Introduction
A number of studies have demonstrated that calcium
and phosphorus metabolism disorders that promote vascular
calcification (VC) and mediate the development of cardiovascular
disease, affect the survival of patients with chronic kidney
disease (CKD) (1–3). VC used to be considered a passive
process of deposition of calcium in the extracellular matrix;
however, more studies (4,5) have demonstrated that VC is a similar
process to bone formation, which is regulated by various factors
and its central point is the change of the phenotype of vascular
smooth muscle cells (VSMCs) to osteogenic cells. High phosphorus
(HP) is one of the most important risk factors and contributors of
VC in CKD condition (6).
VC of media, also known as Monckeberg's
calcification, is the characteristic VC that appears in patients
with CKD, which results in hardening of the whole vasculature,
decreased blood vessel elasticity and hemodynamic alterations
(7). Furthermore, patients with
CKD frequently present with calcified heart valves and
calciphylaxis (8).
Peroxisome proliferator-activated receptor γ (PPAR
γ) is a nuclear receptor, which is involved in fatty acid and
energy metabolism. A PPAR γ agonist is a type of
insulin-sensitizing agent and the range of clinical uses has
increased in recent years. The activation of PPAR γ can regulate
metabolism, reduce inflammation, affect the balance of immune
cells, inhibit apoptosis, oxidative stress and improve endothelial
cell function (9). PPAR γ agonists
have many potential therapeutic effects, including regulation of
bone remodeling, due to their pleiotropic activity (10). A previous study identified that
upregulation of the activity of PPAR γ receptor could reduce VC
induced by diabetes (11).
Pioglitazone (PIO) is a novel generation of PPAR γ agonist. The
present study aimed to investigate whether PIO could alleviate
calcification of VSMCs induced by HP and to elucidate its possible
mechanism.
Materials and methods
Reagents
PIO and the PPAR γ inhibitor GW9662 were bought from
Selleck Chemicals (Houston, TX, USA). The calcium deposition assay
(Calcium Assay kit) was from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). β-glycerophosphate (β-GP) was purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Antibodies for
α-smooth muscle actin (α-SMA; cat. no. 14395-1-AP), runt-related
transcription factor 2 (Runx2; cat. no. 20700-1-AP), and GAPDH
(cat. no. 10494-1-AP) were from Wuhan Sanying Biotechnology (Wuhan,
China). Antibodies for bone morphogenetic protein-2 (BMP2; cat. no.
ab14933), glycogen synthase kinase-3β (GSK-3β; cat. no. ab32391),
phosphorylated (p)-GSK-3β (cat. no. ab30619) and β-catenin (cat.
no. ab16051) were from Abcam (Cambridge, UK). The antibody against
cyclin D1 (cat. no. 2922) was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Anti-histone deacetylase
(HDAC1; cat. no. PA1-860) was from Thermo Fisher Scientific, Inc.,
(Waltham, MA, USA). The horseradish peroxidase-conjugated secondary
antibody (cat.no. ZB-2301) was from OriGene Technologies, Inc.
(Beijing, China).
Cell culture
The rat VSMC A7r5 cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA). The VSMCs
were cultured serially in Dulbecco's modified Eagle medium (DMEM;
Gibco, Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA),
100 U/ml penicillin and 0.1 mg/ml streptomycin. Cultures were
maintained at 37°C in a humidified atmosphere of 5% CO2
and 95% air. The VSMCs were divided into 5 groups: i) Control,
which was cultured in ordinary medium; ii) control +
dimethylsuphoxide (DMSO; 25 mg/ml); iii) HP, which was cultured in
media with 10 mM β-GP; iv) HP + DMSO (25 mg/ml); v) HP + PIO, HP
group with 5, 10, 15 or with 20 µM PIO was dissolved in DMSO
respectively; and vi) HP + PIO + GW9662, culture medium containing
10 mM β-GP, 20 µM PIO and 20 µM GW9662. When VSMCs were at 70–80%
confluence in ordinary media, the cells were switched to the
calcification medium, i.e., DMEM growth medium containing 10 mM
β-GP, 50 µg/ml vitamin C and 1×10−7 mol/l insulin
(Sigma-Aldrich; Merck KGaA) for 12 days. The medium was replaced
every 3 days.
Detection of VSMC calcification
Following 12 days of the VSMCs being cultured, the
deposition of calcium in cells was detected using 2% Alizarin red
staining for 2 min at 4°C. To determine the calcium concentrations
in the VSMCs, cells were decalcified with 0.6 M HCl for 24 h at
37°C. Calcium content of culture supernatant was tested by the
o-cresolphthalein complexone and then normalized to protein
content.
