Introduction
Central nervous system (CNS) ischemic injury is a
major cause of morbidity and mortality. Reperfusion injury is
caused by the recovery of blood supply to the tissues following a
period of ischemia. The shortage of blood supply creates a
condition of nutrient and oxygen insufficiency, and the restoration
of blood circulation results in oxidative damage in the tissues
(1). Astrocytes are glial cells in
the CNS and serve important roles in numerous CNS functions,
including neuronal regeneration, apoptosis regulation, blood-brain
barrier (BBB) maintenance and extracellular ion homeostasis
(2).
Edema is among the most prominent features in spinal
cord injury (SCI) and has been attributed to astrocyte dysfunction
(3). Under conditions of cellular
edema, astrocytes have been reported to fail to exert their
physiological functions to support neuronal activity (4). Following SCI in humans, the recovery
process has been demonstrated to involve the rapid transition of
astrocytes from an aquaporin-4 (AQP4)-positive to an AQP4-negative
state, which has been associated with the development of spinal
cord edema (5). AQP4 is expressed
by astrocytic end-processes that encircle microvessels along the
BBB and the brain-cerebrospinal fluid interface (6). AQP4 is a water channel that is
functionally enriched on astrocytic end-processes at the
perivascular space and the subpial surface of the glia limitans
(7). AQP4 selectively conducts
water molecules in and out of astrocytes during edema formation and
resolution, while preventing the passage of ions and other solutes
(8). Enhanced AQP4 expression has
been associated with increased CNS water contents and the
development of edema following ischemia (9). However, contradictory reports have
demonstrated that AQP4 mitigates the damage and suppresses the
retrograde degeneration of rubrospinal neurons, via promoting the
resolution of edema following SCI (10). AQP4 has also been reported to
facilitate the clearance of excess water following
contusion-induced SCI (11). The
regulation of AQP4 expression is critical for tissue water
transport during repair processes in the CNS following ischemic
injury, in the maintenance of the BBB and in the release and uptake
of neurotransmitters (12).
Methylprednisolone sodium succinate (MPSS) has been
used as a treatment for acute SCI (13); however, high doses of MPSS have
been associated with deleterious adverse effects (14), such as enhancing the avascular
necrosis of nerve fibers (15).
The use of MPSS in the clinical treatment of acute SCI was prompted
by its protective effects on neurological damage (16). However, high doses of MPSS have
been associated with adverse effects, including infections, sepsis,
respiratory complications and an increase in mortality (17). Reduction of the administered MPSS
dose, or its substitution with alternative neuroprotective agents,
may have potential as strategies to avoid the adverse effects of
MPSS during the treatment of SCI (18).
Ischemia-reperfusion (IR) injury causes edema that
further limits the blood supply to the tissues and results in
hypoxia (19). Under hypoxic
conditions, the synthesis of erythropoietin (EPO) by interstitial
cells of the renal cortex is potentiated. EPO has been reported to
exert neuroprotective effects against SCI (20); however, EPO has been associated
with adverse effects, such as increases in hematocrit and blood
viscosity, and thrombosis. EPO has been identified as a novel
neuroprotective agent against neurological injury (21). EPO and its receptor (EPO-R) are
expressed throughout the CNS, including astrocytes, and have been
reported to exert neuroprotection in cell lines and animal models
of CNS disorders (22). Previous
studies demonstrated that intravenous administration of EPO within
8 h following non-hemorrhagic cerebral infarction significantly
improved prognosis in rats (23).
Notably, Gorio et al (24)
suggested that MPSS combined with EPO may suppress the protective
effects of EPO against neuronal injury, via blocking the EPO-R,
increasing vascular EPO clearance and inhibiting the EPO-mediated
synthesis of neurotrophic factors. However, contradictory reports
demonstrated that combination treatment with EPO and MPSS following
nerve injury potentiated the recovery of neurological function and
improved tissue pathology (25).
The present study aimed to investigate the effects of the
combination of EPO with MPSS in the treatment of SCI, via assessing
their effects on AQP4 expression in astrocytes following ischemia
and reperfusion in vitro.
Materials and methods
Primary astrocyte culture
The present study obtained ethical approval by the
Soochow University Ethics Committee (Suzhou, China). Newborn female
Sprague-Dawley rats (age, 1–3 days) were purchased from the
Laboratory Animal Center of Soochow University and housed under
controlled conditions of temperature (22°C), humidity, and light
(light/dark cycle, 14/10 h). Rats were exclusively breastfed until
treatment. Postnatal day (P) 3 female Sprague-Dawley rats were
sacrificed by decapitation. The hypothalamus was isolated, minced
and washed with PBS to remove the meninges and residual blood, and
then digested and dissociated using 0.25% trypsin for 3 min (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a 37°C water
bath. Dissociated cells were filtered through a 30-µm nylon mesh,
and then seeded onto poly-L-lysine (10 µg/ml; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) pretreated 75-cm2 culture
dishes. Cells were cultured in Dulbecco's modified Eagle's
medium/Nutrient Mixture F-12 (DMEM/F12; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.) and
maintained at 37°C in a 5% CO2 incubator. The culture
medium was replaced every 2–3 days, until the cells achieved the
desired confluence following ~2 weeks in culture, as previously
described (26). Microglial cells
and oligodendrocyte progenitors were dissociated following
agitation in a 37°C incubator at 260 rpm for 2 h and at 200 rpm
overnight. The microglial cells and oligodendrocytes grow on the
surface of the astrocytes and were easier to detach by agitation;
the astrocytes remained attached to the plate. Cells of the 3rd
passage were prepared for testing and cells were purified after 2–3
passages.
