Introduction
MicroRNAs (miRNAs) are short noncoding RNA
molecules, usually between 22 and 23 nucleotides in length, which
regulate the expression of protein-coding genes at the
post-transcriptional level by interfering with the translation of
mRNAs or by inducing their degradation (1). In humans, miRNAs have been proposed
to regulate ~60% of all protein-coding genes and fulfill regulatory
functions, as established by their involvement in numerous
processes and diseases (2,3). In addition, previous studies have
reported that miRNAs are involved in the self-renewal and cell-fate
decisions of stem cells, control of the cell cycle, and maintenance
of the balance of keratinocyte proliferation, differentiation and
apoptosis, whereas their aberrant expression can lead to disease
development (4,5). For example, our previous study
(6) observed them iRNA expression
profiles of epidermal cells at various stages of differentiation;
concluding that the expression of 191 miRNAs was significantly
altered, the target genes of which are closely correlated with cell
proliferation, differentiation, apoptosis and migration.
Furthermore, Liu et al (7)
detected a significant differential miRNA expression profile in
cutaneous wounds between diabetic rats and normal rats, which may
be closely associated with the mechanisms underlying diabetic wound
healing. Sonkoly et al (8)
reported that upregulation of miR-203 in human keratinocytes may be
required for their differentiation, which is dependent on
activation of the protein kinase C (PKC)/activator protein-1 (AP-1)
pathway. Conversely, pretreatment with the specific PKC inhibitor,
GF109203X, not only suppressed 12-O-tetradecanoylphorbol-13-acetate
(TPA)-induced miR-203 expression, but also suppressed it to below
the basal level. A downstream target for PKC action in
keratinocytes is AP-1, which is a transcription factor that
consists of homodimers or heterodimers of the Jun and Fos families
of nuclear proteins, and serves essential roles in the regulation
of keratinocyte growth and differentiation (8). miRNAs regulate keratinocyte
differentiation by activating the PKC signaling pathway; however,
to the best of our knowledge, there are currently no reports on the
differential miRNA expression profiles of keratinocytes following
treatment with the specific PKC inhibitor, GF109203X.
PKC was initially discovered in 1977 as a
proteolytically activated protein kinase. Later, it was verified as
a Ca2+-activated, phospholipid-dependent Ser/Thr kinase,
firmly associated with signal transduction (9). PKC family isoforms are divided into
three subgroups: The calcium- and phorbol ester-dependent
‘classical/conventional’ subgroup (PKCα, βI, βII, γ), the
calcium-independent ‘novel’ subgroup (PKC σ, δ, ε, η, θ) and the
calcium- and phorbol ester-independent ‘atypical’ subgroup (PKC ζ,
ι, λ) (9). The rapid activation of
PKC enzymes forms part of the signal transduction pathways elicited
by numerous hormones, and their phosphorylation of target proteins
leads to various cellular responses, including cell proliferation,
differentiation and apoptosis (10). In keratinocytes, several cellular
functions are also mediated by signaling via PKC, including
translocation of the desmoyokin/AHNAK protein, inhibition of
proliferation, and differentiation (11). GF109203X is a specific inhibitor of
PKC, which competes at the ATP-binding site and regulates the
development of keratinocytes. Le Panse et al (12) indicated that GF109203X inhibited
c-Fos and c-Jun mRNA expression; in keratinocytes these
proto-oncogenes are involved in the cellular differentiation
process rather than in cellular proliferation. In addition, it has
been verified that GF109203X effectively inhibits granular cell
differentiation marker expression when used at 1 and 5 µM
concentrations; however, it does not alter keratin (K)1 or
K14expression (13). GF109203X has
also been reported to block TPA-induced tumor susceptibility gene
101 protein and K10 upregulation during early keratinocyte
differentiation (14).
Furthermore, keratinocyte differentiation is preceded by a
commitment to irreversible cell cycle withdrawal, and GF109203X may
induce marked protection from loss of growth potential in human
keratinocytes (15). GF109203X may
also suppress the ultraviolet B-induced reduction of cell survival,
caspase-9 activation, downregulation of human inhibitor of
apoptosis protein-1, X-linked inhibitor of apoptosis proteinand PKB
(but not myeloid cell leukemia-1), and upregulation of
glucose-regulated protein 78 in HaCaT cells (16). Overall, these data indicated that
GF109203X may have influence on keratinocyte differentiation.
However, the miRNA profiles and biological characteristics of
keratinocytes induced by specific PKC inhibitors have yet to be
elucidated.
The present study aimed to explore the differential
miRNA expression profile and biological characteristics of
keratinocytes treated with the specific PKC inhibitor, GF109203X.
The findings of the present study may provide a novel approach for
wound healing and regeneration of skin tissues.
Materials and methods
Sample collection
Prepuce samples were obtained from 5 male patients
(age, 16–30 years) who were healthy patients except their prepuce
was too long and underwent circumcision at the Department of
Urology Surgery, The First Affiliated Hospital of Nanchang
University (Nanchang, China) between March 2014 and April 2014. The
present study was conducted in accordance with the Declaration of
Helsinki, with approval obtained from the Nanchang University
Ethics Committee. Written informed consent was obtained from all
participants.
Cell culture and identification
The epidermis was digested with trypsin (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 4°C in the
dark for 8 h. Rapid adhesion to collagen IV (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was used to isolate human differentiated
keratinocytes from epidermal stem cells, as previously described
(6). The differentiated
keratinocytes were cultured in vitro in keratinocyte serum-free
medium supplemented with 10 µg/l epidermal growth factor and 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in a humidified chamber with 5% CO2 for 2 days, and were then
divided into two groups. In the experimental group (EXP), the
primary keratinocytes were treated with GF109203X (Selleck
Chemicals, LLC, Houston, TX, USA) for 2 days, at a final
concentration of 10 µM. In the control group (CON), the primary
keratinocytes were treated with dimethyl sulfoxide (DMSO;
Sigma-Aldrich; Merck KGaA) for 2 days, at a final concentration of
10 µM. The cellular morphology of the two groups was observed under
an inverted phase contrast microscope (CTR6000; Leica Microsystems
GmbH, Wetzlar, Germany). Immunostaining of integrin β1 (catalog no.
AW5254), cytokeratin (CK)19 (catalog no. AM8477b), CK1 (catalog no.
AP9695c) and CK10 (catalog no. AP6704c; Abgent Inc., San Diego, CA,
USA) was used for cell identification, which was performed
according to the manufacturer's protocols.
Extraction of total RNA
Total RNA was isolated using TRIzol (Invitrogen;
Thermo Fisher Scientific. Inc.) and purified with the RNeasy mini
kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's
protocol. RNA quality and quantity were measured using a NanoDrop
spectrophotometer (ND-1000; NanoDrop; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA) and RNA integrity was determined by
electrophoresis on a denaturing agarose gel, which was prepared in
house. On the denaturing gel, the 28S and 18S ribosomal RNA bands
were visible, which suggested that the extracted total RNA was
complete, RNA degradation and contamination were low and the
extracted total RNA exhibited high levels of purity.
miRNA labeling and array
hybridization
After quality control, miRNA was labeled with the
miRCURY™ Power Labeling kit (Exiqon A/S, Vedbaek, Denmark)
according to the manufacturer's protocol. Briefly, 5 µl calf
intestine phosphatase (CIP) reaction solution (1 µl total RNA, 0.5
µl CIP buffer, 0.5 µl CIP and 3 µl ddH2O) was incubated at 37°C for
30 min, and then at 95°C for 5 min to terminate the reaction.