Western blot analysis
Cells were lysed in lysis buffer, containing 25 mM
Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 5 mM
EGTA, 10 mM aprotinin, 10 mM leupeptin and 100 mM
phenylmethylfluoride (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). Protein concentration was determined using a bicinchoninic
acid protein assay (Nanjing KeyGen Biotech Co., Ltd.). Equal
amounts of extracted protein samples (60 µg) were separated by 10%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA). The membranes were blocked
with 5% non-fat dry milk in TBS 0.02% Tween-20 (TBST) for 2 h at
room temperature and then incubated overnight at 4°C with the
following primary antibodies: Anti-α-SMA, anti-BMP2, anti-GSK-3β,
anti-p-GSK-3β, anti-β-catenin, anti-cyclin D1, anti-HDAC (dilution,
1:1,000), anti-Runx2 (dilution, 1:500), anti-β-catenin (dilution,
1:2,000) and anti-GAPDH (dilution, 1:5,000). The membranes were
then incubated for 1 h at room temperature under agitation with the
secondary antibody (dilution, 1:8,000). The membranes were washed 3
times for 10 min each with TBST at room temperature, and protein
bands were visualized with enhanced chemiluminescence (Thermo
Fisher Scientific, Inc.) on a Bio-Rad imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Blots were semi-quantified
by densitometry using Image Lab software version 3.0 (Bio-Rad
Laboratories, Inc.). All experiments were repeated at least three
times.
Statistical analysis
Statistical analyses were performed using SPSS
software version 19.0 (SPSS, Inc., Chicago, IL, USA). All
quantitative data were presented as the mean ± standard deviation.
Multiple comparisons were evaluated using one-way analysis of
variance and significant differences between two groups were
analyzed using the Student-Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference.
Results
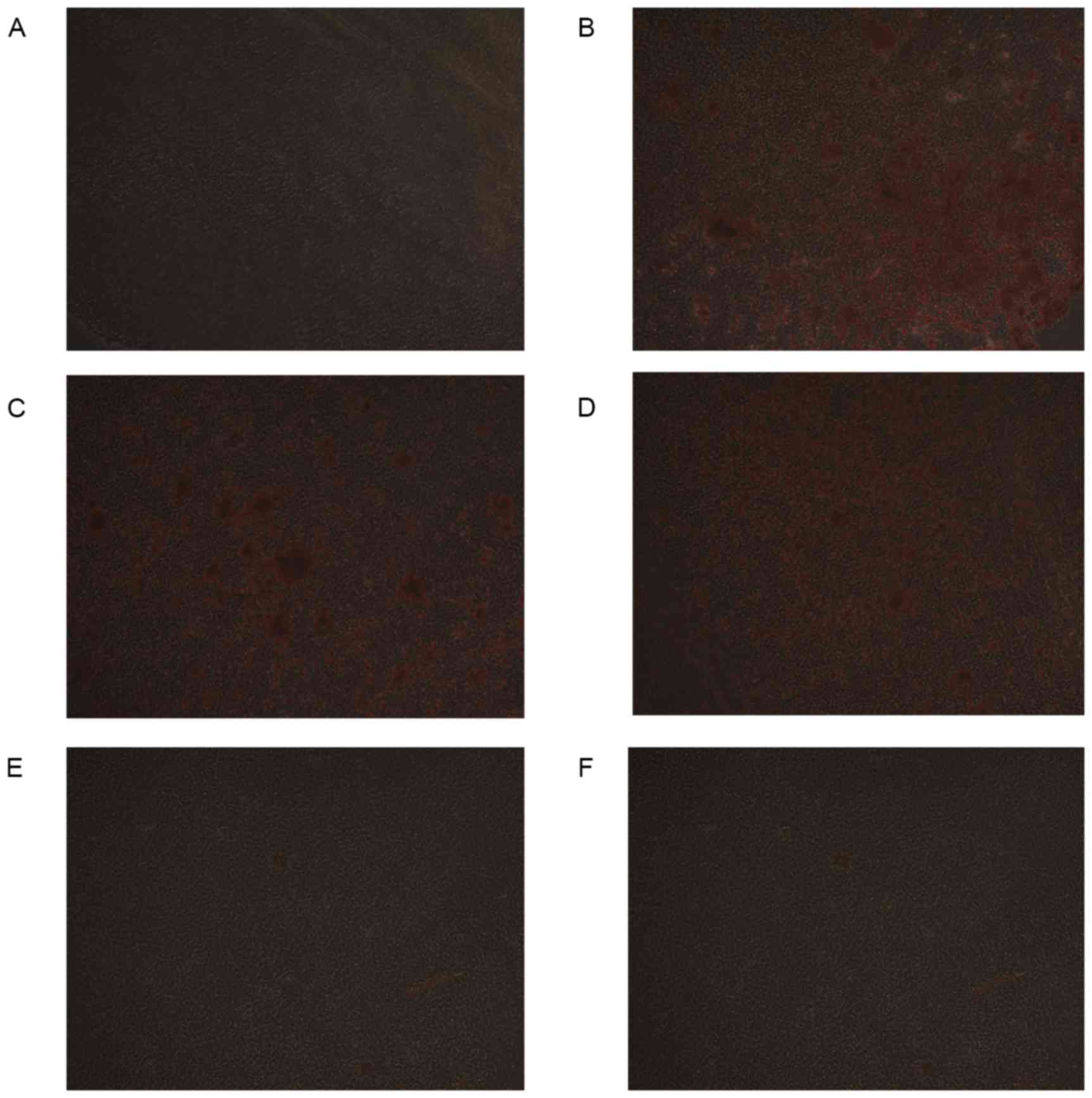
Calcification of VSMCs
Following culture for 12 days, Alizarin red staining
demonstrated that the cell matrix was not colored and the reaction
of calcified nodules was negative in the control group, while
Alizarin red staining verified the formation of mineralized nodules
in the HP group. DMSO itself had no effect on calcification.
