Introduction
Fibromyalgia syndrome (FMS) is a complex disorder
that is characterized by chronic widespread pain and muscle
tenderness, and may be accompanied by disturbances in sleep,
fatigue, anxiety and other clinical manifestations, such as
depression, gastrointestinal symptoms and headache (1). FMS affects ≥2% of the adult
population, with females significantly more affected than males
(2). The etiology of FMS varies
among subjects, but may include neurogenic inflammation caused by
allergen-induced inflammatory response, bacterial or viral
infections, irritants or chemical exposure, as well as oxidative
and emotional stressors (3,4).
Inflammation often includes increased levels of cytokines,
neuropeptides, growth factors and neurotransmitters; however, many
of these are abnormal in patients with FMS (5–7).
Although progress has been made in understanding the
disease mechanism of FMS, its pathophysiology has not been clearly
established. Genetic factors may influence susceptibility to FMS,
but no specific gene has been identified. As FMS often occurs in
several family members, there may be a genetic component. Several
previous studies have reported candidate FMS polymorphisms, such as
in the serotoninergic system genotype (8), the catechol-O-methyltransferase gene
(9) and the D4 dopamine receptor
(10). However, many of the
previously identified associations have been weak or
inconsistent.
Endothelin 1 (EDN1) is a peptide produced by
endothelial and vascular smooth muscle cells and is a potent
vasoconstrictor (11). Owing to
the vasoconstrictive and hypertrophic actions on blood vessels,
EDN1 has been linked to the development of hypertension (12). The human EDN1 gene is 5.5 kb
in length, with 5 exons and 4 introns (13). Patients with FMS express high
levels of EDN1 (14,15), have a high prevalence of insulin
resistance (16) and may have
increased body fat for a given weight (17). These data suggested that the EDN
system may be activated in these patients, and the associations
between EDN1 polymorphisms and EDN1 levels with the
development of FMS may be more prominent compared with those in the
general population. The prevalence of the EDN1 SNP rs1800541
and its association with EDN1 levels in patients with FMS has not
yet been investigated. The T1370G single-nucleotide polymorphism
(SNP; rs1800541) is in the EDN1 promoter region and may
affect to EDN1 expression levels, which may be a potential
intermediate hypertension phenotype (18).
The present study examined whether the rs1800541 SNP
occurs more frequently in patients with FMS than in the local
general population without FMS, and whether plasma EDN1 levels may
be associated with susceptibility to FMS or the clinical
variables.
Materials and methods
Subjects and clinical assessment
This study included a total of 88 patients with FMS
(83 female and five male; age, 48.02±11.30 years; weight, 58.63 ±
0.99 kg; mean ± standard error of the mean) and 87 healthy controls
(all female; age, 40.87±6.21 years; weight, 57.17±0.84 kg) without
a history of FMS or chronic widespread pain (19). Collected data also included height
and weight measurements that were used to assess body mass index
(BMI). Biospecimens used by the present study were provided by the
Biobank of the College of Medicine, Soonchunhyang University
(Cheonan, Korea). This study was approved by the Ethics Review
Committee of the Biobank of the College of Medicine, Soonchunhyang
University (SCHIRB-BIO-150006); written informed consent was
received from all patients prior to the study.
Clinical assessment
The presence of tender points was assessed according
to the standardized manual tender point survey (20). The number of tender points was
counted at 18 specific sites on the body, and the intensity of each
tender point was assessed as follows: 0, no tenderness; 1, light
tenderness (confirmed answer when asked); 2, moderate tenderness
(spontaneous verbal response); and 3, severe tenderness (moving
away). Therefore, the possible number of tender points ranged
between 0 and 18, and the possible total score ranged between 0 and
54. Clinical disease activity and severity of FMS were assessed
using various tools to diagnose FMS. The Korean version of the
Fibromyalgia Impact Questionnaire (FIQ) was used to assess
functional abilities (21); the
Brief Fatigue Inventory (BFI) was used to assess fatigue severity
(22); the Beck Depression
Inventory (BDI) was used to assess depression severity (23); the Medical Outcomes Study 36-item
Short-Form Health Survey, which comprises eight items, including
physical health (physical functioning, role-physical, bodily pain
and general health) and mental health (vitality, social
functioning, role-emotional and mental health) (24), was used to assess quality of life;
and the State-Trait Anxiety Inventory (STAI)-1 and STAI-2 was used
to assess anxiety (Tables I and
II) (25).
 | Table I.Clinical features of FMS and control
groups. |
Table I.
Clinical features of FMS and control
groups.
|
| Control (n=87) | FMS (n=88) |
|
|---|
|
|
|
|
|
|---|
| Parameter | Mean | SE | Mean | SE | P-value |
|---|
| Age | 40.87 | 0.67 | 48.02 | 1.20 | N/A |
| Male, n (%) | 0 (0.00) |
| 5 (5.68) |
| N/A |
| Female, n (%) | 87 (100.00) |
| 83 (94.32) |
|
|
| FIQ | 0.00 | 0.00 | 59.03 | 1.98 | <0.001 |
| BFI | 23.36 | 1.66 | 51.92 | 2.14 | <0.001 |
| BDI | 27.38 | 0.61 | 40.05 | 1.18 | <0.001 |
| PCS | 76.44 | 1.44 | 45.42 | 0.19 | <0.001 |
| MCS | 75.22 | 1.49 | 50.33 | 2.31 | <0.001 |
| STAI1 | 44.21 | 0.70 | 42.45 | 0.71 | 0.081 |
| STAI2 | 45.24 | 0.69 | 49.31 | 0.74 | <0.001 |
 | Table II.Clinical assessments by genotype and
allele in patients with FMS. |
Table II.
