube formation
and distribution of MSCs in vivo. (A) HUVECs stained with
DiI were co-cultured with three MSC cell lines, and tube formation
was observed under a fluorescence microscope at 5 h (magnification,
×100). (B) Tube formation was significantly decreased in the group
co-cultured with LV-sFlt-1-MSCs (12.2±0.9) compared with the group
co-cultured with LV-NC-MSCs (41.0±1.2) or MSCs (41.4±1.3).
*P<0.05 vs. LV-NC-MSCs. (C) SMMC-7721 stained with DiI (10 µM)
were used to establish a subcutaneous hepatocellular carcinoma
mouse model. LV-NC-MSCs marked with GFP were intravenously injected
into tumor-bearing mice. Following 7 days, LV-NC-MSCs had
predominantly migrated to the subcutaneous tumor mass, rather than
other organs (including the brain, spleen an heart). GFP, green
fluorescent protein; HUVEC, human umbilical vein endothelial cells;
LV, lentivirus; MSCs, mesenchymal stem cells; NC, negative control;
sFlt-1, soluble fms-like tyrosine kinase-1. |
Tropism of MSCs for HCC in vivo
MSCs are capable of homing to the tumor
microenvironment following systemic injection. To investigate the
distribution of MSCs in vivo, SMMC-7721 cells stained with
DiI (10 µM) were subcutaneously injected into the rear of mice. A
total of 2 weeks later, LV-NC-MSCs marked with GFP were
intravenously injected into the tail vein tumor-bearing mice. At 7
days, the subcutaneous tumor tissues, livers, lungs, hearts,
kidneys and brains were removed and cut into fresh-frozen sections,
and examined under a fluorescence microscope. LV-NC-MSCs primarily
migrated to the subcutaneous tumor mass, rather than other organs,
including the heart, spleen and brain (Fig. 2C).
LV-sFlt-1-MSCs inhibit tumor growth
and prolong survival in an HCC mouse model
To evaluate the efficacy of LV-sFlt-1-MSCs in
inhibiting the progress of HCC, SMMC-7721 cells were used to
establish a xenograft model as aforementioned (Fig. 3A). The average tumor volume in the
control group treated with PBS was 457.7±41.9 mm3
(Fig. 3B). Tumor size in the mice
treated with LV-sFlt-1-MSCs (86.6±6.0 mm3) was
significantly decreased compared with tumors in the control group
(P<0.05). However, the tumor volume in the mice treated with
LV-NC-MSCs (505.4±30.4 mm3) or LV-sFlt-1 (383.8±12.9
mm3) was not significantly different compared with the
tumor volume in the control group (both P>0.05). The tumor
weight in the LV-sFlt-1-MSCs group was significantly decreased
compared with the PBS group (0.120±0.008 vs. 0.326±0.010 g;
P<0.05); whereas the tumor weight in the group treated with
LV-NC-MSCs was 0.322±0.008 g (P>0.05 vs. PBS), and the weight in
the control group LV-sFlt-1 was 0.286±0.012 g (P>0.05 vs. PBS).
The results of the present study suggested that systemic injection
of LV-sFlt-1-MSCs may inhibit tumor growth in an HCC mouse
model.