Compared with the HP group, the extent of calcification of the
extracellular matrix in HP + 5 µM PIO group and HP + 10 µM PIO
group was less than that of HP group. The HP + 15 µM PIO group and
HP + 20 µM PIO group almost demonstrated no calcium deposition
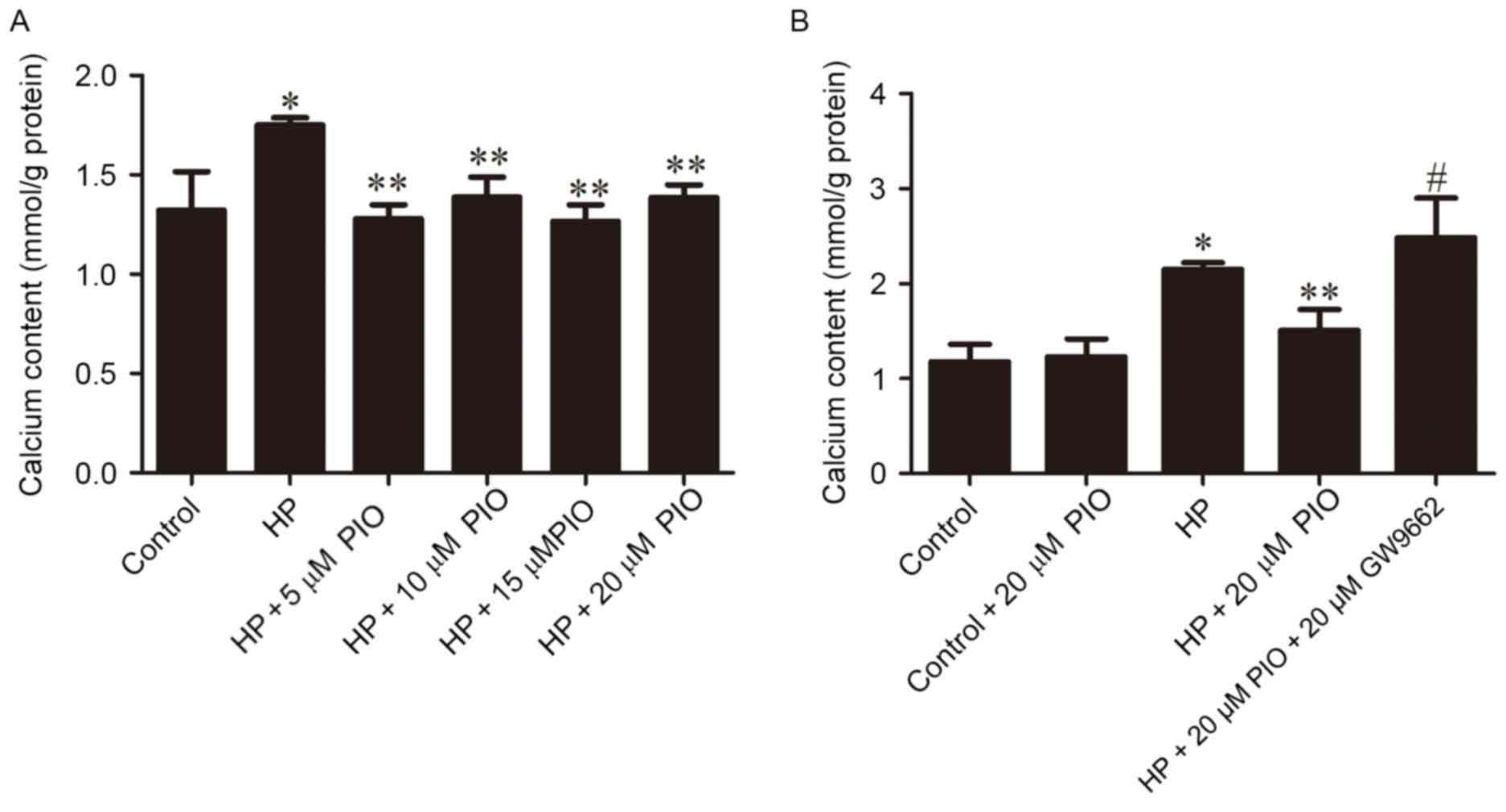
(Fig. 1). Following 12 days of
culture, the calcium content of the extracellular matrix (mmol/g)
in HP group (1.7509±0.0364) was increased compared with the control
group (1.3209±0.19567). Following treatment with 5, 10, 15 and 20
µM PIO, the calcium content of the extracellular matrix was
1.2791±0.0694, 1.3873±0.0996, 1.2660±0.0828 and 1.3857±0.0634
respectively, which were all decreased compared with the HP group.
