Introduction
8-Oxoguanine (8-oxoG) is an oxidized form of a DNA
base and is considered to be a cellular marker for oxidative
stress-induced DNA damage (1). The
8-oxoG may pair with adenine and cytosine at an almost equal ratio,
resulting in guanine to adenine and cytosine to thymine base
substitutions during replication (2). Removal of 8-oxoG is a multi-step
process that relies on three proteins encoded by the mutT homolog 1
(MTH1), mutY DNA glycosylase (MUTYH) and 8-oxoG DNA
glycosylase (OGG1) genes (3). The MTH1 protein hydrolyzes
8-oxoG-triphospate to produce 8-oxoG-monophosphate. This strategy
avoids incorporation of oxidized foreign nucleotides into the
genome. Human OGG1 may efficiently catalyze the splitting of an
N-glycosidic bond between a deoxyribose sugar and the damaged
8-oxoG base (4–7). Therefore, OGG1 excises 8-oxoG
mis-paired with adenine during DNA replication (3). MUTYH excises the adenine inserted
opposite 8-oxoG in the template strand (4). OGG1 localizes to the nucleus and
mitochondria, and is the predominant enzyme responsible for base
excision repair (BER) of 8-oxoG lesions (8). The promoter of the OGG1 gene
contains a binding site for nuclear factor erythroid 2-related
factor 2 (Nrf2), a transcription factor that upregulates the
expression of a variety of antioxidant enzymes (9).
Plasma, which is known as the fourth fundamental
state of matter, refers to a partially ionized gas in physics
(10). Plasma produces ions and
electrons, in addition to uncharged neutral atoms, free radicals
and electrically excited atoms, which exhibit a high chemical
reactivity and emit UV radiation (11). Previous reports have demonstrated
that non-thermal plasma may be used to promote cell proliferation
and wound healing, and to treat cancer (12–15).
It is hypothesized that these properties of plasma are partially
due to the formation of reactive oxygen species (ROS) (15–18).
Our recent study demonstrated that non-thermal
dielectric barrier discharge (DBD) plasma generates ROS and
promotes various types of damage to cellular components, including
lipid membrane peroxidation, DNA breaks and protein carboxylation
(19). These findings suggested
that DBD plasma exerts cytotoxic effects via oxidative
stress-induced damage to these components. The present study
examined whether oxidative stress induced by DBD plasma exposure
might affect the formation of 8-oxoG in HaCaT human
keratinocytes.
Materials and methods
Reagents
N-acetyl cysteine (NAC), avidin-tetramethylrhodamine
isothiocyanate (avidin-TRITC) and actin antibody (A2066; 1:2,000; a
rabbit polyclonal antibody) were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). OGG1/2 (H-300) antibody
(sc-33181; 1:1,000; a rabbit polyclonal antibody), Nrf2 (C-20)
antibody (sc-722; 1:1,000; a rabbit polyclonal antibody) and mouse
anti-rabbit IgG-FITC conjugated secondary antibody (sc-2359; 1:100)
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The phospho-Nrf2 antibody (Ser40; 2073-1; 1:1,000; a rabbit
monoclonal antibody) was purchased from Epitomics (Burlingame, CA,
USA). The TATA box binding protein (TBP) antibody (ab818; 1:2,000;
a mouse monoclonal antibody) was purchased from Abcam Inc.
(Cambridge, MA, USA). The phospho-Akt (Ser473; 9271; 1:1,000; a
rabbit polyclonal antibody) and Akt (9272; 1:1,000; a rabbit
polyclonal antibody) antibodies were purchased from Cell Signaling
Technology (Danvers, MA, USA). The goat anti-rabbit IgG-horseradish
peroxidase conjugated secondary antibody (G21234; 1:10,000) and
goat anti-mouse IgG-horseradish peroxidase conjugated secondary
antibody (G21040; 1:10,000) were purchased from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). All other chemicals and
reagents were of analytical grade.
Cell culture and plasma exposure
The HaCaT human keratinocytes were obtained from
Amore Pacific Corporation (Yongin, Korea) and maintained at 37°C in
an incubator with 5% CO2. Cells were grown in Dulbecco's
modified Eagle's medium (Thermo Fisher Scientific Inc., Waltham,
MA, USA) containing 10% heat-inactivated fetal calf serum (Thermo
Fisher Scientific Inc.), 100 µg/ml streptomycin and 100 U/ml
penicillin. Non-thermal DBD plasma was applied as described
previously (19). The cell
suspension was adjusted to a concentration of 2×105
cells/ml and 11 ml was placed into 60 mm dishes. Cells were exposed
to DBD plasma for 1, 2 and 3 min. Positive controls were treated
with 1 mM NAC 30 min prior to DBD plasma exposure. Following plasma
exposure, cells were incubated for 24 h prior to subsequent
experiments.
