Introduction
Successful pregnancy relies on the subtle regulation
of the maternal immune system to allow for the tolerance of the
semi-allogenic fetus at the maternal-fetal interface. The
maternal-fetal interface is thought to be the origin of
preeclampsia (PE), a complex illness involving multiple organ
dysfunction that occurs during human gestation, affecting 4–5% of
all pregnancies (1). PE is
characterized by serious hypertensive disorder and proteinuria that
can result in restriction of fetal growth, premature birth, and
increase the risk of fetal and maternal morbidity and mortality.
Previous studies have demonstrated that proper immuno-regulation is
crucial during pregnancy and that maternal-fetal tolerance is
required for successful pregnancy (2,3).
Naive CD4+ T cells possess a high level
of plasticity and differentiate into T helper (Th)1 and Th2 and T
regulatory (Treg) cells to execute their immunological functions
(4,5). Specific master transcription factors
T-bet and GATA binding protein 3 (GATA3) control the Th1 and Th2
differentiation, respectively. Predominant Th1 type immunity has
been reported to be correlated with PE pregnancies; increased
Th1-type cytokines, including tumor necrosis factor (TNF)-α and
interferon (IFN)-γ, are detected in PE plasma (6). Th2 cells, a novel CD4+
lymphocyte subpopulation, produce anti-inflammatory cytokines
against pro-inflammatory cytokines released from Th1 cells to
maintain the immune tolerance state and accommodate the
semiallogeneic fetus at the maternal-fetal interface (7). Previous studies identified a new
subset of CD4+ helper cells, the Th17 lineage, with the
receptor-related orphan receptor (ROR)γt as the master
transcription factor and expressing pro-inflammatory cytokines
(8), including interleukin (IL)-17
and IL-22. In PE, the increased level of IL-17 in serum is
correlated with high blood pressure and immune disorders (9). Treg cells are forkhead box (FOX)
P3+CD4+CD25+ cells, along with
other transcription factors. FOXP3 is the master regulator
responsible for the development, maintenance and suppressive
function of Treg cells. Regarded as one of the most important
subsets of suppressor CD4+ T cells, Treg cells are
crucial for anti-inflammatory responses and confer immune-tolerance
during pregnancy (10).
T-bet, GATA3, RORγt, FOXP3 are the major T-cell
transcription factors that regulate the differentiation of T
lymphocyte subsets. Their coordinated regulation is crucial for
maintaining immune homeostasis during pregnancy (11). Previous studies have demonstrated
that over-expression of Th1 cells and an imbalance of Th1/Th2 cells
are predominant factors in the development of PE (12,13).
However, more recent findings have implied a less significant role
for Treg cells in the activation of inflammatory responses in PE.
Instead, it is hypothesized that Th1 and Th17 immunity can act
through the increased expression of IL-23 from dendritic cells
(14–16). To further define the precise roles
of Th1, Th2, Th17 and Treg, the present study used specific small
interfering (si)RNAs (17) to
inhibit RORγt and T-bet in PE CD4+ T lymphocytes and
thus investigate whether the siRNA-mediated knockdown of these
transcription factors may be able to correct the immune imbalance
and alleviate inflammation responses present in PE.
Materials and methods
Study participants
30 PE patients were recruited from the Department of
Obstetrics at the Nantong Women and Children Health Care Hospital
between October 2015 and January 2016 (mean age 27.8±2.5 years;
mean gestational age 34.1±2.3 weeks). Then 30 healthy pregnant
women (mean age 27.7±3.1 years; mean gestational age 33.7±1.1
weeks) were recruited simultaneously as a control group. There were
no significant differences between the two groups in terms of
maternal and gestational ages. PE diagnosis was defined as severe
gestational hypertension (systolic blood pressure of at least 140
mmHg and/or diastolic blood pressure of at least 90 mmHg on 2
occasions at least 4 h apart) and proteinuria (>30 mg/dl in at
least 2 random urine specimens) after 20 weeks' gestation. Patients
had no other obstetric or medical complications or histories of
autoimmune disorders. All experimental procedures using human
samples were performed with the approval of the Nantong Women and
Children Health Care Hospital Ethics Committee and all the
participants provided informed consent.
Cell preparation
Blood samples (10 ml) were collected from each
participant, peripheral blood mononuclear cells (PBMCs) were
isolated by Ficoll density gradient centrifugation at 2,000 × g for
20 min at 4°C (GE Healthcare Life Sciences, Little Chalfont, UK)
and CD4+ T cells were sorted by immune-magnetic beads
coated with anti-human CD4 antibodies (Miltenyi Biotec GmbH,
Bergisch Gladbach, Germany). CD4+ T cells were washed
twice and resuspended in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), placed in 24-well plates in
the presence of anti-CD3 (cat. no. 14-0037-82) monoclonal
antibodies (mAbs) 20 µl (diluted 1:10; eBioscience; Thermo Fisher
Scientific, Inc.) and anti-CD28 mAbs (cat. no. 16-0288-81) 10 µl
(diluted 1:2; eBioscience; Thermo Fisher Scientific, Inc.), and
cultured at 37°C in 5% CO2 to induce cell
differentiation. On day 3, 500,000 U/l IL-2 (Changsheng Gene
Pharmaceutical Co., Ltd., Changchun, China), was added to maintain
cell growth and half of the medium was renewed. On day 6, suspended
cells were collected.
siRNA design
siRNAs were designed using Primer 3 and the
specificity was tested using the Primer-BLAST program (http://www.ncbi.nlm.nih.gov/tools/primer-blast/).