Subsequently, 3.0 µl labeling buffer, 1.5 µl fluorescent label
(Hy3™), 2.0 µl DMSO and 2.0 µl labeling enzyme were added to the
mixture. The system was incubated at 16°C for 1 h, and subsequently
at 65°C for 15 min to terminate the labeling reaction. The
Hy3™-labeled samples were hybridized on the miRCURY™ LNA Array
(v.18.0) (Exiqon A/S) according to the manufacturer's protocol.
Briefly, 25 µl Hy3™-labeled samples were mixed with 25 µl
hybridization buffer and were denatured for 2 min at 95°C, after
which the samples were incubated on ice for 2 min and hybridized to
the microarray for 16–20 h at 56°C in a 12-Bay Hybridization system
(Roche Nimblegen, Inc., Madison, WI, USA). Following hybridization,
the slides were obtained and washed several times using a wash
buffer kit (Exiqon A/S). Finally, the slides were scanned using the
Axon GenePix 4000B Microarray Scanner (Axon Instruments; Molecular
Devices, LLC, Sunnyvale, CA, USA).
Data processing and analysis
Scanned images were then imported into GenePix Pro
6.0 software (Axon Instruments; Molecular Devices, LLC) for grid
alignment and data extraction. Replicated miRNAs were averaged and
miRNAs withintensities ≥30 in all samples were chosen for
calculating the normalization factor. Expressed data were
normalized using the median normalization. Following normalization,
significant differentially expressed miRNAs between the two groups
were identified through fold change and P-value (fold change >2
and P<0.05). Differential miRNA expression between the two cell
groups was analyzed using a Student's t-test. Finally, hierarchical
clustering was performed to detect distinguishable miRNA expression
profiling among samples.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and miRNA target
prediction
According to the microarray results, the expression
levels of hsa-miR-1-3p were upregulated and the expression levels
of hsa-miR-31-5p were downregulated in the experimental group
compared with the control group, exhibiting strong original signals
and clear differences. Therefore, both of these miRNAs were
selected for RT-qPCR verification. In RT-qPCR, small nuclear
(sn)RNA U6 was used as an endogenous control. Firstly, cDNA was
synthesized using a Gene Amp PCR system 9700 (Applied Biosystems,
Thermo Fisher Scientific, Inc.). RT was performed in a 20 µl
reaction containing 200 ng total RNA, 0.3 µl 1 µM RT primer, 2 µl
2.5 mM dNTP (HyTest Ltd, Turku, Finland), 2 µl 10× RT buffer
(Epicentre; Illumina, Inc., San Diego, CA, USA), 1 µl 50 U/µl RT
enzyme (Epicentre; Illumina, Inc.), 0.3 µl 40 U/µl RNase inhibitor
(Epicentre; Illumina, Inc.), 20 µl nuclease free water and 0.2 µl
MMLV High Performance Reverse Transcriptase (Epicentre; Illumina,
Inc.). The stem-loop RT reaction was performed at 16°C for 30 min,
followed by 42°C for 30 min and 85°C for 5 min. A total of 2 µl RT
reaction was then used with 1 µl specific primers for each of the
hsa-miR-1-3p and hsa-miR-31-5p in triplicate wells for PCR on an
Applied Biosystems ViiA 7 Real-time PCR system (Applied Biosystems,
Thermo Fisher Scientific, Inc.). The thermal cycling parameters
were as follows: An initial predenaturation step at 95°C for 10
min, followed by 40 cycles of denaturation at 95°C for 10 sec,
annealing at 60°C for 60 sec, followed by 95°C for 10 sec, 60°C for
60 sec and 95°C for 15 sec. The primers, which were synthesized by
Bioligo Life Technology (Shanghai, China) and the sequences are
presented in Table I. Expression
levels were calculated using the comparative quantitative cycle
(Cq) method (17). RT-qPCR was
performed in triplicate for each treatment group. To demonstrate
the function of differential miRNAs, target gene prediction and
functional analysis were conducted. The following websites:
http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/ and
http://www.targetscan.org/were used to
predict target genes of the differentially expressed miRNAs. In
addition, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway analysis were used to identify the roles of
these target genes in biological pathways or GO terms, which were
accessed from the databases of http://www.geneontology.org/ and http://www.genome.jp/kegg/, respectively.
 | Table I.Primer sequences used for
quantitative polymerase chain reaction. |
Table I.
Primer sequences used for
quantitative polymerase chain reaction.
| miRNA | Sequences | Annealing
temperature (°C) | Product length
(bp) |
|---|
| U6 |
F:5′GCTTCGGCAGCACATATACTAAAAT3′ |
|
|
|
|
R:5′CGCTTCACGAATTTGCGTGTCAT3′ | 60 | 89 |
| hsa-miR-1-3p |
GSP:5′GGGGCTGGAATGTAAAGAAGT3′ |
|
|
|
|
R:5′GTGCGTGTCGTGGAGTCG3′ | 60 | 65 |
| hsa-miR-31-5p |
F:5′GGAGGCAAGATGCTGGC3′ |
|
|
|
|
R:5′CAGTGCGTGTCGTGGAGT3′ | 60 | 64 |
Results
Biological characteristics of the
cells
Non-adherent cells were irregular in shape, size and
distribution after culturing for 2 days, and were loosely attached
to the plate wells with no clones detected under an inverted
microscope. These results suggested that the characteristics of
non-adherent cells were in accordance with terminally
differentiating epidermal keratinocytes. In the experimental group,
parts of the keratinocytes induced by GF109203X attached to the
well and formed clones, and the expression of CK19, CK14 and
integrin β1 was positive, whereas CK10 expression was negative,
which is in agreement with the characteristics of epidermal-like
stem cells (data not shown). However, in the control group, the
number of cells was significantly decreased with no clones
detected, and the expression of CK10 was positive, whereas the
expression of CK19, CK14 and integrin β1 was negative, which is in
accordance with the characteristics of terminally differentiating
epidermal keratinocytes.