Characterization of astrocytes
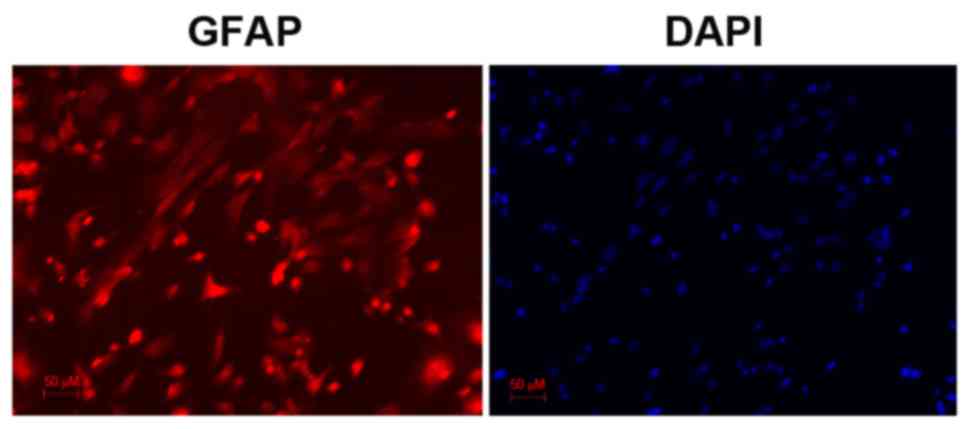
Astrocytes were identified by immunocytochemical
examination using anti-glial fibrillary acidic protein (GFAP)
antibody, which revealed obvious astrocytic morphology. Cell nuclei
were counterstained with DAPI. Astrocyte purity was assessed based
on the % of GFAP-positive cells to DAPI-positive cells and was
revealed to be >95%.
Briefly, cells were seeded (1–5×106
cells/ml) into 6-well plates, fixed with 4% paraformaldehyde at
22°C for 20 min, permeabilized with 0.01% Triton X-100 and blocked
in 5% goat serum (G9023, Sigma-Aldrich; Merck KGaA) for 2 h at 4°C.
Cells were then incubated with rat anti-GFAP primary antibody
overnight at 4°C (1:1,000; sc-9065; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), and with sheep anti-rat Cyanine 3-conjugated
secondary antibody [1:100; D111042; Sangon Biotech (Shanghai) Co.,
Ltd., Shanghai, China] at 24°C for 1 h. Cells were counterstained
with the fluorescent dye DAPI for 15 min, and then dehydrated by
graded concentrations of ethanol. Stained cells were observed under
an epifluorescence microscope (Olympus Corporation, Tokyo, Japan)
and photomicrographs were captured. Three randomly selected fields
of view/slide were used for cell counting by Image Pro Plus 6.0
(National Institutes of Health, Bethesda, MD, USA).
IR injury
IR injury includes two phases: An acute phase of
ischemic injury, and a delayed phase of reperfusion injury. To
simulate ischemic conditions, primary astrocyte cultures were
transiently deprived of oxygen and glucose. The medium was replaced
with oxygen-depleted, glucose-, serum- and phenol red-free DMEM
(Gibco; Thermo Fisher Scientific, Inc.) and the cells were
incubated in a 92% N2, 5% CO2 and 1%
O2 incubator at 37°C. Following 4 h of oxygen and
glucose deprivation, reperfusion was simulated. The medium was
restored to DMEM/F12 supplemented with 10% FBS and cells were
cultured in a normoxic incubator (5% CO2) at 37°C, as
previously described (27). Normal
cells were not subjected to oxygen and glucose deprivation and were
maintained in a normoxic incubator at 37°C.
Experimental groups
The following treatment groups were used in the
present study: Normal group, untreated astrocytes maintained in
normoxic conditions; Control group, cultured astrocytes that were
subjected to 4 h of oxygen and glucose deprivation, and
subsequently cultured in regular medium under normoxic conditions;
EPO group, cultured astrocytes that were subjected to 4 h of oxygen
and glucose deprivation, followed by reperfusion in the presence of
10 U/ml EPO (Kirin brewery Company, Ltd., Tokyo, Japan); MPSS
group, cultured astrocytes that were subjected to 4 h of oxygen and
glucose deprivation, followed by reperfusion in the presence of 10
µg/ml MPSS (Pfizer Inc., New York, NY, USA); EPO + MPSS group,
cultured astrocytes that were subjected to 4 h of oxygen and
glucose deprivation, followed by reperfusion in the presence of 10
U/ml EPO and 10 µg/ml MPSS.
MTT assay
The MTT assay was used to determine the viability of
astrocytes according to the measured absorbance or optical density
(OD) value. Cells were seeded (5×104 cells/ml) into
96-well plates in culture medium (200 µl/well). A total of 20 µl
MTT solution was added to each well and cells were incubated for an
additional 4 h. Subsequently, 150 µl dimethyl sulfoxide were added
to each well and plates were agitated gently for 10 min to fully
solubilize the formazan crystals. The absorbance of each well was
measured at 490 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
concentration and purity of RNA was evaluated by spectrometry at
260 and 280 nm. Total RNA was reverse transcribed into cDNA using a
SYBR Green Real-Time PCR Master Mix (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The amplification
conditions were as follows: AQP4, 45 sec at 94°C followed by 30
cycles of 30 sec at 94°C, 30 sec at 52°C and 60 sec at 72°C;
β-actin, 45 sec at 94°C followed by 30 cycles of 30 sec at 94°C, 30
sec at 58°C and 60 sec at 72°C. Primers sequences, designed by
Primer-Express V3.0 (Thermo Fisher Scientific, Inc.), were as
follows: AQP4 forward, 5′-TTGGACCAATCATAGGCGC-3′ and reverse,
5′-GGTCAATGTCGATCACATGC-3′ (product, 213 bp); β-actin forward,
5′-GAGAGGGAAATCGTGCGTGAC-3′ and reverse, 5′-CATCTGCTGGAAGGTGGACA-3′
(product, 457 bp). The experiments were performed in triplicate and
the comparative Cq method (28)
was used to perform relative quantification. Differential
expression was calculated by analysis of the target gene
amplification following normalization to the rat β-actin endogenous
reference.
Western blot analysis
Cells from the various treatment groups were
collected and lysed using Western/IP Cell Lysis Buffer (Beyotime
Institute of Biotechnology, Haimen, China) for 1 h at 0°C. Protein
concentration was measured by bicinchoninic acid assay. Equal
amounts of extracted protein samples (50 µg) were separated by 10%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
The membranes were blocked with 2% skimmed milk and TBS-0.1%
Tween-20 (TBST). Primary polyclonal antibodies against AQP4
(sc-9888; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) diluted
(1:500) in TBST were used. Membranes were incubated with the
primary antibodies at 4°C overnight, or with anti-GAPDH [1:1,000;
AB10016; Sangon Biotech (Shanghai) Co., Ltd] at 4°C for 2 h. Blots
were subsequently incubated with horseradish peroxidase-conjugated
rabbit anti-goat IgG secondary antibodies [1:1,000; D111047; Sangon
(Shanghai) Biotech Co., Ltd.] for 2 h at 4°C. Protein bands were
visualized on X-ray film using enhanced chemiluminescence (luminol
chemiluminescence substrate, SF-2; Jiangsu Sunlant Bioengineering
Co., Ltd.). Developed films were digitized and semi-quantified by
densitometry using a gel imaging system (Smartview-2001 Version 5;
Shanghai Furi Science & Technology Co., Ltd., Shanghai,
China).
