Introduction
Schwann cells (SCs) are the predominant glial cells
in the peripheral nervous system and serve a vital role in the
growth and regeneration of peripheral nerves (1). Following nerve injury, the denervated
SCs begin to dedifferentiate, proliferate and migrate, and
subsequently form linear arrays in the original endoneurial tubes
termed bands of Büngner; these arrays provide a remote channel for
axon regeneration (2). Previous
studies have indicated that nerve regenerative potential may be
enhanced if cells, particularly SCs, cover the bio-artificial tube
(3,4). The complex of scaffolding and SCs
acts as the core of tissue-engineered nerves and SCs are the most
important seed cells of tissue-engineered nerves (5). Therefore, developing an efficient
method of SC culture is essential for the success of nerve repair
techniques.
The major obstacle to SC purification is the removal
of fibroblasts. Numerous fibroblast removal strategies have been
reported including serum-free medium treatment (6), anti-mitotic treatment (7), immunoselective methods (8) and differential detachment methods
(9), amongst others. Another
method is based on pre-degeneration following peripheral nerve
injury (10). Pre-degeneration may
be performed in vivo and in vitro. In vivo
(compared with in vitro) pre-degeneration is more cumbersome
to perform as it requires two surgical interventions and takes
longer to accomplish (11). This
method is not applicable to humans for ethical reasons; therefore,
the present study focused on developing an in vitro
pre-degeneration method. During in vitro pre-degeneration,
harvested nerve pieces were placed in a specialized medium prior to
enzymatic digestion. The purpose of this procedure was to stimulate
the proliferation of SCs, and to promote fibroblast migration from
the nerve pieces. A previous study has indicated that, compared
with immediate culture, performing in vitro pre-degeneration
prior to cell culture of the harvested cells may increase SC purity
and yield (11). The conditions of
pre-degeneration may impact the purity and yield of cultivated SCs
(12). Kraus et al
(13) demonstrated that in
vitro pre-degeneration for 7 days increased the yield of SCs by
~50%; however, different periods of pre-degeneration had limited
effect on the purity of the SCs. Furthermore, pituitary extracts
(14) and neuregulins (15) were shown to stimulate SC
proliferation.
Based on previous experience using multiple factors
as SC proliferation promoters (16), basic fibroblast growth factor
(b-FGF), heregulin and forskolin were selected to aid nerve
pre-degeneration and SC culture, which was performed over a 7-day
period. The present study reported a novel technique for obtaining
an enriched population of SCs from mature Rhesus monkey nerves,
using in vitro pre-degeneration of these nerves in the
presence of SC proliferation promoters.
Materials and methods
Ethics statement
The present study was approved by the ethics
committee of Shanghai Jiao Tong University School of Medicine
(Shanghai, China). All surgical interventions, treatments and
postoperative animal care procedures were performed in accordance
with the Guide for the Care and Use of Laboratory Animals. Three
adult Rhesus monkeys (4-year-old males, weighing 5.88–8.24 kg) were
purchased from Ping'an Animal Reproduction Center of Chengdu
(reproduction license no. SCXK 2008–013; Chengdu, China). All
monkeys were individually housed at the Department of Laboratory
Animal Sciences at Shanghai Jiao Tong University School of
Medicine, at a temperature of 21°C with 55% humidity under a 12-h
light/dark cycle with free access to food and water.
Materials
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from Hyclone (GE Healthcare Life
Sciences, Little Chalfont, UK). Collagenase NB4 was obtained from
Serva Electrophoresis GmbH (Heidelberg, Germany). Neutral protease
Dispase II was from Roche Applied Science (Madison, WI, USA).
Heregulin-β1 and b-FGF were sourced from PeproTech, Inc. (Rocky
Hill, NJ, USA). Forskolin was purchased from Cayman Chemical
Company (Ann Arbor, MI, USA). Cytosine-B-arabinoside hydrochloride
(Ara-C), penicillin-streptomycin and 0.25% trypsin-EDTA were
obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). All the culture plates were BD Falcon; BD Biosciences
(Franklin Lakes, NJ, USA). The compositions of the culture media
used for SC isolation are presented in Table I. The following primary antibodies
were used for immunofluorescence and flow cytometry: Rabbit
anti-S100 calcium binding protein B (S100β; cat no. Z0311)
polyclonal antibody (Dako; Agilent Technologies, Santa Clara, CA,
USA), anti-glial fibrillary acidic protein (GFAP; cat no. ab7260)
polyclonal antibody and anti-nerve growth factor receptor (P75NTR;
cat no. ab8874) polyclonal antibody (Abcam, Cambridge, UK). The
Alexa Fluor 488-conjugated goat anti-rabbit-IgG secondary antibody
(cat no. R37116) was purchased from Invitrogen (Thermo Fisher
Scientific, Inc.).
 | Table I.Culture media composition. |
Table I.
Culture media composition.