The four different concentrations of PIO all significantly reduced
the calcification of rat VSMCs (P<0.01; Fig. 2A). The calcium content increased
significantly in the group treated with HP + PIO + GW96620 compared
with the control (P<0.01; Fig.
2B).
Expression levels of α-SMA, BMP2 and
Runx2
Compared with the control group, the protein level
of α-SMA in the HP group was significantly reduced. Compared with
the HP group, the expression of α-SMA in the group treated with 20
µM PIO increased (Fig. 3A). The
expression levels of BMP2 and Runx2 in the HP group were increased
compared with the control group. Compared with the HP group, levels
of BMP2 and Runx2 in the group treated with 20 µM PIO decreased
relatively (Fig. 3B and C).
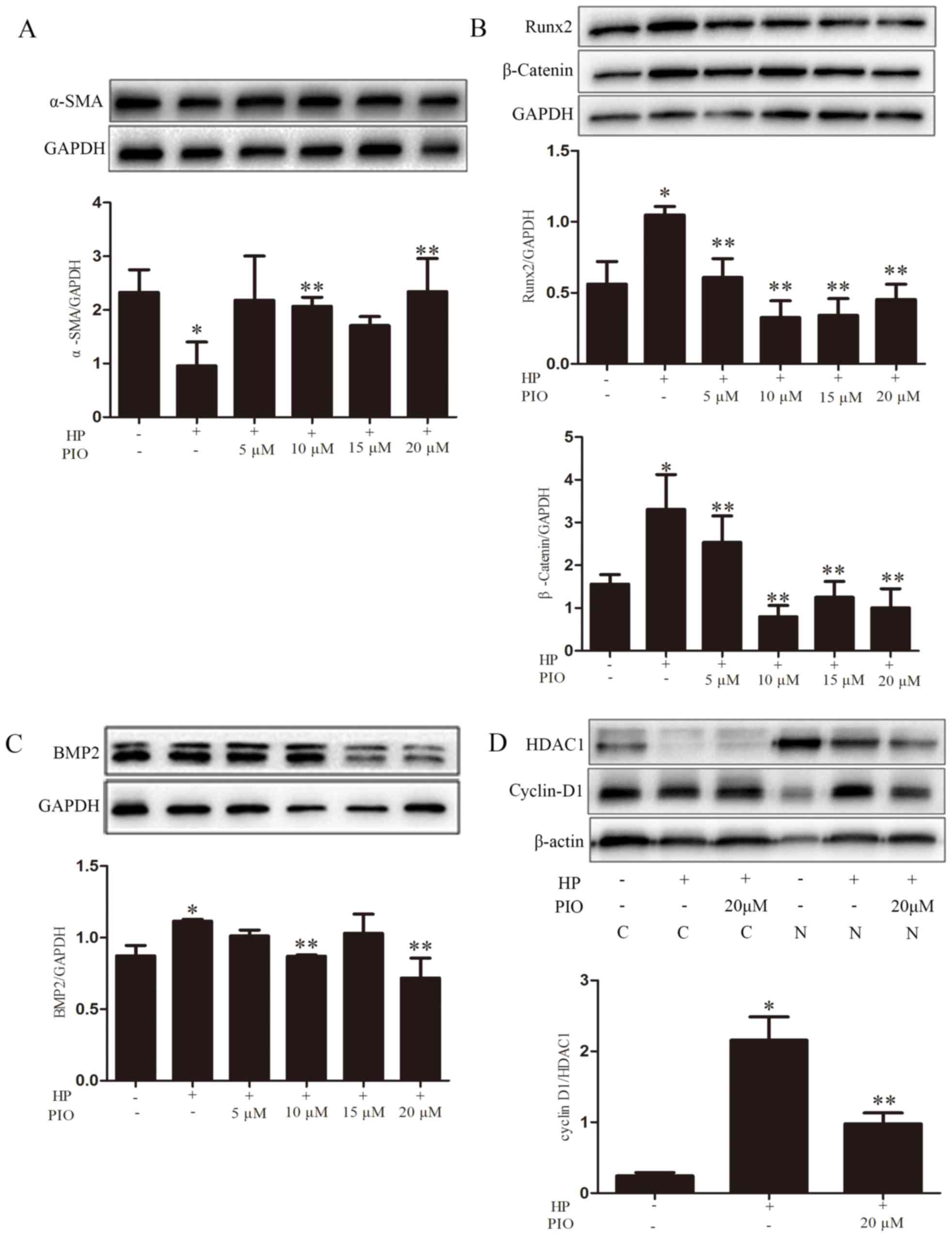 | Figure 3.α-SMA, Runx2 and BMP2 expression were
analyzed by western blotting using GAPDH as the loading control.