Extraction and qualification of total
RNA
The A260/A280 ratio of RNA is a method used to
detect RNA purity; samples ~2.0 are considered to represent pure
RNA. A ratio <1.8 indicates sample contamination. A ratio
>2.0 indicates RNA hydrolysis. Therefore, a ratio range between
1.8 and 2.1 is considered acceptable. In addition, the A260/A230
ratio should be >1.8 for pure RNA. As demonstrated in Table II, the extracted RNAs conformed to
the quality standards and therefore qualified for the subsequent
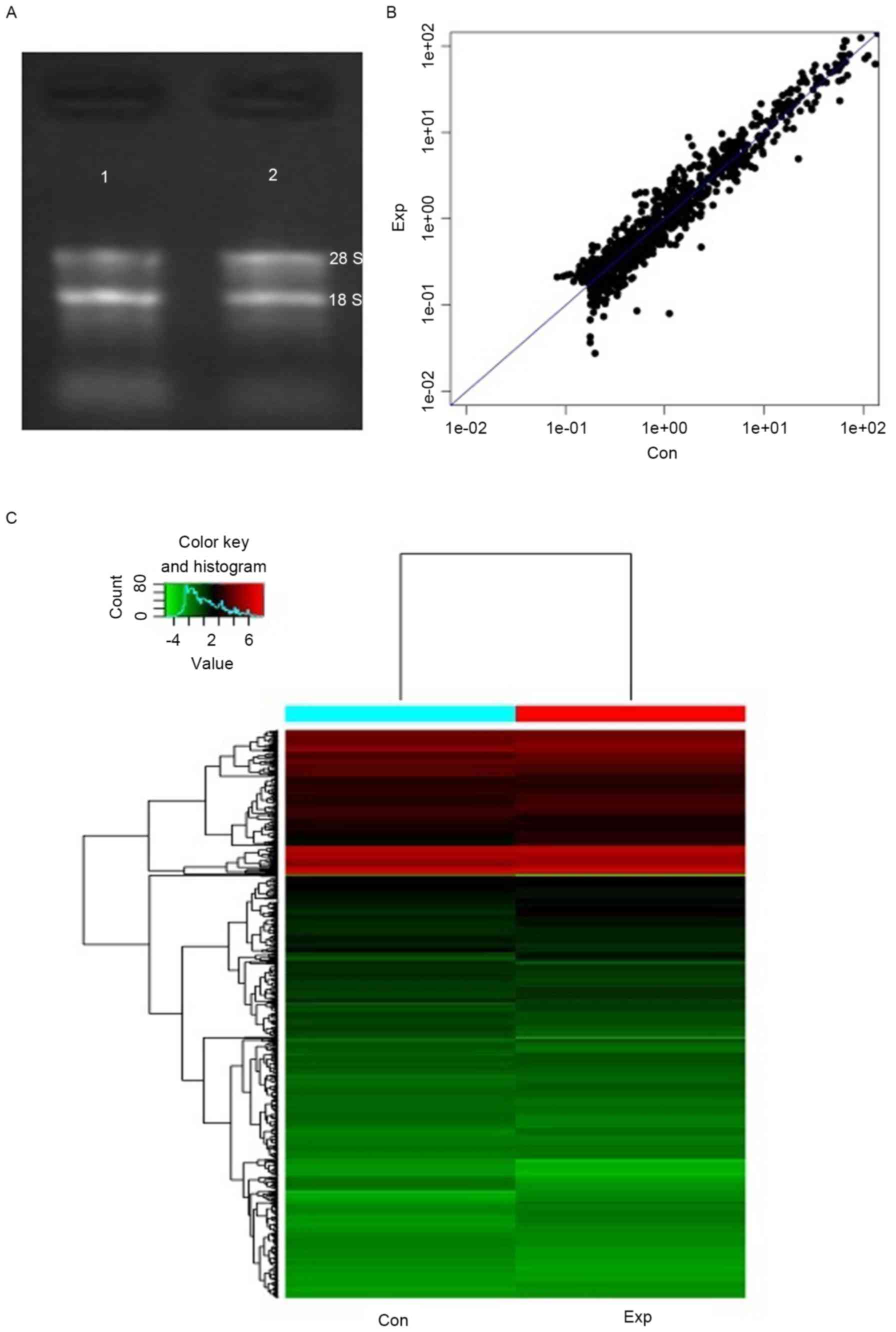
miRNA experiments. On the denaturing electrophoresis gel (Fig. 1), the 18S and 28S rRNA bands were
clearly visible in the RNA samples, suggesting good integrity.
 | Table II.RNA quantification and quality
assurance, as determined by NanoDropND-1000. |
Table II.
RNA quantification and quality
assurance, as determined by NanoDropND-1000.
| Group | OD260/280
ratio | OD260/230
ratio | Concentration
(ng/µl) | Quantity (ng) | Result |
|---|
| EXP | 1.85 | 2.12 | 101.82 | 1018.2 | Pass |
| CON | 1.86 | 2.06 | 337.7 | 3377 | Pass |
Differential miRNA expression
The miRNA expression variations and patterns between
the two groups are presented in Fig.
1B and C. According to data processing and analysis, a total of
45 miRNAs were upregulated, whereas 34 miRNAs were downregulated in
the experiment group compared with expression in the control group
(Table III). The miRNAs with the
greatest upregulation and downregulation were hsa-miR-1-3p
(5.0265-fold) and hsa-miR-31-5p (13.9011-fold), respectively.
 | Table III.Differential expression of
miRNAs. |
Table III.
Differential expression of
miRNAs.
| miRNA probe ID | miRNA | EXP/CON |
|---|
| Upregulated |
|
|
|
10916 | hsa-miR-1-3p | 5.026537 |
|
42660 | hsa-miR-144-5p | 3.747800 |
|
145844 |
hsa-miR-374a-5p | 3.715758 |
|
148228 | hsa-miR-3656 | 3.560661 |
|
168635 | hsa-miR-378e | 3.138800 |
|
147755 | hsa-miR-378c | 2.817634 |
|
42654 | hsa-miR-483-5p | 2.683051 |
|
46944 | hsa-miR-1297 | 2.674556 |
|
147851 | hsa-miR-3201 | 2.632528 |
|
147604 | hsa-miR-4285 | 2.624769 |
|
29577 |
hsa-miR-374a-3p | 2.570440 |
|
168935 |
hsa-miR-4687-3p | 2.562501 |
|
146072 | hsa-miR-1469 | 2.484515 |
|
168944 |
hsa-miR-4707-5p | 2.460301 |
|
17752 | hsa-let-7f-5p | 2.456365 |
|
11053 | hsa-miR-32-5p | 2.405171 |
|
33596 | hsa-miR-126-5p | 2.382427 |
|
147926 | hsa-miR-4329 | 2.377924 |
|
27536 |
hsa-miR-190a-5p | 2.370629 |
|
42782 |
hcmv-miR-UL148D | 2.338837 |
|
42640 | hsa-miR-20b-5p | 2.337647 |
|
11004 |
hsa-miR-203a-3p | 2.295231 |
|
169221 | hsa-miR-4748 | 2.294769 |
|
169230 |
hsa-miR-4747-3p | 2.281731 |
|
17503 | hsa-miR-590-5p | 2.268278 |
|
147840 | hsv2-miR-H9-3p | 2.262449 |
|
4040 | hsa-miR-9-5p | 2.239972 |
|
169395 | hsa-miR-4484 | 2.239785 |
|
