Introduction
Allergy is an acquired hypersensitivity reaction of
the immune system in response to normally innocuous environmental
substances, which manifests in several forms ranging from minor
urticaria, allergic rhinitis and conjunctivitis, and asthma to
life-threatening anaphylaxis (1,2),
causing a substantial socio-economic burden. Several therapeutic
trials have been performed to modulate allergy, however, with
limited success. These have included the prevention of Th2
responses, enhancement of Th1 responses and decreasing of IgE
concentrations (3).
Glucocorticoids are important and effective for the treatment of
anaphylactic disease. However, it is well known that the prolonged
use of high doses of glucocorticoids causes a range of side effects
(4). Therefore, the development of
novel and effective therapies for anaphylactic disease is
required.
Mast cells are important in allergic responses. An
allergic response is triggered by allergen sensitization, which
elicits Th2 immune responses, including an increase in levels of
interleukin (IL)-4 and IL-13, leading to immunoglobulin class
switching in B cells to produce predominantly IgE (5). Upon activation, mast cells undergo
degranulation and release a range of biologically active
substances, which are important in host defense and allergic
reactions. Among the immune mediators released from the mast cells,
histamine is one of the most well characterized and potent
mediators (1,6).
Bacillus Calmette-Guerin extract (BCGE), comprising
predominantly lipopolysaccharide (77.8%) and nucleic acids
(16.67%), is extracted from BCG using phenol. It has been shown
that the polysaccharide in BCGE promotes the maturation of
dendritic cells and the secretion of IL-12, which causes the
transformation from T0 to T1 and the secretion of interferon
(IFN)-γ (7). A previous study
indicated that nucleic acids from BCG can activate natural killer
cells, enhances the production of IFN-γ and IL-12, and inhibits the
production of IgE (8–10). In China, BCGE has been shown to be
clinically effective for regulating immunity, which enhances the
resistance of the body to anaphylactic disease, infectious diseases
and cancer (11–13).
However, the mechanisms remain to be fully
elucidated. Previous studies demonstrated that BCGE was able to
inhibit the production of IL-4 and IgE (14–16),
and impede the transcriptional activity of nuclear factor-κB
(16). The present study aimed to
investigate the effects and mechanisms of BCGE on IgE-mediated
anaphylaxis using in vivo and in vitro models.
Materials and methods
Animals
A total of 60 female Kunming mice aged 6–8 weeks,
weighing 18–22 g, 10 female Hartley guinea pigs aged 8–10 weeks,
weighing 350–450 g, and 56 female Wistar rats aged 8–10 weeks,
weighing 350–450 g, were purchased from the Center of Experimental
Animals of China Medical University (Shenyang, China). All the
animals were maintained under controlled conditions (temperature,
20–25°C; humidity 40–70%) with daily 12/12-h light/dark cycles. All
the animals were provided with food and water ad libitum.
All procedures were performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (17).
All animal experiments were performed in accordance
with the Institutional Animal Ethics Committee and Animal Care
Guidelines for Experimental Animals of China Medical
University.
Ovalbumin (OVA)-induced passive
cutaneous anaphylaxis (PCA)
The rats (n=8) were sensitized by intramuscular
injection of OVA (10 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and DPT vaccine (2 ml per rat; Chengdu Institute of
Biological Products Co., Ltd., Chengdu, China). Following
sensitization for 14 days, the whole blood samples were obtained
and placed at room temperature for 2 h, then were centrifuged at
1,000 × g for 20 min at room temperature, and serum was separated
and mixed; following which intradermal injection of another 48 rats
was performed using three titers (1:2, 1:8 and 1:32) in every rat
(0.1 ml at each titer). At 24 h post-injection of sensitized serum
(three titers/rat, 0.1 ml each titer), the rats were challenged by
an intravenous injection of a 1 ml mixture of Evan's blue and OVA
(10 mg OVA; 5 mg Evan's blue). BCGE (0.026, 0.077 and 0.232 mg/kg;
Zhejiang Pharmaceutical Co., Ltd., Zhejiang, China) or vehicle
(normal saline; NS) was administered by intravenous injection every
other day for 21 days prior to challenge. Evan's blue was extracted
from the skin tissues in 5 ml acetone and sodium chloride solution
(3:7, V/V) for 48 h and measured using a spectrophotometer (WF2
W-2000; Unico, Dayton, NY, USA) at 590 nm.
Dextran T40-induced scratching
behavior
Scratching behavior was induced in the mice by
intravenous injection of 1.25 mg/kg dextran T40. BCGE (0.025, 0.075
and 0.225 mg/kg) or vehicle (NS) was administered by intravenous
injection every other day for 21 days prior to treatment with
dextran T40. Dextran T40 was administered 1 h following the final
administration of BCGE or NS. The scratching frequency and duration
were detected within 30 min of induction by dextran T40. The
anticoagulant whole blood samples were centrifuged at 1,000 × g for
15 min at room temperature and plasma was obtained. Histamine
levels in plasma were also determined within 30 min of induction by
dextran T40 using a histamine ELISA kit (cat. no. EA31; Oxford
Biomedical Research, MI, USA).
OVA-induced intestinal tube
contraction
The guinea pigs were actively sensitized using OVA,
and the intestinal canal was prepared and placed into a Magnus bath
with tyrode solution and O2 at 37°C. Subsequently, one
end of the intestinal canal was connected to a hook at the other
end of the transducer with a 2 g load. The intestinal canal was
preincubated with BCGE (10−6, 10−5,
10−4, 10−3 and 10−2 mg/ml) or
vehicle control (tyrode) for 15 min prior to challenge with 1 mg/ml
OVA. Contractions were monitored over a period of 30 min.