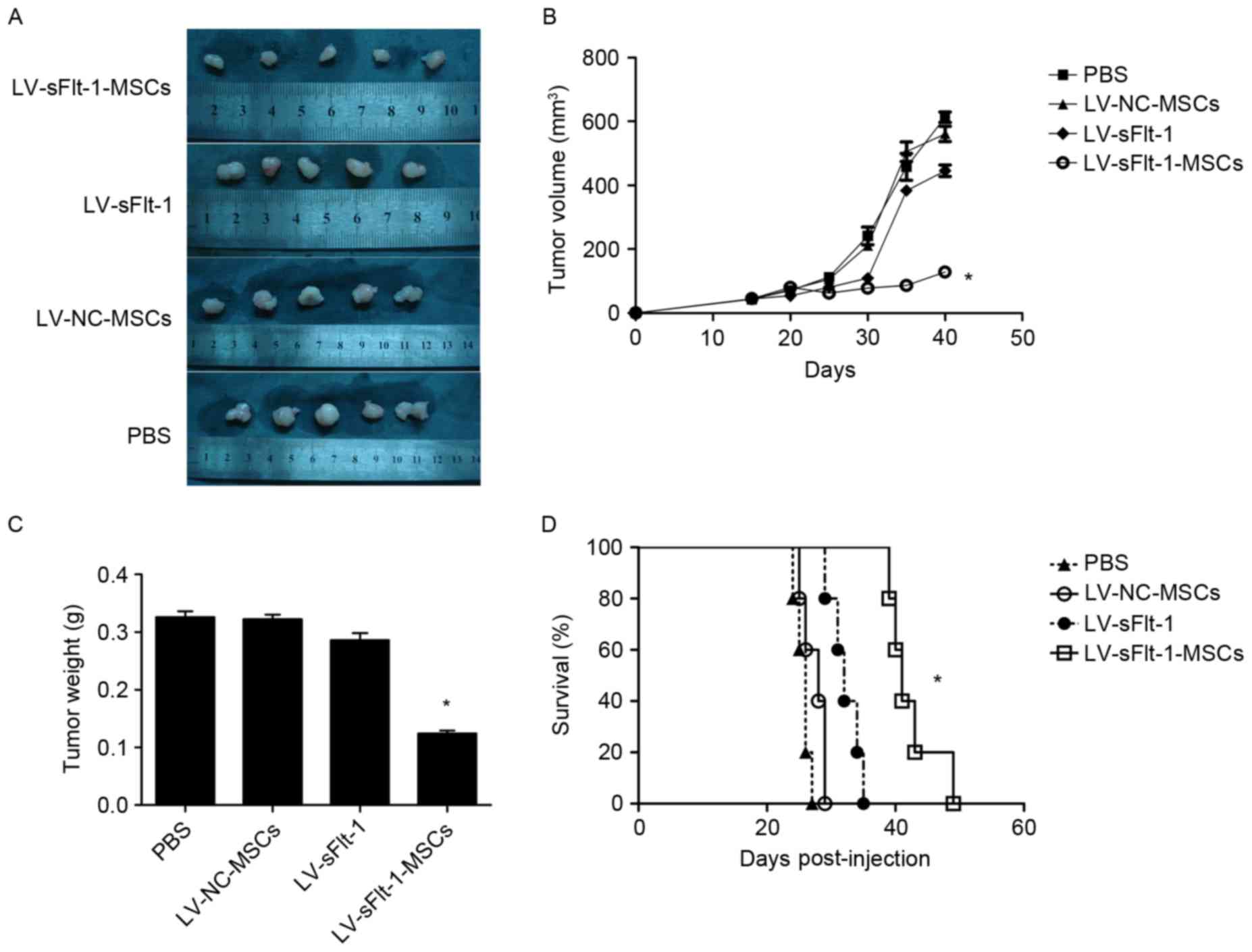 | Figure 3.Therapeutic effects of LV-sFlt-1-MSCs
in a subcutaneous HCC model. SMMC-7721 cells were used to establish
a subcutaneous HCC model. (A) When the diameter of the tumor nodule
reached 0.5 cm, tumor-bearing mice received an injection of PBS,
LV-NC-MSCs, LV-sFlt-1 or LV-sFlt-1-MSCs. Following treatment for 3
weeks, the mice were sacrificed and images were captured. (B) When
the diameter of the tumor nodule reached 0.5 cm, (on day 15
post-implantation) tumor volumes were measured every 5 days. (C)
Tumor tissues were removed, and tumor weights were measured. The
tumor weight in the LV-sFlt-1-MSCs group was decreased compared
with the PBS group. (D) Survival curves were constructed using the
Kaplan-Meier method, and a log-rank test was used to evaluate
significant differences. *P<0.05 vs. PBS. HCC, hepatocellular
carcinoma; LV, lentivirus; MSCs, mesenchymal stem cells; NC,
negative control; sFlt-1, soluble fms-like tyrosine kinase-1. |
The remaining tumor-bearing mice (n=5/group) were
monitored to examine the survival rate (Fig. 3D). The survival rate of
tumor-bearing mice was monitored from the date that mice were
treated with PBS, LV-NC-MSCs, LV-sFlt-1 or LV-sFlt-1-MSCs (days
post-injection). The median survival of mice treated with
LV-sFlt-1-MSCs was significantly increased compared with the
survival in the control group treated with PBS (41 vs. 26 days;
P<0.05), whereas no significant differences were identified when
comparing the median survival of mice treated with LV-NC-MSCs (28
days; P>0.05) or LV-sFlt-1 (32 days; P>0.05) with the
survival of mice in the control group. In the present study, the
results suggested that LV-sFlt-MSCs prolonged the survival time of
tumor-bearing mice compared with the control group.