The quantification of the expression levels of (A) α-SMA, (B) Runx2
and β-catenin, (C) BMP2, and (D) cyclin D1/HDAC1 were represented
as the mean ± standard error (n=5/group) for each group in its
respective column. *P<0.05 vs. control group; **P<0.05 vs. HP
group. HP, high phosphate; α-SMA, α-smooth muscle actin; Runx2,
runt-related transcription factor 2; BMP2, bone morphogenetic
protein-2; PIO, pioglitazone; HDAC1, histone deacetylase 1; C,
cytoplasmic; N, nuclear. |
PIO and the Wnt/β-catenin signaling
pathway
To investigate whether PIO reduced VC via the
Wnt/β-catenin signaling pathway, the expression of the associated
proteins β-catenin, p-GSK-3β, GSK-3β and cyclin-D1 was observed.
The levels of β-catenin, p-GSK-3β/GSK-3β and cyclin-D1 increased in
the HP group compared with the controls; while the expressions of
the above proteins were decreased in the HP + 20 µM PIO group
compared with those of the HP group (Figs. 3 and 4).
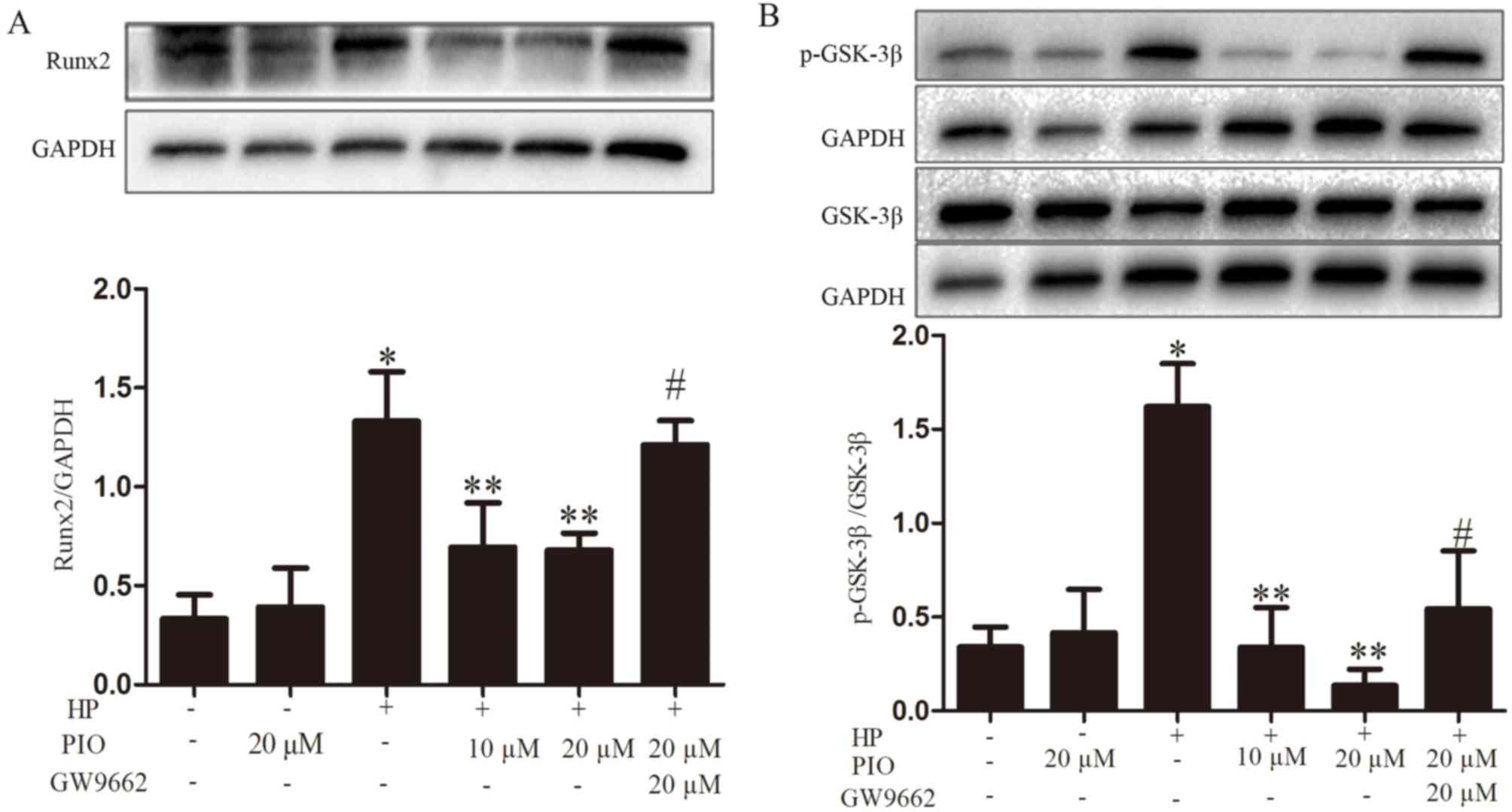
Effect of PPAR γ inhibitor GW9662 on
calcification
The calcium content of HP + PIO + GW9662 group
increased compared with 20 µM PIO intervention group (Fig. 2B). The levels of Runx2 and
p-GSK-3β/GSK-3β in HP + PIO + GW9662 group were significantly
increased compared with those of 20 µM PIO intervention group
(P<0.01; Fig. 4).
Discussion
VC in patients with CKD is common and considered to
be associated with an increased risk of mortality (12). The progression of calcification in
patients with CKD is linked to the serum phosphorus level,
phosphate is an integral part of hydroxyapatite and also an
important signaling cascade trigger factor in VC (13). The present study demonstrated that
10 mM β-GP induced calcification of rat VSMCs: Alizarin red
staining revealed calcified nodules, and the calcium content of
extracellular matrix increased. In addition, compared with control
group, the expression of α-SMA decreased, while BMP2 and Runx2
increased, consistent with previous study results (14,15).
Previous studies suggested that PPAR γ was an
important regulatory factor in bone remodeling, which served the
role of molecular switch for the differentiation of mesenchymal
stem cells (MSCs) into adipocyte and osteoblasts. Increasing the
expression of PPAR γ and enhancing its activity, may inhibit the
differentiation of MSCs to the osteogenic cells (10). Embryonic stem cells with a
homozygous PPAR γ defection cannot differentiate into fat cells;
however, can spontaneously differentiate into osteoblasts (16). A number of studies confirmed that
PPAR γ could reduce VC induced by high fat and glucose (17,18).