Clinical assessments by genotype and
allele in patients with FMS.
|
| FMS
genotype/allele |
Genotype/allele |
|---|
|
|
|
|
|---|
| Assessment | TT | TG | GG | P-value | T | G | P-value |
|---|
| n | 35 | 40 | 13 |
| 110 | 66 |
|
| FIQ | 61.06±2.85 | 56.80±3.26 | 60.42±4.75 | 0.593 | 59.51±1.74 | 58.23±2.36 | 0.663 |
| BFI | 55.29±3.25 | 49.80±3.12 | 49.38±6.55 | 0.447 | 53.29±1.85 | 49.64±2.58 | 0.252 |
| BDI | 39.03±1.77 | 40.68±1.70 | 40.85±3.87 | 0.785 | 39.63±1.00 | 40.74±1.46 | 0.531 |
| PCS | 45.52±2.72 | 46.90±2.97 | 40.64±5.86 | 0.559 | 46.02±1.62 | 44.43±2.42 | 0.586 |
| MCS | 47.95±3.44 | 52.83±3.29 | 49.05±7.77 | 0.612 | 49.72±1.95 | 51.34±2.89 | 0.644 |
| STAI1 | 43.31±1.11 | 42.80±1.05 | 39.08±1.79 | 0.133 | 43.13±0.62 | 41.33±0.83 | 0.086 |
| STAI2 | 48.47±1.11 | 50.23±1.10 | 48.69±2.26 | 0.526 | 49.12±0.64 | 49.62±0.90 | 0.652 |
| Tender points | 17.34±0.22 | 16.60±0.40 | 17.62±0.21 | 0.139 | 17.00±0.26 | 17.07±0.18 | 0.817 |
| Total score | 29.77±1.19 | 27.70±1.26 | 27.85±1.52 | 0.442 | 27.76±0.86 | 29.02±0.70 | 0.259 |
Measurement of plasma EDN1 levels
Plasma was extracted from fresh whole blood samples
with EDTA. The blood was centrifuged at 13,200 × g for 10 min and
stored at −80°C. Plasma EDN1 levels were determined using an
Endothelin ELISA kit (cat no. 583151; Cayman Chemical, Ann Arbor,
MI, USA) with 50 µl plasma from each subject, according to the
manufacturer's protocol.
Genotyping
DNA was extracted from fresh whole-blood samples
(300 µl) using a DNA purification kit (Nanohelix Co., Ltd., Seoul,
Korea), according to the manufacturer's protocol. SNPs were
identified by polymerase chain reaction-high-resolution melting
(PCR-HRM) curve analysis and the SensiFAST HRM Kit (Bioline,
Taunton, MA, USA) as previously described (26). The EDN1 gene primers were: Forward,
5′-CAGAATGACCCGGTGACACT-3′ and reverse, 5′-CATTGGCTTTTTCCGCTAGT-3′.
Cycling conditions were as follows: Activation of polymerase at
95°C for 2 min; followed by 43 cycles of 95°C for 5 sec, 60°C for
10 sec and 72°C for 15 sec; and the HRM step at 95°C for 15 sec;
55°C for 15 sec; and 95°C for 5 sec.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) was assessed using
SNP Stats (http://bioinfo.iconcologia.net/index.php) and SPSS
18.0 software (SPSS Inc., Chicago, IL, USA). Associations between
the SNP and patients with FMS were estimated by computing odds
ratios (ORs) and 95% confidence intervals (CIs) by logistic
regression analyses with age and sex controlled as covariates.
Models assuming co-dominant (where relative disease hazard differs
between subjects with one minor allele and those with two minor
alleles), dominant (subjects with one or two minor alleles
demonstrate the same relative hazard), recessive (individuals with
two minor alleles are at increased risk of the disease), or
over-dominant inheritance (assumes the heterozygote has the
strongest impact and compares major alleles/major alleles + minor
alleles/minor allele vs. major alleles/minor allele) were used in
the logistic regression analysis for the SNP. Genotypic and allelic
frequencies of the SNP were compared between the patients with FMS
and controls using the χ2 test for the case-control
association study. The difference between patients with FMS and
controls was adjusted for age and sex as covariables. P<0.05 was
considered to indicate a statistically significant difference.
Results
The relationship between the SNP and the clinical
features of patients with FMS was assessed, including number of
tender points, FIQ, BFI, BDI, STAI-I and STAI-II scores (Tables I and II). Most of the parameters were
significantly different between the control and FMS group (Table I). None of the parameters assessed
in the patients with FMS differed among the SNP genotypes or
alleles (Table II).
The EDN1 SNP T1370G genotype was detected
successfully by PCR-HRM analysis in all subjects (n=175, 100%).