Measurement of cytokine and OVA-sIg E
levels
The rats were sensitized by intramuscular injection
of OVA (10 mg/kg) and a DPT vaccine (2 ml per rat). BCGE (0.026,
0.077 and 0.232 mg/kg) or vehicle (NS) was administered by
intravenous injection 7 days prior to sensitization every other day
for 21 days. On days 7, 14 and 21 post-sensitization, the whole
blood samples were obtained and placed at room temperature for 2 h,
then were centrifuged at 1,000 × g for 20 min at room temperature,
and rat serum was collected for detection of the levels of IL-4 and
IFN-γ using an ELISA kit (cat. nos. R4000 and RIF00; R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol. The level of OVA-s IgE in the serum was
detected on the day 14 post-sensitization using an ELISA kit (cat.
no. E110-117; Bethyl Laboratories, Inc., Montgomery, TX, USA)
according to the manufacturer's protocol.
OVA-induced mast cell
degranulation
On day 14 following sensitization by OVA, the rats
were sacrificed and 10 ml Hank's solution was injected into the
abdominal cavity. Following gentle massaging of the abdomen for 3
min, the abdominal cavity was opened and peritoneal fluid was
collected. The peritoneal fluid was placed on ice for 10 min,
centrifuged at 120 × g for 5 min at 4°C, and the supernatant
discarded. The cells were suspended in Hank's solution
(2×105/ml. The mast cell suspension was placed into a
tube containing BCGE (10−6, 10−5,
10−4, 10−3 and 10−2 mg/ml) or
vehicle control (Hank's solution) and OVA for 5 min at 37°C. The
reaction was terminated on ice, and the mast cell solution was
placed onto a slide treated with 0.025% neutral red ethanol
solution to stain for 3 min. Following staining, 100 mast cells
were selected at random for calculating the percentage of
degranulation.
Measurement of cyclic adenosine
monophosphate (cAMP) levels in mast cells
The mast cell suspension was prepared as described
above, and was placed into a tube containing BCGE (10−6,
10−5, 10−4, 10−3 and
10−2 mg/ml) or vehicle control (Hank's solution) and OVA
for 10 min at 37°C, with the reaction terminated using frozen
acidified ethanol. The mixture was then placed in liquid nitrogen
for 4 min and melted in room temperature, which was repeated five
times and followed by centrifugation at 120 × g for 5 min at 4°C.
The supernatant was collected (0.9 ml), unwatered by decompression
and then dissolved in 1 ml PBS. The level of cAMP was detected
using a radioimmunoassay kit according to the manufacturer's
protocol.
Western blot analysis
Proteins were extracted from the mast cells treated
as above using a Nuclear and Cytoplasmic Protein Extraction kit
(Beyotime Institute of Biotechnology, Inc., Haimen, China). The
extracts were boiled for 5 min with loading buffer. The protein