Then, 3 pairs of RORγt or T-bet specific double-stranded siRNAs
were designed to target distinct sets of RNA sequences and the
siRNAs were chemically synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China). Details are presented in Table I.
 | Table I.siRNA sequences designed for T-bet
and RORγt with scrambled siRNA as the negative control. |
Table I.
siRNA sequences designed for T-bet
and RORγt with scrambled siRNA as the negative control.
| siRNA | Sense | Antisense |
|---|
|
siT-bet-918/940 |
UUAGUUUCCCAAAUGAAACUU |
GUUUCAUUUGGGAAACUAAAG |
|
siT-bet-1124/1146 |
UGUAAUCUCGGCAUUCUGGUA |
CCAGAAUGCCGAGAUUACUCA |
|
siT-bet-1837/1859 |
AAUAACACUGUUUCUGUUCCU |
GAACAGAAACAGUGUUAUUAG |
|
siRORγt-368/390 |
CCCGAGAUGCUGUCAAGUUTT |
AACUUGACAGCAUCUCGGGTT |
|
siRORγt-629/651 |
CCUCAUAUUCCAACAACUUTT |
AAGUUGUUGGAAUAUGAGGTT |
|
siRORγt-713/735 |
GGCAGAGAGAGCUUCUAUATT |
UAUAGAAGCUCUCUCUGCCTT |
| Scrambled
siRNA |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
Transfection of cells with siRNA
To establish a stable transfection and evaluate
transfection efficiency, 6-FAM-siRNA (Shanghai GenePharma Co.,
Ltd.) was transfected into cells simultaneously to measure the
efficiency of transfection. In all, 3 conditions were tested on
2.5×104 cells in each well of a 24-well plate with 30
pmol siRNA and 1, 2 or 3 µl DMRIE-C (Life Technologies; Thermo
Fisher Scientific, Inc.); following 6 h transfection, efficiency
was ascertained by fluorescence microscopy.
The medium was removed and cells were washed with
Optimem® I (Gibco; Thermo Fisher Scientific, Inc.) 1 day
prior to transfection. Cells were then suspended at a concentration
of 5×104/ml and 2.5×104 cells were seeded in
a 24-well plate and incubated at 37°C in 5% CO2 in a
humidified incubator. After 24 h and at 80% confluence the cells
were transfected with siRNA using DMRIE-C (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. There
were 5 groups: Blank control (BC) transfected with blank liposome,
negative control (NC) transfected with scrambled-siRNA, group 1
transfected with T-bet-siRNA, group 2 transfected with RORγt-siRNA
and group 3 transfected with T-bet-siRNA and RORγt-siRNA. DMRIE-C
reagent was diluted in Optimem® I medium in a 0.5 ml
Eppendorf tube, mixed at room temperature for 10 min and then
diluted siRNA was added to the mixture and incubated for 30 min to
form the siRNA/transfection reagent complex. The complex was then
added to the cells and cultured in serum-free medium. Following
transfection for 4–5 h at 37°C in 5% CO2, the medium in
each well was replaced with complete RPMI 1640 without antibiotics
and incubated for 48 h at 37°C prior to subsequent experiments.
SYBR Green reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen, Thermo Fisher Scientific, Inc.) and
complementary DNA (cDNA) was synthesized from 1 µg RNA using the
RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.). RT-qPCR was conducted with the aid of the
Rotor-gene Q Realtime PCR Platform and SYBR Green PCR Master Mix
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols. Oligonucleotide primer pairs used for the analysis were
as follows: T-bet, sense AAT GTG ACC CAG ATG ATT GTG C and
anti-sense ATG CTG GTG TCA ACA GAT GTG TAC; GAT A3, sense ACC GGC
TTC GGA TGC AA and anti-sense TGC TCT CCT GGC TGC AGA C; RORγt,
sense TAA CCA AAA ATG GAT GGG ATG and anti-sense, AAG CTG TGG CCT
CAA GGA T; FOXP3, sense GAG AAG GAG AAG CTG AGT GCC AT and
anti-sense AGC AGG AGC CCT TGT CGG AT; GAP DH, sense ACG GCA AATTC
AAC GGG ACA GTC A and anti-sense TGG GG GCA TCG GCA GAA GG. Primers
were synthesized by Shanghai GenePharma Co., Ltd. The
amplifications were conducted in a 36-well plate in a 20 µl
reaction volume containing 1X Real-time PCR Master Mix, 0.2 µM
primer, 1 U rTaq polymerase, 2 µl cDNA samples and RNase-free
water. The samples were run in triplicate for the target and for
house-keeping genes using the following conditions: Initial
denaturation for 3 min at 95°C, followed by a total of 40 cycles
(denaturation 12 sec at 95°C and annealing 40 sec at 62°C) and
extension for 30 sec at 72°C. Statistical analysis of the mean
value as the representative of each investigated gene and the
2−ΔΔCq method were used to analyze relative changes in
gene expression (18).
Western blot analysis
Cells were homogenized in lysate buffer containing
10 mM Tris-HCl (pH 7.4), 1% Triton X-100, 1% sodium deoxycholate, 5
mM EDTA, 1 mM PMSF, 10 mg/l aprotinin and 50 mg/l leupeptin. The
homogenate was then centrifuged at 12,000 × g for 10 min at 4°C to
collect the supernatant. Protein concentration was determined using
a BCA protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The supernatant was diluted in 5X SDS loading buffer and was
boiled. Then 20 µg of protein were subjected to SDS polyacrylamide
gel electrophoresis (5–10% gradient gels) and transferred to
polyvinylidine diflouride filter membranes. The membrane was then
blocked with 5% nonfat milk for 2 h at room temperature, and probed
with the following monoclonal antibodies: Anti-T-bet mAbs, cat. no.
sc21749; anti-GATA3-mAbs, cat. no. 268×; anti-RORγt mAbs, cat. no.
Sc6062; anti-FOXP3 mAbs, cat. no. sc-166212, at a dilution of
1:100. Membranes were washed in Tris-buffered saline containing
0.5% Tween-20 and incubated with 5% nonfat dry milk overnight at
4°C. Following incubation with the appropriate horseradish
peroxidase conjugated anti-human IgG monoclonal anti-body (diluted
1:100, cat. no. sc-2453), All monoclonal antibodies were purchased
from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Proteins were
detected with an enhanced chemiluminescent system (Pierce, Thermo
Fisher Scientific, Inc.).