Distribution of the EDN1 T1370G genotype was consistent with
HWE in the control and FMS subjects (P>0.05). In total, 88
patients with FMS (age, 48.02±11.30 years) were genotyped, of whom
83 (94.3%) were female. A total of 87 healthy control patients were
genotyped (age, 40.87±6.21 years), which were significantly younger
compared with the patients with FMS, and which were all female;
therefore, all results were adjusted for age and sex. The average
body weight in the patients with FMS was 58.63±0.99 kg, and in the
control group was 57.17±0.84 kg, which was not significantly
different (P=0.260). The average BMI of the subjects was 23.33±0.36
(FMS) and 22.17±0.43 (control), which was slightly different
(P=0.040); however, the BMI score was not identified as related to
FMS genotype and plasma EDN1 levels.
Of the patients with FMS, 35 out of 88 (39.80%) had
the TT genotype, 40 (45.50%) had TG and 13 (14.80%) had the GG
genotype of the EDN1 SNP. TG and GG genotype frequencies
were 23.00 and 13.80% in the control group and 45.50 and 14.80% in
the FMS group for the co-dominant model (OR, 3.14 and 1.70; 95% CI,
1.59–6.23 and 0.70–4.15, respectively; P=0.004; Table III), respectively. The GG
genotype was associated with an increased risk for FMS. FMS
susceptibility was significantly associated in the over-dominant
model (TG + GG vs. TT; OR, 2.79; 95% CI, 1.45–5.36; P=0.002;
Table III), indicating that
absence of the G allele (TT) decreased the risk for FMS compared
with the presence of the G allele (GG or TG). Allelic frequency was
also associated with susceptibility to FMS (OR, 0.56; 95% CI,
0.36–0.89; P=0.014; Table III).
The T allele frequency was lower in the FMS group (62.0%) compared
with in the control group (75.0%).