concentration was determined using a bicinchoninic protein assay
kit (Beyotime Institute of Biotechnology). For western blotting
analysis, an equal quantity of total protein (30 µg/lane) was
loaded, and separated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis on a 12% gel and transferred onto polyvinylidene
difluoride membranes. The membranes were washed with PBST and 5%
skim milk for 1 h at room temperature. Following three washes with
PBST, the membranes were incubated with primary antibodies against
protein kinase A (PKA; cat. no. ab211265; 1:1,000 dilution) and
GAPDH (cat. no. ab9485; 1:2,500 dilution; Abcam, Cambridge, UK) at
4°C overnight. Following three further washes, the blots were
subsequently incubated with a with horseradish
peroxidase-conjugated secondary antibody (cat. no. E030120-02;
dilution, 1:2,000; EarthOx Life Sciences, Millbrae, CA USA) for 1 h
at room temperature. Following three final washes, the blots were
visualized using Beyo ECL Plus reagent (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol.
Densitometric analysis of the western blots was achieved using
ImageJ v1.48 software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Statistical analyses were performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation. One-way analysis of variance followed by
Student-Newman-Keuls test was used to determine significant
differences between treatment groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
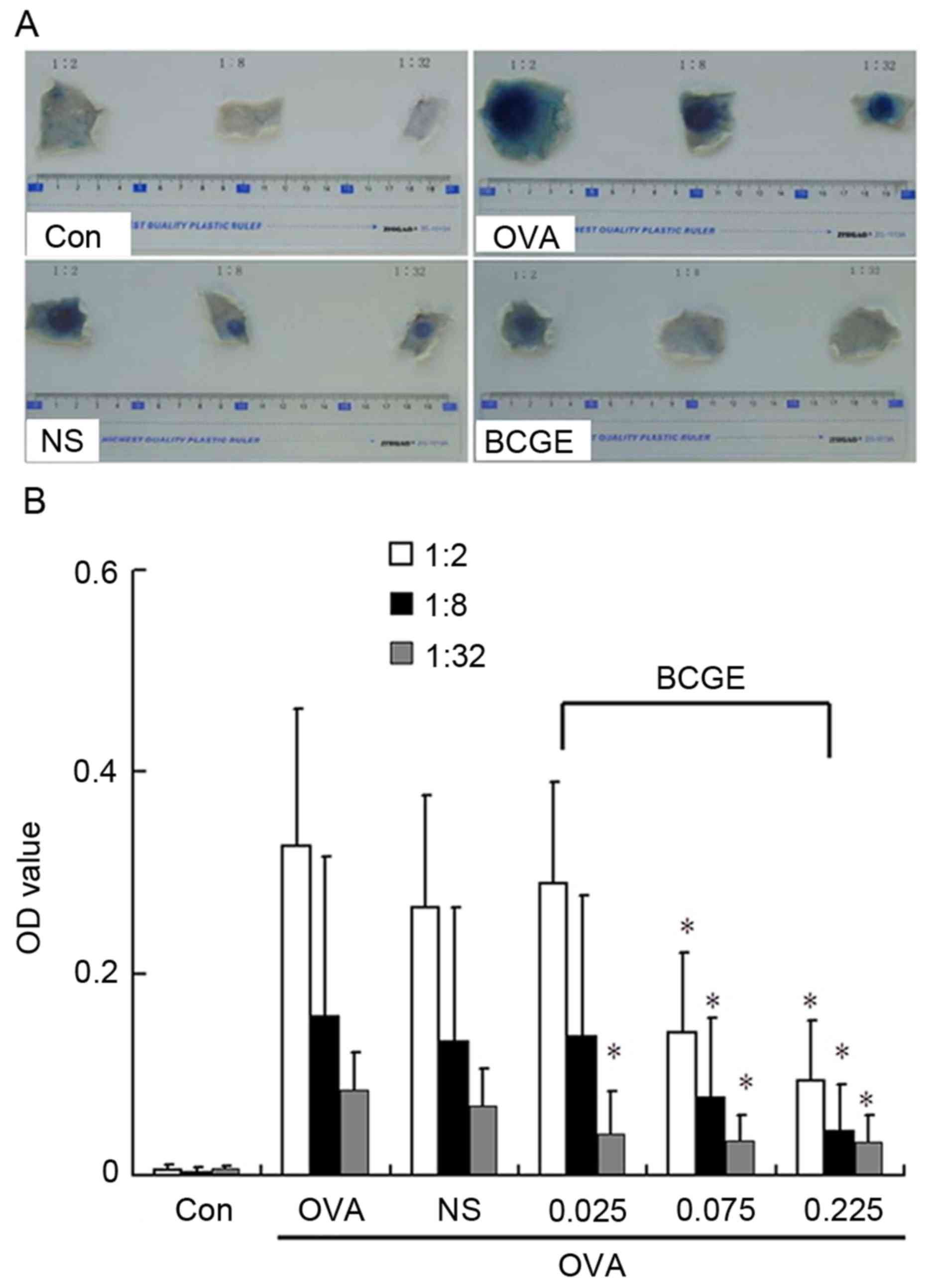
BCGE suppresses OVA-induced PCA
The rats were sensitized with an intradermal
injection of serum from OVA-sensitized rats and challenged with
intravenous OVA, After 30 min, Evan's blue dye resulted in intense
blue staining at the intradermal injection sites, compared with the
control (Fig. 1A). BCGE (0.026,
0.077 and 0.232 mg/kg) significantly suppressed OVA-induced PCA
vascular permeability (P<0.05) in a dose-dependent manner,
compared with the NS control (Fig. 1A
and B). Taken together, these data indicated that BCGE was able
to suppress mast cell-mediated anaphylactic reactions in
vivo.
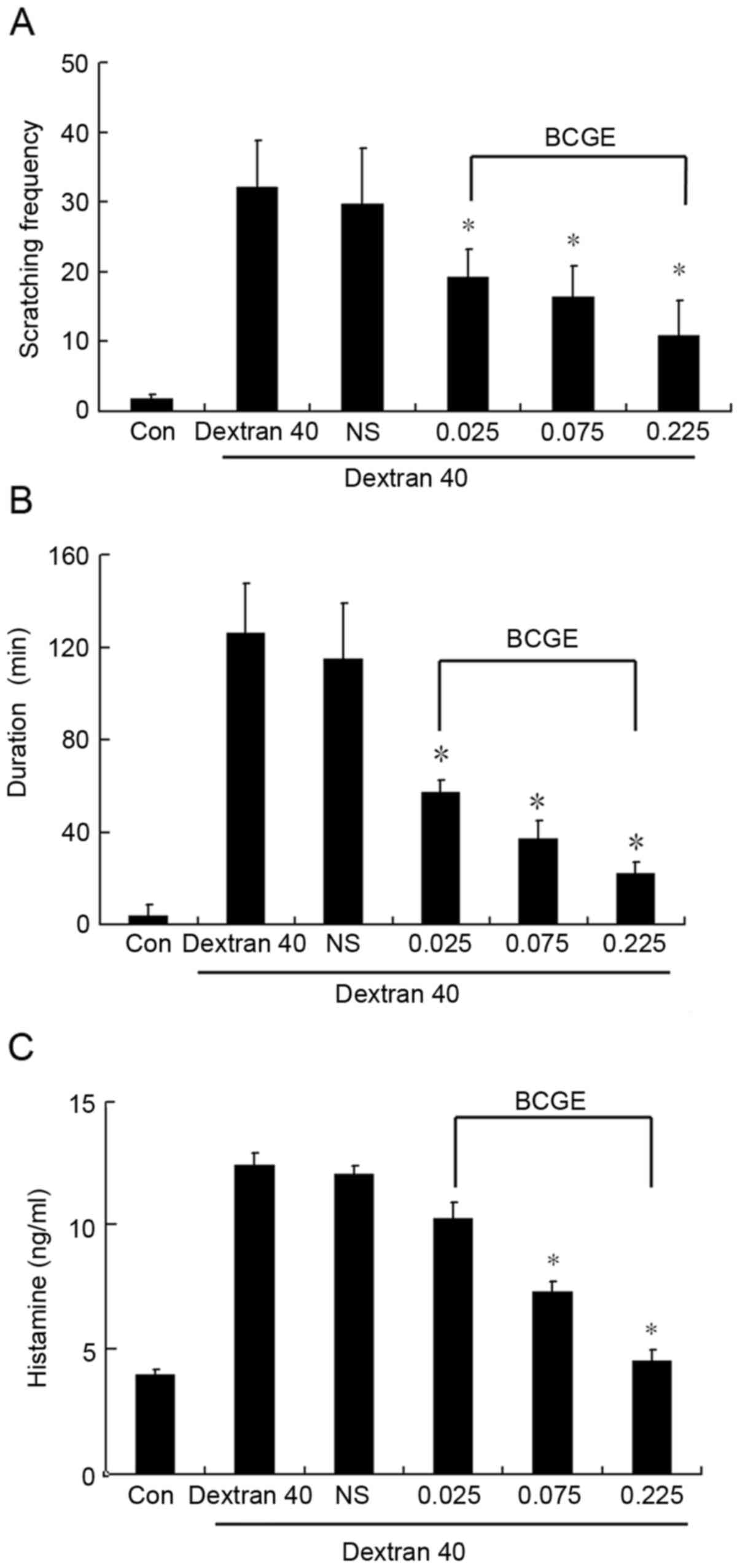
BCGE suppresses scratching behavior
induced by dextran T40
The mice treated with dextran T40 developed
significant scratching behavior and the duration of scratching
behavior was prolonged within the 30 min period (Fig. 2A and B), BCGE pretreatment (0.026,
0.077 and 0.232 mg/kg) significantly prevented scratching behavior
in a dose-dependent manner, compared with the NS control. In
addition, plasma histamine levels were significantly increased
following treatment with dextran T40, whereas BCGE caused a
dose-dependent suppression of the rise in plasma histamine levels,
compared with the NS control (Fig.