Flow cytometry
For intracellular cytokine staining, lymphocytes
were collected and cultured in 96-well plates (200 µl and
2×109 cells per well). 500 ng/ml Ionomycin (Invitrogen;
Thermo Fisher Scientific, Inc.) and 10 ng/ml phorbol 12-myristate
13-acetate (PMA; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
were added and cultured at 37°C in 5% CO2 for 3 h, then
treated with 2.5 µmol monensin for 2 h. Cells were collected and
the supernatant was removed by centrifugation at 1,500 × g for 5
min at room temperature; surface staining was performed by
incubation with the corresponding fluorescently labelled antibodies
on ice for 15 min in the dark. Mouse monoclonal antibodies (mAb)
were used at a dilution of 1:20 and provided by eBioscience (Thermo
Fisher Scientific, Inc.). The antibodies were as follows:
fluoresein isothiocyanate (FITC)-labelled anti-human IL-17A (IgG1,
cat. no. 11-7179), anti-human interferon-γ (IgG1, cat no. 53-7319),
anti-human FOXP3 (IgG2a, cat no. 11-4776-41), phycoerythrin
(PE)-labelled IL-10 (IgG1, cat no. 53-7108-41), perCy5.5-labeled
anti-human CD8 (IgG1, cat no. 45-0088-41). Cells were washed twice
with staining buffer and fixed in 500 µl of 40 g/l paraformaldehyde
(PFA) at 4°C for 30 min. Cells were washed twice using staining
buffer, then intracellular staining was performed with the
Cytofix-Cytoperm buffer kit (BD Biosciences, Franklin Lakes, NJ,
USA). Following cell membrane permeation for 30 min at 4°C, the
supernatant was removed and incubated with fluorescently labeled
antibodies (BD Biosciences) against intracellular cytokines.
Following a final wash, 300 µl of 40 g/l PFA was added and flow
cytometric measures were performed on a FACSCalibur flow cytometer
(and analyzed using FlowJo software version 7.6.1; FlowJo, LLC.,
Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism v.5.0a (GraphPad Software, Inc. La Jolla, CA, USA). All data
were expressed as the mean ± standard error. Comparison between two
groups were performed by paired or unpaired Student's t-test, as
appropriate. One-way analysis of variance followed by Dunnett's
test were used for comparisons among multiple treatment groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of T-bet and RORγt mRNA is
increased in PE
The expression levels of T-bet and RORγt mRNA in
patients with PE were significantly higher compared with normal
pregnancies (P<0.05; Table
II), while no difference was observed in GATA3 and FOXP3 mRNA
levels among the groups. The ratio of T-bet: GATA3 and RORγt:FOXP3
mRNA in PBMC of patients with PE were significantly higher compared
with normal pregnancy patients (P<0.05), indicating a Th1 and
Th17-shift.
 | Table II.mRNA expression of transcription
factors in peripheral blood mononuclear cells from normal pregnancy
and PE. |
Table II.
mRNA expression of transcription
factors in peripheral blood mononuclear cells from normal pregnancy
and PE.
| Groups | n | T-bet | GATA3 | T-bet/GATA3 | RORγt | FOXP3 | RORγt/FOXP3 |
|---|
| Normal | 30 |
0.029±0.007 |
0.052±0.012 |
0.556±0.095 |
0.018±0.004 |
0.036±0.017 |
0.535±0.124 |
| PE | 30 |
0.057±0.011a |
0.037±0.010 |
1.695±0.746a |
0.026±0.003a |
0.031±0.007 |
0.896±0.203a |
Transfection efficiency
Activated CD4+ T cells transfected with