148620 | hsa-miR-454-3p | 2.196783 |
|
42800 | hsa-miR-582-5p | 2.188416 |
|
17315 |
kshv-miR-K12-3-3p | 2.175560 |
|
169399 |
hsa-miR-4750-5p | 2.144243 |
|
10923 | hsa-miR-107 | 2.143846 |
|
169183 | hsa-miR-4644 | 2.128833 |
|
169170 | hsa-miR-4472 | 2.104725 |
|
146089 | hsv1-miR-H8-5p | 2.094303 |
|
168696 | hsa-miR-4739 | 2.094056 |
|
168893 | hsa-miR-4505 | 2.093298 |
|
169272 | hsa-miR-4419b | 2.080859 |
|
42496 |
hsa-miR-181c-5p | 2.041768 |
|
169110 | hsa-miR-4497 | 2.040852 |
|
168670 |
hsa-miR-4694-5p | 2.031255 |
|
146086 | hsa-miR-30a-5p | 2.030778 |
|
148509 | hsa-miR-328-5p | 2.028205 |
|
169375 | Has-miR-660-3p | 2.005712 |
| Downregulated |
|
|
|
11052 | hsa-miR-31-5p | 13.901180 |
|
42668 | hsa-let-7c-3p | 7.137004 |
|
42959 |
hsa-miR-514a-3p | 6.094606 |
|
169159 | hsa-miR-4521 | 4.968959 |
|
147809 |
hsa-miR-514b-3p | 4.793510 |
|
17848 |
hsa-miRPlus-A1087 | 4.497239 |
|
42686 | hsa-miR-136-3p | 4.108723 |
|
148402 | hsa-miR-3920 | 3.275565 |
|
145689 | hsa-miR-543 | 2.614642 |
|
42516 |
kshv-miR-K12-12-5p | 2.564381 |
|
147842 |
hsv2-miR-H11-5p | 2.556539 |
|
11023 | hsa-miR-222-3p | 2.505895 |
|
145838 |
hsa-miR-125b-1-3p | 2.407407 |
|
11140 | hsa-miR-508-3p | 2.396755 |
|
11139 | hsa-miR-507 | 2.307986 |
|
29379 | hsa-miR-452-5p | 2.302862 |
|
168958 |
hsa-miR-2681-5p | 2.283967 |
|
11037 | hsa-miR-299-3p | 2.269868 |
|
145914 |
hsa-miR-135b-5p | 2.266023 |
|
168606 |
hsa-miR-4633-5p | 2.253791 |
|
169239 |
hsa-miR-4732-5p | 2.236971 |
|
46789 |
hsa-miR-513b-5p | 2.213730 |
|
169379 |
hsa-miR-4694-3p | 2.194923 |
|
147501 | hsa-miR-98-3p | 2.165957 |
|
46917 | hsa-miR-205-5p | 2.142624 |
|
145751 | hsa-miR-23b-5p | 2.138643 |
|
148278 |
hsa-miR-138-2-3p | 2.126352 |
|
168963 |
hsa-miR-664b-5p | 2.123429 |
|
146111 | hsa-miR-767-5p | 2.105775 |
|
168953 |
hsa-miR-4704-5p | 2.097161 |
|
146165 | hsa-miR-1973 | 2.089585 |
|
29190 | hsa-miR-708-5p | 2.072155 |
|
17818 | hsa-miR-27a-5p | 2.064144 |
|
31076 | hsa-miR-559 | 2.018320 |
Verification of the microarray data by
RT-qPCR
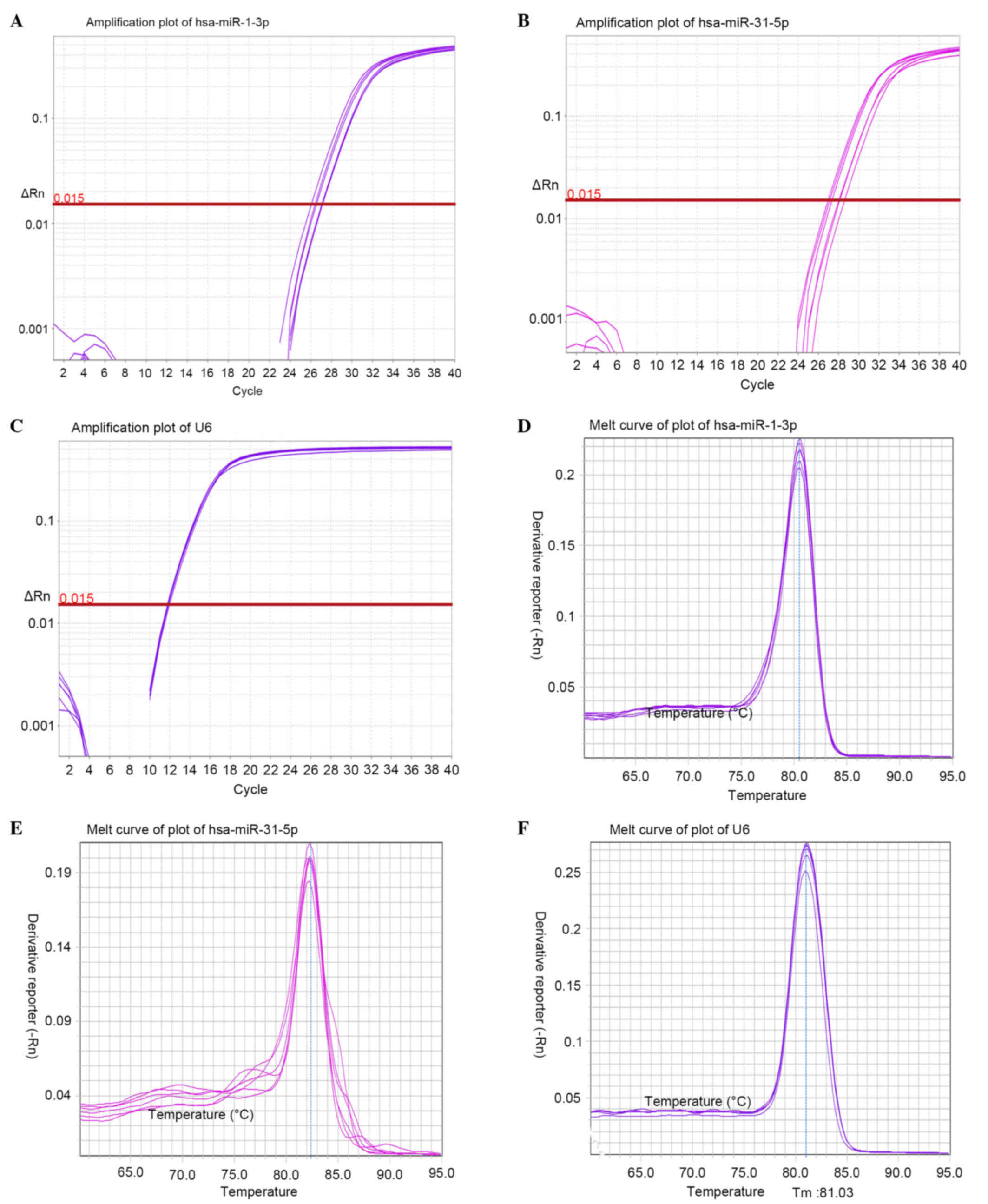
In order to verify the microarray results, RT-qPCR
assays were performed on selected miRNAs (hsa-miR-1-3p and
hsa-miR-31-5p) in the EXP and CON groups. Amplification and
dissociation curve charts for hsa-miR-1-3p, hsa-miR-31-5p and snRNA
U6 were generated (Fig. 2). The
2−ΔΔCq value of the miRNAs was calculated according to
the relative quantitative method. The 2−ΔΔCq analysis
revealed an upregulation of hsa-miR-1-3p (1.724) and downregulation
of hsa-miR-31-5p (0.458), which is consistent with the microarray
results, thus suggesting that the microarray data were
reliable.
Prediction of target genes
To demonstrate the function of differential miRNAs,
target gene prediction and functional analysis were conducted.