 | Table III.Genotype and allele frequencies of
endothelin 1 single-nucleotide polymorphisms in healthy control
patients and patients with fibromyalgia syndrome. |
Table III.
Genotype and allele frequencies of
endothelin 1 single-nucleotide polymorphisms in healthy control
patients and patients with fibromyalgia syndrome.
|
| Control (n=87) | FMS (n=88) |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
|
Genotype/allele | Freq. | % | Freq. | % | Model | OR (95% CI) | P-value |
|---|
| TT | 55 | 63.20 | 35 | 39.80 | Co-dominant | 3.14
(1.59–6.23) | 0.004 |
| TG | 20 | 23.00 | 40 | 45.50 |
| 1.70
(0.70–4.15) |
|
| GG | 12 | 13.80 | 13 | 14.80 | Dominant | 2.60
(1.41–4.79) | 0.002 |
|
|
|
|
|
| Recessive | 1.08
(0.46–2.53) | 0.850 |
|
|
|
|
|
| Overdominant | 2.79
(1.45–5.36) | 0.002 |
| T | 130 | 0.75 | 110 |
0.62 |
|
|
|
| G | 44 | 0.25 | 66 |
0.38 |
| 0.56
(0.36–0.89) | 0.014 |
Association between EDN1 gene
polymorphisms and plasma EDN1 levels in healthy control patients
and patients with FMS
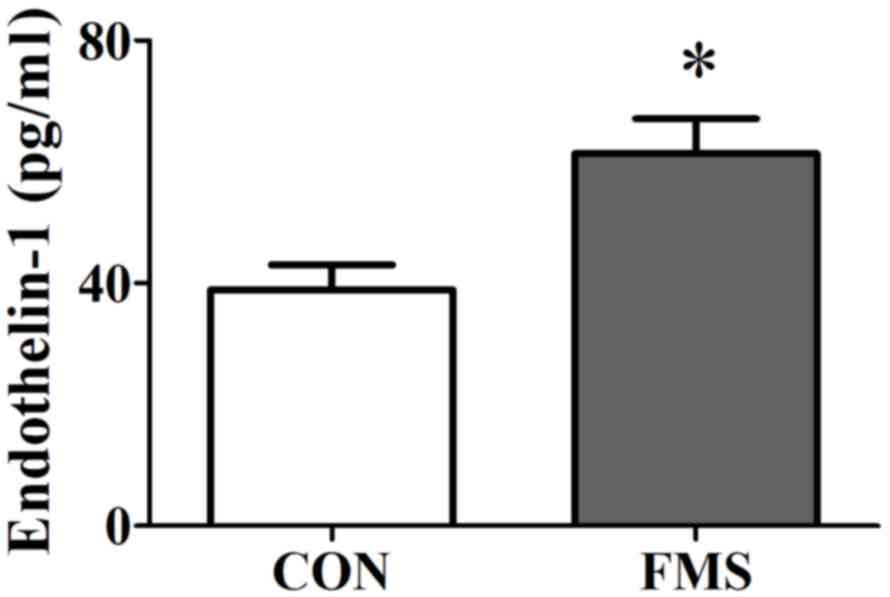
Plasma EDN1 levels increased significantly in
patients with FMS compared with patients in the control group (mean
± standard error of the mean; 38.89±5.87 vs. 61.34±8.14 pg/ml;
P=0.027; Fig. 1). Plasma EDN1
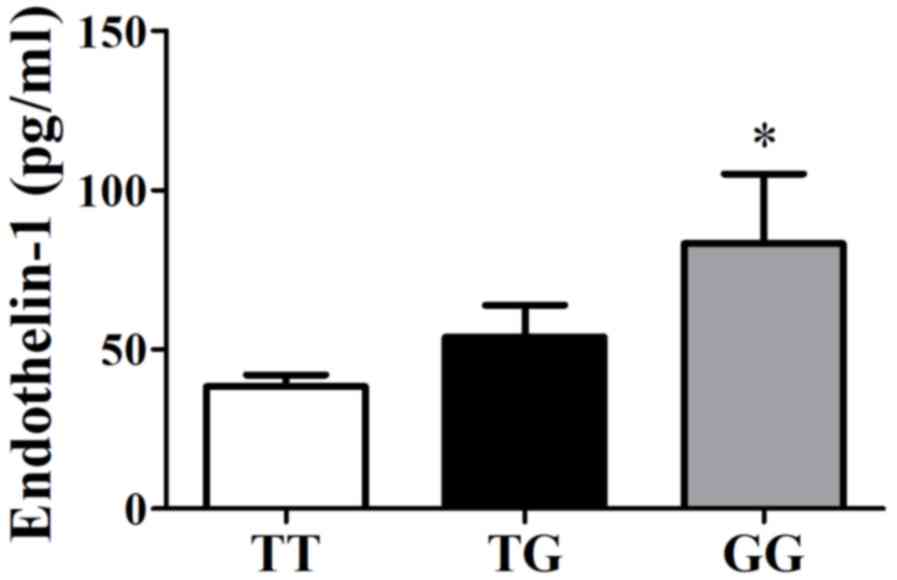
levels increased significantly in patients with the GG genotype
(83.30±21.93 pg/ml) compared with those with the TG genotype
(53.91±10.04 pg/ml) or TT genotype (38.50±3.56 pg/ml; P=0.011;
Fig. 2) in all patients. Plasma
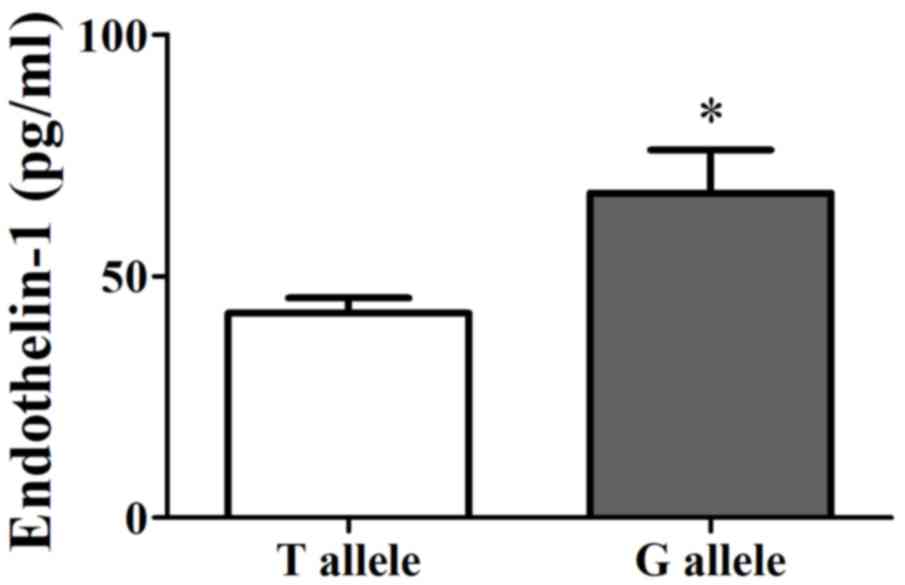
EDN1 levels in the G allele group (67.27±8.93 pg/ml) was relatively
higher than that in the T allele group (42.35±3.15 pg/ml) among all
patients (P<0.05; Fig. 3).