| Culture medium | Composition |
|---|
| Basal medium | DMEM |
|
| 10% FBS |
|
| 1%
penicillin/streptomycin |
| Schwann cell
culture medium | DMEM |
|
| 10% FBS |
|
| 1%
penicillin/streptomycin |
|
| 2 µM forskolin, 20
ng/ml |
|
| heregulin-β1 and 20
ng/ml |
|
| b-FGF |
| Purification
medium | DMEM |
|
| 2.5% FBS/10 µM
Ara-C |
|
| 1%
penicillin/streptomycin |
|
| 2 µM forskolin, 20
ng/ml |
|
| heregulin-β1 and 20
ng/ml |
|
| b-FGF |
Harvesting of common peroneal nerve
and in vitro pre-degeneration
All surgical procedures were performed under sterile
surgical conditions and general anesthesia. The animals were
anesthetized by intramuscular injection of a mixture of ketamine
(20 mg/kg) and xylazine (2 mg/kg). Briefly, the bilateral common
peroneal nerve was exposed with an incision that extended from the
lower interface along the biceps femoris distally towards the
popliteal fossa. Two 7-cm segments of the common peroneal nerves
were then excised. The nerves were immediately washed three times
with ice-cold phosphate-buffered saline (PBS, pH 7.2) supplemented
with 2% FBS. The epineurium was gently stripped off using
fine-pointed forceps and microscissors under an anatomical
microscope. The common peroneal nerve segments were cut into short
pieces (2–3 mm in length), placed into one 6-well plate and
cultured first in basal medium (BM) for 3 h and then switched to
Schwann cell culture medium (SCCM). The cells were kept in a
humidified atmosphere of 5% CO2 at 37°C. The nerve
pieces were monitored for evidence of in vitro
pre-degeneration on the 2nd, 5th and 7th day of culture. The
finalized process of SC isolation and purification is summarized
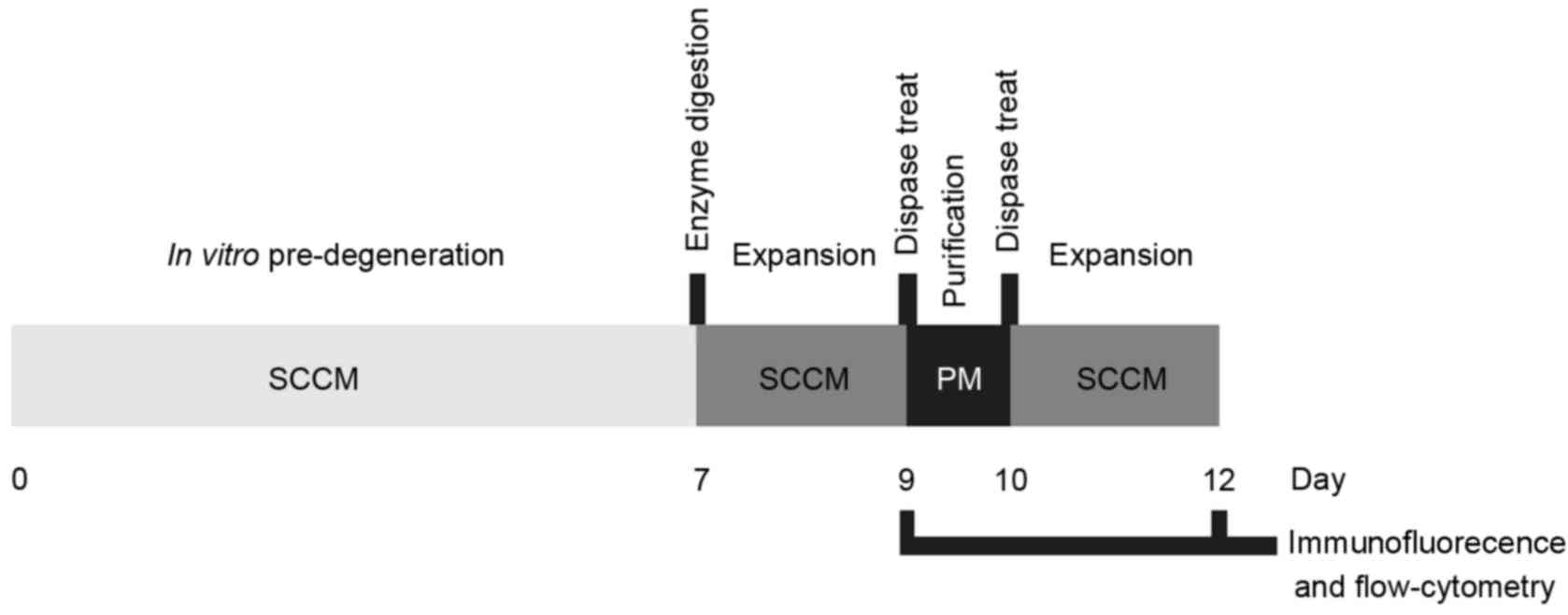
and presented in Fig. 1.
Primary culture
For dissociation of the nerve pieces, the
pre-degeneration medium was discarded, and the nerve fragments were
transferred to a 15-ml conical tube containing 0.2% (0.27 U/ml)
collagenase NB4 in DMEM and incubated at 37°C for 45–60 min until
the cells were dispersed into a homogeneous suspension. Following
centrifugation of the suspension at 600 × g for 5 min at room
temperature, the supernatant was removed and discarded, and the
cells were re-suspended in SCCM. The cells were subsequently seeded
onto one laminin-coated six-well plate and cultivated at 37°C in 5%
CO2 for 48 h prior to isolation of the SCs.
Purification of SCs
Differential detachment methods were applied as
previously described (10).
Following cultivation in the six-well plate for 48 h, the SCCM was
replaced with 0.1 ml/cm2 of Dispase II (1.25 U/ml)
diluted in DMEM. The cells were then incubated at 37°C for 10–15
min and subsequently shaken horizontally for 1 min to release
rounded-up cells. The suspended cells were transferred to a 15-ml
conical tube and centrifuged at 600 × g for 5 min at room
temperature. The supernatant was discarded, and the cells were
re-suspended in purification medium (PM) to reduce the fibroblast
contamination, and cultured for 24 h. After another 24-h
cultivation period, the cells were subjected once more to the
aforementioned purification steps (Dispase II treatment) and
re-suspended in SCCM (Fig. 1).
Immunostaining of S100β, P75NTR and
GFAP
In order to characterize the isolated cells, S100β,
P75NTR and GFAP immunostaining was performed. The cells were fixed
to slides with 4% paraformaldehyde (PFA) for 15 min at room
temperature, washed three times with PBS, treated with 0.3% Triton
X-100 to permeabilize the membranes, washed again in PBS and
blocked with 10% bovine serum album (Sigma-Aldrich; Merck KGaA) at
37°C for 30 min. The slides were subsequently incubated with rabbit
anti-S100 polyclonal antibody (1:200), rabbit anti-P75NTR
polyclonal antibody (1:500) or rabbit anti-GFAP polyclonal antibody
(1:500) at 37°C for 1 h. The slides were washed three times with
PBS and incubated with a FITC-conjugated goat anti-rabbit IgG
secondary antibody (1:500; cat no. F2765) for 1 h at 37°C, and
finally incubated with 4′, 6′-diamidino-2-phenylindole
dihydrochloride (DAPI; 1:500; Invitrogen; Thermo Fisher Scientific,
Inc.) for 2 min at room temperature to stain the cell nuclei.