2C). These results suggested that BCGE inhibited mast
cell-mediated anaphylactic reactions in vivo.
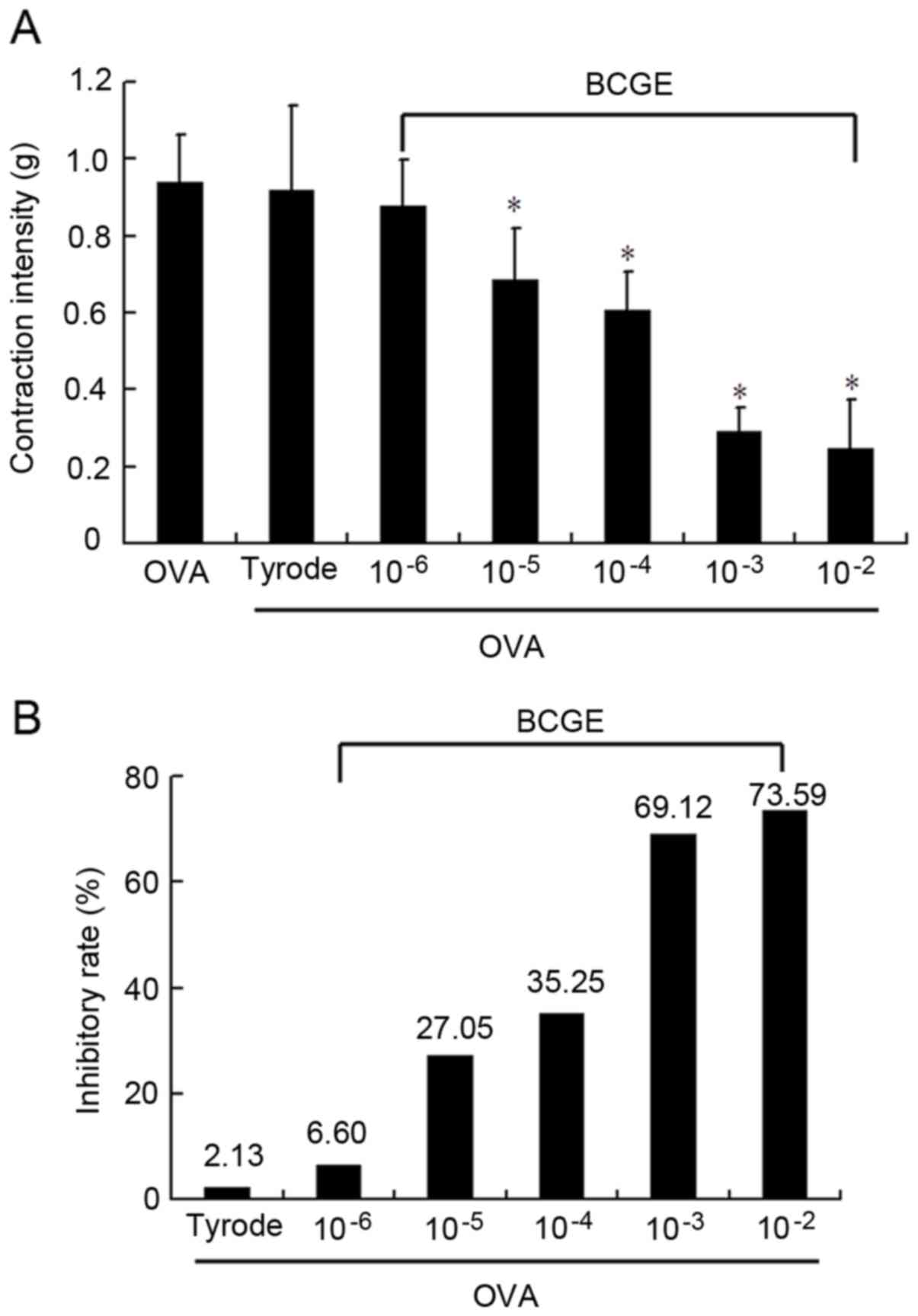
BCGE attenuates OVA-induced intestine
anaphylactic contraction
OVA at a concentration of 1 mg/ml induced strong and
sustained anaphylactic contractions of the sensitized guinea pig
intestinal tube, reaching 0.919±0.22 g within 30 min. BCGE
pretreatment (10−5-10−2 mg/ml) significantly
suppressed OVA-induced intestinal tube anaphylactic contraction, as
reflected by the reduction in contraction intensity in a
dose-dependent manner, compared with the tyrode solution control
(Fig. 3A and B). These findings
demonstrated the inhibitory effect of BCGE on inhibiting mast
cell-mediated anaphylactic reactions in an in vitro model of
anaphylaxis.