Statistical analysis
Data are expressed the mean ± standard deviation.
Statistical analysis was performed with one-way analysis of
variance and the Student-Newman-Keuls post hoc test were performed
using SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Astrocyte characterization
Astrocytes of the third passage were observed by
microscopy. Astrocytes exhibited ‘vascular processes‘, which
connected them to the outer surface of the capillary walls in close
proximity. Astrocytic processes were revealed to envelope neuronal
synapses. Individual synapses were viewed as slender structures
that were interwoven into a mesh. Astrocytes were demonstrated to
extend processes in order to form the glial limiting membrane.
Following immunostaining for GFAP, an astrocyte marker, cells
exhibited a characteristic astrocytic morphology and GFAP-positive
staining (Fig. 1). DAPI was used
to counterstain the nuclei of living cells (Fig. 1). The purity of the cultures was
calculated as the % of GFAP-positive cells over the total number of
cells (based on DAPI staining). Astrocytes were revealed to amount
for ~95% of the culture.
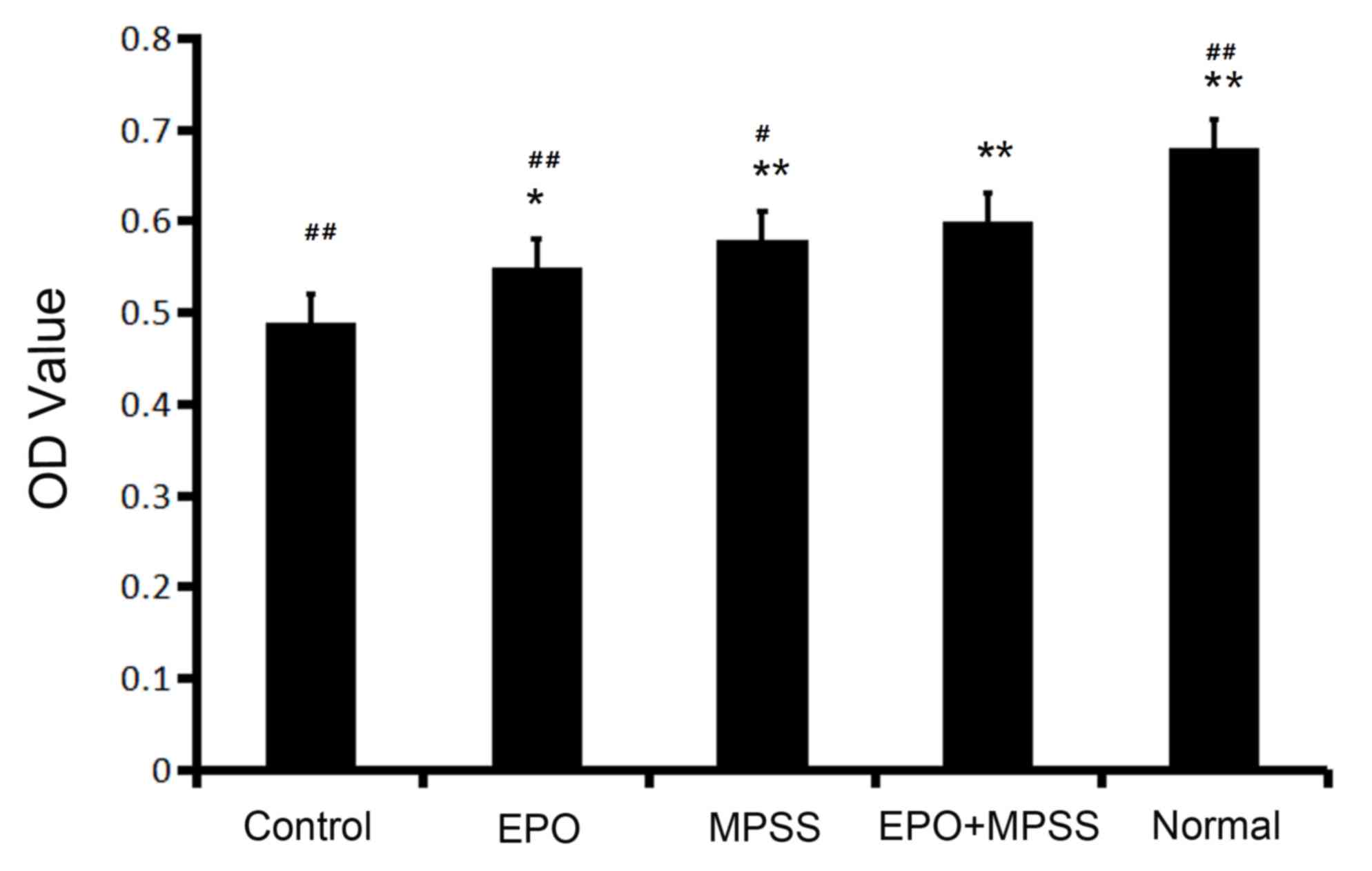
Astrocyte viability
The cell viability of astrocytes in the five
experimental groups tested in the present study was evaluated by
MTT assay. The total numbers of viable cells were significantly
increased in all the drug intervention groups (Fig. 2). The results demonstrated that
treatment with EPO, MPSS or their combination significantly
promoted viability of astrocytes compared with the control
(Fig. 2). Of note, the cell
viability of the EPO + MPSS group, was significantly increased
compared with EPO or MPSS groups alone (Fig. 2).