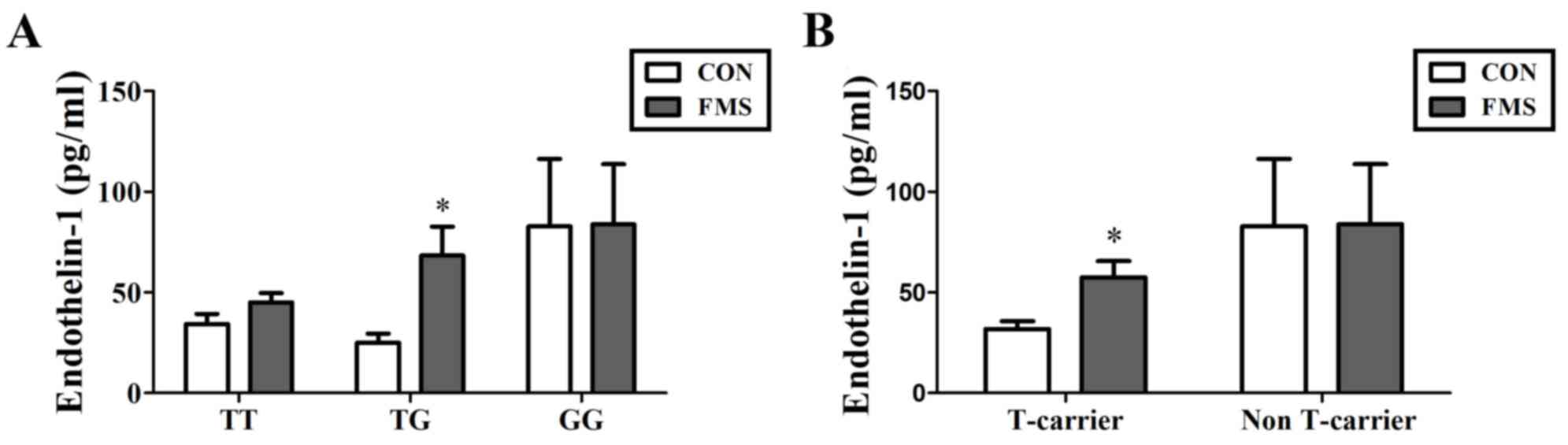
The relationship between plasma EDN1 level and EDN1
polymorphism was further compared within the patients. Plasma EDN1
levels in patients with FMS with the TG genotype were significantly
higher compared with EDN1 levels in healthy control patients with
the TG genotype (68.32±14.41 vs. 25.08±4.51 pg/ml; P=0.006;
Fig. 4A). In addition, the level
of T-carrier group (TT + TG) in FMS patients was significantly
higher compared with healthy controls (57.45±8.05 vs. 31.87±3.85
pg/ml; P=0.002; Fig. 4B). It was
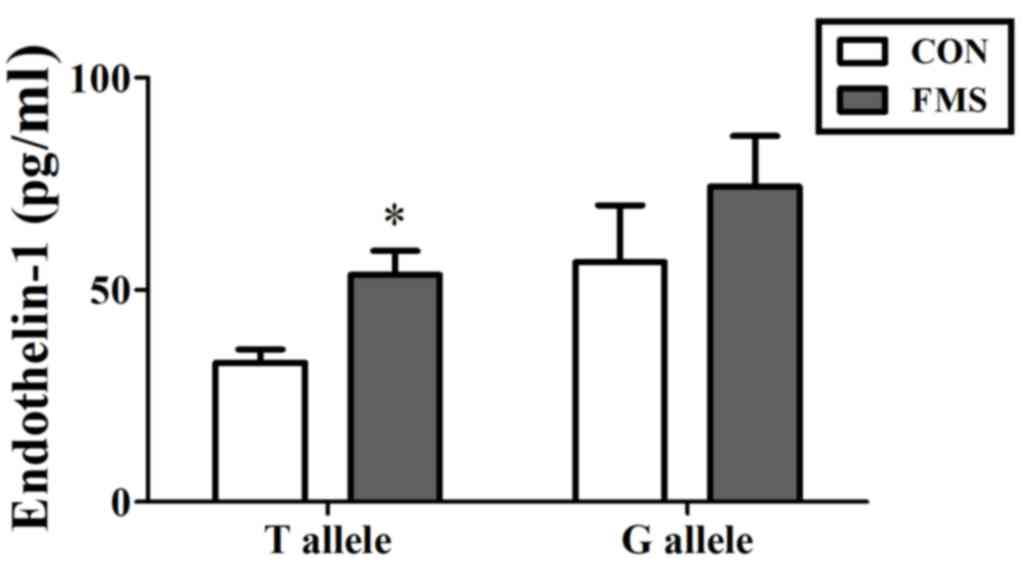
also demonstrated that patients with FMS with the T allele
exhibited higher levels of EDN1 in the plasma compared with healthy
controls (53.50±5.70 vs. 32.91±3.05 pg/ml; P=0.002; Fig. 5). However, the levels of EDN1
expression were not significantly different between controls and
FMS patients with G allele (P>0.05).
Discussion
The present study investigated the association
between plasma EDN1 expression levels and the EDN1 SNP in patients
with FMS. Genotypic and allelic distributions of EDN1 SNP in
patients with FMS were significantly different from those in the
healthy control patient group, which suggested an association
between EDN1 SNP and FMS. In addition, plasma EDN1 levels in
patients with FMS were demonstrated to be higher than those of
healthy controls.
Vascular endothelial cells are able to modulate
local vascular tone by secreting relaxing factors, such as nitric
oxide, and constrictive factors such as EDN1. Other than its direct
vasoconstrictive effect, EDN1 may also increase the sensitivity of
blood vessels to other circulating vasoconstrictive hormones,
including noradrenaline, serotonin and angiotensin II (27). EDN1 expression was previously
demonstrated to be increased in certain vascular beds, such as in
the heart (28) and the kidney
(29), following tissue ischemia.
Increased EDN1 production has also been described in other vascular
and rheumatologic diseases, including vasospastic syndrome,
multiple sclerosis, giant cell arteries and systemic lupus
erythematosus (30,31). It has been reported that patients
with FMS have significantly higher levels of brachial-ankle
pulse-wave velocity (baPWV) compared with healthy controls
(32). BaPWV is correlated with
disease severity assessed by FIQ (32). Another study reported that plasma
EDN1 levels in German patients with FMS were significantly higher
compared with those in healthy controls (n=21/group) (31). The present study analyzed plasma
EDN1 expression levels and SNPs to validate these results in a
relatively large Korean population (n=175 total), and demonstrated
that plasma EDN1 expression levels were increased in patients with
FMS. In addition, subjects with G allele had higher EDN1 levels
compared with T allele. Previous studies suggested a possible
effect of EDN1 in distinctive vascular cold-response of patients
with FMS (33,34), in which repeated relative ischemia
might increase EDN1 level. An elevated EDN1 level, in turn, may
further enhance vasospasms.