The aim of the present study was to investigate whether regulating
the activity of PPAR γ could reduce the VC induced by HP levels.
The results demonstrated that PIO intervention could decrease the
calcium content of extracellular matrix to the level where Alizarin
red staining was negative. The expression level of α-SMA increased
while the levels of BMP2 and Runx2 decreased when treated with PIO.
These results demonstrated that PIO could reduce the calcification
of rats VSMCs induced by HP and inhibit the differentiation of
VSMCs into osteoblast-like cells.
Wnt signaling pathway has a key role in bone
formation. Low density lipoprotein receptor (LRP)-related protein 5
is a co-receptor of Wnt signaling pathways (19). Mouse models of the activation of
the Wnt signaling pathways demonstrate that it can increase bone
mass (20). Further studies
demonstrated that inhibiting or reducing the antagonists of the Wnt
signaling pathways, including secreted frizzled-related protein 1
(sfrp1), adenomatosus polyposis coli protein and dickkopf-related
protein 1 (Dkk1) can increase the trabecular bone (20–24);
while overexpression of antagonists including Dkk1 may reduce bone
mineral density (24–26). The key process of VC is the
phenotype transformation from VSMCs to osteoblast (4,5). The
aim of the present study was to investigate whether there was an
association between the Wnt signaling pathway and VC.
The canonical Wnt signaling pathways are also known
as the Wnt/β-catenin signaling pathways. When Wnt ligands bind to
frp, it can interact with disheveled, a cytoplasmic protein that
acts upstream of β-catenin and GSK-3β. Then GSK-3β becomes
phosphorylated and the complex dissociates. β-catenin cannot be
degraded in the cytoplasm and therefore steadily accumulates and
then enters the nucleus, combining with transcription
factor-4/lymphoid enhancer-binding factor 1 (TCF/LEF, respectively)
and activates the expression of Wnt signaling pathway targeted
genes, and participates in a variety of physiological mechanisms
(27). The downstream targeted
genes of the canonical Wnt signaling pathways includes several
associated with cell proliferation, such as protooncogene c-myc and
cyclin D1 (28). The expression
level of cyclin D1 can reflect the activity of the Wnt signaling
pathway. Woldt et al (17)
demonstrated that a PPAR γ agonist could reduce the VC induced by
LRP1 via inhibiting the Wnt 5a signaling pathway. A PPAR γ agonist
could activate Wnt 5a signaling pathway antagonist sfrp2. β-catenin
in canonical Wnt signaling pathways is encoded by the CTNNB1 gene.