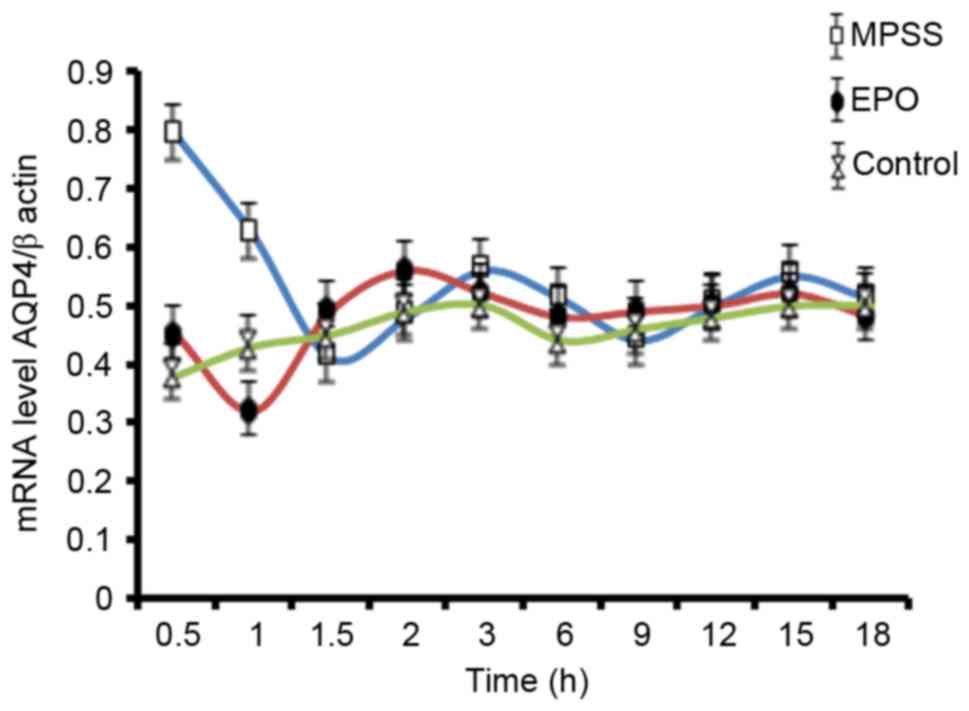
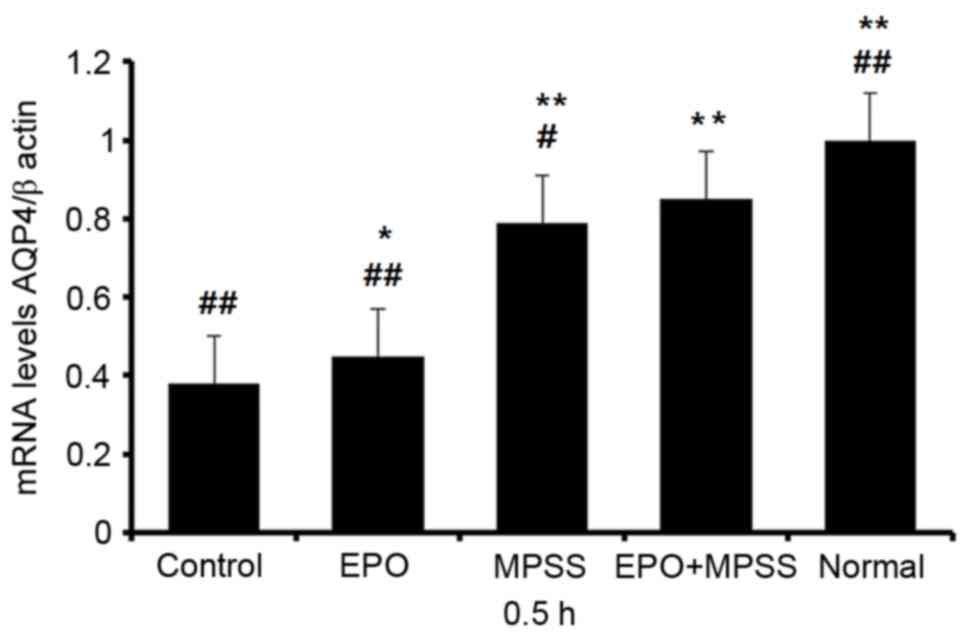
AQP4 mRNA expression levels
To analyze the changes of AQP4 gene expression as a
function of time, RT-qPCR analysis was performed. A time effect
curve was constructed from results up to 18 h following reperfusion
(Fig. 3). AQP4 mRNA levels had the
most obvious changes at 0.5 h following reperfusion, and therefore
this time point was chosen for RT-qPCR analysis of all the five
experimental groups (Fig. 4).
Notably, AQP4 expression following EPO or MPSS intervention was
significantly upregulated at 0.5 h, compared with the control group
(Fig. 4). In addition, the EPO +
MPSS group had significantly higher AQP4 mRNA expression levels
compared with the control group, and compared with either
intervention alone (Fig. 4),
indicating a synergistic action of the two drugs in upregulating
AQP4 expression.