Elevated tissue or plasma concentrations of EDN1 may
occur in a variety of pathological states, such as metastasized
prostate and breast cancer cells (35), following cutaneous injury (36). Plasma EDN1 levels may also increase
following ischemic injury related to acute respiratory distress
syndrome, sepsis and disseminated intravascular coagulation
(37,38). EDN1 expression contributes to pain
in many of these pathologies; inflammation leads to the release of
substances that may excite or sensitize primary afferent nerve
fibers and cause pain and/or hyperalgesia (39,40),
and EDN1 has been reported to be significantly oversecreted in
inflammatory conditions (41).
EDN1 levels in synovial fluid are elevated in patients with
inflammation-related diseases, such as rheumatoid arthritis (RA),
osteoarthritis and gout. Plasma EDN1 levels in patients with active
RA were demonstrated to be higher than the levels in patients with
non-active RA, whereas EDN1-like immunoreactivity in synovial fluid
was revealed to be several-fold higher than in plasma (42). Endothelial dysfunction may induce
vascular inflammation by producing vasoconstricting agents,
adhesion molecules and growth factors (43,44).
Patients with cardiovascular disease exhibit increased tissue
expression and plasma levels of inflammatory markers and mediators,
including C-reactive protein (CRP) and adhesion molecules, such as
intercellular adhesion molecule 1 (ICAM1), selectins and vascular
cell adhesion molecule 1 (VCAM1) (45,46).
In addition, patients with hypertension have been reported to
exhibit increased plasma concentrations of tumor necrosis factor-α
(a primary inflammatory cytokine), interleukin 6 (a secondary
inflammatory cytokine), ICAM1, VCAM1, selectin E, von Willebrand
factor and CRP (47). However,
high concentrations of inflammatory mediators may be independent
risk factors for the development of hypertension (48,49).
Additional studies are required to clarify the relationship between
elevated plasma EDN1 levels and the aforementioned markers of
vascular inflammation in patients with FMS to draw definite
conclusions.
Results from the present study suggested that EDN1
may have a potential effect on disease susceptibility in patients
with FMS. However, whether high EDN1 plasma levels contributed to
the etiology and how expression affected pain and psychiatric
problems related to FMS remains unclear.
The present study, to the best of our knowledge, was
the first to demonstrate that patients with the EDN1 TG genotype
may have an elevated risk of FMS in the Korean population. In
addition, patients with FMS with the EDN1 T allele exhibited
significantly higher plasma EDN1 levels compared with healthy
controls. The results revealed that patients with the TG genotype
were more susceptible to FMS with increased plasma EDN1 levels.
Functional analysis of EDN1 SNP rs1800541 in the future may help to
clarify the potential biological mechanism of FMS.
In conclusion, plasma EDN1 levels were significantly
increased in Korean patients with FMS compared with those in
healthy controls. EDN1 SNP was revealed to be associated with
susceptibility to FMS. The power of sample size was calculated
using a genetic power calculator (http://zzz.bwh.harvard.edu/gpc). In this study, the
genetic power was calculated to be 0.5322 for the EDN1 SNP (number
of case: 88; control-to-case ratio: 0.988; number of cases for 80%
power: 165); it was insufficiently powerful to determine a positive
association. Owing to the relatively small number of subjects,
these results should be validated by additional studies using
larger sample sizes.
References
|
1
|
Mease P: Fibromyalgia syndrome: Review of
clinical presentation, pathogenesis, outcome measures, and
treatment. J Rheumatol. 75:6–21. 2005.
|
|
2
|
Gran JT: The epidemiology of chronic
generalized musculoskeletal pain. Best Pract Res Clin Rheumatol.
17:547–561. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Russell IJ and Larson AA:
Neurophysiopathogenesis of fibromyalgia syndrome: A unified
hypothesis. Rheum Dis Clin North Am. 35:421–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russell IJ, Orr MD, Littman B, Vipraio GA,
Alboukrek B, Michalek JE, Lopez Y and MacKillip F: Elevated
cerebrospinal levels of substance P in patients fibromyalgia
syndrome. Arthritis Rheum. 37:1593–1601. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giovengo SL, Russell IJ and Larson AA:
Increased concentrations of nerve growth factor in cerebrospinal
fluid of patients with fibromyalgia. J Rheumatol. 26:1564–1569.
1999.PubMed/NCBI
|
|
6
|
Ozgocmen S, Ozyurt H, Sogut S and Akyol H:
Current concepts in the pathophysiology of fibromyalgia: The
potential role of oxidative stress and nitric oxide. Rheumatol Int.