Images were captured under a fluorescence microscope (Olympus
Corporation, Tokyo, Japan) and processed using Image-Pro Plus
version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Cells in
three randomly selected fields were counted to calculate the SC
purity.
Flow cytometric analysis
To further analyze SC purity using specific marker
molecules, P0 (before purification) cells and P2 (after
purification) cells were collected after 1 min incubation with
0.25% trypsin-EDTA at 37°C, washed with and suspended in blocking
buffer (2% FBS in PBS) and incubated with rabbit anti-P75NTR
antibody (1:500) for 30 min at 4°C. The cells were subsequently
washed twice with blocking buffer and incubated with an Alexa Fluor
488-conjugated goat anti-rabbit-IgG secondary antibody (1:1,000)
for 15 min at 4°C. Cells were analyzed using an EPICS Altra flow
cytometer (Beckman Coulter, Inc., Fullerton, CA, USA). Cells
without primary antibody treatment were used as a blank
control.
SC purity and cell yield
SCs were morphologically identified from fibroblasts
under a phase-contrast microscope and counted in three randomly
selected fields to obtain an average number. Cells with a bipolar
or tripolar shape were identified as SCs and flat or polygonal
cells were identified as fibroblasts. Following trypsinization, all
cells (SCs and fibroblasts) were counted with a hemocytometer to
determine the total yield. Purity was defined as the percentage of
SCs relative to the total number of counted cells. Cell yields were
expressed as the mean of 106 cells per biopsy. Cell
number was presented as the mean ± standard deviation (SD).
Statistical analysis
Data were presented as the mean values ± SD. For
quantitative comparison and analysis, an unpaired Student's t-test
and one way analysis of variance followed by Dunnett's post hoc
test were used to compare differences between groups. All
statistical analyses were performed using SPSS version 18.0 (SPSS,
Inc., Chicago, IL, USA). P-values <0.05 were considered
statistically significant.
Results
Cell yield and SC purity following in
vitro pre-degeneration
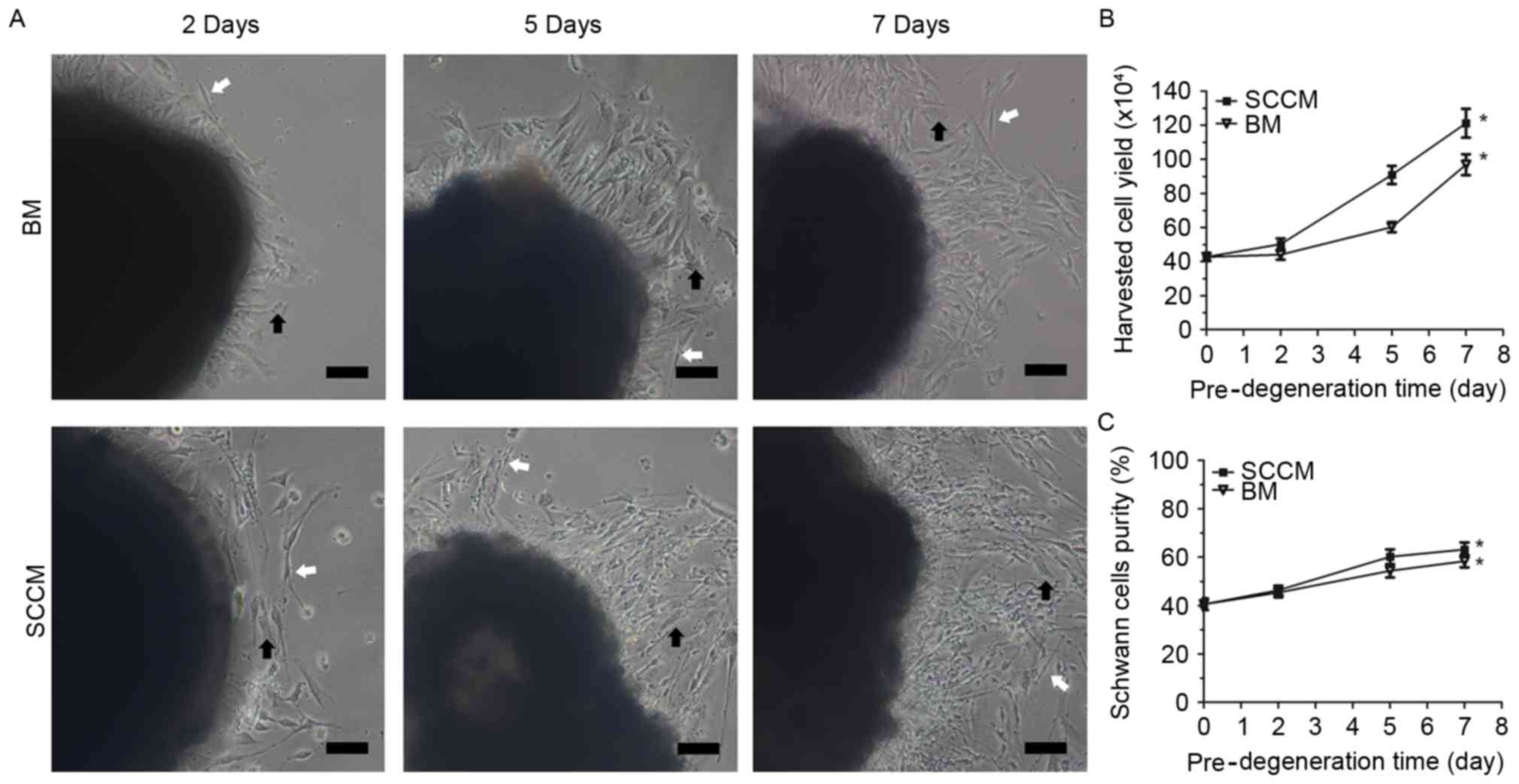
Following 2 days of pre-degeneration treatment,
several cells began to migrate out of the nerve explants and attach
to the culture dish. The number of cells that migrated out of the
nerve explants increased with increased treatment time. The cells
migrating into the BM were predominantly fibroblasts and rarely SCs
(Fig. 2A). Following 2, 5 and 7
days of pre-degeneration, the nerve explants were subjected to
enzymatic digestion and examined to determine SC yield prior to
seeding the cells onto laminin-coated six-well plates. The cell
yield (Fig. 2B) was
50±8.2×104 cells/well after 2 days of pre-degeneration
in SCCM, and 44±7.2×104 cells/well after 2 days of
pre-degeneration in BM. The cell yield in SCCM
(120±21×104 cells/well) was 1.25-fold higher compared
with the BM media (96±1.5×104 cells/well) after 7 days
of pre-degeneration, and was 2.84 times higher compared with the
immediate culture cells that had not undergone pre-degeneration
(43±1.5×104 cells/well). In addition, the purity of the
SCs was slightly higher after 7 days of pre-degeneration and
culture in SCCM (63.17±7.1%) compared with 7 days of
pre-degeneration and culture in BM (58.33±6.2%) (Fig. 2C). These results demonstrated that
SC purity and yield were greater than the fibroblast purity and
yield following in vitro pre-degeneration in SCCM.