Databases of identified target genes can be accessed to compile
potential targets for differential miRNAs, due to the development
of numerous computational algorithms (18). The present study obtained all the
target genes of the 79 differentially expressed miRNAs according to
three public databases. Subsequently, GO and KEGG analysis were
used to identify the biological functions of these target
genes.
The most enriched GO terms of the three ontologies
are listed in Tables IV–VI. Transcription process, apoptosis
process and cell proliferation process were among the most
significantly enriched in terms of biological process; the cellular
component GO analysis demonstrated that the target genes were
associated with the nucleus, cytoplasm and cytosol; and protein
binding, DNA binding, ATP binding and transcription factor binding
were significantly enriched in terms of molecular function. Taken
together, these results suggested that the target genes of
differentially expressed miRNAs may be involved in cell
proliferation, division, mitosis, apoptosis and differentiation.
Finally, KEGG analysis indicated that 77 pathways were associated
with the target genes of differentially expressed miRNAs; 15
significantly enriched pathways are presented in Table VII, including the
phosphoinositide 3-kinase (PI3K)-Akt signaling pathway, the
mitogen-activated protein kinase (MAPK) signaling pathway, protein
processing in endoplasmic reticulum, focal adhesion and mammalian
target of rapamycin (mTOR) signaling, which are associated with
cell growth, differentiation, apoptosis and migration.
 | Table IV.Enriched terms in GO biological
process. |
Table IV.
Enriched terms in GO biological
process.
| Term ID | Term name | Hit number |
|---|
| GO:0006351 | Transcription,
DNA-dependent | 514 |
| GO:0006355 | Regulation of
transcription, DNA-dependent | 328 |
| GO:0045944 | Positive regulation
of transcription from RNA polymerase II promoter | 259 |
| GO:0007165 | Signal
transduction | 243 |
| GO:0006915 | Apoptotic
process | 216 |
| GO:0045893 | Positive regulation
of transcription, DNA-dependent | 200 |
| GO:0000122 | Negative regulation
of transcription from RNA polymerase II promoter | 198 |
| GO:0010467 | Gene
expression | 181 |
| GO:0045892 | Negative regulation
of transcription, DNA-dependent | 161 |
| GO:0043066 | Negative regulation
of apoptotic process | 143 |
| GO:0008285 | Negative regulation
of cell proliferation | 123 |
| GO:0006366 | Transcription from
RNA polymerase II promoter | 112 |
| GO:0008284 | Positive regulation
of cell proliferation | 105 |
| GO:0008283 | Cell
proliferation | 99 |
| GO:0051301 | Cell division | 94 |
| GO:0007049 | Cell cycle | 85 |
| GO:0006357 | Regulation of
transcription from RNA polymerase II promoter | 79 |
| GO:0006367 | Transcription
initiation from RNA polymerase II promoter | 73 |
| GO:0016055 | Wnt receptor
signaling pathway | 65 |
| GO:0001525 | Angiogenesis | 65 |
| GO:0043065 | Positive regulation
of apoptotic process | 64 |
| GO:0007173 | Epidermal growth
factor receptor signaling pathway | 62 |
| GO:0006917 | Induction of
apoptosis | 60 |
| GO:0007050 | Cell cycle
arrest | 58 |
| GO:0007067 | Mitosis | 58 |
| GO:0000082 | G1/S
transition of mitotic cell cycle | 47 |
| GO:0007243 | Intracellular
protein kinase cascade | 43 |
| GO:0007219 | Notch signaling
pathway | 43 |
| GO:0006260 | DNA
replication | 42 |
| GO:0030335 | Positive regulation
of cell migration | 41 |
| GO:0016477 | Cell migration | 40 |
| GO:0019827 | Stem cell
maintenance | 40 |
 | Table VI.Enriched terms in GO cell
component. |
Table VI.
Enriched terms in GO cell
component.
| Term Id | Term name | Hit number |
|---|
| GO:0005634 | Nucleus | 1,321 |
| GO:0005737 | Cytoplasm | 1,104 |
| GO:0005829 | Cytosol | 668 |
| GO:0005730 | Nucleolus | 459 |
| GO:0005654 | Nucleoplasm | 303 |
| GO:0005794 | Golgi
apparatus | 213 |
| GO:0016020 | Membrane | 198 |
| GO:0005783 | Endoplasmic
reticulum | 168 |
| GO:0005789 | Endoplasmic
reticulum membrane | 167 |
| GO:0048471 | Perinuclear region
of cytoplasm | 150 |
| GO:0000139 | Golgi membrane | 139 |
| GO:0043231 | Intracellular
membrane-bounded organelle | 110 |
| GO:0005667 | Transcription
factor complex | 83 |
| GO:0005925 | Focal adhesion | 50 |
| GO:0031965 | Nuclear
membrane | 50 |
| GO:0005765 | Lysosomal
membrane | 43 |
| GO:0005938 | Cell cortex | 40 |
| GO:0005741 | Mitochondrial outer
membrane | 39 |
| GO:0005911 | Cell-cell
junction | 36 |
| GO:0005819 | Spindle | 34 |
| GO:0000790 | Nuclear
chromatin | 32 |
| GO:0000151 | Ubiquitin ligase
complex | 25 |
| GO:0017053 | Transcriptional
repressor complex | 22 |
 | Table VII.Pathways associated with the
differentially expressed miRNAs. |
Table VII.
Pathways associated with the
differentially expressed miRNAs.