Analysis of 8-oxoG level
Cellular DNA was isolated and purified using the
genomic DNA purification kit (Promega Corporation, Madison, WI,
USA) and quantified using a spectrophotometer. The quantity of
8-hydroxy-2′-deoxyguanosine (8-OHdG), a nucleoside of 8-oxoG, in
the DNA was determined using the Bioxytech 8-OHdG ELISA kit (Oxis
International, Tampa, FL, USA), according to the manufacturer's
protocol. The quantity of 8-OHdG was considered proportional to
8-oxoG. In addition, the quantity of 8-oxoG was determined via a
fluorescent binding assay using avidin, which binds to 8-oxoG with
high affinity (20). The cells
were fixed and permeabilized with ice-cold methanol for 15 min, and
subsequently incubated with avidin-TRITC for 1 h at room
temperature. 8-oxoG was visualized using a confocal microscope and
LSM 5 PASCAL laser scanning software version 3.5 (Zeiss GmbH, Jena,
Germany).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from cells using the total
RNA extraction kit (Intron Biotechnology, Seongnam, Korea) and cDNA
was amplified using reverse transcription reagent kit according to
the manufacturer's protocol (25021; Intron Biotechnology). PCR
amplifications of OGG1 and GAPDH (a housekeeping
gene) were performed as follows: An initial pre-denaturation step
at 94°C for 2 min, 35 cycles of denaturation at 94°C for 20 sec,
annealing at 58°C for 30 sec and extension at 72°C for 1 min, and a
final extension step at 72°C for 5 min. Primer pairs (Bioneer
Corporation, Daejeon, Korea) were as follows: Sense,
5′-CTGCCTTCTGGACAATCTTT-3′ and antisense, 5′-TAGCCCGCCCTGTTCTTC-3′
for human OGG1; and sense, 5′-TCAAGTGGGGCGATGCTGGC-3′ and
antisense, 5′-TGCCAGCCCCAGCGTCAAAG-3′ for human GAPDH.
Amplified products were resolved by electrophoresis and
photographed under UV light.
Transient transfection and OGG1
promoter luciferase assay
Cells were cultured at a density of 1×106
cells per 60 mm dish to maintain approximately 60–80% confluence.
Cells were transiently transfected with a plasmid harboring the
OGG1 promoter using the transfection reagent Lipofectamine
(Invitrogen; Thermo Fisher Scientific Inc.), according to the
manufacturer's protocol. The OGG1 promoter-luciferase
construct was a generous gift from Professor Ho Jin You (Chosun
University, Gwangju, Korea). Transfected cells were exposed to DBD
plasma for 2 min, with or without pretreatment with 1 mM NAC for 30
min. Cells were incubated for 24 h prior to analysis. Subsequently,
cells were lysed with reporter lysis buffer (Promega Corporation),
and the lysate was mixed with the luciferase assay reagent (Promega
Corporation). The mixture was detected by a luminometer.
Protein extraction and western blot
analysis
Cells were seeded in 100-mm culture plates at a
density of 2×105 cells/ml. To extract total protein,
cells were lysed on ice for 30 min using 150 µl lysis reagent
(Intron Biotechnology), and centrifuged at 13,000 × g at 4°C
for 30 min. To extract nuclear proteins, cells were lysed using the
subcellular protein fractionation kit (Thermo Fisher Scientific
Inc.). Protein concentrations of the cellular and nuclear extracts
were determined using the Bradford assay. Aliquots of the lysates
(20 µg protein) were boiled for 5 min, electrophoresed on 10%
SDS-polyacrylamide gels, and transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Hercules, CA, USA). Membranes were
incubated with the appropriate primary antibody for 2 h at room
temperature, followed by a horseradish peroxidase-conjugated IgG
secondary antibody for 1 h at room temperature. Protein bands were
visualized by developing the blots with an enhanced
chemiluminescence detection kit (GE Healthcare Life Sciences,
Chalfont, UK) and exposing the membranes to autoradiography film
(Thermo Fisher Scientific Inc.).
Immunocytochemistry
Cells were plated onto coverslips, fixed with 1%
paraformaldehyde for 30 min and permeabilized with 2% triton X-100
in PBS for 30 min (21).
Subsequently, cells were blocked with 1% bovine serum albumin in
PBS for 1 h, incubated with OGG1 or Nrf2 primary antibodies for 2 h
at room temperature and probed with a FITC-conjugated secondary
antibody at 1:200 for 1 h at 37°C. Following washing with PBS,
cells were transferred onto microscope slides in mounting medium
containing DAPI (Vector Laboratories, Burlingame, CA, USA). Slides
were examined using a confocal microscope and images were captured
using LSM 5 PASCAL laser scanning software version 3.5 (Zeiss
GmbH).