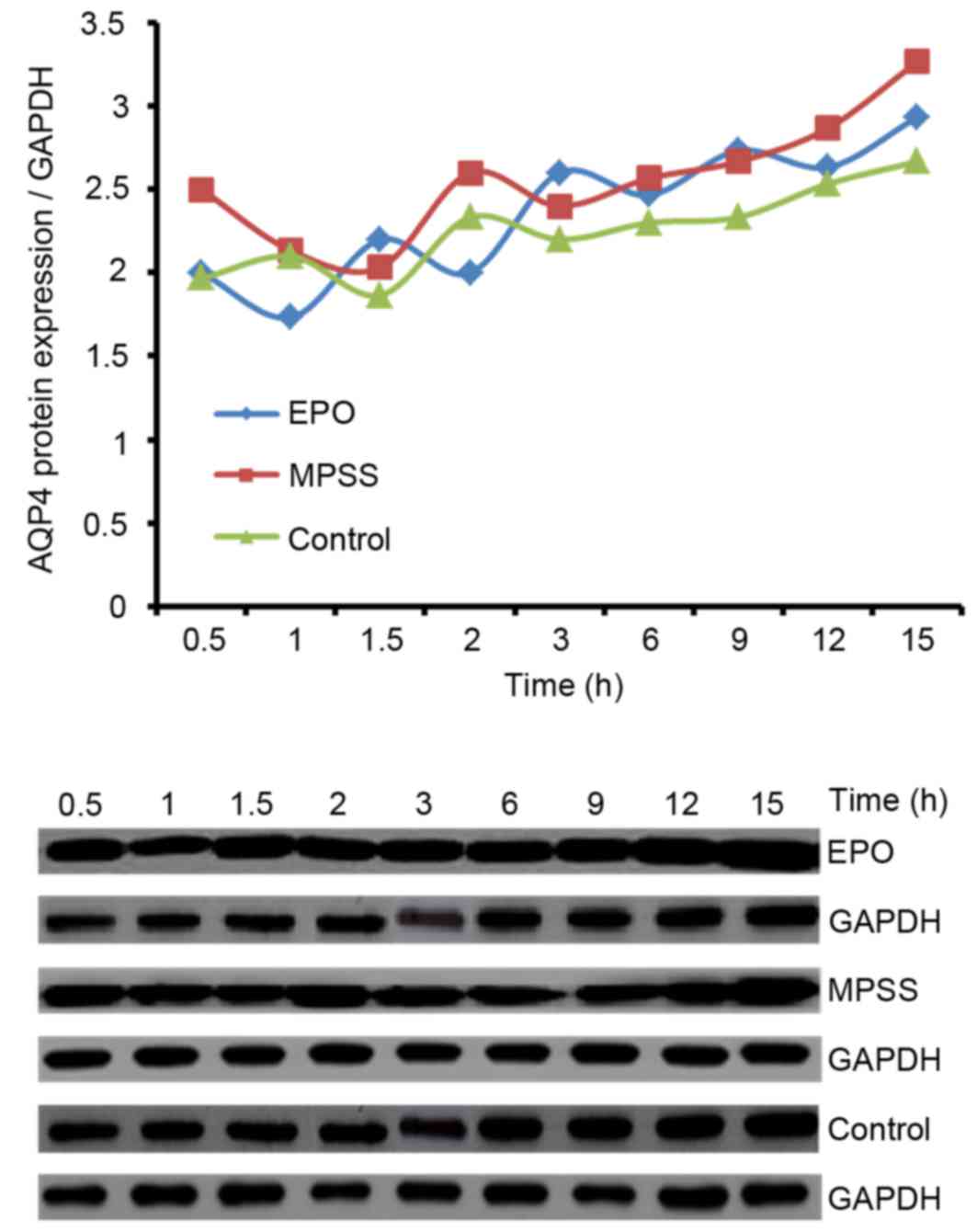
AQP4 protein expression levels
Western blot analysis was used in order to examine
the time effect curve of AQP4 protein expression levels for 15 h
following reperfusion. Levels of AQP4 protein were upregulated
beginning at 12 h following reperfusion with drug interventions
(Fig. 5). Thus, the time of 12 h
after drug intervention was selected for further analysis of the
five experimental groups (Fig. 6).
Compared with the normal group, astrocyte AQP4 protein expression
levels in the control group decreased significantly following
oxygen and glucose deprivation (Fig.
6). Following reperfusion, AQP4 protein levels increased
significantly by treatment with EPO, MPSS or their combination,
compared with control (Fig. 6).
Notably, the AQP4 protein levels were significantly higher in the
EPO + MPSS group compared with either drug alone (Fig. 6), confirming a synergistic action
between EPO and MPSS in the regulation of AQP4 expression.
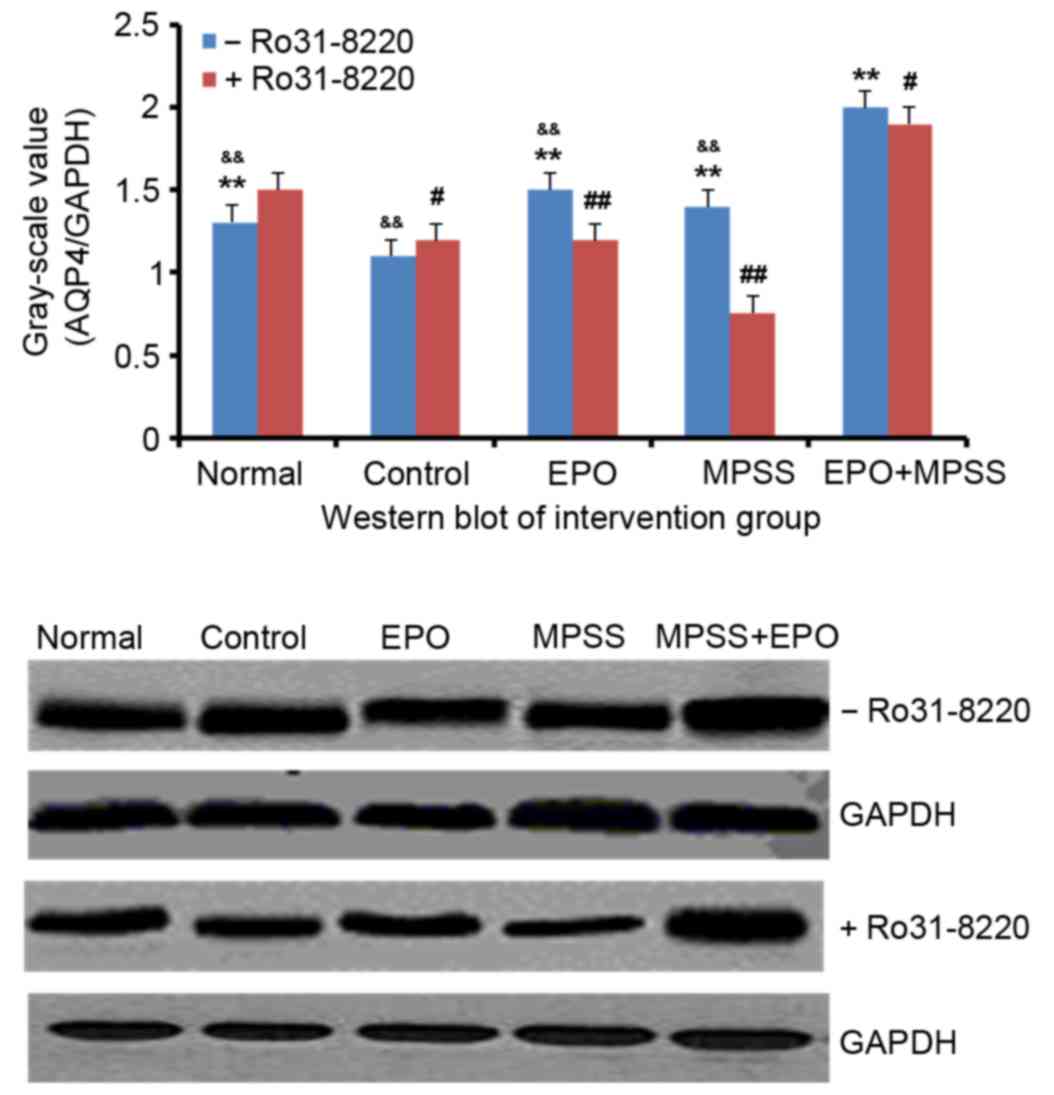
Ro 31–8220 inhibits the EPO and
MPSS-mediated AQP4 upregulation
Cultured astrocytes were subjected to oxygen and
glucose deprivation for 4 h, and then pretreated with the protein
kinase C (PKC) inhibitor Ro 31–8220 (100 nM, Medchemexpress) for 10
min followed by reperfusion. Western blot analysis demonstrated
that the protein expression levels of AQP4 were significantly
decreased in the control compared with the normal group (P<0.05;
Fig. 6), and PKC inhibition had no
effect in the control group. However, in the EPO and the MPSS
groups, Ro 31–8220 pretreatment significantly decreased the AQP4
protein expression levels, compared with cells treated with EPO or
MPSS alone (P<0.01; Fig. 6).