Anti-angiogenesis effects of
LV-sFlt-1-MSCs in vivo
LV-sFlt-1-MSCs was demonstrated to suppress HCC
growth through the inhibition of angiogenesis. As presented in
Fig. 4, a significant decrease in
MVD was observed in the group treated with LV-sFlt-1-MSCs compared
with the control group treated with PBS (38.3±1.7 vs. 96.1±2.0;
P<0.05).
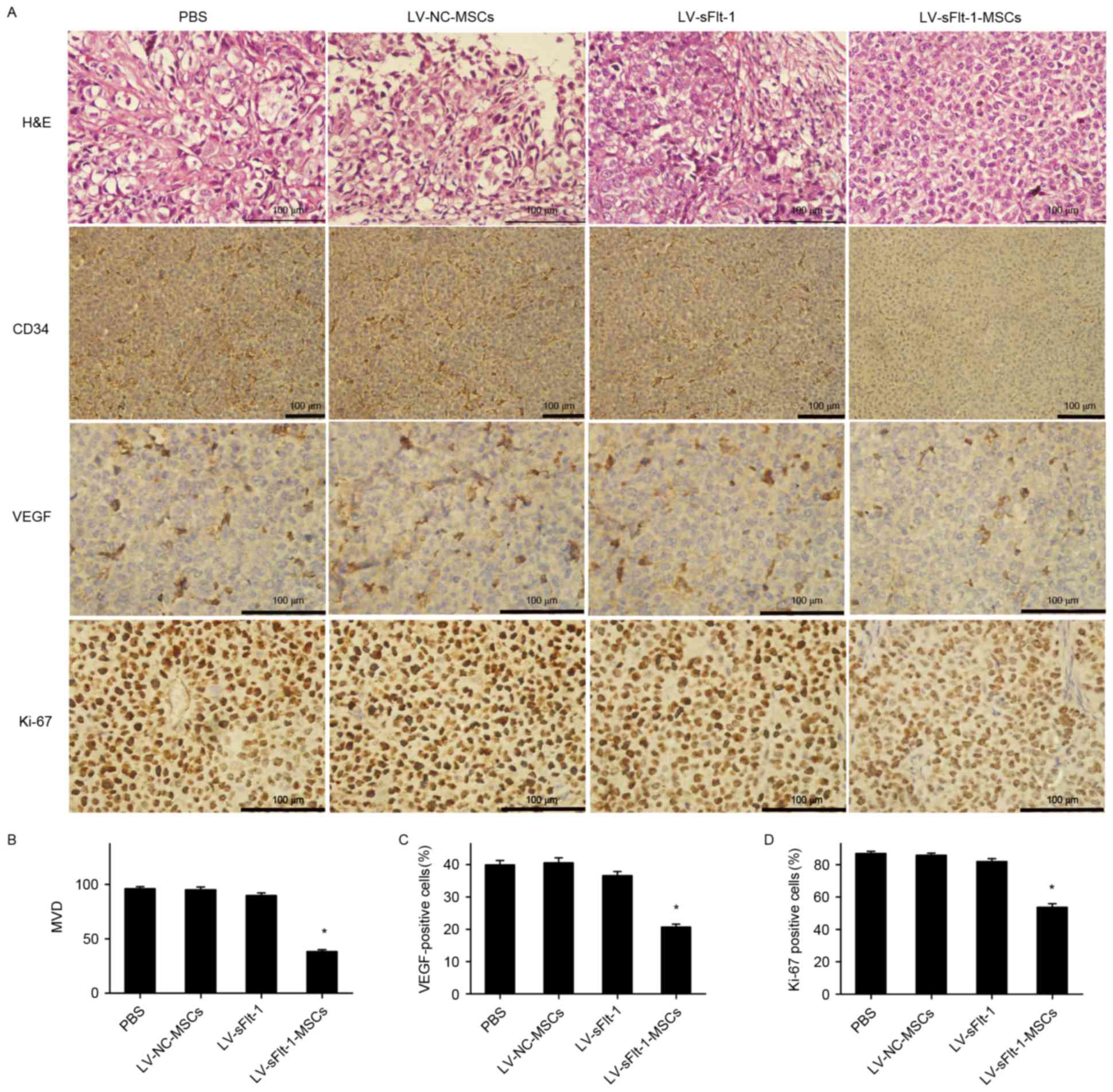 | Figure 4.Immunohistochemical analysis of CD34,
Ki-67 and VEGF expression. (A) Tumor sections were stained with
H&E (magnification, ×400), CD34 antibody (magnification, ×200),
Ki-67 antibody (magnification, ×400) and VEGF antibody
(magnification, ×400). Representative images are shown. (B) MVD was
analyzed by calculating the number of CD34-positive microvessels
from five randomly selected fields. (C) Protein expression of VEGF
was suppressed in the mice treated with LV-sFlt-1-MSCs compared
with the control group. (D) The proliferation index was determined
as the number of Ki-67-positive (brown) cells/the number of total
cells × 100 in five randomly selected fields. *P<0.05 vs. PBS.