Statistical analysis
Statistical significance was determined using
analysis of variance and Tukey's tests with version 3.5 of
SigmaStat software (Systat Software Inc., San Jose, CA, USA). All
values are presented as the mean ± standard error. P<0.05 was
considered to indicate a statistically significant difference.
Results
Exposure to non-thermal DBD plasma
increases 8-oxoG levels in HaCaT cells
Our previous study demonstrated that exposure of
HaCaT cells to non-thermal DBD plasma for 2 min at 9.8 J/ml reduced
cell viability by 50% (19).
Therefore, the same plasma dose was utilized in the present study.
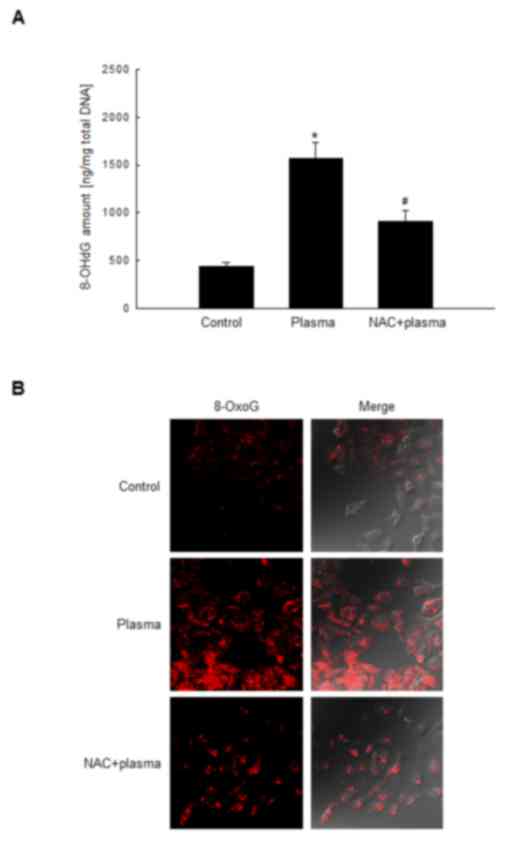
The quantity of 8-OHdG in DBD plasma-exposed HaCaT cells was
investigated using an ELISA kit, and was considered to be
proportional to the quantity of 8-oxoG. The 8-OHdG level in HaCaT
cells exposed to DBD plasma were 1,571 ng/mg, which was
significantly greater compared with control cells (442 ng/mg);
however, this increase was significantly suppressed in cells that
were pretreated with the antioxidant NAC prior to plasma exposure
(Fig. 1A). In addition, 8-oxoG was
analyzed by confocal microscopy using avidin-TRITC (20). The fluorescence intensity of
avidin-TRITC bound to 8-oxoG was elevated in DBD plasma-exposed
cells compared with control cells; however, pretreatment of DBD
plasma-exposed cells with NAC markedly reduced this (Fig. 1B). These results indicated that
exposure of HaCaT cells to DBD plasma generated the oxidized DNA
base 8-oxoG.
Non-thermal DBD plasma attenuates
expression of OGG1 in HaCaT cells
The glycosylase enzyme OGG1 is responsible for the
excision of 8-oxoG (22). mRNA
expression level of OGG1 in HaCaT cells was reduced by DBD
plasma exposure (Fig. 2A). In
addition, the transcriptional activity of the OGG1 promoter
was reduced in DBD plasma-exposed cells; however, pretreatment of
cells with NAC attenuated this significantly (Fig. 2B). Furthermore, OGG1 protein
expression was reduced by exposure to DBD plasma in a
time-dependent manner (Fig. 2C),
and NAC pretreatment reversed this decrease (Fig. 2D). Subsequently, immunocytochemical
analysis of the control, plasma-exposed and NAC-pretreated
plasma-exposed cells was performed to determine the cellular
distribution of the OGG1 protein. This revealed that OGG1
expression in HaCaT cells exposed to DBD plasma was reduced
compared with control cells; this decrease was attenuated in
NAC-pretreated cells (Fig. 2E).
These results indicated that DBD plasma exposure reduces the
expression of OGG1 at the mRNA and protein level in HaCaT cells;
however, pretreatment with NAC suppresses this effect.