6-FAM-siRNA were observed using fluorescence microscopy. Cells
emitting a green fluorescent signal were considered to be
transfected successfully. The optimum transfection conditions
involved mixing 30 pmol siRNA with 2 µl DMRIE-C, giving a
transfection efficiency of ~73.6%.
siRNA efficiency
RT-qPCR and western blot analysis were performed to
screen for the most effective siRNAs. Gene and protein expression
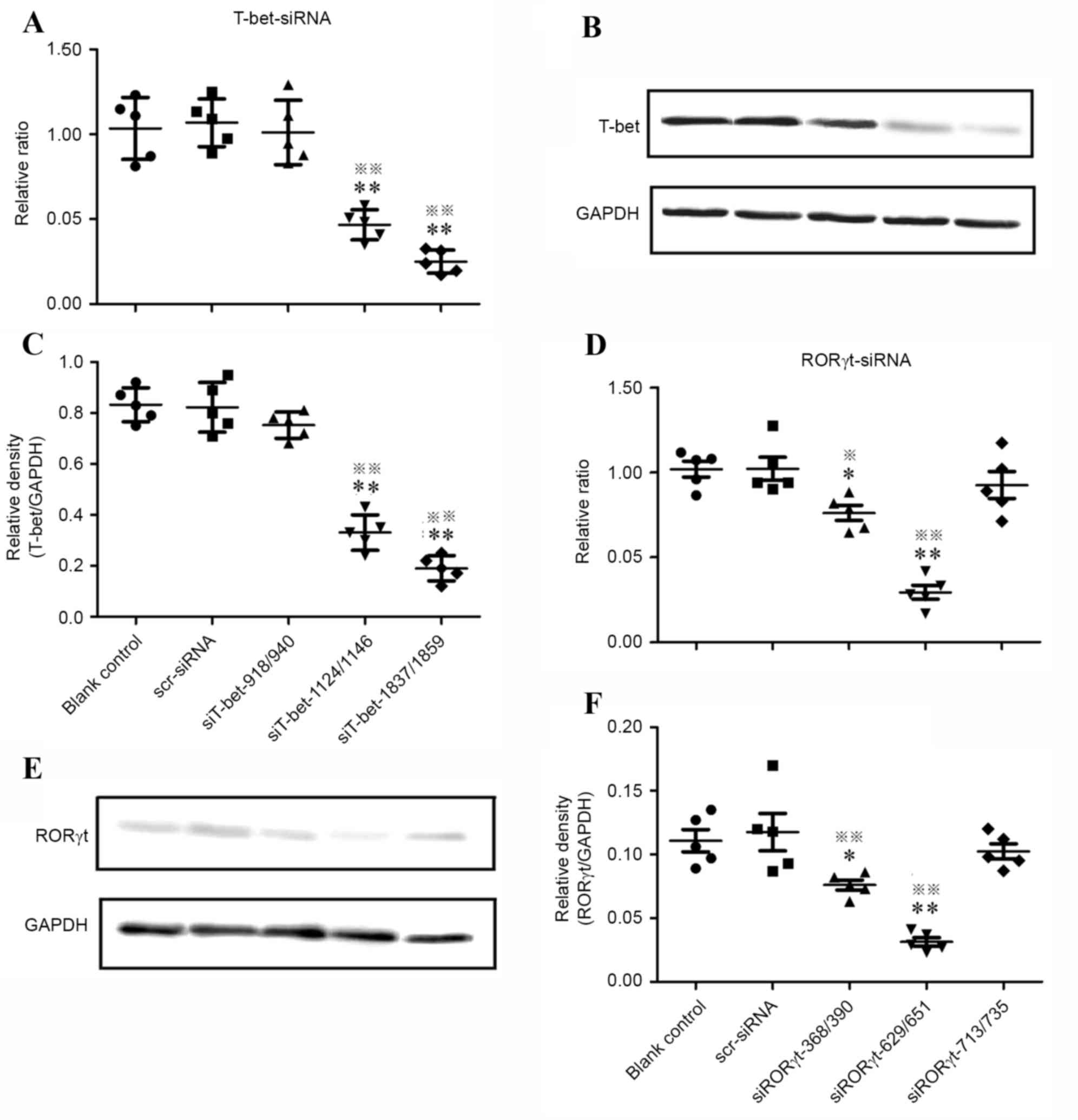
were normalized to GAPDH. As demonstrated in Fig. 1, in all the T-bet-specific siRNA
treated groups, no statistically significant differences were
observed in the levels of T-bet mRNA between the blank control and
nonspecific siRNA control (P>0.05). In the siT-bet-1837/1859
treated group, T-bet mRNA knockdown efficiency decreased by 75% and
the relative ratio was 0.250±0.068; western blot analysis
demonstrated that the relative density of T-bet protein was
0.250±0.068, significantly lower compared with the blank and
nonspecific siRNA controls (P<0.01; Fig. 1A-C).
Of all RORγt-specific siRNAs treated groups, the
siRORγt-629/651 group demonstrated over 70% knockdown efficiency
and the relative ratio was 0.293±0.091; western blot analysis
demonstrated that the level of RORγt protein expression was
0.049±0.013, significantly lower compared with the blank and
nonspecific siRNA controls (P<0.01; Fig. 1D-F), while no significant
difference was observed between the blank and nonspecific siRNA
controls.
These data suggested that siRORγt-629/651 and
siT-bet-1837/1859 were the most efficient and specific siRNAs to
downregulate mRNA levels, silence genes and inhibit protein
expression.
siRORγt-629/651 and siT-bet-1837/1859
affect the expression of T lymphocyte transcription factors
It was next investigated whether
siRORγt-629/651 or siT-bet-1837/1859 alone or in combination
affected T lymphocyte subsets. As demonstrated in Fig. 2, T-bet mRNA levels were decreased
by 70% and western blot analysis demonstrated a clear decrease in
protein expression (25–40%) in the T-bet knockdown and T-bet/RORγt
double knockdown groups compared with the BC and NC groups.
Comparison of mRNA and protein levels of the transcription factor
GATA3 demonstrated no significant differences among the 5 groups. A
decreased ratio of T-bet: GATA3 mRNA was observed in the T-bet and
T-bet/RORγt double knockdown groups compared with the BC and NC
groups. Knockdown of T-bet or RORγt alone led to a significant
decrease (30 and 60%, respectively) in RORγt gene expression along
with a significant increase (40%) in FOXP3 gene expression under
the same experimental conditions. The T-bet/RORγt double knockdown
demonstrated a marked decrease (75%) in RORγt gene expression and a
marked increase (50%) in FOXP3 gene expression compared with the BC
and NC groups. The ratio of RORγt: FOXP3 mRNA notably increased in
each siRNA group. To confirm these results, T lymphocyte cell
expression of transcription factors was analyzed by western blot
analysis and it was determined that T-bet knockdown assays led to a
decrease in RORγt and an increase in FOXP3 protein expression. This
change was more evident in the T-bet/RORγt double knockdown group.
It was also confirmed that treatment with siRNA did not alter the
expression of GATA3.