CD34, cluster of differentiation 34; H&E, hematoxylin and
eosin; Ki-67, proliferation marker protein Ki-67; LV, lentivirus;
MSCs, mesenchymal stem cells; MVD, microvessel density; NC,
negative control; sFlt-1, soluble fms-like tyrosine kinase-1; VEGF,
vascular endothelial growth factor. |
VEGF serves a major role in angiogenesis. Previous
results suggested that sFlt-1 may inhibit tube formation and
angiogenesis (10), and the
present study evaluated the effects of sFlt-1 on VEGF expression by
immunohistochemical analysis. The ratio of VEGF-positive staining
cells to non-staining cells in the mice treated with LV-sFlt-1-MSCs
was significantly decreased compared with the ratio in the control
group treated with PBS (20.7±0.9 vs. 39.9±1.4%, P<0.05; Fig. 4C).
Effects of LV-sFlt-1-MSCs on cell
proliferation in HCC
To determine whether LV-sFlt-1-MSCs were able to
inhibit cell proliferation, the proliferation index, which was
measured by Ki-67 staining, was examined by immunohistochemical
analysis. The average proliferation index in five random fields was
53.7±2.3% in the group treated with LV-sFlt-1-MSCs, 86.9±1.3% in
the control group, 85.9±1.2% in the group treated with LV-NC-MSCs
and 81.9±1.9% in the group treated with LV-sFlt-1 (Fig. 4D). A significant decrease in the
proliferation rate was observed in the group treated with
LV-sFlt-1-MSCs compared with the control group (P<0.05).
Discussion
The present study demonstrated that MSCs engineered
to secrete sFlt-1 were able to secrete sFlt-1 efficiently and
inhibit tube formation of HUVECs in vitro. Additionally,
in vivo experiments indicated that, following intravenous
injection, LV-sFlt-1-MSCs migrated to tumor tissues and inhibited
tumor growth through anti-angiogenesis effects.
The weight of mice did not decrease following
systemic injection of LV-sFlt-1-MSCs, whereas the weight of mice in
the LV-sFlt-1 treatment group exhibited a transient decline. An
apparent advantage of MSCs as delivery vehicles is immune
privilege, which is a prerequisite for allogeneic treatment. The
immune privilege of MSCs may be attributed to decreased expression
of MHC class I surface markers and a lack of the co-stimulatory
molecules CD40, CD80 and CD86 (25). Therefore, MSCs modified by
therapeutic genes may not induce host immune response.
In the present investigation, MSCs were demonstrated
to migrate to tumor tissues rather than other organs, such as the
heart, spleen or brain. In addition, the intratumoral level of
sFlt-1 expression in the LV-sFlt-1-MSCs group was increased
compared with the LV-sFlt-1 group, whereas the plasma level of
sFlt-1 in the LV-sFlt-1-MSCs group was decreased. The results of
the present study further confirmed the tropism of MSCs towards
tumor sites. Despite previous studies that MSCs are able to migrate
to tumor sites (26–28), the molecular mechanisms underlying
that migration have not been completely elucidated. Several
previous studies have indicated that the tropism of MSCs may be
associated with a variety of growth factors and cytokines,
including hepatocyte growth factor, interleukin-6, monocyte
chemotactic protein-1 and stromal-derived growth factor-1 (29–31).