Primary culture and phase-contrast
photomicrographs of SCs
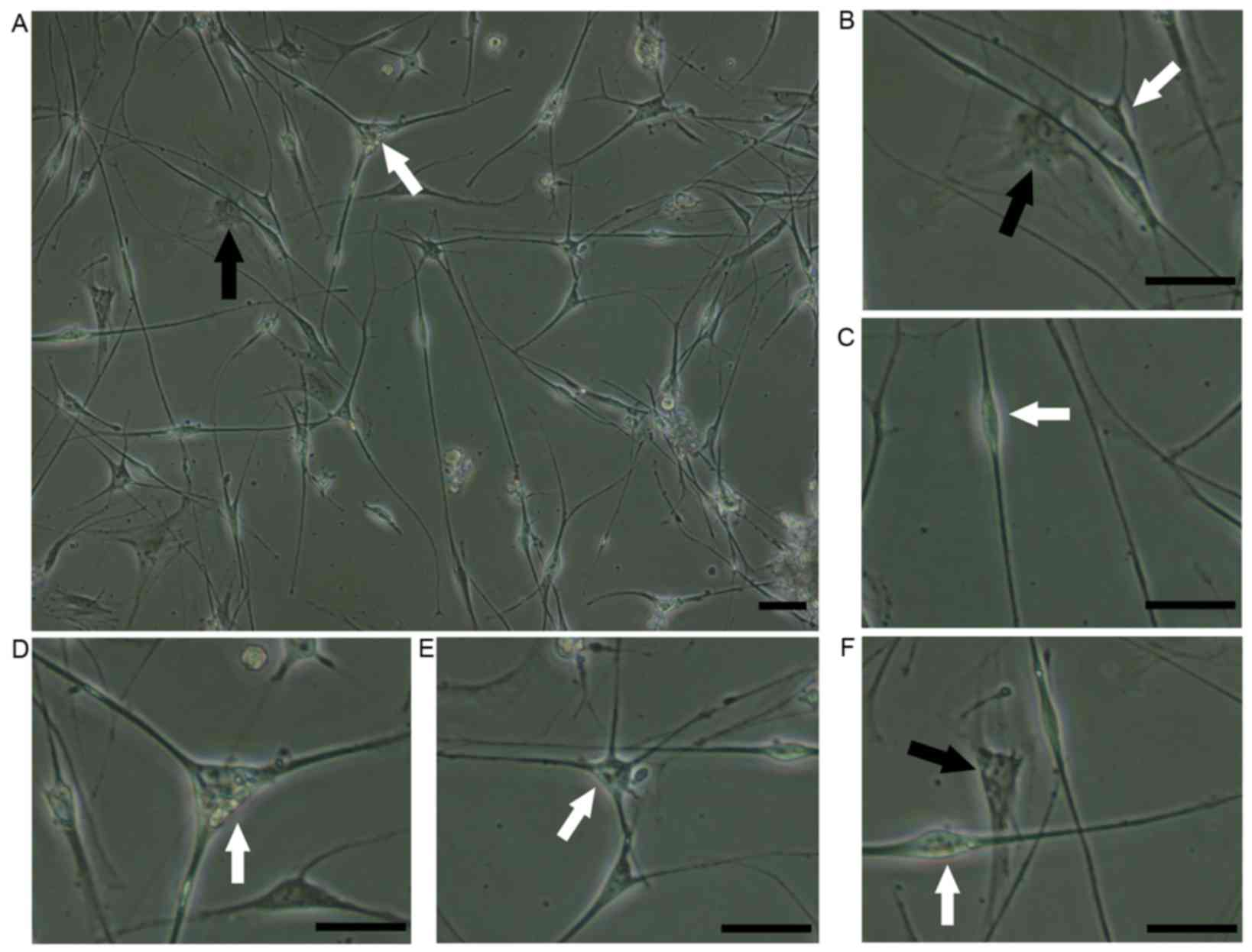
The majority of cells suspended in SCCM adhered to
the laminin-coated flasks within 24 h and exhibited two distinct
morphologies: SCs and fibroblasts (Fig. 3A). Fibroblasts were characterized
by a regular polygonal shape and ovoid nucleus (Fig. 3B). The SCs were characterized as
small, elongated bipolar (Fig.
3C), tripolar (Fig. 3D) or
multipolar (Fig. 3E) cells with a
bright nucleus. The fibroblasts were tightly attached to the cell
plate and adhered under the SCs (Fig.
3F), which facilitated the removal of the SCs.