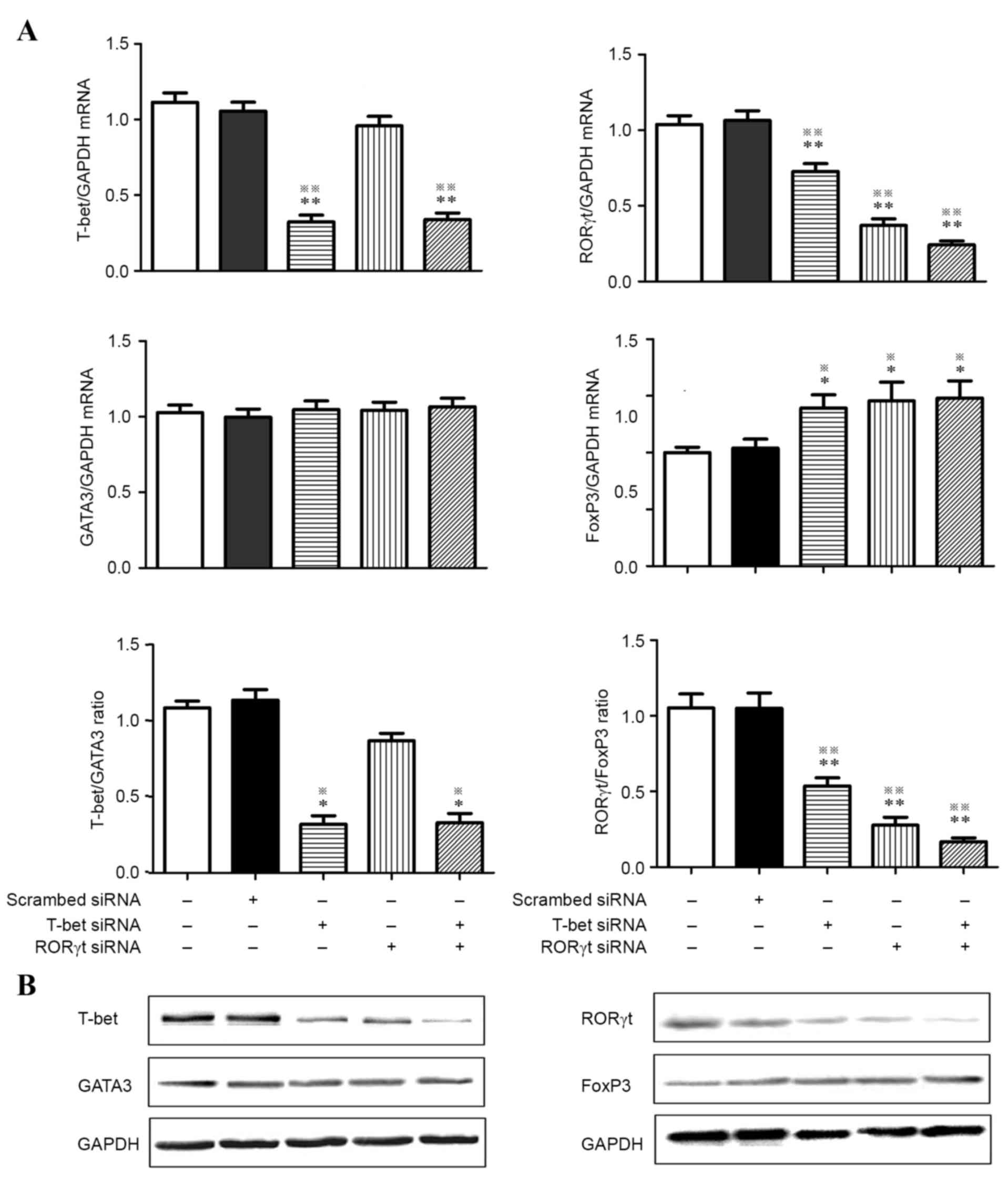 | Figure 2.RT-qPCR and western blot analysis were
performed to detect the expression of T-bet, GATA3, RORγt and FOXP3
in activated CD4+ T cells treated with T-bet-specific
siRNA and RORγt-specific siRNA alone or in combination. (A) Gene
expression of T-bet, GATA3, RORγt, FOXP3, T-bet:GATA3 ratio and
RORγt:FOXP3 ratio from activated CD4+ T cells of
preeclampsia. (B) Western blot analysis confirmed the results of
RT-qPCR. GAPDH served as internal control. *P<0.05, vs. blank
control; **P<0.01, vs. blank control; ※P<0.05, vs.
scr-siRNA; ※※P<0.01, vs. scr-siRNA. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; GATA3, GATA
binding protein 3; RORγt, retinoic acid receptor-related orphan
receptor γt; FOX, forkhead box; si, small interfering. |
RNAi knockdown of RORγt and T-bet
affects T lymphocyte cytokine expression
To investigate whether siRORγt-629/651 and
siT-bet-1837/1859 would influence the expression of cytokines by T
lymphocytes, flow cytometry was employed to identify
IFN-γ+ (Th1), IL-4+ (Th2), IL-17+
(Th17) and FOXP3+ IL-10+ (Treg); all of which
are cytokines secreted by subsets of CD4+ T cells.
Multi-cytokine analysis demonstrated that compared with the control
group and scrambled-siRNA, knockdown of T-bet alone
demonstrated a marked decrease in IFN-γ and IL-17 levels and an
increase in IL-10 levels. Knockdown of RORγt alone led to a marked
decrease in IL-17 and an increase in IL-10. In the T-bet/RORγt
double knockdown group, a marked decrease of IFN-γ and IL-17 and a
significant increase in IL-10 was observed. Notably, no significant
differences in IL-4 were detected among the 5 groups (Fig. 3).