MSCs are thought to express chemokine receptors, including C-C
chemokine receptor type 2, C-X-C chemokine receptor type 4 and
hepatocyte growth factor receptor, which may be associated with the
migratory property of MSCs (31–33).
The possible chemotactic factors that may be involved merit further
investigation.
Solid tumor growth is primarily dependent on
angiogenesis. VEGF, a major angiogenic factor, has been identified
to stimulate endothelial cell proliferation, migration and tube
formation by interacting with VEGFR (34). VEGFR-1 is considered to be a decoy
that negatively regulates the activation of VEGFR-2 by binding to
intact VEGF (8). Targeting the
VEGF/VEGFR signaling pathway has been used in anti-angiogenesis
therapies (35,36). It has been reported that sFlt-1 may
suppress angiogenesis by forming inactive heterodimers with VEGFR-2
or sequestering free ligands (37). Consequently, sFlt-1 has been used
as a therapeutic agent for inhibiting angiogenesis (12,38).
In the present study, it was demonstrated that LV-sFlt-1-MSCs
suppressed tube formation of HUVECs in vitro. Spontaneous
metastasis rarely happens when an HCC subcutaneous model is
established (39). In previous
studies, researchers monitored the survival time of
subcutaneously-implanted tumor-bearing mice (40,41)
and revealed that subcutaneously-implanted tumor-bearing mice could
also die because of the tumor burden (42). Notably, MSCs engineered to secrete
sFlt-1 exhibited marked antitumor effects through the inhibition of
angiogenesis, and prolonged survival in an HCC mouse model.
In conclusion, the results of the present study
suggested that MSCs engineered to secrete sFlt-1 exerted potent
anti-angiogenic effects in HCC. The strategy for using MSCs as
delivery vehicles to treat HCC has numerous prospective
applications. In addition to sFlt-1, additional therapeutic agents
may also be delivered to treat HCC by modifying the MSCs.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of Fujian Province (grant no.
2014J01323) and The Science and Technology Project of Fujian
Province (grant no. 2014Y0057).
References
|
1
|
Fernández M, Semela D, Bruix J, Colle I,
Pinzani M and Bosch J: Angiogenesis in liver disease. J Hepatol.
50:604–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miura H, Miyazaki T, Kuroda M, Oka T,
Machinami R, Kodama T, Shibuya M, Makuuchi M, Yazaki Y and Ohnishi
S: Increased expression of vascular endothelial growth factor in
human hepatocellular carcinoma. J Hepatol. 27:854–861. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamaguchi R, Yano H, Iemura A, Ogasawara
S, Haramaki M and Kojiro M: Expression of vascular endothelial
growth factor in human hepatocellular carcinoma. Hepatology.
28:68–77. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Terman BI, Carrion ME, Kovacs E, Rasmussen
BA, Eddy RL and Shows TB: Identification of a new endothelial cell
growth factor receptor tyrosine kinase. Oncogene. 6:1677–1683.
1991.PubMed/NCBI
|
|
6
|
Pajusola K, Aprelikova O, Korhonen J,
Kaipainen A, Pertovaara L, Alitalo R and Alitalo K: FLT4 receptor
tyrosine kinase contains seven immunoglobulin-like loops and is
expressed in multiple human tissues and cell lines. Cancer Res.
52:5738–5743. 1992.PubMed/NCBI
|
|
7
|
Shibuya M: Structure and dual function of
vascular endothelial growth factor receptor-1 (Flt-1). Int J
Biochem Cell Biol. 33:409–420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shibuya M and Claesson-Welsh L: Signal
transduction by VEGF receptors in regulation of angiogenesis and
lymphangiogenesis. Exp Cell Res. 312:549–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kendall RL, Wang G and Thomas KA:
Identification of a natural soluble form of the vascular
endothelial growth factor receptor, FLT-1, and its
heterodimerization with KDR. Biochem Biophys Res Commun.