Cell yield and SC purity following
purification
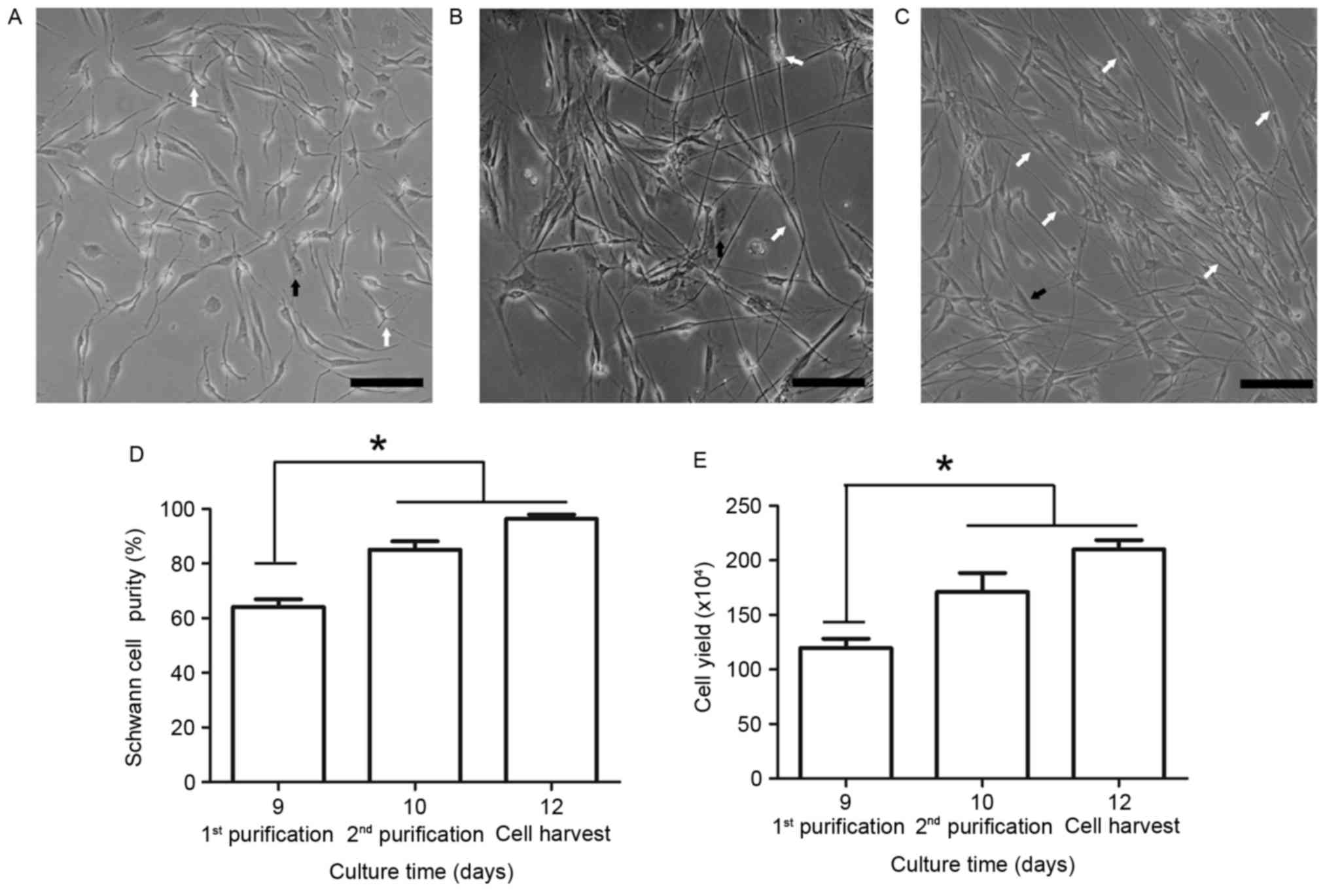
Following the first dispase treatment, the SCs
appeared to proliferate faster than the fibroblasts (Fig. 4A); however, the fibroblasts
subsequently became the predominant cell type. Therefore, Ara-C was
used to inhibit fibroblast growth during the 48–72 h period.
Following the first round of SC purification on day 9 and the use
of Ara-C (Fig. 4B), SC purity
markedly increased to 85.1±7.4%, determined by cell counting based
on morphological differences. However, the remaining fibroblasts
continued to proliferate rapidly and an additional round of
purification was performed on day 10. Following the second round of
purification and subsequent 48 h culture, SC purity reached
96.3±3.9% (Fig. 4C and D). A
significant difference in SC purity was observed between days 9 and
12. To determine the final cell yield, the enriched SCs were
identified by cell counting using a hematocytometer on days 9, 10
and 12. The cell yield increased and reached 2.1±0.2×106
cells/well following the second round of purification (Fig. 4E). These results demonstrated that
cell yield and SC purity were significantly increased following two
rounds of purification.