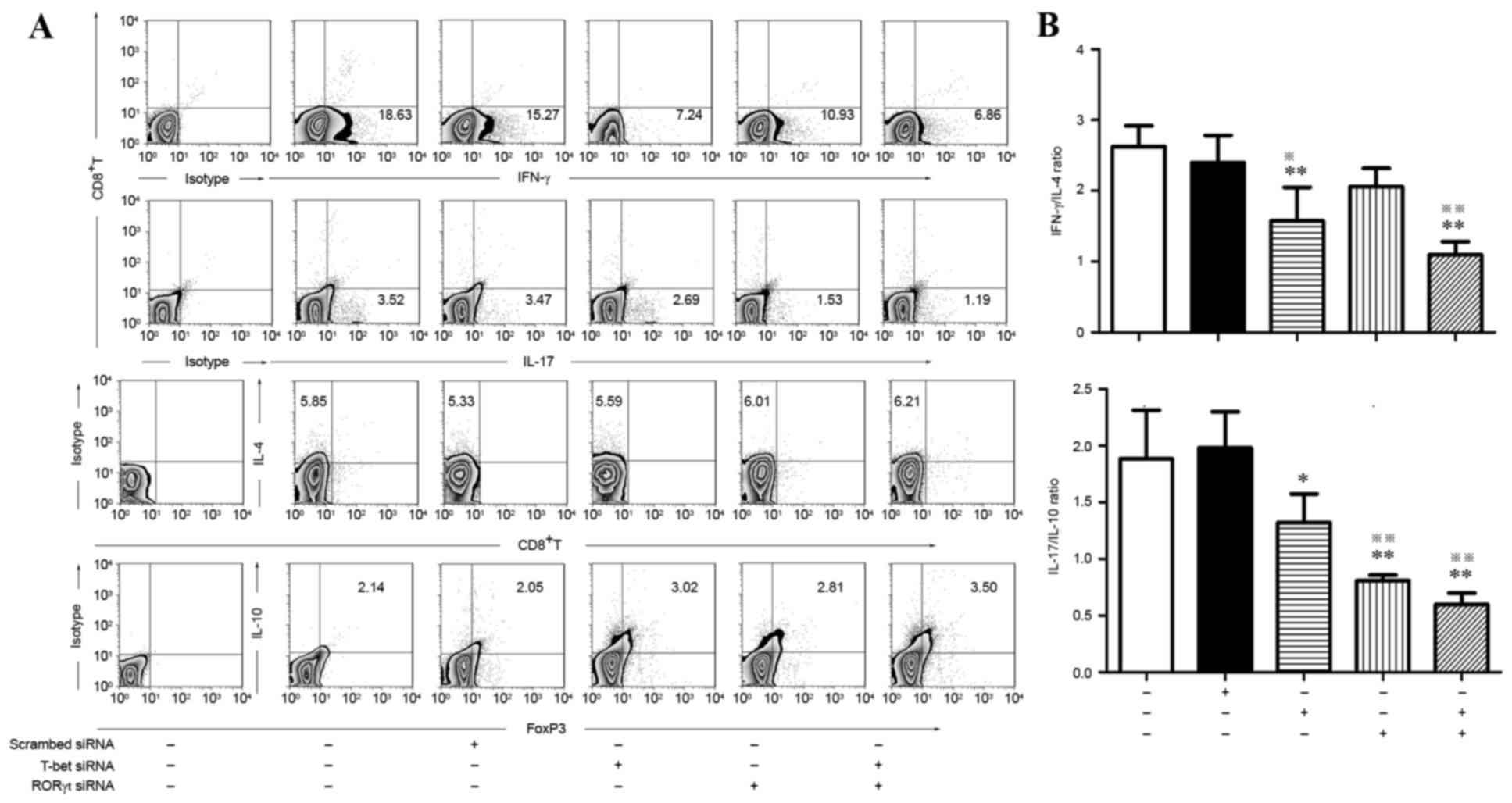 | Figure 3.Flow cytometric analysis of
IFN-γ+ (Th1), IL-4+ (Th2), IL-17+
(Th17) and FOXP3+ IL-10+ (Treg) subsets in
activated CD4+ T cells treated by T-bet-specific siRNA
and RORγt-specific siRNA alone or in combination. (A) Typical
results for IFN-γ+ Th1, IL-4+ Th2,
IL-17+ Th17 and IL-10+ Treg subsets detected
by flow cytometry. (B) Comparison of IFN-γ/IL-4 and IL-17/IL-10
between different groups. *P<0.05, vs. blank control;
**P<0.01, vs. blank control; ※P<0.05, vs.
scr-siRNA; ※※P<0.01, vs. scr-siRNA. IFN, interferon;
IL, interleukin; FOX, forkhead box; si, small interfering; RORγt,
retinoic acid receptor-related orphan receptor γt; scr,
scrambled. |
The ratio of IFN-γ:IL-4 and IL-17:IL-10 was analyzed
in each group and it was identified that the ratio of IFN-γ:IL-4
was significantly decreased in the T-bet knockdown and T-bet/RORγt
double knockdown groups compared with the control and
scramble-siRNA groups. The ratio of IL-17:IL-10 was significantly
lower in the siRORγt, siT-bet and combination groups compared with
the control and scramble-siRNA groups (Fig. 3).
Discussion
PE has been reported to result from an excessive
maternal response toward existing inflammation originating at the
maternal-fetal interface. A longitudinal study of the changes in
cytokine expression during pregnancy identified a general trend
toward enhanced expression of counter-regulatory cytokines and a
dampening of inflammatory cytokine expression (19), implying that changes in cytokine
balance may underlie the development of PE. Previous studies also
have demonstrated that increased expression of Th1 and
Th17-specific cytokines may be responsible for activating the
inflammatory response characteristic of this disorder (20,21).
Certain transcription factors are known to act as
‘master regulators’ in controlling the differentiation of T
lymphocyte subpopulations. The present study compared Th1, Th2,
Th17 and Treg cell transcription factor mRNA expression in normal
pregnancy and PE and identified that the expression of a
Th1-specific transcription factor, T-bet, and a Th17-specific
transcription factor, RORγt, were increased in PE; consistent with
previous studies suggesting a pro-inflammatory state (14,22).
Little is known about the function of transcription
factors in regulating T lymphocyte subsets. The present study
hypothesized that downregulation of transcription factor expression
in CD4+ T cells would affect the expression and balance
of T lymphocyte subsets. High-efficiency siRNA was used to inhibit
T-bet or RORγt gene expression and it was identified that RORγt
levels decreased not only following RORγt siRNA treatment, however
also in the T-bet siRNA treatment group and in the combination
treatment group, accompanied by marked increases of FOXP3
expression. A number of studies have demonstrated that Th17 cells
contribute to disease predominantly via changes to Th1 cells and
that suppression of T-bet ameliorates the severity of disease by
limiting the differentiation of Th17 cells via regulation of IL-23R
(16,23). These results imply that
IL-12-driven Th1 cells and IL-23-driven Th17 cells may be
associated and may arise from the same T-bet-expressing precursor.