226:324–328. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koga J, Matoba T, Egashira K, Kubo M,
Miyagawa M, Iwata E, Sueishi K, Shibuya M and Sunagawa K: Soluble
Flt-1 gene transfer ameliorates neointima formation after wire
injury in flt-1 tyrosine kinase-deficient mice. Arterioscler Thromb
Vasc Biol. 29:458–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bagley RG, Kurtzberg L, Weber W, Nguyen
TH, Roth S, Krumbholz R, Yao M, Richards B, Zhang M, Pechan P, et
al: sFLT01: A novel fusion protein with antiangiogenic activity.
Mol Cancer Ther. 10:404–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krishnan B, Torti FM, Gallagher PE and
Tallant EA: Angiotensin-(1–7) reduces proliferation and
angiogenesis of human prostate cancer xenografts with a decrease in
angiogenic factors and an increase in sFlt-1. Prostate. 73:60–70.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Owen LA, Uehara H, Cahoon J, Huang W,
Simonis J and Ambati BK: Morpholino-mediated increase in soluble
Flt-1 expression results in decreased ocular and tumor
neovascularization. PLoS One. 7:e335762012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tipnis S, Viswanathan C and Majumdar AS:
Immunosuppressive properties of human umbilical cord-derived
mesenchymal stem cells: Role of B7-H1 and IDO. Immunol Cell Biol.
88:795–806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kidd S, Spaeth E, Dembinski JL, Dietrich
M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M and Marini FC:
Direct evidence of mesenchymal stem cell tropism for tumor and
wounding microenvironments using in vivo bioluminescent imaging.
Stem Cells. 27:2614–2623. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sasportas LS, Kasmieh R, Wakimoto H,
Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza
RL, Weissleder R and Shah K: Assessment of therapeutic efficacy and
fate of engineered human mesenchymal stem cells for cancer therapy.
Proc Natl Acad Sci USA. 106:4822–4827. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loebinger MR, Kyrtatos PG, Turmaine M,
Price AN, Pankhurst Q, Lythgoe MF and Janes SM: Magnetic resonance
imaging of mesenchymal stem cells homing to pulmonary metastases
using biocompatible magnetic nanoparticles. Cancer Res.
69:8862–8867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren C, Kumar S, Chanda D, Chen J, Mountz
JD and Ponnazhagan S: Therapeutic potential of mesenchymal stem
cells producing IFN-alpha in a mouse melanoma lung metastasis
model. Stem Cells. 26:2332–2338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura K, Ito Y, Kawano Y, Kurozumi K,
Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y and Hamada H:
Antitumor effect of genetically engineered mesenchymal stem cells
in a rat glioma model. Gene Ther. 11:1155–1164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Y, Yao A, Zhang W, Lu S, Yu Y, Deng L,
Yin A, Xia Y, Sun B and Wang X: Human mesenchymal stem cells
overexpressing pigment epithelium-derived factor inhibit
hepatocellular carcinoma in nude mice. Oncogene. 29:2784–2794.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Lu Y, Huang W, Xu H, Chen X, Geng Q,
Fan H, Tan Y, Xue G and Jiang X: In vitro effect of
adenovirus-mediated human gamma interferon gene transfer into human
mesenchymal stem cells for chronic myelogenous leukemia. Hematol
Oncol. 24:151–158. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Q, Cheng P, Yin T, He H, Yang L, Wei
Y and Chen X: Therapeutic potential of bone marrow-derived
mesenchymal stem cells producing pigment epithelium-derived factor
in lung carcinoma. Int J Mol Med. 30:527–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng Y, Opeskin K, Goad J and Williams ED:
Tumor-induced activation of lymphatic endothelial cells via
vascular endothelial growth factor receptor-2 is critical for
prostate cancer lymphatic metastasis. Cancer Res. 66:9566–9575.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kidd S, Spaeth E, Klopp A, Andreeff M,
Hall B and Marini FC: The (in) auspicious role of mesenchymal
stromal cells in cancer: Be it friend or foe. Cytotherapy.
10:657–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SM, Oh JH, Park SA, Ryu CH, Lim JY,
Kim DS, Chang JW, Oh W and Jeun SS: Irradiation enhances the tumor
tropism and therapeutic potential of tumor necrosis factor-related
apoptosis-inducing ligand-secreting human umbilical cord
blood-derived mesenchymal stem cells in glioma therapy. Stem Cells.