| Pathway | Function | Related
differentially expressed miRNAs |
|---|
| PI3K-Akt signaling
pathway | Activated by many
types of cellular stimuli or toxic insults; regulates fundamental
cellular functions, including transcription, translation,
proliferation, growth and survival | hsa-miR-181c-5p,
hsa-miR-20b-5p, hsa-miR-1297, hsa-miR-378e, hsa-miR-378c |
| MAPK signaling
pathway | Highly conserved
module, involved in various cellular functions, including cell
proliferation, differentiation and migration | hsa-miR-181c-5p,
hsa-miR-20b-5p, hsa-miR-374a-5p, hsa-miR-590-5p, hsa-let-7f-5p,
hsa-miR-378c, hsa-miR-299-3p |
| Protein processing
in endoplasmic reticulum | Promotes cell
apoptosis | hsa-miR-181c-5p,
hsa-miR-1297, hsa-miR-374a-5p, hsa-miR-299-3p, hsa-miR-20b-5p |
| Focal adhesion | Serves essential
roles in important biological processes, including cell motility,
cell proliferation, cell differentiation, regulation of gene
expression and cell survival | hsa-miR-1-3p,
hsa-miR-181c-5p, hsa-miR-20b-5p, hsa-miR-374a-5p, hsa-miR-378e,
hsa-miR-4644, hsa-miR-378c |
| Hippo signaling
pathway | Promotes Mats
localization in the cytoplasm, leading to cell apoptosis and
restricting organ size overgrowth | hsa-miR-20b-5p,
hsa-miR-374a-5p, hsa-miR-4644 |
| Wnt signaling
pathway | Required for basic
developmental processes, including cell-fate specification,
progenitor-cell proliferation and control of asymmetric cell
division | hsa-miR-20b-5p,
hsa-miR-1297, hsa-miR-374a-5p, hsa-miR-222-3p, hsa-miR-135b-5p |
| Cell cycle | Regulation of cell
mitosis | hsa-miR-1-3p,
hsa-miR-20b-5p, hsa-miR-1297 |
| TGF-β signaling
pathway | TGF-β family
members are involved in a wide spectrum of cellular functions,
including proliferation, apoptosis, differentiation and
migration | hsa-miR-181c-5p,
hsa-miR-20b-5p, hsa-miR-374a-5p, hsa-miR-590-5p, hsa-miR-454-3p,
hsa-miR-135b-5p |
| Adherens
junction | Important for
maintaining tissue architecture and cell polarity, and can limit
cell movement and proliferation | hsa-miR-181c-5p,
hsa-miR-1-3p, hsa-miR-20b-5p, hsa-miR-378e, hsa-miR-4644,
hsa-miR-378c |
| p53 signaling
pathway | p53 activation is
induced by a number of stress signals, including DNA damage,
oxidative stress and activated oncogenes, thus resulting in three
major outputs: Cell cycle arrest, cellular senescence and
apoptosis | hsa-miR-1297 |
| Apoptosis | Apoptosis is a
genetically controlled mechanism of cell death involved in the
regulation of tissue homeostasis | hsa-miR-20b-5p |
| Hedgehog signaling
pathway | Involved in control
of stem cell proliferation in adult tissues | hsa-miR-4644 |
| mTOR signaling
pathway | Regulates cell
growth and cell differentiation | hsa-miR-181c-5p,
hsa-miR-20b-5p, hsa-miR-454-3p |
| ErbB signaling
pathway | Regulates diverse
biological responses, including proliferation, differentiation,
cell motility and survival | hsa-miR-181c-5p,
hsa-miR-378e, hsa-miR-4644, hsa-miR-378c |
| VEGF signaling
pathway | Mediates the
proliferation and migration of endothelial cells, and promotes
their survival and vascular permeability | hsa-miR-1-3p,
hsa-miR-4644 |
Discussion
In the present study, following incubation with
GF109203X, some of the surviving keratinocytes reverted from a
differentiated to a dedifferentiated state, as evidenced by the
high colony-forming efficiency and expression of biological markers
of keratinocyte stem cells, including intergrin β1, CK19 and CK14.
However, in the CON group these alterations were not detected.
These findings suggested that terminally differentiating epidermal
keratinocytes may acquire some stem cell characteristics by
modulation with GF109203X treatment. Therefore, dedifferentiation
of human terminally differentiating keratinocytes may be induced by
GF109203X in vitro. Mature cell dedifferentiation is a
popular phenomenon, in which terminally differentiating epidermal
cells can revert to their ancestor cells by dedifferentiation;
i.e., epidermal cells can revert from the ‘old’ differentiated
state to the not fully differentiated ‘young’ state, or even the
‘naive’ state with the characteristics of epidermal stem cells.
Previous studies have confirmed that keratinocytes can be
dedifferentiated into their progenitor cells, and have identified
that dedifferentiated young epidermal cells may be used to treat
severe wounds (19,20). In addition, Sun et al
(21) demonstrated that
dedifferentiation of human terminally differentiating keratinocytes
into their precursor cells may be induced by basic fibroblast
growth factor. Another study indicated that dedifferentiated
epidermal cells are able to form clones and generate a complete
epithelium following migration to cutaneous wounds (22). Zhao et al (23) demonstrated that LiCl and glycogen
synthase kinase-3β inhibitor-induced cells are able to regenerate
skin, in a manner equivalent to that of epidermal stem cells. These
findings suggested that dedifferentiation is a promising method for
the production of abundant epidermal stem cells, which may be used
to bioengineer skin equivalents and as stem cell-based therapies in
cutaneous repair and regeneration. It is well known that poor wound
healing after trauma, surgery, acute illness or chronic disease
conditions affects millions of people worldwide each year (24), and the cost of non-healing wounds
is a great burden to health care systems (25). The efficacy of conventional
approaches to treating cutaneous wounds is limited; dressings,
periodic debridement, eliminating causative factors and innovations
in surgical autologous grafting techniques are inherently limited
to the size of available donor sites and are insufficient for
global burn injuries (26).
Therefore, the present study offers a potential novel strategy for
the treatment of cutaneous wounds. Furthermore, dedifferentiated
cells are readily available in large quantities with the use of
simple methods, and are considered moral and ethical alternatives
for disease therapy, with no risk of genetic incompatibility or
tissue rejection.
The present study used microarray hybridization to
comparably observe the expression of miRNAs between EXP and CON
groups. The results detected 45 upregulated miRNAs and 34
downregulated miRNAs when keratinocytes began exhibiting the
characteristics of epidermal-like stem cells. In the present study,
hsa-miR-1-3p was the most significantly upregulated miRNA and
hsa-miR-31-5p was the most significantly downregulated miRNA.
Hsa-miR-1-3p is also known as miR-1, which is significantly
positively correlated with expression of the proliferation marker
Ki67, and is involved in proliferation (27). A previous study demonstrated that
inhibition of PKC prevented the upregulation of miR-1 induced by
constitutively active Gαi2, demonstrating a role for PKC in the
regulation of muscle-specific miRNA (28). In the present study, miR-1 was
upregulated in keratinocytes treated with the PKC inhibitor
GF109203X, which may also serve an important role in proliferation.
Hsa-miR-31-5p is also known as miR-31, which has been implicated as
a key regulator of keratinocyte differentiation and proliferation.
Peng et al (29) indicated
that miR-31 is an endogenous negative regulator of factor
inhibiting hypoxia-inducible factor-1 expression, which results in
keratinocyte differentiation by enhancing Notch signaling; this
finding is in accordance with the results of the present study.
Furthermore, nuclear factor-κB-induced miR-31 promotes keratinocyte
proliferation by suppressing protein phosphatase 6 in psoriasis
(30). Recently, in human
metastatic cutaneous squamous cells, the increased expression of
miR-31 was revealed to promote migration, invasion and colony
forming ability (31). Taken
together, these findings suggested that miR-31 is a multifunctional
miRNA that serves important roles in physiological and pathological
conditions of epidermal keratinocytes; however, the molecular
mechanisms of PKC and miR-31 remain poorly characterized and
require further study. In addition, the present study demonstrated
that miR-181c-5p and miR-374a were predominantly expressed in
GF109203X-induced keratinocytes, which is in accordance with our
previous observation that these miRNAs were upregulated in native
keratinocyte stem cells (6).
Hsa-miR-181c-5p has functional relevance in the maintenance of
stemness, which may regulate cell proliferation and cell cycle
progression via the Notch signaling pathway and bone morphogenetic
protein pathway in cancer stem cells (32). In addition, miR-374a has been
reported to promote the proliferation of osteosarcoma cells by
targeting Axin2 (33). Overall,
these data indicated that these miRNAs may promote proliferation
and maintain the undifferentiated state when keratinocytes were
induced to re-express the biological characteristics of
epidermal-like stem cells by GF109203X.