T-bet not only serves an important role in regulating IL-12-driven
Th1 cells, however can additionally influence the differentiation
of IL-23-driven Th17 cells (24,25).
Functional associations between Treg and Th17 cells have been
reported and they are suggested to arise from common precursors in
a reciprocal manner and conduct diametrically opposing functions
(26). FOXP3 has been demonstrated
to bind to RORα and RORγt and inhibit their biological activity;
this can be blocked by exposure to TGF-β and lead to rapid
induction of RORγt (27,28). The results of the present study
support a reciprocal pathway between the Th1/Th17 and Th17/Treg
subsets, indicating a mechanism in support of the increased
expression of FOXP3 and decreased expression of RORγt observed in
the siT-bet group.
Cytokines are important mediators of immune
responses and their expression profiles are used to categorize the
functional status of the immune system. The present study has
demonstrated that Th1 and Th17-type immunity is present in PE. The
increased production of pro-inflammatory cytokines promotes
systemic inflammation and pro-inflammatory cytokines, such as
TNF-α, IL-6, IFN-γ and IL-17, which are produced by activated Th1
and Th17 cells, have been identified to contribute to widespread
vascular endothelial malfunction and vasospasm in PE. In the
vasculature, increased cytokines promote the expression of the
potent vasoconstrictor ET-1 and stimulate B-cell secrete agonistic
autoantibody to the Ang II to further increase the blood pressure
(29). In addition,
pro-inflammatory cytokines also can promote the expression of
adhesion molecules in the vasculature and lead to increased
vascular permeability that aggravates the symptoms of this disorder
(30). In the placenta,
overexpression of TNF-α and IL-6 leads to a significant reduction
in the activities of caspases in trophoblasts, suggesting that
excess and/or aberrant trophoblast death can be induced by
pro-inflammatory cytokines (31).
In contrast, anti-inflammatory cytokines produced by Th2 and Treg
cells, including IL-4, IL-5 and IL-10, can have protective effects
in pregnancy; their decreased expression may cause abnormal
trophoblast differentiation and invasion, immune maladaptation, and
exaggerated systemic inflammatory response, all of which have been
proposed to be responsible for the development of PE (32). In the present study, knockdown of
RORγt was identified to inhibit IL-17A expression, which was
followed by an increase in IL-10 expression. Knockdown of T-bet may
reverse the ratio of IL-17A:IL-10 and IFN-γ:IL-4 by depressing
IFN-γ and IL-17A expression and promoting IL-10 expression.
Knockdown of T-bet in combination with RORγt significantly
increased the expression of IL-10 and inhibited IL-17A. Based on
the propensity of Tregs to convert to a Th17 phenotype following
exposure to inflammatory cytokines, it has been proposed that
overexpression of pro-inflammatory cytokines may suppress the
tolerance system and thus shift the T lymphocyte balance from
pro-inflammatory to anti-inflammatory and ameliorate the imbalance
observed in PE (33).
In summary, the present study identified increased
expression of pro-inflammatory cytokines accompanied by enhanced
expression of the transcription factors T-bet and RORγt in patients
with PE compared with normal pregnant controls. In addition,
diminished anti-inflammatory cytokine expression was observed,
possibly accentuating the systemic inflammation observed in PE.
These results, along with previous observations led to the
hypothesis that restoration of an impaired immune response in PE
may be possible by regulating transcription factor expression. The
results of the present study demonstrated that siRORγt-629/650 and
siT-bet-1837/1859 were effective in silencing RORγt and T-bet gene
expression, respectively, suggesting that siRNA-mediated knockdown
of T-bet in combination with RORγt may be an effective therapeutic
treatment for regulating immune imbalance in PE. Further studies
will be required to elucidate the mechanism of CD4+ T
cell transcription factors in PE with the aim of developing
interventions to ameliorate the adverse outcomes of the
disease.
References
|
1
|
Sachan R, Patel ML, Dhiman S, Gupta P,
Sachan P and Shyam R: Diagnostic and prognostic significance of
serum soluble endoglin levels in preeclampsia and eclampsia. Adv
Biomed Res. 5:1192016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan DX, Duan J, Li MQ, Xu B, Li DJ and Jin
LP: The decidual gamma-delta T cells up-regulate the biological
functions of trophoblasts via IL-10 secretion in early human
pregnancy. Clin Immunol. 141:284–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao Y and Wang PL: Increased CD56(+) NK
cells and enhanced Th1 responses in human unexplained recurrent
spontaneous abortion. Genet Mol Res. 14:18103–18109. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
LaMarca B, Cornelius DC, Harmon AC, Amaral
LM, Cunningham MW, Faulkner JL and Wallace K: Identifying immune
mechanisms mediating the hypertension during preeclampsia. Am J
Physiol Regul Integr Comp Physiol. 311:R1–R9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slack M, Wang T and Wang R: T cell
metabolic reprogramming and plasticity. Mol Immunol. 68:507–512.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szabo SJ, Kim ST, Costa GL, Zhang X,
Fathman CG and Glimcher LH: Pillars article: A novel transcription
factor, T-bet, directs Th1 lineage commitment. Cell. 2000. 100:
655–669. J Immunol. 194:2961–2975. 2015.PubMed/NCBI
|
|
7
|
Tindemans I, Serafini N, Di Santo JP and
Hendriks RW: GATA-3 function in innate and adaptive immunity.
Immunity. 41:191–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ivanov II, McKenzie BS, Zhou L, Tadokoro
CE, Lepelley A, Lafaille JJ, Cua DJ and Littman DR: The orphan
nuclear receptor RORgammat directs the differentiation program of
proinflammatory IL-17+ T helper cells. Cell.