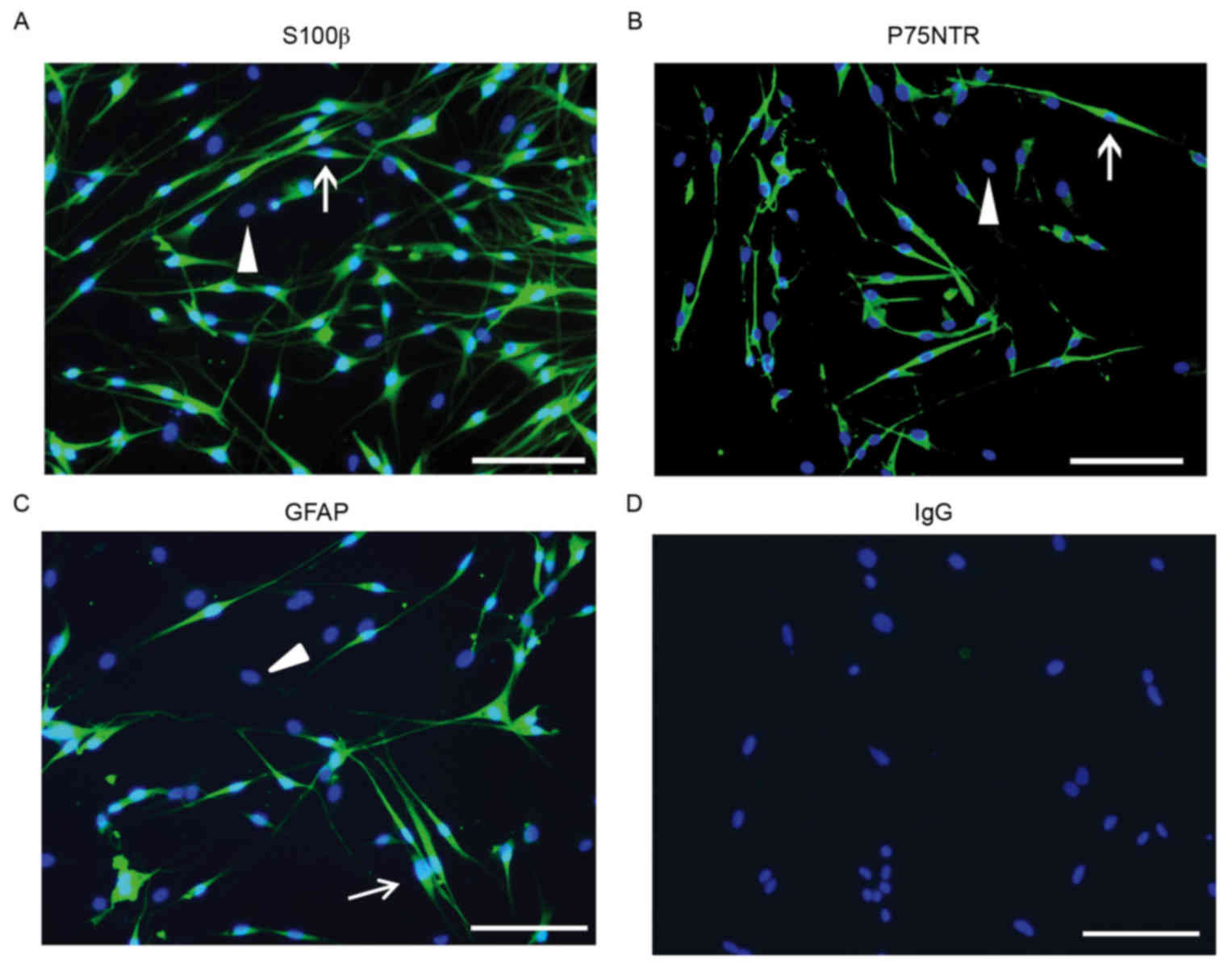
Immunostaining of SC markers
Immunostaining for S100β protein (predominantly
expressed in SC nuclei), P75NTR (primarily expressed on the cell
surface and may be used as a marker in flow cytometric analysis)
and GFAP (expressed in cells lacking fibronectin) were used to
differentiate SCs from fibroblasts. Purified SCs exhibited typical
bipolar or tripolar morphology and oval nuclei and unlike
fibroblasts, were immunopositive for S100β,P75NTR and GFAP
(Fig. 5).
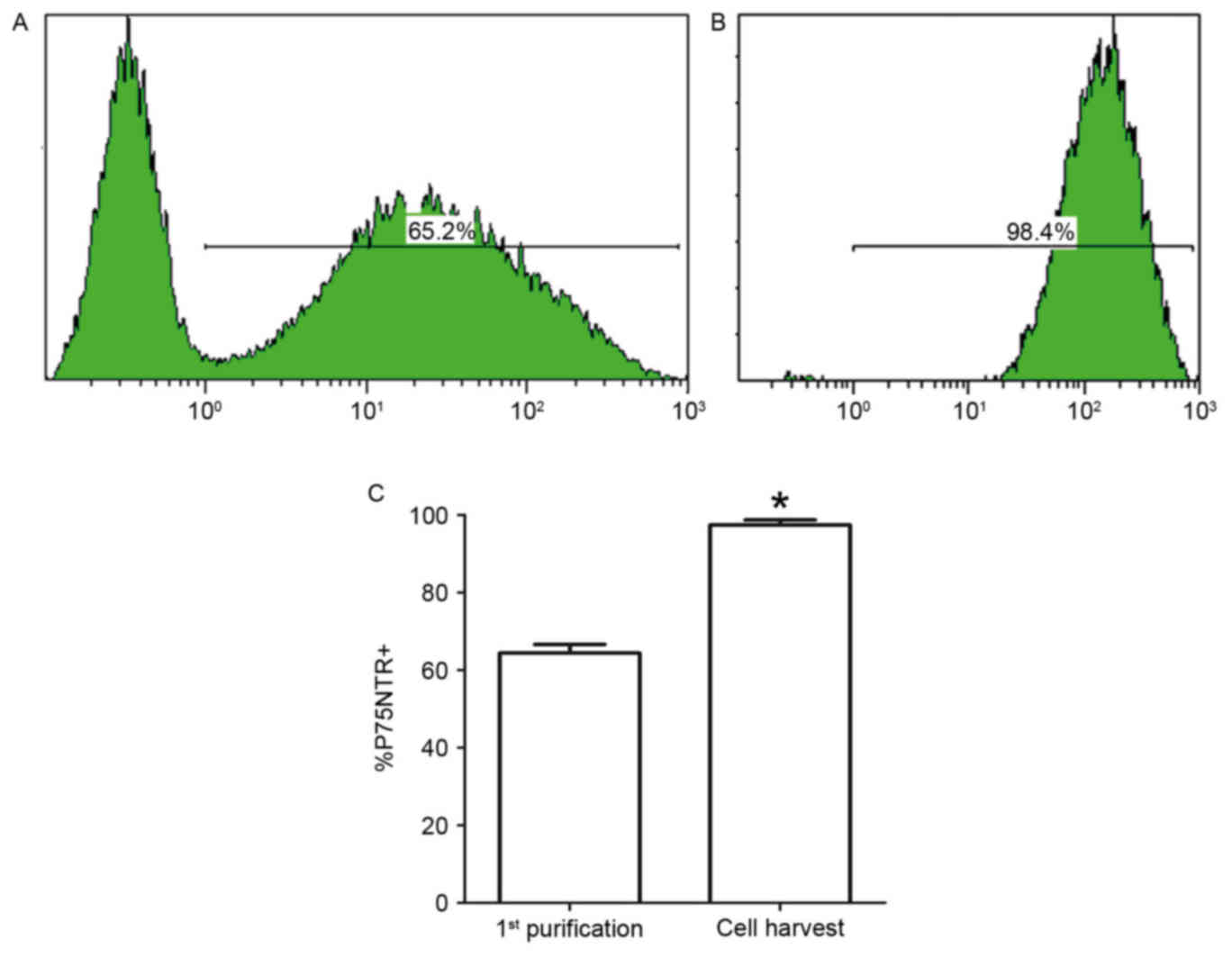
Flow cytometric analysis
To provide further quantitative evidence of SC
purity, the cells were analyzed before and after purification by
flow cytometry with a P75NTR-specific rabbit antibody and tagged
with an anti-rabbit IgG antibody (Alexa Fluor 488). Following
pre-degeneration, cell suspensions obtained from collagenase
digestion of the nerve explants were incubated for 48 h,
immunostained for P75NTR, and analyzed by flow cytometry. The
percentage of P75NTR-positive SCs was 65.2±1.3% at 48 h and
98.4±1.5% following one purification cycle. There was a significant
difference in cell percentages (Fig.