28:2217–2228. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou W, Zheng H, He TC, Chang J, Fu YX and
Fan W: LIGHT delivery to tumors by mesenchymal stem cells mobilizes
an effective antitumor immune response. Cancer Res. 72:2980–2989.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui
KM and Lam PY: Human bone marrow-derived mesenchymal stem cells
suppress human glioma growth through inhibition of angiogenesis.
Stem Cells. 31:146–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wels J, Kaplan RN, Rafii S and Lyden D:
Migratory neighbors and distant invaders: Tumor-associated niche
cells. Genes Dev. 22:559–574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Studeny M, Marini FC, Dembinski JL,
Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE and Andreeff
M: Mesenchymal stem cells: Potential precursors for tumor stroma
and targeted-delivery vehicles for anticancer agents. J Natl Cancer
Inst. 96:1593–1603. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Son BR, Marquez-Curtis LA, Kucia M,
Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ and
Janowska-Wieczorek A: Migration of bone marrow and cord blood
mesenchymal stem cells in vitro is regulated by stromal-derived
factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves
matrix metalloproteinases. Stem Cells. 24:1254–1264. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song C and Li G: CXCR4 and matrix
metalloproteinase-2 are involved in mesenchymal stromal cell homing
and engraftment to tumors. Cytotherapy. 13:549–561. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klopp AH, Spaeth EL, Dembinski JL,
Woodward WA, Munshi A, Meyn RE, Cox JD, Andreeff M and Marini FC:
Tumor irradiation increases the recruitment of circulating
mesenchymal stem cells into the tumor microenvironment. Cancer Res.
67:11687–11695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shibuya M: Vascular endothelial growth
factor and its receptor system: Physiological functions in
angiogenesis and pathological roles in various diseases. J Biochem.
153:13–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeng Z, Huang WD, Gao Q, Su ML, Yang YF,
Liu ZC and Zhu BH: Arnebin-1 promotes angiogenesis by inducing
eNOS, VEGF and HIF-1α expression through the PI3K-dependent
pathway. Int J Mol Med. 36:685–697. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SL, Lee ST, Trang KT, Kim SH, Kim IH,
Lee SO, Kim DG and Kim SW: Parthenolide exerts inhibitory effects
on angiogenesis through the downregulation of VEGF/VEGFRs in
colorectal cancer. Int J Mol Med. 33:1261–1267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takei Y, Mizukami H, Saga Y, Yoshimura I,
Hasumi Y, Takayama T, Kohno T, Matsushita T, Okada T, Kume A, et
al: Suppression of ovarian cancer by muscle-mediated expression of
soluble VEGFR-1/Flt-1 using adeno-associated virus serotype
1-derived vector. Int J Cancer. 120:278–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Justiniano SE, Elavazhagan S, Fatehchand
K, Shah P, Mehta P, Roda JM, Mo X, Cheney C, Hertlein E, Eubank TD,
et al: Fcγ receptor-induced soluble vascular endothelial growth
factor receptor-1 (VEGFR-1) production inhibits angiogenesis and
enhances efficacy of antitumor antibodies. J Biol Chem.
288:26800–26809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Killion JJ, Radinsky R and Fidler IJ:
Orthotopic models are necessary to predict therapy of
transplantable tumors in mice. Cancer Metastasis Rev. 17:279–284.
1999. View Article : Google Scholar
|
|
40
|
Wang J, Xu L, Zeng W, Hu P, Zeng M, Rabkin
SD and Liu R: Treatment of human hepatocellular carcinoma by the
oncolytic herpes simplex virus G47delta. Cancer Cell Int.
14:832014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schmitz V, Raskopf E, Gonzalez-Carmona MA,
Vogt A, Rabe C, Leifeld L, Kornek M, Sauerbruch T and Caselmann W:
Plasminogen fragment K1-5 improves survival in a murine
hepatocellular carcinoma model. Gut. 56:271–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu LM, Shi DM, Dai Q, Cheng XJ, Yao WY,
Sun PH, Ding Y, Qiao MM, Wu YL, Jiang SH and Tu SP: Tumor
suppressor XAF1 induces apoptosis, inhibits angiogenesis and
inhibits tumor growth in hepatocellular carcinoma. Oncotarget.
5:5403–5415. 2014. View Article : Google Scholar : PubMed/NCBI
|