Certain researchers demonstrated that the mRNA levels of CTNNB1
were downregulated in adipocytes and murine adipose tissue
following treatment with a PPAR γ agonist, and β-catenin was also a
transcriptionally targeted gene of PPAR γ (29). All the above results suggested that
the PPAR γ agonist inhibited the expression of CTNNB1. Liu et
al (30) confirmed that PPAR γ
and β-catenin exhibited a direct interactive effect. The authors
suggested that PPAR γ can suppress Wnt signaling pathways in normal
cells by directing p-β-catenin to the proteasome through a process
involving its catenin binding domain. By contrast, oncogenic
β-catenin resists proteasomal degradation by inhibiting PPAR γ
activity, which requires its TCF/binding domain. In the present
study addition of 20 mM PIO in the HP medium, resulted in the
expression of β-catenin and cyclin D1 and the ratio of
p-GSK-3β/GSK-3β all to be decreased. In addition, the present study
also demonstrated that the effect of PIO on calcification and the
Wnt/β-catenin signaling pathway was reduced when adding the PPAR γ
antagonist GW9662. Therefore, it was inferred that PIO alleviated
VC induced by HP levels, and its mechanism was through its
activation of PPAR γ and downregulation of the activation of the
Wnt/β-catenin signaling pathway.
In conclusion, the present study confirmed that PIO
can suppress the calcification of VSMCs induced by HP via
downregulation of the activation of the Wnt/β-catenin signaling
pathway. These results provided a novel strategy for the prevention
and treatment of VC in CKD. The specific and detailed molecular
mechanism of how PPAR γ affected Wnt/β-catenin signaling pathway
remain unknown and requires further study in vivo.
Acknowledgements
The present study was funded by the Special
Foundation for Clinical Science and Technology of Jiangsu Province
(grant no. BL2014080), the Six Talent Peaks Project in Jiangsu
Province (grant no. WSN-056) and the Priority Academic Program
Development of Jiangsu Higher Education Institutions.
References
|
1
|
Shanahan CM, Crouthamel MH, Kapustin A and
Giachelli CM: Arterial calcification in chronic kidney disease: Key
roles for calcium and phosphate. Circ Res. 109:697–711. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schlieper G, Schurgers L, Brandenburg V,
Reutelingsperger C and Floege J: Vascular calcification in chronic
kidney disease: An update. Nephrol Dial Transplant. 31:31–39. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Six I, Maizel J, Barreto FC, Rangrez AY,
Dupont S, Slama M, Tribouilloy C, Choukroun G, Mazière JC,
Bode-Boeger S, et al: Effects of phosphate on vascular function
under normal conditions and influence of the uraemic state.
Cardiovasc Res. 96:130–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Persy V and D'Haese P: Vascular
calcification and bone disease: The calcification paradox. Trends
Mol Med. 15:405–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steitz SA, Speer MY, Curinga G, Yang HY,
Haynes P, Aebersold R, Schinke T, Karsenty G and Giachelli CM:
Smooth muscle cell phenotypic transition associated with
calcification: Upregulation of Cbfa1 and downregulation of smooth
muscle lineage markers. Circ Res. 89:1147–1154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giachelli CM: The emerging role of
phosphate in vascular calcification. Kidney Int. 75:890–897. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lanzer P, Boehm M, Sorribas V, Thiriet M,
Janzen J, Zeller T, St Hilaire C and Shanahan C: Medial vascular
calcification revisited: Review and perspectives. Eur Heart J.