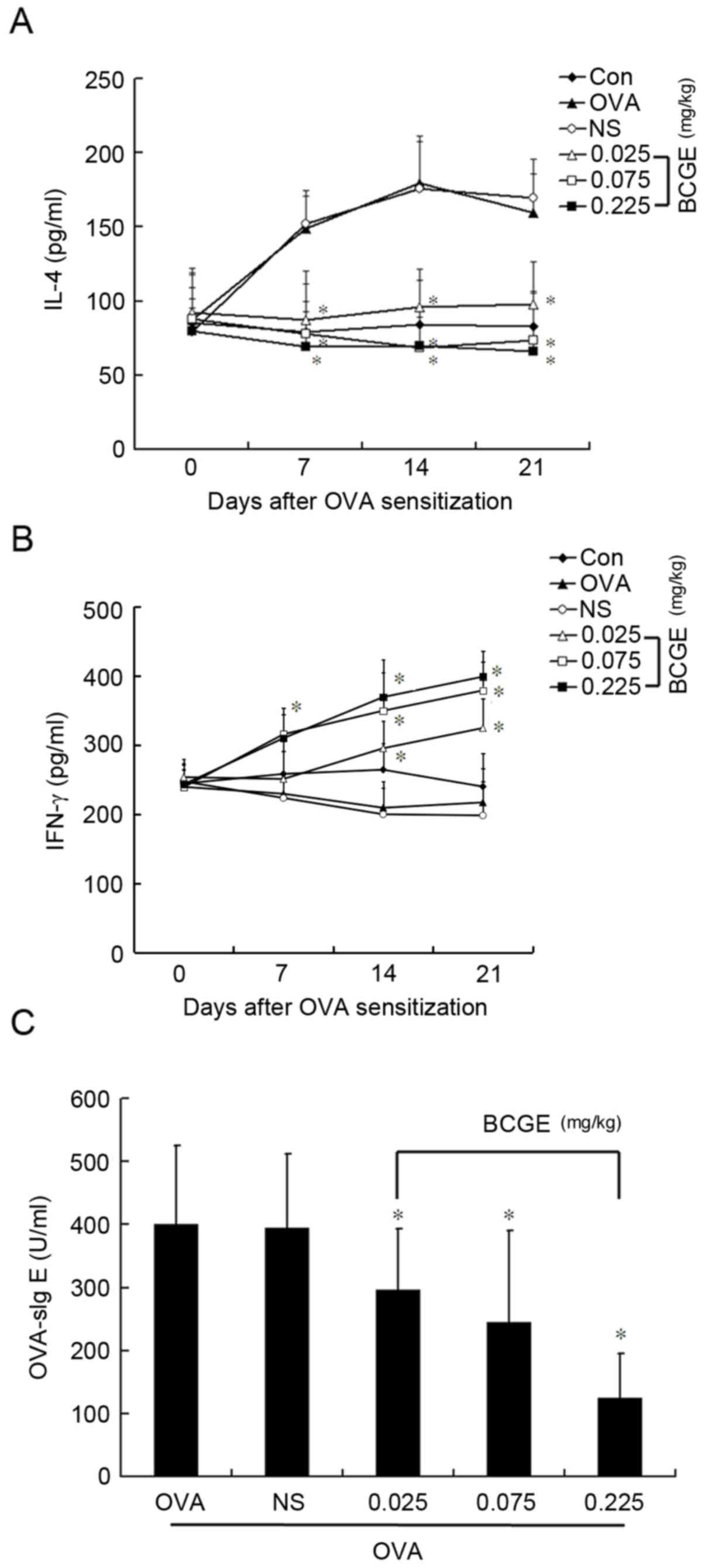
BCGE regulates OVA-induced cytokine
secretion and levels of OVA-sIg E
To obtain further insights into the molecular
mechanisms involved in the effect of BCGE on mast cell-mediated
anaphylaxis, the levels of IL-4 and IFN-γ were measured in the
plasma of OVA-sensitized rats. The level of IL-4 increased
significantly 7, 14 and 21 days following sensitization
(P<0.05), whereas no significant change in IFN-γ was found.
Intramuscular application of BCGE markedly reduced the OVA-induced
increase in IL-4 (P<0.05), whereas the level of IFN-γ increased
in a dose-dependent manner (Fig. 4A
and B). Furthermore, the intramuscular injection of BCGE
significantly reduced the level of OVA-sIg E 21 days following
sensitization (Fig. 4C).
BCGE suppresses OVA-induced mast cell
degranulation
The mast cells stimulated by OVA showed significant
degranulation, compared with the unstimulated mast cells. BCGE
(10−3 and 10−2) significantly prevented
OVA-induced mast cell degranulation in a concentration-dependent
manner (P<0.05; Fig. 5A and B).
To elucidate the mechanism underlying the effect of BCGE on
inhibiting OVA-induced immediate mast cell degranulation, the level
of cAMP and the expression of PKA were determined upon OVA
stimulation of the mast cells. The level of cAMP and expression of
PKA were decreased significantly 10 min following stimulation by
OVA. BCGE (10−3 and 10−2) significantly
inhibited the OVA-induced decrease of cAMP and PKA in a
concentration-dependent manner, compared with the vehicle control
(Fig. 5C and D).