Finally, the EPO + MPSS group exhibited the highest protein
expression levels for AQP4 following reperfusion, and PKC
inhibition partially but significantly inhibited this AQP4
upregulation (P<0.05; Fig.
6).
Discussion
In the present study, primary astrocyte cultures
were established following the isolation of astrocytes from the rat
cerebral cortex; P3 rats were used, as the yield of viable neurons
in cell suspension is the highest at this stage. Astrocytes
isolated from female rats have been reported to be more resistant
to oxygen and glucose deprivation compared with male astrocytes
(29). In the present study,
astrocytes were obtained from P3 female rats and were subjected to
oxygen and glucose deprivation followed by reperfusion, in
accordance with a widely-used model to imitate ischemic conditions
in vitro (30).
In the present study, EPO and MPSS in combination
attenuated the ischemia-induced downregulation of AQP4 expression,
and were revealed to be more effective compared with the
administration of EPO or MPSS therapy alone. The mRNA expression
levels of AQP4 were significantly downregulated in astrocytes
following ischemia, thus suggesting that the decreased expression
of AQP4 may limit water influx so as to maintain the physiological
morphology and preserve the viability of astrocytes under ischemic
conditions.
In the early stages of cytotoxic edema, AQP4
expression is rapidly increased, leading to water influx following
reperfusion, and the development of cytotoxic edema. The
upregulation in AQP4 expression causes excess water influx into the
cells, thus resulting in cell swelling and elevated pressure
(31). In the present study, the
results demonstrated that in the initial 30 min of reperfusion, the
oxygen and glucose deprivation-mediated downregulation in AQP4
expression was inhibited by EPO or MPSS administration. In
addition, the mRNA and protein expression levels of AQP4 in the EPO
+ MPSS group were significantly higher compared with the control
group. The present results were in accordance with previous animal
studies using experimental models of SCI, which reported that when
EPO was administered 30 min post-injury, it could improve the
neurological outcome (32).
The upregulation of AQP4 expression has been
observed in hypoxic-ischemic neonatal rats following treatment with
EPO (33). Conversely, EPO was
demonstrated to significantly attenuate the upregulation in AQP4
expression induced by oxygen-glucose deprivation followed by
reoxygenation (34). The
protective effects of EPO in astrocytes may be mediated through the
activation of c-Jun N-terminal kinase, mitogen-activated protein
kinase and phosphatidylinositol-4,5-bisphosphate 3-kinase/Akt
signaling pathways (35,36). EPO treatment has been demonstrated
to preserve the neurological function and to attenuate neuronal
cell damage in a mouse model of spinal cord IR injury (37), and it has been reported to
significantly suppress edema formation and prevent neurological
injury following spinal cord ischemia in rats (38). Furthermore, EPO administration has
been reported to protect the integrity of the BBB, and its
beneficial effects during BBB disruption have been associated with
increased levels of AQP4 (39–41).
In the present study, the mRNA expression levels of
AQP4 were significantly increased following treatment with MPSS
compared with the control group. EPO inhibited the rapid increase
in AQP4 expression more potently compared with MPSS. By contrast,
MPSS inhibited the rapid decrease more effectively than did EPO.
Previous studies have reported that the inhibition of the initial
upregulation of AQP4 prevents the development of cellular edema
(42). The AQP4 mRNA level is
biphasic following reperfusion. Triggered by SCI, an early
upregulation of AQP4 expression has been reported, suggested to
facilitate edema formation, followed by a later downregulation
after the onset of edema (43).
The present study demonstrated that EPO and MPSS displayed
protection within 30 min.
PKC has been reported to regulate AQP4 protein
expression, and PKC activation by EPO can alter plasma membrane
water permeability (34). Low
doses of MPSS have been demonstrated to elicit a biological
response through the activation of the same signaling pathway
(44). The activation of PKC
causes a decrease in AQP4 expression, thus reducing cellular water
permeability and alleviating ischemia-induced swelling (34). In the present study, in order to
investigate the roles of PKC in the mechanisms underlying the
effects of EPO and MPSS on IR injury, astrocytes were pretreated
with the PKC inhibitor Ro 31–8220 prior to reperfusion. The present
results revealed that PKC inhibition partly counteracted the
effects of EPO on AQP4 expression following ischemia. Notably, Ro
31–8220 significantly inhibited the MPSS-induced AQP4 upregulation,
thus suggesting that EPO and MPSS may act through a common
mechanism implicating the PKC pathway. However, it is possible that
other pathways may also be implicated in the regulation of osmotic
pressure in astrocytes, and further studies are required to fully
elucidate the molecular mechanisms of EPO and MPSS action.