The enrichment analysis of the differentially
expressed miRNAs demonstrated that hsa-miR-181c-5p, hsa-miR-378c
and hsa-miR-20b-5p are involved in numerous KEGG pathways that
regulate cell proliferation, differentiation and motility, which
may serve important roles in dedifferentiation. Hsa-miR-181c-5p,
hsa-miR-20b-5p, hsa-miR-374a-5p, hsa-miR-590-5p, hsa-let-7f-5p and
hsa-miR-378c are involved in the MAPK signaling pathway, which has
been reported to be involved in cell proliferation,
differentiation, inflammation and tumor growth (34,35).
PKCδ/p38δ MAPK signaling, which is a key controller of keratinocyte
proliferation and differentiation, increases p21 (Cip1) expression
to suppress keratinocyte proliferation (36). A further study demonstrated that
PKCδ/p38δ MAPK signaling suppresses methylosome protein 50
expression, leading to reduced H3/H4 arginine dimethylation at the
p21 (Cip1) promoter; this was associated with enhanced p21 (Cip1)
expression and reduced cell proliferation (37). Previous research has indicated that
the MAPK signaling pathway may increases p21 (Cip1) expression to
suppress keratinocyte proliferation, which indicated that
hsa-miR-181c-5p, hsa-miR-20b-5p, hsa-miR-374a-5p, hsa-miR-590-5p,
hsa-let-7f-5p and hsa-miR-378c may be associated with p21 (Cip1)
expression and keratinocyte proliferation. In addition, the
PI3K-Akt and mTOR signaling pathways may regulate the growth and
differentiation of keratinocytes (38,39),
whereas the Hedgehog signaling pathway is a critical regulator of
lineage-specific stem cells that maintains specialized sensory
compartments in the epidermis (40) which may serve a key role in
re-expression of biological characteristics in induced
keratinocytes.
The present study aimed to determine whether the
differentially expressed miRNAs are associated with
dedifferentiation of keratinocytes induced by GF109203X. It is well
known that Oct-3/4, sex determine region Y-box 2 (Sox2), Nanog,
c-Myc and Kruppel-like factor 4 (KLF4) are associated with
dedifferentiation (41,42). In cancer cells and mouse embryonic
stem cells, zinc finger E-box binding homeobox 1 links
epithelial-mesenchymal transition activation and maintenance of
stemness by suppressing stemness-inhibiting miRNAs, including
miR-200c, miR-203 and miR-183, which cooperate to suppress
expression of stem cell factors, such as Sox2 and KlLF4 (43). In addition, miR-134, miR-296 and
miR-470, which are upregulated during retinoicacid-induced
differentiation of mouse embryonic stem cells, target the amino
acid coding sequence of Nanog, Oct4 and Sox2 genes, leading to
transcriptional and morphological alterations characteristic of
differentiating mouse embryonic stem cells, and resulting in a
novel phenotype (44). Lauschke
et al (45) identified that
miRNAs are important drivers of hepatic dedifferentiation. Taken
together, dedifferentiation is a process associated with modulation
of numerous genes, in which miRNAs may have an important role; this
may explain why were so many differentially expressed miRNAs were
detected during GF109203X-induced keratinocyte
dedifferentiation.
In conclusion, when treated with the PKC inhibitor
GF109203X, keratinocytes exhibited a series of alterations,
including altered morphology, expression of epidermal cell-specific
markers and differentially expressed miRNAs. Bioinformatics
analysis of the differentially expressed miRNAs indicated that
inhibition of PKC signaling was associated with cell proliferation,
differentiation and dedifferentiation. Considering that
pre-clinical and clinical studies have demonstrated that modulation
of miRNA expression by administration of specific miRNA mimics or
inhibitors may be beneficial for treating diseases (46), the present study may offer novel
miRNAs for regulation of the PKC pathway. However, the exact
mechanisms underlying the differentially expressed miRNAs remain
unclear and require further study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460293), the
Science and Technology Planning Project of Jiangxi Province, China
(grant no. 20133BBG70026) and the Special Fund for Graduate
Innovation Project of Jiangxi Province, China (grant no.
YC2015-S086).
References
|
1
|
Moreno-Moya JM, Vilella F and Simón C:
MicroRNA: Key gene expression regulators. Fertil Steril.
101:1516–1523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki HI: Dissecting microRNA biogenesis
and microRNA-mediated regulation of gene network. Seikagaku.
87:413–421. 2015.(In Japanese). PubMed/NCBI
|
|
3
|
He L and Sedwick C: Lin He: ‘Junk’ DNA
isn't. J Cell Biol. 211:4–5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin EC, Qureshi AT, Dasa V, Freitas MA,
Gimble JM and Davis TA: MicroRNA regulation of stem cell
differentiation and diseases of the bone and adipose tissue:
Perspectives on miRNA biogenesis and cellular transcriptome.
Biochimie. 124:98–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao S: MicroRNA biogenesis and their
functions in regulating stem cell potency and differentiation. Biol
Proced Online. 18:82016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song Z, Liu D, Peng Y, Li J, Zhang Z and
Ning P: Differential microRNA expression profile comparison between
epidermal stem cells and differentiated keratinocytes. Mol Med Rep.
11:2285–2291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu YF, Ding M, Liu DW, Liu Y, Mao YG and
Peng Y: MicroRNA profiling in cutaneous wounds of diabetic rats.
Genet Mol Res. 14:9614–9625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sonkoly E, Wei T, Loriè E Pavez, Suzuki H,
Kato M, Törmä H, Ståhle M and Pivarcsi A: Protein kinase
C-dependent upregulation of miR-203 induces the differentiation of
human keratinocytes. J Invest Dermatol. 130:124–134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gutcher I, Webb PR and Anderson NG: The
isoform-specific regulation of apoptosis by protein kinase C. Cell
Mol Life Sci. 60:1061–1070. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Diaz-Meco MT and Moscat J: The atypical
PKCs in inflammation: NF-κB and beyond. Immunol Rev. 246:154–167.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitev V and Miteva L: Signal transduction
in keratinocytes. Exp Dermatol. 8:96–108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Panse R, Coulomb B, Mitev V, Bouchard
B, Lebreton C and Dubertret L: Differential modulation of human
fibroblast and keratinocyte growth by the protein kinase C
inhibitor GF 109203X. Mol Pharmacol. 46:445–451. 1994.PubMed/NCBI
|
|
13
|
Lee YS, Yuspa SH and Dlugosz AA:
Differentiation of cultured human epidermal keratinocytes at high
cell densities is mediated by endogenous activation of the protein
kinase C signaling pathway. J Invest Dermatol. 111:762–766. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
You HL, Eng HL, Hsu SF, Chen CM, Ye TC,
Liao WT, Huang MY, Baer R and Cheng JT: A PKC-Sp1 signaling pathway
induces early differentiation of human keratinocytes through
upregulation of TSG101. Cell Signal. 19:1201–1211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tibudan SS, Wang Y and Denning MF:
Activation of protein kinase C triggers irreversible cell cycle
withdrawal in human keratinocytes. J Invest Dermatol.
119:1282–1289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park YK and Jang BC: UVB-induced
anti-survival and pro-apoptotic effects on HaCaT human
keratinocytes via caspase- and PKC-dependent downregulation of PKB
HIAP-1, Mcl-1, XIAP andER stress. Int J Mol Med. 33:695–702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dweep H and Gretz N: miRWalk2. 0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kormos B, Belso N, Bebes A, Szabad G,
Bacsa S, Széll M, Kemény L and Bata-Csörgo Z: In vitro
dedifferentiation of melanocytes from adult epidermis. PLoS One.