6).
Discussion
The SC-coated scaffold is an effective strategy to
repair peripheral nerve defects (17). Therefore, populations of highly
purified SCs may be needed in sufficient quantity and on short
notice. Researchers have investigated a variety of methods to
satisfy this requirement, including repeated explantation methods
(8), differential adhesion and
detachment methods (18),
immunoselective methods (19) and
serum tapering (20), amongst
others. Although they are effective at purifying SCs, these methods
have limitations (10,21,22),
including the requirement for special equipment, complicated
procedures and high cost.
Experimentally, embryonic or newborn mice and rats
are currently the predominant source of non-myelinating SCs, which
have a strong capacity to proliferate (23). However, in most treatment
strategies, SCs are obtained from adult animals. Casella et
al (24,25) demonstrated that the culture and
amplification of SC populations becomes more difficult as the age
of the animal increases. Therefore, the development of a method for
culturing SCs from adult animals may be clinically more relevant as
nerve injury frequently occurs in adults. Monkeys are ideal models
for studying diseases of the human nervous system. Newborn and
adult monkeys have fully mature neural structures with nerve
bundles surrounded by epineurium; however, this makes SC migration
from nerve explants difficult. In the present study,
pre-degeneration of the common peroneal nerve in vitro
resulted in SC proliferation and migration. The SCs were
subsequently enzymatically dissociated from the nerve pieces and
purified. Cell yield and SC purity were increased following nerve
explant pre-degeneration in vitro in the presence of
mitogen-activated protein (heregulin-β1) and an activator of
adenylate cyclase (forskolin).
During in vivo pre-degeneration, SCs
dedifferentiate and proliferate following peripheral nerve
transaction (20). A period of
time must pass in order to allow for Wallerian degeneration to
occur and SCs can subsequently be enzymatically dissociated from
the nerve. In vivo pre-degeneration procedures are
cumbersome to perform, and the requirement for two independent
surgical procedures means this process is not ethically acceptable
in human research. Therefore, the present study investigated the
in vitro approach. Different pre-degeneration conditions may
impact upon the yield and purity of SCs (10). Kraus et al (13) compared SC yield and purity after 2,
7 and 14 days of in vitro pre-degeneration, and demonstrated
a doubling of the yield after 7 days when compared with 2 days. It
is of note that marked contamination of the culture by fibroblasts
was observed after longer pre-degeneration periods. Therefore, they
recommended the 7-day pre-degeneration period, which is in
accordance with the method used in the present study. Levi et
al (22) demonstrated that
heregulin-β stimulates the heregulin receptors erb-b2 receptor
tyrosine kinase (erbB) 2 and erbB3 in SCs and leads to the
activation of the mitogen activated protein kinase pathway, and
demonstrated that forskolin significantly enhances the expression
level of a group of genes associated with cell division. SC
proliferation requires long-term simultaneous exposure to heregulin
plus forskolin as well as bFGF (an independent activator of the
extracellular signal-related kinase pathway, which is important for
SC proliferation) (26).
In the present study, in vitro
pre-degeneration was performed in SCCM or BM. Following
pre-degeneration for 7 days, the cell yield was 1.25 times higher
in SCCM compared with BM, and 2.84 times higher in SCCM compared
with non-pre-degeneration conditions. SC purity was also markedly
higher in SCCM compared with non-pre-degeneration conditions. Based
on the present observations, the migration rate of SCs out of the
nerve explants appeared to reach its maximum after 14 days (data
not shown). However, the level of fibroblast proliferation was also
significantly increased, which reduced the purity of the SCs.
Therefore, the shorter 7-day pre-degeneration period may be optimal
to maximize SC purity.
The primary obstacle to SC purification is
fibroblast contamination. Anti-mitotic and low-serum treatments at
the appropriate time may be an effective method to inhibit
fibroblast proliferation. The present study developed several
strategies to reduce fibroblast contamination: i) Removal of the
epineurium as completely as possible; ii) application of PM at the
appropriate time, in this study this was after 48 h (the time when
the proliferation rate is higher in fibroblasts compared with SCs);
and iii) use of differential detachment methods. This series of
purification steps increased SC purity from 65.2 to 98.4%.
In conclusion, the newly established protocols in
the present study provide a rapid and efficient method for
obtaining highly enriched populations of SCs from mature Rhesus
monkey nerves. These cells may be used to provide support for
regenerating peripheral or central nerves.
Acknowledgements
The current study was supported by the National
Natural Science Foundation (grant nos. 31170932 and 81000522) and
the Lanzhou Personnel Entrepreneurship and Innovation Project
(grant no. 2015-RC-74). The animal studies were performed in the
Department of Laboratory Animal Science, Shanghai Jiaotong
University School of Medicine. The authors would like to thank
Norton Healthcare (Louisville, KY, USA) for their ongoing support.