The results of the present study suggested that the
combination of EPO with MPSS may have a therapeutic effect on
traumatic SCI. EPO and MPSS alone and in combination significantly
upregulated the mRNA and protein expression of AQP4 in astrocytes,
which was decreased following oxygen and glucose deprivation. In
addition, MPSS alone and in combination with EPO enhanced the
viability of astrocytes following IR. The effects of EPO and MPSS
on AQP4 protein expression were mitigated by blocking PKC
signaling, thus suggesting that EPO and MPSS may modulate the
expression of AQP4 through PKC-dependent pathways.
The concentration of EPO in the brain is closely
related to hypoxia (45). In the
present study, western blot analysis demonstrated that treatment
with EPO or MPSS alone or in combination following ischemia,
resulted in significantly enhanced AQP4 protein expression 12 h
following reperfusion. These results suggested that EPO and MPSS
may modulate water transport across the cell membrane, through the
regulation of AQP4 expression, and thus prevent the onset of edema.
Furthermore, EPO in combination with MPSS was revealed to protect
cortical astrocytes against IR injury, as suggested by the results
of the MTT assay, which indicated an increase in cell
viability.
The present study demonstrated that routine
recommended clinical doses had a clear effect on protein expression
of AQP4. The findings suggested that combination therapy of IR
injury with EPO and MPSS may have potential as a novel approach to
reduce the administered doses of MPSS, and thus prevent the
appearance of adverse effects. However, further studies are
required to investigate the efficacy and safety of the EPO + MPSS
combination therapy in eliminating edema following SCI.
Acknowledgements
The present study was supported by the Medical
Science and Technology Development Foundation of the Jiangsu
Province Department of Health (grant no. H200718), and the Suzhou
City Social Development Fund (grant no. SSY0625).
References
|
1
|
Foreman B: The pathophysiology of delayed
cerebral ischemia. J Clin Neurophysiol. 33:174–182. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perez-Alvarez A, Navarrete M, Covelo A,
Martin ED and Araque A: Structural and functional plasticity of
astrocyte processes and dendritic spine interactions. J Neurosci.
34:12738–12744. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heuser K, Szokol K and Taubøll E: The role
of glial cells in epilepsy. Tidsskr Nor Laegeforen. 134:37–41.
2014.(In English, Norwegian). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nesic O, Guest JD, Zivadinovic D, Narayana
PA, Herrera JJ, Grill RJ, Mokkapati VU, Gelman BB and Lee J:
Aquaporins in spinal cord injury: The janus face of aquaporin 4.
Neuroscience. 168:1019–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stokum JA, Kurland DB, Gerzanich V and
Simard JM: Mechanisms of astrocyte-mediated cerebral edema.
Neurochem Res. 40:317–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clasadonte J, Dong J, Hines DJ and Haydon
PG: Astrocyte control of synaptic NMDA receptors contributes to the
progressive development of temporal lobe epilepsy. Proc Natl Acad
Sci USA. 110:17540–17545. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papadopoulos MC and Verkman AS: Aquaporin
water channels in the nervous system. Nat Rev Neurosci. 14:265–277.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rajkowska G, Hughes J, Stockmeier CA,
Miguel-Hidalgo J Javier and Maciag D: Coverage of blood vessels by
astrocytic endfeet is reduced in major depressive disorder. Biol
Psychiatry. 73:613–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mao L, Wang HD, Pan H and Qiao L:
Sulphoraphane enhances aquaporin-4 expression and decreases spinal
cord oedema following spinal cord injury. Brain Inj. 25:300–306.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Q, Zhang YJ, Gao JY, Li XM, Kong H,
Zhang YP, Xiao M, Shields CB and Hu G: Aquaporin-4 mitigates
retrograde degeneration of rubrospinal neurons by facilitating
edema clearance and glial scar formation after spinal cord injury
in mice. Mol Neurobiol. 49:1327–1337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimura A, Hsu M, Seldin M, Verkman AS,
Scharfman HE and Binder DK: Protective role of aquaporin-4 water
channels after contusion spinal cord injury. Ann Neurol.
67:794–801. 2010.PubMed/NCBI
|
|
12
|
Genc S, Zadeoglulari Z, Oner MG, Genc K
and Digicaylioglu M: Intranasal erythropoietin therapy in nervous
system disorders. Expert Opin Drug Deliv. 8:19–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Evaniew N, Noonan VK, Fallah N, Kwon BK,
Rivers CS, Ahn H, Bailey CS, Christie SD, Fourney DR, Hurlbert RJ,
et al: Methylprednisolone for the treatment of patients with acute
spinal cord injuries: A propensity score-matched cohort study from
a Canadian multi-center spinal cord injury registry. J Neurotrauma.
32:1674–1683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilson JR, Forgione N and Fehlings MG:
Emerging therapies for acute traumatic spinal cord injury. CMAJ.
185:485–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tesiorowski M, Potaczek T, Jasiewicz B,
Sapa J and Zygmunt M: Methylprednisolone-acute spinal cord injury,
benefits or risks? Postepy Hig Med Dosw (Online). 67:601–609.
2013.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chikuda H, Yasunaga H, Takeshita K,
Horiguchi H, Kawaguchi H, Ohe K, Fushimi K and Tanaka S: Mortality
and morbidity after high-dose methylprednisolone treatment in
patients with acute cervical spinal cord injury: A
propensity-matched analysis using a nationwide administrative
database. Emerg Med J. 31:201–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jongen PJ, Stavrakaki I, Voet B,
Hoogervorst E, van Munster E, Linssen WH, Sinnige LG, Verhagen WI,
Visser LH, Van Der Kruijk R, et al: Patient-reported adverse
effects of high-dose intravenous methylprednisolone treatment: A
prospective web-based multi-center study in multiple sclerosis
patients with a relapse. J Neurol. 263:1641–1651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schroeder GD, Kwon BK, Eck JC, Savage JW,
Hsu WK and Patel AA: Survey of cervical spine research society
members on the use of high-dose steroids for acute spinal cord
injuries. Spine (Phila Pa 1976). 39:971–977. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schäfer R, Mueller L, Buecheler R, Proksch
B, Schwab M, Gleiter CH and Danielyan L: Interplay between
endothelin and erythropoietin in astroglia: The role in protection
against hypoxia. Int J Mol Sci. 15:2858–2875. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Merelli A, Czornyj L and Lazarowski A:
Erythropoietin: A neuroprotective agent in cerebral hypoxia,
neurodegeneration, and epilepsy. Curr Pharm Des. 19:6791–6801.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ugurluer G, Cebi A, Mert H, Mert N, Serin
M and Erkal HS: Neuroprotective effects of erythropoietin against
oxidant injury following brain irradiation: An experimental study.