26:585–597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maes M, Libbrecht I, Van Hunsel F, Lin AH,
De Clerck L, Stevens W, Kenis G, de Jongh R, Bosmans E and Neels H:
The immune-inflammatory pathophysiology of fibromyalgia: Increased
serum soluble gp130, the common signal transducer protein of
various neurothrophic cytokines. Psychoneuroendocrinology.
24:371–383. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bondy B, Spaeth M, Offenbaecher M,
Glatzeder K, Stratz T, Schwarz M, de Jonge S, Krüger M, Engel RR,
Färber L, et al: The T102C polymorphism of the 5-HT2A-receptor gene
in fibromyalgia. Neurobiol Dis. 6:433–439. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vargas-Alarcón G, Fragoso JM, Cruz-Robles
D, Vargas A, Vargas A, Lao-Villadóniga JI, García-Fructuoso F,
Ramos-Kuri M, Hernández F, Springall R, et al:
Catechol-O-methyltransferase gene haplotypes in Mexican and Spanish
patients with fibromyalgia. Arthritis Res Ther. 9:R1102007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buskila D, Cohen H, Neumann L and Ebstein
RP: An association between fibromyalgia and the dopamine D4
receptor exon III repeat polymorphism and relationship to novelty
seeking personality traits. Mol Psychiatr. 9:730–741. 2004.
View Article : Google Scholar
|
|
11
|
Vane JR, Anggård EE and Botting RM:
Regulatory functions of the vascular endothelium. N Engl J Med.
323:27–36. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schiffrin EL and Thibault G: Plasma
endothelin in human essential hypertension. Am J Hypertens.
4:303–308. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arinami T, Ishikawa M, Inoue A, Yanagisawa
M, Masaki T, Yoshida MC and Hamaguchi H: Chromosomal assignments of
the human endothelin family genes: The endothelin-1 gene (EDN1) to
6p23-p24, the endothelin-2 gene (EDN2) to 1p34 and the endothelin-3
gene (EDN3) to 20q13.2-q13.3. Am J Hum Genet. 48:990–996.
1991.PubMed/NCBI
|
|
14
|
Pache M, Schwarz HA, Kaiser HJ, Wüest P,
Klöti M, Dubler B and Flammer J: Elevated plasma endothelin-1
levels and vascular dysregulation in patients with rheumatoid
arthritis. Med Sci Monit. 8:CR616–CR619. 2002.PubMed/NCBI
|
|
15
|
Kuryliszyn-Moskal A, Klimiuk PA,
Sierakowski S and Ciolkiewicz M: A study on vascular endothelial
growth factor and endothelin-1 in patients with extra-articular
involvement of rheumatoid arthritis. Clin Rheumatol. 25:314–319.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
La Montagna G, Cacciapuoti F, Buono R,
Manzella D, Mennillo GA, Arciello A, Valentini G and Paolisso G:
Insulin resistance is an independent risk factor for
atherosclerosis in rheumatoid arthritis. Diab Vasc Dis Res.
4:130–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stavropoulos-Kalinoglou A, Metsios GS,
Koutedakis Y, Nevill AM, Douglas KM, Jamurtas A, van Zanten JJ,
Labib M and Kitas GD: Redefining overweight and obesity in
rheumatoid arthritis patients. Ann Rheum Dis. 66:1316–1321. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panoulas VF, Douglas KM, Smith JP, Taffé
P, Stavropoulos-Kalinoglous A, Toms TE, Elisaf MS, Nightingale P
and Kitas GD: Polymorphisms of the endothelin-1 gene associate with
hypertension in patients with rheumatoid arthritis. Endothelium.
15:203–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wolfe F, Smythe HA, Yunus MB, Bennett RM,
Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M,
Clark P, et al: The American College of rheumatology 1990 criteria
for the classification of fibromyalgia. Report of the multicenter
criteria committee. Arthritis Rheum. 33:160–170. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okifuji A, Turk DC, Sinclair JD, Starz TW
and Marcus DA: A standardized manual tender point survey. I.
Development and determination of a threshold point for the
identification of positive tender points in fibromyalgia syndrome.
J Rheumatol. 24:377–383. 1997.PubMed/NCBI
|
|
21
|
Kim YA, Lee SS and Park K: Validation of a
Korean version of the fibromyalgia impact questionnaire. J Korean
Med Sci. 17:220–224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mendoza TR, Wang XS, Cleeland CS,
Morrissey M, Johnson BA, Wendt JK and Huber SL: The rapid
assessment of fatigue severity in cancer patients: Use of the brief
fatigue inventory. Cancer. 85:1186–1196. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Richter P, Werner J, Heerlein A, Kraus A
and Sauer H: On the validity of the beck depression inventory. A
review. Psychopathology. 31:160–168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ware JE Jr and Sherbourne CD: The MOS
36-item short-form health survey (SF-36). I. Conceptual framework
and item selection. Med Care. 30:473–483. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JT and Shin DG: A standardization
study of State-Trait Anxiety Inventory in Korea. New Med J.