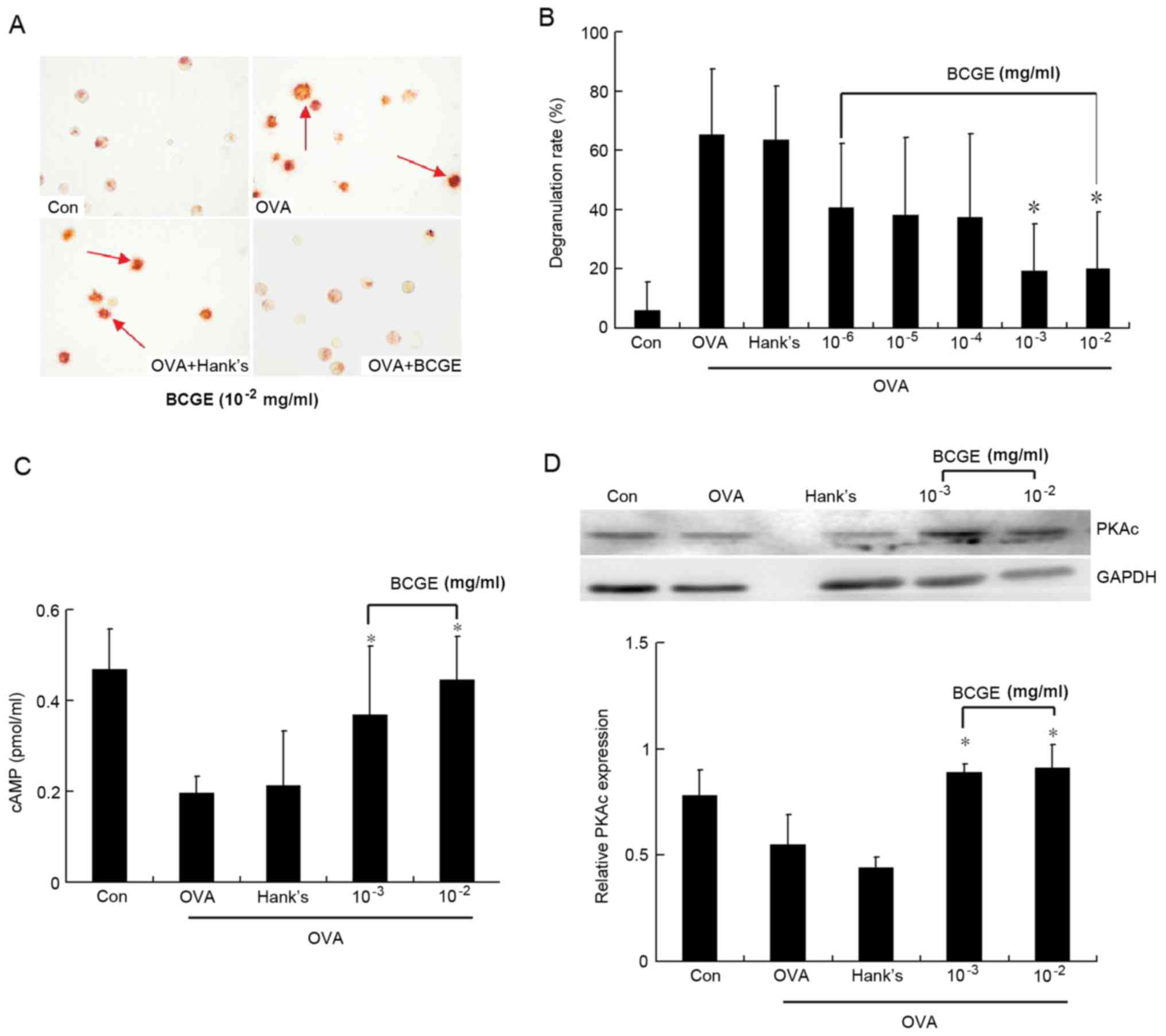 | Figure 5.Effects of BCGE on OVA-induced mast
cell degranulation. Mast cell degranulation was measured. (A)
Photomicrographs of the Con, OVA, OVA+Hank's and OVA+ BCGE
(10−2) groups are representative of three separate
experiments, magnification, ×20; red arrows indicate the
degranulation of mast cell. (B) Data are expressed as a percentage
of total cellular contents. (C) cAMP levels were detected using a
radioimmunoassay kit according to the manufacturer's protocol. (D)
Expression of PKA was detected using western blot analysis and
immunoblots shown are representative of three separate experiments
with similar patterns of results. Values are expressed as the mean
± standard deviation of three separate experiments. *P<0.05, vs.
Hank's. OVA, ovalbumin; BCGE, bacillus Calmette-Guerin extract;
Con, negative control; cAMP, cyclic adenosine monophosphate; PKA,
protein kinase A. |
Discussion
IgE-induced mast cell degranulation is important in
the development of allergic diseases. Mast cell-derived vasoactive
mediators, including histamine, serotonin and peptidoleukotrienes,
are rapidly released into the extracellular milieu (18,19).
Histamine appears to be most important to the manifestation of
vasodilation, increased vascular permeability, pruritus and smooth
muscle contraction in allergic anaphylaxis (18,19).
Strategies to prevent IgE-induced mast cell degranulation are
central to the identification of drugs for the treatment of
allergic diseases. For the first time, to the best of our
knowledge, the present study demonstrated that BCGE possessed
anti-allergic activity in mast cell-dependent in vivo and
in vitro models of anaphylaxis. BCGE inhibited IgE-induced
mast cell degranulation, likely via an increase in levels of cAMP
levels and expression of PKA.