Arch Med Sci. 12:1348–1353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Genc S, Koroglu TF and Genc K:
Erythropoietin and the nervous system. Brain Res. 1000:19–31. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuen CM, Leu S, Lee FY, Yen CH, Lin YC,
Chua S, Chung SY, Chai HT, Sheu JJ, Ko SF, et al: Erythropoietin
markedly attenuates brain infarct size and improves neurological
function in the rat. J Investig Med. 58:893–904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gorio A, Madaschi L, Di Stefano B, Carelli
S, Di Giulio AM, De Biasi S, Coleman T, Cerami A and Brines M:
Methylprednisolone neutralizes the beneficial effects of
erythropoietin in experimental spinal cord injury. Proc Natl Acad
Sci USA. 102:16379–16384. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alibai E, Zand F, Rahimi A and
Rezaianzadeh A: Erythropoietin plus methylprednisolone or
methylprednisolone in the treatment of acute spinal cord injury: A
preliminary report. Acta Med Iran. 52:275–279. 2014.PubMed/NCBI
|
|
26
|
Boussicault L, Hérard AS, Calingasan N,
Petit F, Malgorn C, Merienne N, Jan C, Gaillard MC, Lerchundi R,
Barros LF, et al: Impaired brain energy metabolism in the BACHD
mouse model of Huntington's disease: Critical role of
astrocyte-neuron interactions. J Cereb Blood Flow Metab.
34:1500–1510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He L, Zhang X, Wei X and Li Y:
Progesterone attenuates aquaporin-4 expression in an astrocyte
model of ischemia/reperfusion. Neurochem Res. 39:2251–2261. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fairbanks SL, Young JM, Nelson JW, Davis
CM, Koerner IP and Alkayed NJ: Mechanism of the sex difference in
neuronal ischemic cell death. Neuroscience. 219:183–191. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong W, Xiao S, Cheng M, Ye X and Zheng G:
Minocycline induces protective autophagy in vascular endothelial
cells exposed to an model of ischemia/reperfusion-induced injury.
Biomed Rep. 4:173–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rao KV, Reddy PV, Curtis KM and Norenberg
MD: Aquaporin-4 expression in cultured astrocytes after fluid
percussion injury. J Neurotrauma. 28:371–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Robertson CS, Cherian L, Shah M, Garcia R,
Navarro JC, Grill RJ, Hand CC, Tian TS and Hannay HJ:
Neuroprotection with an erythropoietin mimetic peptide (pHBSP) in a
model of mild traumatic brain injury complicated by hemorrhagic
shock. J Neurotrauma. 29:1156–1166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brissaud O, Villega F, Konsman J Pieter,
Sanchez S, Raffard G, Franconi JM, Chateil JF and Bouzier-Sore AK:
Short-term effect of erythropoietin on brain lesions and
aquaporin-4 expression in a hypoxic-ischemic neonatal rat model
assessed by magnetic resonance diffusion weighted imaging and
immunohistochemistry. Pediatr Res. 68:123–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang Z, Sun X, Huo G, Xie Y, Shi Q, Chen
S, Wang X and Liao Z: Protective effects of erythropoietin on
astrocytic swelling after oxygen-glucose deprivation and
reoxygenation: Mediation through AQP4 expression and MAPK pathway.
Neuropharmacology. 67:8–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miljus N, Heibeck S, Jarrar M, Micke M,
Ostrowski D, Ehrenreich H and Heinrich R: Erythropoietin-mediated
protection of insect brain neurons involves JAK and STAT but not
PI3K transduction pathways. Neuroscience. 258:218–227. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwon MS, Kim MH, Kim SH, Park KD, Yoo SH,
Oh IU, Pak S and Seo YJ: Erythropoietin exerts cell protective
effect by activating PI3K/Akt and MAPK pathways in C6 cells. Neurol
Res. 36:215–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smith PD, Puskas F, Fullerton DA, Meng X,
Cho D, Cleveland JC Jr, Weyant MJ and Reece TB: Attenuation of
spinal cord ischemia and reperfusion injury by erythropoietin. J
Thorac Cardiovasc Surg. 141:256–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hwang J, Huh J, Kim J, Jeon Y, Cho S and
Han S: Pretreatment with erythropoietin attenuates the neurological
injury after spinal cord ischemia. Spinal Cord. 50:208–212. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chu H, Ding H, Tang Y and Dong Q:
Erythropoietin protects against hemorrhagic blood-brain barrier
disruption through the effects of aquaporin-4. Lab Invest.
94:1042–1053. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Uzüm G, Diler A Sarper, Bahcekapili N and
Ziylan Y Ziya: Erythropoietin prevents the increase in blood-brain
barrier permeability during pentylentetrazol induced seizures. Life
Sci. 78:2571–2576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martinez-Estrada OM, Rodriguez-Millán E,
González-De Vicente E, Reina M, Vilaró S and Fabre M:
Erythropoietin protects the in vitro blood-brain barrier against
VEGF-induced permeability. Eur J Neurosci. 18:2538–2544. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang C, Chen J and Lu H: Expression of
aquaporin-4 and pathological characteristics of brain injury in a
rat model of traumatic brain injury. Mol Med Rep. 12:7351–7357.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang G and Yang GY: Aquaporin-4: A
potential therapeutic target for cerebral edema. Int J Mol Sci.
17:pii: E1413. 2016. View Article : Google Scholar
|
|
44
|
Corsini E, Pinto A, Galbiati V, Viviani B,
Galli CL, Marinovich M and Racchi M: Corticosteroids modulate the
expression of the PKC-anchoring protein RACK-1 and cytokine release
in THP-1 cells. Pharmacol Res. 81:10–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang CY, Zhang JX, Lü XT and Li BY:
Effects of intermittent hypoxic exposure on the parameter of
erythrocyte and serum hypoxia inducible factor-1 alpha and
erythropoietin levels. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
25:932–934. 2009.(In Chinese). PubMed/NCBI
|