21:1223–1229. 1978.
|
|
26
|
Lee H, Kim HK, Won H, Im J, Kwon JT and
Kim HJ: Genetic relationship between an endothelin 1 gene
polymorphism and lead-related high blood pressure. Mol Cell
Toxicol. 12:111–116. 2016. View Article : Google Scholar
|
|
27
|
Bauer V and Sotníková R: Nitric oxide-the
endothelium-derived relaxing factor and its role in endothelial
functions. Gen Physiol Biophys. 29:319–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goodwin AT, Smolenski RT, Gray CC,
Jayakumar J, Amrani M and Yacoub MH: Role of endogenous endothelin
on coronary reflow after cardioplegic arrest. J Thorac Cardiovasc
Surg. 122:1167–1173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Forbes JM, Jandeleit-Dahm K, Allen TJ,
Hewitson TD, Becker GJ and Jones CL: Endothelin and endothelin A/B
receptors are increased after ischaemic acute renal failure. Exp
Nephrol. 9:309–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flammer J, Pache M and Resink T:
Vasospasm, its role in the pathogenesis of diseases with particular
reference to the eye. Prog Retin Eye Res. 20:319–349. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pache M, Ochs J, Genth E, Mierau R, Kube T
and Flammer J: Increased plasma endothelin-1 levels in fibromyalgia
syndrome. Rheumatology (Oxford). 42:493–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SK, Kim KS, Lee YS, Park SH and Choe
JY: Arterial stiffness and proinflammatory cytokines in
fibromyalgia syndrome. Clin Exp Rheumatol. 28 6 Suppl 63:S71–S77.
2010.PubMed/NCBI
|
|
33
|
Bennett RM, Clark SR, Campbell SM, Ingram
SB, Burckhardt CS, Nelson DL and Porter JM: Symptoms of Raynaud's
syndrome in patients with fibromyalgia. A study utilizing the
Nielsen test, digital photoplethysmography and measurements of
platelet a2-adrenergic receptors. Arthritis Rheum. 34:264–269.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lapossy E, Gasser P, Hrycaj P, Dubler B,
Samborski W and Muller W: Cold-induced vasospasm in patients with
fibromyalgia and chronic low back pain in comparison to healthy
subjects. Clin Rheumatol. 13:442–445. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nelson JB, Chan-Tack K, Hedican SP,
Magnuson SR, Opgenorth TJ, Bova GS and Simons JW: Endothelin-1
production and decreased endothelin B receptor expression in
advanced prostate cancer. Cancer Res. 56:663–668. 1996.PubMed/NCBI
|
|
36
|
Ahn GY, Butt KI, Jindo T, Yaguchi H,
Tsuboi R and Ogawa H: The expression of endothelin-1 and its
binding sites in mouse skin increased after ultraviolet B
irradiation or local injection of tumor necrosis factor alpha. J
Dermatol. 25:78–84. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Druml W, Steltzer H, Waldhäusl W, Lenz K,
Hammerle A, Vierhapper H, Gasic S and Wagner OF: Endothelin-1 in
adult respiratory distress syndrome. Am Rev Respir Dis.
148:1169–1173. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Voerman HJ, Stehouwer CD, van Kamp GJ, van
Schijndel RJ Strack, Groeneveld AB and Thijs LG: Plasma endothelin
levels are increased during septic shock. Crit Care Med.
20:1097–1101. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kidd BL and Urban LA: Mechanisms of
inflammatory pain. Br J Anaesth. 87:3–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schaible HG, Ebersberger A and Von Banchet
GS: Mechanisms of pain in arthritis. Ann NY Acad Sci. 966:343–354.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rae GA and Henriques MG: Endothelins in
inflammationPro-Inflammatory and Anti-Inflammatory Peptides. Said
S: Marcel Dekker; New York, NY: pp. 163–202. 1998
|
|
42
|
Miyasaka N, Hirata Y, Ando K, Sato K,
Morita H, Shichiri M, Kanno K, Tomita K and Marumo F: Increased
production of endothelin-1 in patients with inflammatory
arthritides. Arthritis Rheum. 35:397–400. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Savoia C and Schiffrin EL: Inhibition of
the renin angiotensin system: Implications for the endothelium.
Curr Diab Rep. 6:274–278. 2016. View Article : Google Scholar
|
|
44
|
Libby P: Current concepts of the
pathogenesis of the acute coronary syndromes. Circulation.
104:365–372. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Blake JG and Ridker PM: Novel clinical
markers of vascular wall inflammation. Circ Res. 89:763–771. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sesso HD, Buring JE, Rifai N, Blake GJ,
Gaziano JM and Ridker PM: C-reactive protein and the risk of
developing hypertension. JAMA. 290:2945–2951. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Preston RA, Ledford M, Materson BJ,
Baltodano NM, Memon A and Alonso A: Effects of severe, uncontrolled
hypertension on endothelial activation: soluble vascular cell
adhesion molecule-1, soluble intercellular adhesion molecule-1 and
von Willebrand factor. J Hypertens. 20:871–877. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Blake GJ, Rifai N, Buring JE and Ridker
PM: Blood pressure, C-reactive protein, and risk of future
cardiovascular events. Circulation. 108:2993–2999. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thorand B, Löwel H, Schneider A, Kolb H,
Meisinger C, Fröhlich M and Koenig W: C-reactive protein as a
predictor for incident diabetes mellitus among middle-aged men:
Results from the MONICA Augsburg cohort study, 1984–1998. Arch
Intern Med. 163:93–99. 2003. View Article : Google Scholar : PubMed/NCBI
|