PCA and skin itching are commonly used in in
vivo animal models to demonstrate IgE-mediated mast cell
degranulation and to evaluate potential anti-allergy compounds
(18,19). PCA is a localized cutaneous
allergic response, which results from allergen-induced vascular
hyperpermeability and plasma extravasation, representing clinical
features of urticaria. Skin itching is a generalized allergic
reaction manifested as skin itching resembling the clinical
features of anaphylaxis. Histamine, a preformed mediator rapidly
released upon mast cell degranulation, is critical to anaphylaxis
by binding to histamine receptors, leading to widening of
intercellular gap junctions via endothelial cell retraction and
vasodilation via increased production of nitric oxide (20,21).
In the present study, BCGE prevented OVA-induced cutaneous vascular
hyperpermeability in rats and skin itching in mice, and caused
elevation in levels of plasma histamine in mice. Allergen-induced
anaphylactic intestinal smooth muscle contraction is a widely used
in vitro model to demonstrate immediate mast cell
degranulation and the rapid release of spasmogens, including
histamine and peptidoleukotrienes, causing intestinal contraction.
In the present study, BCGE significantly suppressed OVA-induced
guinea pig intestine smooth muscle anaphylactic contraction over a
30 min interval.
To elucidate its anti-allergy mechanisms of action,
the present study investigated the direct effects of BCGE on the
degranulation of mast cells sensitized by OVA. BCGE significantly
reduced the number of degranulated mast cells in a
concentration-dependent manner. These findings indicated that BCGE
inhibited OVA-induced mast cell degranulation. Mast cell activation
and histamine release are tightly regulated by IgE from B cells,
and serum levels of IgE are elevated in proportion to the
development of allergic diseases (22–24).
In the present study, OVA-induced total IgE levels in serum were
reduced by BCGE. From these results, it was hypothesized that BCGE
alleviated OVA-induced mast cell degranulation through the
inhibition of IgE production. IgE synthesis by B cells is regulated
by Th2 cytokines, particularly IL-4 and IL-13. In humans, the
overproduction of IL-4 is a critical factor in allergic diseases.
Sensitization to an allergen reflects the ability of an allergen to
elicit a Th2 cell response, in which IL-4 and IL-13 drive the
production of IgE by promoting class-switch recombination in B
cells (1). In the results of the
present study, BCGE decreased levels of IL-4 and IL-13, which are
important in isotype switching to IgE. These results indicated that
BCGE reduced the serum levels of IgE by suppressing the Th2
response, particularly the production of IL-4 and IL-13.
The molecular mechanism underlying mast cell
degranulation is a complex process by which several proteins are
mediated. Each link is precisely controlled, with calcium,
calmodulin, three phosphoinositide signaling pathways, two glycerol
pathways and intracellular cyclic nucleotide signals involved in
signal transduction from the activation of FcεR to particle fusion
of vesicle rupture. cAMP in mast cells is involved in the
regulation of the degranulation process, and is correlated with the
physiological and pathological, cyclic chronic urticarial and
treatment outcomes, which can stabilize the mast cell membrane and
inhibit the release of allergic mediators (25,26).
PKA is a cAMP-dependent protein kinase, which can catalyze the
phosphorylation of serine and threonine residues in proteins
(27). It has been shown that the
activity of PKA is affected in the process of mast cell
degranulation, and certain drugs can inhibit the degranulation of
mast cells, partly due to regulation of the activity of PKA
(28,29). In the present study, it was found
that BCGE significantly reversed the OVA-induced reduction of cAMP
and expression of PKA in mast cells, which suggested that BCGE
suppressed mast cell degranulation by increasing the levels of cAMP
and the expression of PKA.
In conclusion, the present study demonstrated that
the intramuscular application of BCGE inhibited the development of
OVA-induced anaphylaxis in vitro and in vivo. The
inhibitory effect of BCGE was mediated by inhibiting IgE-mediated
mast cell degranulation. The mechanism underlying this therapeutic
effect of BCGE was mediated predominantly by a reduction in the
level of IL-4, an increase in the level of IFN-γ and the activation
of cAMP-PKA. These results suggested that BCGE may offer potential
as a therapeutic candidate for OVA-induced anaphylaxis.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of the People's Republic of
China (grant nos. 71273279 and 81501346).
References
|
1
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simons FE: Anaphylaxis. J Allergy Clin
Immunol. 125 2 Suppl 2:S161–S181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi EJ, Lee S, Hwang JS, Im SH, Jun CD,
Lee HS and Kim SH: DA-9601 suppresses 2, 4-dinitrochlorobenzene and
dust mite extract-induced atopic dermatitis-like skin lesions. Int
Immunopharmacol. 11:1260–1264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furue M, Terao H, Rikihisa W, Urabe K,
Kinukawa N, Nose Y and Koga T: Clinical dose and adverse effects of
topical steroids in daily management of atopic dermatitis. Br J
Dermatol. 148:128–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leung DY, Jain N and Leo HL: New concepts
in the pathogenesis of atopic dermatitis. Curr Opin Immunol.
15:634–638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galli SJ, Nakae S and Tsai M: Mast cells
in the development of adaptive immune responses. Nat Immunol.
6:135–142. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caramalho I, Lopes-Carvalho T, Ostler D,
Zelenay S, Haury M and Demengeot J: Regulatory T cells selectively
express toll-like receptors and are activated by
lipopolysaccharide. J Exp Med. 197:403–411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto S, Kuramoto E, Shimada S and
Tokunaga T: In vitro augmentation of natural killer cell activity
and production of interferon-alpha/beta and -gamma with
deoxyribonucleic acid fraction from Mycobacterium bovis BCG. Jpn J
Cancer Res. 79:866–873. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamamoto S, Yamamoto T, Kataoka T,
Kuramoto E, Yano O and Tokunaga T: Unique palindromic sequences in
synthetic oligonucleotides are required to induce IFN [correction
of INF] and augment IFN-mediated [correction of INF] natural killer
activity. J Immunol. 148:4072–4076. 1992.PubMed/NCBI
|
|
10
|
Fujieda S, Iho S, Kimura Y, Sunaga H,
Igawa H, Sugimoto C, Yamamoto S and Saito H: DNA from mycobacterium
bovis bacillus Calmette-Guérin (MY-1) inhibits immunoglobulin E
production by human lymphocytes. Am J Respir Crit Care Med.