126:1121–1133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maddur MS, Miossec P, Kaveri SV and Bayry
J: Th17 cells: Biology, pathogenesis of autoimmune and inflammatory
diseases, and therapeutic strategies. Am J Pathol. 181:8–18. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kassan M, Wecker A, Kadowitz P, Trebak M
and Matrougui K: CD4+CD25+FOXP3 regulatory T
cells and vascular dysfunction in hypertension. J Hypertens.
31:1939–1943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jianjun Z, Yali H, Zhiqun W, Mingming Z
and Xia Z: Imbalance of T-cell transcription factors contributes to
the Th1 type immunity predominant in pre-eclampsia. Am J Reprod
Immunol. 63:38–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tarnowska-Madra U, Leibschang J, Kowalska
B, Filipp E, Kozar A, Nimer A and Maciejewski T: Levels of
immunoreactive cytokines in serum of women with preeclampsia or
severe pregnancy hypertension. Ginekol Pol. 81:192–196.
2010.PubMed/NCBI
|
|
13
|
Zhang Z, Liu H, Shi Y, Xu N, Wang Y, Li A
and Song W: Increased circulating Th22 cells correlated with Th17
cells in patients with severe preeclampsia. Hypertens Pregnancy.
36:100–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Darmochwal-Kolarz D, Kludka-Sternik M,
Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B and
Oleszczuk J: The predominance of Th17 lymphocytes and decreased
number and function of Treg cells in preeclampsia. J Reprod
Immunol. 93:75–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Figueiredo AS and Schumacher A: The T
helper type 17/regulatory T cell paradigm in pregnancy. Immunology.
148:13–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng T, Qin H, Wang L, Benveniste EN,
Elson CO and Cong Y: Th17 cells induce colitis and promote Th1 cell
responses through IL-17 induction of innate IL-12 and IL-23
production. J Immunol. 186:6313–6318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gish RG, Yuen MF, Chan HL, Given BD, Lai
CL, Locarnini SA, Lau JY, Wooddell CI, Schluep T and Lewis DL:
Synthetic RNAi triggers and their use in chronic hepatitis B
therapies with curative intent. Antiviral Res. 121:97–108. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Denney JM, Nelson EL, Wadhwa PD, Waters
TP, Mathew L, Chung EK, Goldenberg RL and Culhane JF: Longitudinal
modulation of immune system cytokine profile during pregnancy.
Cytokine. 53:170–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Su L, Zhu T and Shen M: Changes in
the subsets of dendritic cells and T cells in peripheral blood of
patients with preeclampsia. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
29:72–75. 2013.(In Chinese). PubMed/NCBI
|
|
21
|
Wang J, Tao YM, Cheng XY, Zhu TF, Chen ZF,
Yao H and Su LX: Dendritic cells derived from preeclampsia patients
influence Th1/Th17 cell differentiation in vitro. Int J Clin Exp
Med. 7:5303–5309. 2014.PubMed/NCBI
|
|
22
|
Vargas-Rojas MI, Solleiro-Villavicencio H
and Soto-Vega E: Th1, Th2, Th17 and Treg levels in umbilical cord
blood in preeclampsia. J Matern Fetal Neonatal Med. 29:1642–1645.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kobayashi T, Okamoto S, Hisamatsu T,
Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A,
Koganei K, et al: IL23 differentially regulates the Th1/Th17
balance in ulcerative colitis and Crohn's disease. Gut.
57:1682–1689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen Y, Langrish CL, McKenzie B,
Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W,
Churakovsa T, Low J, Presta L, et al: Anti-IL-23 therapy inhibits
multiple inflammatory pathways and ameliorates autoimmune
encephalomyelitis. J Clin Invest. 116:1317–1326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bettelli E and Kuchroo VK: IL-12- and
IL-23-induced T helper cell subsets: Birds of the same feather
flock together. J Exp Med. 201:169–171. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng SG: Regulatory T cells vs Th17:
differentiation of Th17 versus Treg, are the mutually exclusive? Am
J Clin Exp Immunol. 2:94–106. 2013.PubMed/NCBI
|
|
27
|
Postigo J, Iglesias M, Álvarez P, Jesús
Augustin J, Buelta L, Merino J and Merino R: Bone morphogenetic
protein and activin membrane-bound inhibitor, a transforming growth
factor β rheostat that controls murine treg cell/Th17 cell
differentiation and the development of autoimmune arthritis by
reducing interleukin-2 signaling. Arthritis Rheumatol.
68:1551–1562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou L, Lopes JE, Chong MM, Ivanov II, Min
R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al:
TGF-beta-induced FOXP3 inhibits T(H)17 cell differentiation by
antagonizing RORgammat function. Nature. 453:236–240. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou CC, Irani RA, Dai Y, Blackwell SC,
Hicks MJ, Ramin SM, Kellems RE and Xia Y: Autoantibody-mediated
IL-6-dependent endothelin-1 elevation underlies pathogenesis in a
mouse model of preeclampsia. J Immunol. 186:6024–6034. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Liu G, Ma J, Zhou L, Zhang Q and Gao
L: Lack of IL-6 increases blood-brain barrier permeability in
fungal meningitis. J Biosci. 40:7–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Siwetz M, Blaschitz A, El-Heliebi A, Hiden
U, Desoye G, Huppertz B and Gauster M: TNF-α alters the
inflammatory secretion profile of human first trimester placenta.
Lab Invest. 96:428–438. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu F, Guo J, Tian T, Wang H, Dong F,
Huang H and Dong M: Placental trophoblasts shifted Th1/Th2 balance
toward Th2 and inhibited Th17 immunity at fetomaternal interface.
APMIS. 119:597–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raghupathy R: Cytokines as key players in
the pathophysiology of preeclampsia. Med Princ Pract. 22 Suppl
1:S8–S19. 2013. View Article : Google Scholar
|