6:e171972011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poloni A, Maurizi G, Anastasi S, Mondini
E, Mattiucci D, Discepoli G, Tiberi F, Mancini S, Partelli S,
Maurizi A, et al: Plasticity of human dedifferentiated adipocytes
toward endothelial cells. Exp Hematol. 43:137–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun X, Fu X, Han W, Zhao Y, Liu H and
Sheng Z: Dedifferentiation of human terminally differentiating
keratinocytes into their precursor cells induced by basic
fibroblast growth factor. Biol Pharm Bull. 34:1037–1045. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li JF, Duan HF, Wu CT, Zhang DJ, Deng Y,
Yin HL, Han B, Gong HC, Wang HW and Wang YL: HGF accelerates wound
healing by promoting the dedifferentiation of epidermal cells
through beta1-integrin/ILK pathway. Biomed Res Int.
2013:4704182013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao Z, Zhang C, Fu X, Yang R, Peng C, Gu
T, Sui Z, Wang C and Liu C: Differentiated epidermal cells regain
the ability to regenerate a skin equivalent by increasing the level
of β-catenin in the cells. Cells Tissues Organs. 196:353–361. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eming SA, Martin P and Tomic-Canic M:
Wound repair and regeneration: Mechanisms, signaling, and
translation. Sci Transl Med. 6:265sr62014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hay RJ, Johns NE, Williams HC, Bolliger
IW, Dellavalle RP, Margolis DJ, Marks R, Naldi L, Weinstock MA,
Wulf SK, et al: The global burden of skin disease in 2010: An
analysis of the prevalence and impact of skin conditions. J Invest
Dermatol. 134:1527–1534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun BK, Siprashvili Z and Khavari PA:
Advances in skin grafting and treatment of cutaneous wounds.
Science. 346:941–945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boštjančič E, Jerše M, Glavač D and Zidar
N: miR-1, miR-133a/b and miR-208a in human fetal hearts correlate
to the apoptotic and proliferation markers. Exp Biol Med (Maywood).
240:211–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Minetti GC, Feige JN, Bombard F, Heier A,
Morvan F, Nürnberg B, Leiss V, Birnbaumer L, Glass DJ and Fornaro
M: Gαi2 signaling is required for skeletal muscle growth,
regeneration, and satellite cell proliferation and differentiation.
Mol Cell Biol. 34:619–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng H, Kaplan N, Hamanaka RB, Katsnelson
J, Blatt H, Yang W, Hao L, Bryar PJ, Johnson RS, Getsios S, et al:
microRNA-31/factor-inhibiting hypoxia-inducible factor 1 nexus
regulates keratinocyte differentiation. Proc Natl Acad Sci USA.
109:14030–14034. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan S, Xu Z, Lou F, Zhang L, Ke F, Bai J,
Liu Z, Liu J, Wang H, Zhu H, et al: NF-κB-induced microRNA-31
promotes epidermal hyperplasia by repressing protein phosphatase 6
in psoriasis. Nat Commun. 6:76522015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang A, Landén NX, Meisgen F,
Lohcharoenkal W, Ståhle M, Sonkoly E and Pivarcsi A: MicroRNA-31 is
overexpressed in cutaneous squamous cell carcinoma and regulates
cell motility and colony formation ability of tumor cells. PLoS
One. 9:e1032062014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sanchez-Diaz PC, Hsiao TH, Chang JC, Yue
D, Tan MC, Chen HI, Tomlinson GE, Huang Y, Chen Y and Hung JY:
De-regulated microRNAs in pediatric cancer stem cells target
pathways involved in cell proliferation, cell cycle and
development. PLoS One. 8:e616222013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu T, Zhang C, Chai MX, An YB and Jia JL:
miR-374a promotes the proliferation of osteosarcoma cell
proliferation by targeting Axin2. Int J Clin Exp Pathol.
8:10776–10783. 2015.PubMed/NCBI
|
|
34
|
Mishra S, Tripathi A, Chaudhari BP,
Dwivedi PD, Pandey HP and Das M: Deoxynivalenol induced mouse skin
cell proliferation and inflammation via MAPK pathway. Toxicol Appl
Pharmacol. 279:186–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Pesakhov S, Weng A, Kafka M, Gocek
E, Nguyen M, Harrison JS, Danilenko M and Studzinski GP: ERK 5/MAPK
pathway has a major role in 1α, 25-(OH)2 vitamin D3-induced
terminal differentiation of myeloid leukemia cells. J Steroid
Biochem Mol Biol. 144:223–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saha K, Adhikary G, Kanade SR, Rorke EA
and Eckert RL: p38δ Regulates p53 to Control p21Cip1 expression in
human epidermal keratinocytes. J Biol Chem. 289:11443–11453. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saha K and Eckert RL: Methylosome protein
50 and PKCδ/p38δ protein signaling control keratinocyte
proliferation via opposing effects on p21Cip1 Gene Expression. J
Biol Chem. 290:13521–13530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xiao X, He Y, Li C, Zhang X, Xu H and Wang
B: Nicastrin mutations in familial acne inversa impact keratinocyte
proliferation and differentiation through Notch and
phosphoinositide 3-kinase/AKT signaling pathways. Br J Dermatol.
174:522–532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Patruno A, Pesce M, Grilli A, Speranza L,
Franceschelli S, De Lutiis MA, Vianale G, Costantini E, Amerio P,
Muraro R, et al: mTOR Activation by PI3K/Akt and ERK signaling in
short ELF-EMF exposed human keratinocytes. PLoS One.
10:e01396442015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiao Y, Thoresen DT, Williams JS, Wang C,
Perna J, Petrova R and Brownell I: Neural Hedgehog signaling
maintains stem cell renewal in the sensory touch dome epithelium.
Proc Natl Acad Sci USA. 112:7195–7200. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakamura Y, Ishikawa H, Kawai K, Tabata Y
and Suzuki S: Enhanced wound healing by topical administration of
mesenchymal stem cells transfected with stromal cell-derived
factor-1. Biomaterials. 34:9393–9400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tao R, Sun TJ, Han YQ, Xu G, Liu J and Han
YF: Optimization of in vitro cell labeling methods for human
umbilical cord-derived mesenchymal stem cells. Eur Rev Med
Pharmacol Sci. 18:1127–1134. 2014.PubMed/NCBI
|
|
43
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tay Y, Zhang J, Thomson AM, Lim B and
Rigoutsos I: MicroRNAs to Nanog, Oct4 and Sox2 coding regions
modulate embryonic stem cell differentiation. Nature.
455:1124–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lauschke VM, Vorrink SU, Moro SM, Rezayee
F, Nordling Å, Hendriks DF, Bell CC, Sison-Young R, Park BK,
Goldring CE, et al: Massive rearrangements of cellular miRNA
signatures are key drivers of hepatocyte dedifferentiation.
Hepatology. 64:1743–1756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sethupathy P: The promise and challenge of
therapeutic MicroRNA silencing in diabetes and metabolic diseases.
Curr Diab Rep. 16:522016. View Article : Google Scholar : PubMed/NCBI
|