160:2056–2061. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng D, Zheng B, Chen W and Chen Z: The
prophylactic effect of BCG polysaccharides nucleic acid on the
acute attack of chronic obstructive pulmonary disease. Hua Xi Yi Ke
Da Xue Xue Bao. 33:121–122. 2002.(In Chinese). PubMed/NCBI
|
|
12
|
Xiong C, Li Q, Lin M, Li X, Meng W, Wu Y,
Zeng X, Zhou H and Zhou G: The efficacy of topical intralesional
BCG-PSN injection in the treatment of erosive oral lichen planus: A
randomized controlled trial. J Oral Pathol Med. 38:551–558. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng T, Liu B, Duan X, Zhang T, Cai C and
Zeng G: Systematic review and cumulative analysis of the
combination of mitomycin C plus Bacillus Calmette-Guérin (BCG) for
non-muscle-invasive bladder cancer. Sci Rep. 7:31722017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin YJ, Song ZY, Hu Y, Qian XB, Wang XY
and He XY: Effects of montelukast and BCG-PSN on the expression of
STAT5b mRNA and IL-4 mRNA in blood mononuclearcells of rats with
asthma. Zhongguo Dang Dai Er Ke Za Zhi. 11:133–137. 2009.(In
Chinese). PubMed/NCBI
|
|
15
|
Zuo CX, Huang JH, Liao ZH, Lu JY and Chen
J: Effects of BCG-PSN on serum levels of IL-4 and IL-12 in patients
with condyloma acuminatum. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
29:690–692. 2004.(In Chinese). PubMed/NCBI
|
|
16
|
Sun M, Wang S, Zhao L, Zhao H, Yao W, Jin
W and Wei M: Suppression of 2,4-dinitrochlorobenzene-induced atopic
dermatitis by extract of Bacillus Calmette-Guerin. Mol Med Rep.
9:689–694. 2014.PubMed/NCBI
|
|
17
|
Cho A and Seok SH: Ethical guidelines for
use of experimental animals in biomedical research. J Bacteriol
Virol. 43:18–26. 2013. View Article : Google Scholar
|
|
18
|
Finkelman FD: Anaphylaxis: Lessons from
mouse models. J Allergy Clin Immunol. 120:506–515; quiz 516–517.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inagaki N and Nagai H: Analysis of the
mechanism for the development of allergic skin inflammation and the
application for its treatment: Mouse models for the development of
remedies for human allergic dermatitis. J Pharmacol Sci.
110:251–259. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Evora PR and Simon MR: Role of nitric
oxide production in anaphylaxis and its relevance for the treatment
of anaphylactic hypotension with methylene blue. Ann Allergy Asthma
Immunol. 99:306–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumar P, Shen Q, Pivetti CD, Lee ES, Wu MH
and Yuan SY: Molecular mechanisms of endothelial hyperpermeability:
Implications in inflammation. Expert Rev Mol Med. 11:e192009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuoka H, Maki N, Yoshida S, Arai M,
Wang J, Oikawa Y, Ikeda T, Hirota N, Nakagawa H and Ishii A: A
mouse model of the atopic eczema/dermatitis syndrome by repeated
application of a crude extract of house-dust mite Dermatophagoides
farinae. Allergy. 58:139–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SH, Jun CD, Suk K, Choi BJ, Lim H,
Park S, Lee SH, Shin HY, Kim DK and Shin TY: Gallic acid inhibits
histamine release and pro-inflammatory cytokine production in mast
cells. Toxicol Sci. 91:123–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang JS, Yoon WK, Han MH, Lee H, Lee CW,
Lee KH, Han SB, Lee K, Yang KH, Park SK and Kim HM: Inhibition of
atopic dermatitis by topical application of silymarin in NC/Nga
mice. Int Immunopharmacol. 8:1475–1480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sullivan TJ, Parker KL, Kulczycki A Jr and
Parker CW: Modulation of cyclic AMP in purified rat mast cells.
III. Studies on the effects of concanavalin A and anti-IgE on
cyclic AMP concentrations during histamine release. J Immunol.
117:713–716. 1976.PubMed/NCBI
|
|
26
|
Kurosawa M, Mori H, Nagai H and Koda A:
Change in the activity of the cyclic AMP-dependent protein kinase
in antigen-stimulated sensitized mast cells and effect of drugs
inhibiting allergic mediator release. Jpn J Pharmacol. 43:454–457.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurosawa M: Studies of phosphorylation in
rat mast cells (sixth report). Cyclic AMP-dependent protein
phosphorylation in rat mast cell granule membranes. Nihon
Yakurigaku Zasshi. 86:87–92. 1985.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sullivan TJ, Parker KL, Eisen SA and
Parker CW: Modulation of cyclic AMP in purified rat mast cells. II.
Studies on the relationship between intracellular cyclic AMP
concentrations and histamine release. J Immunol. 114:1480–1485.
1975.PubMed/NCBI
|
|
29
|
Lau HY and Chan CL: Modulation of
intracellular cyclic AMP in immunologically activated rat
peritoneal mast cells by prostaglandin D2. Inflamm Res. 50 Suppl
2:S61–S62. 2001.PubMed/NCBI
|