35:1515–1525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brandenburg VM, Sinha S, Specht P and
Ketteler M: Calcific uraemic arteriolopathy: A rare disease with a
potentially high impact on chronic kidney disease-mineral and bone
disorder. Pediatr Nephrol. 29:2289–2298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ivanova EA, Parolari A, Myasoedova V,
Melnichenko AA, Bobryshev YV and Orekhov AN: Peroxisome
proliferator-activated receptor (PPAR) gamma in cardiovascular
disorders and cardiovascular surgery. J Cardiol. 66:271–278. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wan Y: PPARγ in bone homeostasis. Trends
Endocrinol Metab. 21:722–728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li F, Cai Z, Chen F, Shi X, Zhang Q, Chen
S, Shi J, Wang DW and Dong N: Pioglitazone attenuates progression
of aortic valve calcification via down-regulating receptor for
advanced glycation end products. Basic Res Cardiol. 107:3062012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morena M, Jaussent I, Dupuy AM, Bargnoux
AS, Kuster N, Chenine L, Leray-Moragues H, Klouche K, Vernhet H,
Canaud B and Cristol JP: Osteoprotegerin and sclerostin in chronic
kidney disease prior to dialysis: Potential partners in vascular
calcifications. Nephrol Dial Transplant. 30:1345–1356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paloian NJ and Giachelli CM: A current
understanding of vascular calcification in CKD. Am J Physiol Renal
Physiol. 307:F891–F900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao L, Sun YT, Sun W, Xu TH, Ren C, Fan X,
Sun L, Liu LL, Feng JM, Ma JF and Wang LN: High phosphorus level
leads to aortic calcification via β-catenin in chronic kidney
disease. Am J Nephrol. 41:28–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Speer MY, Yang HY, Brabb T, Leaf E, Look
A, Lin WL, Frutkin A, Dichek D and Giachelli CM: Smooth muscle
cells give rise to osteochondrogenic precursors and chondrocytes in
calcifying arteries. Circ Res. 104:733–741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
EI'chaninov DV, Akker LV, Fedorova IA and
Popovtseva AV: Bone resorption and formation markers in women with
climacteric syndrome in early postmenopause. Klin Lab Diagn. 21–24.
2009.(In Russian).
|
|
17
|
Woldt E, Terrand J, Mlih M, Matz RL,
Bruban V, Coudane F, Foppolo S, El Asmar Z, Chollet ME, Ninio E, et
al: The nuclear hormone receptor PPARγ counteracts vascular
calcification by inhibiting Wnt5a signalling in vascular smooth
muscle cells. Nat Commun. 3:10772012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou YB, Zhang J, Peng DQ, Chang JR, Cai
Y, Yu YR, Jia MZ, Wu W, Guan YF, Tang CS and Qi YF: Peroxisome
proliferator-activated receptor γ ligands retard cultured vascular
smooth muscle cells calcification induced by high glucose. Cell
Biochem Biophys. 66:421–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boyden LM, Mao J, Belsky J, Mitzner L,
Farhi A, Mitnick MA, Wu D, Insogna K and Lifton RP: High bone
density due to a mutation in LDL-receptor-related protein 5. N Engl
J Med. 346:1513–1521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balemans W, Patel N, Ebeling M, Van Hul E,
Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ,
et al: Identification of a 52 kb deletion down-stream of the SOST
gene in patients with van Buchem disease. J Med Genet. 39:91–97.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bodine PV, Zhao W, Kharode YP, Bex FJ,
Lambert AJ, Goad MB, Gaur T, Stein GS, Lian JB and Komm BS: The Wnt
antagonist secreted frizzled-related protein-1 is a negative
regulator of trabecular bone formation in adult mice. Mol
Endocrinol. 18:1222–1237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glass DA II, Bialek P, Ahn JD, Starbuck M,
Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA and
Karsenty G: Canonical Wnt signaling in differentiated osteoblasts
controls osteoclast differentiation. Dev Cell. 8:751–764. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holmen SL, Zylstra CR, Mukherjee A, Sigler
RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL and Williams BO:
Essential role of beta-catenin in postnatal bone acquisition. J
Biol Chem. 280:21162–21168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morvan F, Boulukos K, Clément-Lacroix P,
Roman S Roman, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho
S, Pognonec P, et al: Deletion of a single allele of the Dkk1 gene
leads to an increase in bone formation and bone mass. J Bone Miner
Res. 21:934–945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Sarosi I, Cattley RC, Pretorius J,
Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, et al:
Dkk1-mediated inhibition of Wnt signaling in bone results in
osteopenia. Bone. 39:754–766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakanishi T, Yamaai T, Asano M, Nawachi K,
Suzuki M, Suqimoto T and Takiqawa M: Overexpression of connective
tissue growth factor/hypertrophic chondrocyte-specific gene product
24 decreases bone density in adult mice and induces dwarfism.
Biochem Biophys Res Commun. 281:678–681. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jansson EA, Are A, Greicius G, Kuo IC,
Kelly D, Arulampalam V and Pettersson S: The Wnt/beta-catenin
signaling pathway targets PPARgamma activity in colon cancer cells.
Proc Natl Acad Sci USA. 102:1460–1465. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo F, Ren X, Dong Y, Hu X, Xu D, Zhou H,
Meng F, Tian W and Zhao Y: Constitutive expression of PPARγ
inhibits proliferation and migration of gastric cancer cells and
down-regulates Wnt/β-catenin signaling pathway downstream target
genes TERT and ENAH. Gene. 584:31–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Wang H, Zuo Y and Farmer SR:
Functional interaction between peroxisome proliferator-activated
receptor gamma and beta-catenin. Mol Cell Biol. 26:5827–5837. 2006.
View Article : Google Scholar : PubMed/NCBI
|