We sincerely thank Mr Weihua Lu (Shanghai Jiao Tong University,
Shanghai, China) for his commitment to animal care.
References
|
1
|
Fawcett JW and Keynes RJ: Peripheral nerve
regeneration. Annu Rev Neurosci. 13:43–60. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dezawa M: Central and peripheral nerve
regeneration by transplantation of Schwann cells and
transdifferentiated bone marrow stromal cells. Anat Sci Int.
77:12–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madduri S and Gander B: Schwann cell
delivery of neurotrophic factors for peripheral nerve regeneration.
J Peripher Nerv Syst. 15:93–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang XN, Jin YQ, Bi H, Wei W, Cheng J, Liu
ZY, Shen Z, Qi ZL and Cao Y: Peripheral nerve repair with epimysium
conduit. Biomaterials. 34:5606–5616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hedayatpour A, Sobhani A, Bayati V,
Abdolvahhabi MA, Shokrgozar MA and Barbarestani M: A method for
isolation and cultivation of adult Schwann cells for nerve conduit.
Arch Iran Med. 10:474–480. 2007.PubMed/NCBI
|
|
6
|
Needham LK, Tennekoon GI and McKhann GM:
Selective growth of rat Schwann cells in neuron- and serum-free
primary culture. J Neurosci. 7:1–9. 1987.PubMed/NCBI
|
|
7
|
Rutkowski JL, Kirk CJ, Lerner MA and
Tennekoon GI: Purification and expansion of human Schwann cells in
vitro. Nat Med. 1:80–83. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pannunzio ME, Jou IM, Long A, Wind TC,
Beck G and Balian G: A new method of selecting Schwann cells from
adult mouse sciatic nerve. J Neurosci Methods. 149:74–81. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin YQ, Liu W, Hong TH and Cao Y:
Efficient Schwann cell purification by differential cell detachment
using multiplex collagenase treatment. J Neurosci Methods.
170:140–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mauritz C, Grothe C and Haastert K:
Comparative study of cell culture and purification methods to
obtain highly enriched cultures of proliferating adult rat Schwann
cells. J Neurosci Res. 77:453–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haastert K, Mauritz C, Matthies C and
Grothe C: Autologous adult human Schwann cells genetically modified
to provide alternative cellular transplants in peripheral nerve
regeneration. J Neurosurg. 104:778–786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dreesmann L, Mittnacht U, Lietz M and
Schlosshauer B: Nerve fibroblast impact on Schwann cell behavior.
Eur J Cell Biol. 88:285–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kraus A, Tager J, Kohler K, Manoli T,
Haerle M, Werdin F, Hoffmann J, Schaller HE and Sinis N: Efficacy
of various durations of in vitro predegeneration on the cell count
and purity of rat Schwann-cell cultures. J Neurotrauma. 27:197–203.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haastert K, Seef P, Stein VM, Tipold A and
Grothe C: A new cell culture protocol for enrichment and genetic
modification of adult canine Schwann cells suitable for peripheral
nerve tissue engineering. Res Vet Sci. 87:140–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rahmatullah M, Schroering A, Rothblum K,
Stahl RC, Urban B and Carey DJ: Synergistic regulation of Schwann
cell proliferation by heregulin and forskolin. Mol Cell Biol.
18:6245–6252. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu J, Qin J, Shen Z, Kretlow JD, Wang X,
Liu Z and Jin Y: Dispase rapidly and effectively purifies Schwann
cells from newborn mice and adult rats. Neural Regen Res.
7:256–260. 2012.PubMed/NCBI
|
|
17
|
Wang G, Cao L, Wang Y, Hua Y, Cai Z, Chen
J, Chen L, Jin Y, Niu L, Shen H, et al: Human eyelid adipose
tissue-derived Schwann cells promote regeneration of a transected
sciatic nerve. Sci Rep. 7:432482017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei Y, Zhou J, Zheng Z, Wang A, Ao Q, Gong
Y and Zhang X: An improved method for isolating Schwann cells from
postnatal rat sciatic nerves. Cell Tissue Res. 337:361–369. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manent J, Oguievetskaia K, Bayer J, Ratner
N and Giovannini M: Magnetic cell sorting for enriching Schwann
cells from adult mouse peripheral nerves. J Neurosci Methods.
123:167–173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang SH, Shin GW, Shin YS, J PK, Kim YR,
Yang HH, Lee EY, Lee EG, Huh NE, Ju OM and Jung TS: Experimental
evaluation of pathogenicity of Lactococcus garvieae in black
rockfish (Sebastes schlegeli). J Vet Sci. 5:387–390.
2004.PubMed/NCBI
|
|
21
|
Tomita K, Hata Y, Kubo T, Fujiwara T, Yano
K and Hosokawa K: Effects of the in vivo predegenerated nerve graft
on early Schwann cell migration: Quantitative analysis using
S100-GFP mice. Neurosci Lett. 461:36–40. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levi AD, Bunge RP, Lofgren JA, Meima L,
Hefti F, Nikolics K and Sliwkowski MX: The influence of heregulins
on human Schwann cell proliferation. J Neurosci. 15:1329–1340.
1995.PubMed/NCBI
|
|
23
|
Niapour N, Mohammadi-Ghalehbin B,
Golmohammadi MG, Gholami MR, Amani M and Niapour A: An efficient
system for selection and culture of Schwann cells from adult rat
peripheral nerves. Cytotechnology. 68:629–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casella GT, Bunge RP and Wood PM: Improved
method for harvesting human Schwann cells from mature peripheral
nerve and expansion in vitro. Glia. 17:327–338. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Casella GT, Wieser R, Bunge RP, Margitich
IS, Katz J, Olson L and Wood PM: Density dependent regulation of
human Schwann cell proliferation. Glia. 30:165–177. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schworer CM, Masker KK, Wood GC and Carey
DJ: Microarray analysis of gene expression in proliferating Schwann
cells: Synergistic response of a specific subset of genes to the
mitogenic action of heregulin plus forskolin. J Neurosci Res.
73:456–464. 2003. View Article : Google Scholar : PubMed/NCBI
|