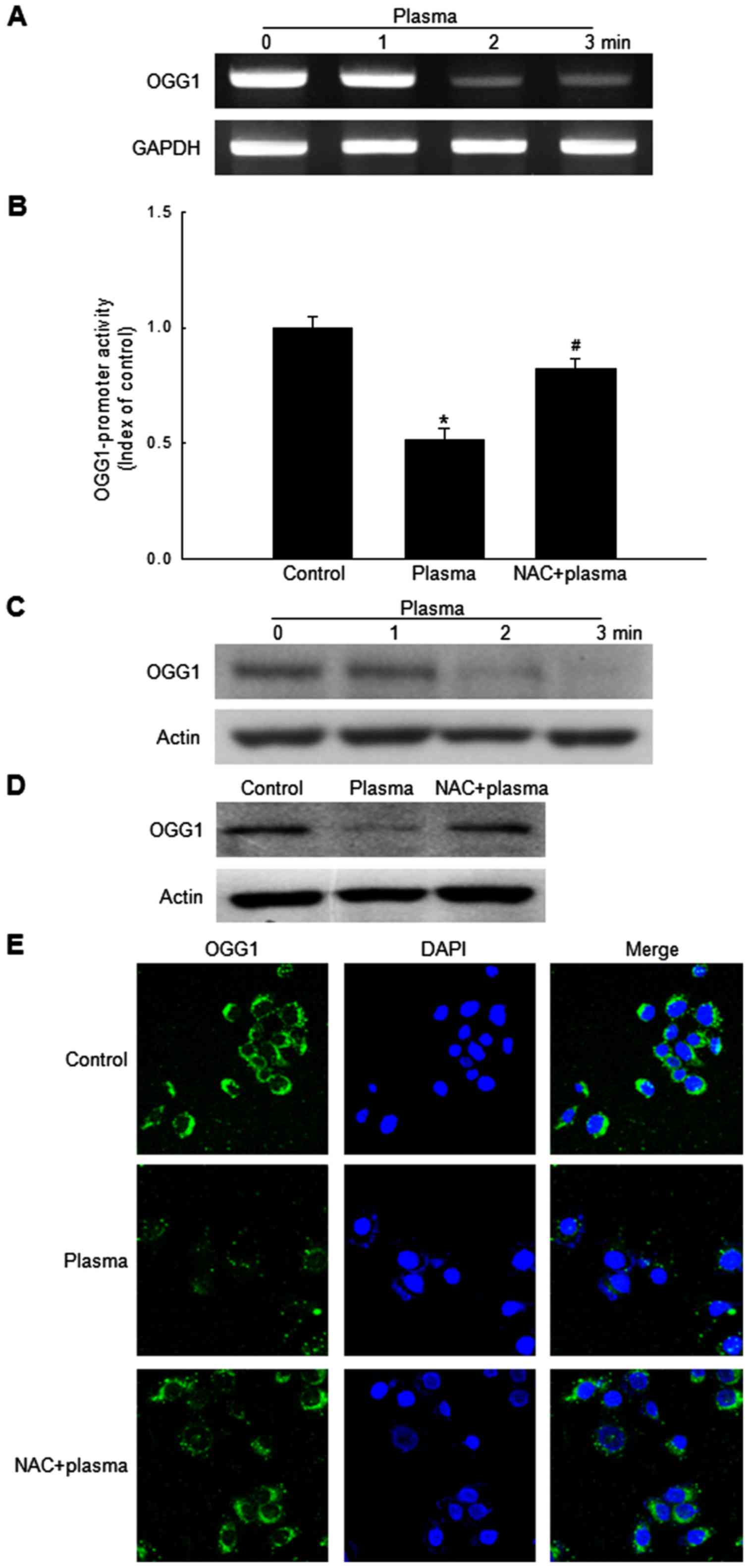 | Figure 2.Exposure to non-thermal DBD plasma
reduces OGG1 expression level in HaCaT cells. (A)
OGG1 and GAPDH mRNA expression levels were analyzed
by RT-PCR in cells exposed to DBD plasma for 1, 2 or 3 min,
followed by incubation for 24 h. (B) Cells were transfected with an
OGG1-containing plasmid using Lipofectamine and exposed to
DBD plasma for 2 min, with or without pretreatment with NAC for 30
min. Cells were incubated for 24 h prior to assessment of
luciferase activity. Data are expressed as the mean ± standard
error of triplicate samples within the same experiment. *P<0.05
vs. control cells; #P<0.05 vs. plasma-exposed cells.
(C) Western blot analysis was utilized to assess OGG1 and actin
(loading control) protein expression levels in cells exposed to DBD
plasma for 1, 2 or 3 min, and incubated for 24 h prior to analysis.
(D) Western blot analysis and (E) confocal microscopy were
performed to assess the distribution of OGG1 protein in control
cells, DBD plasma-exposed cells, and pretreatment of NAC and
plasma-exposed cells. Cells were incubated for 24 h prior to
analysis. (E) Green fluorescence indicates OGG1 and blue
fluorescence indicates nuclei. DBD, dielectric barrier discharge;
OGG1, 8-oxoguanine glycosylase 1; RT-PCR, reverse
transcription-polymerase chain reaction; NAC, N-acetyl
cysteine. |
Non-thermal DBD plasma attenuates
nuclear translocation of Nrf2 in HaCaT cells
Nrf2, a transcription factor that regulates the
expression levels of antioxidant and detoxifying enzymes (23), binds to antioxidant response
elements in the OGG1 promoter and regulates its expression
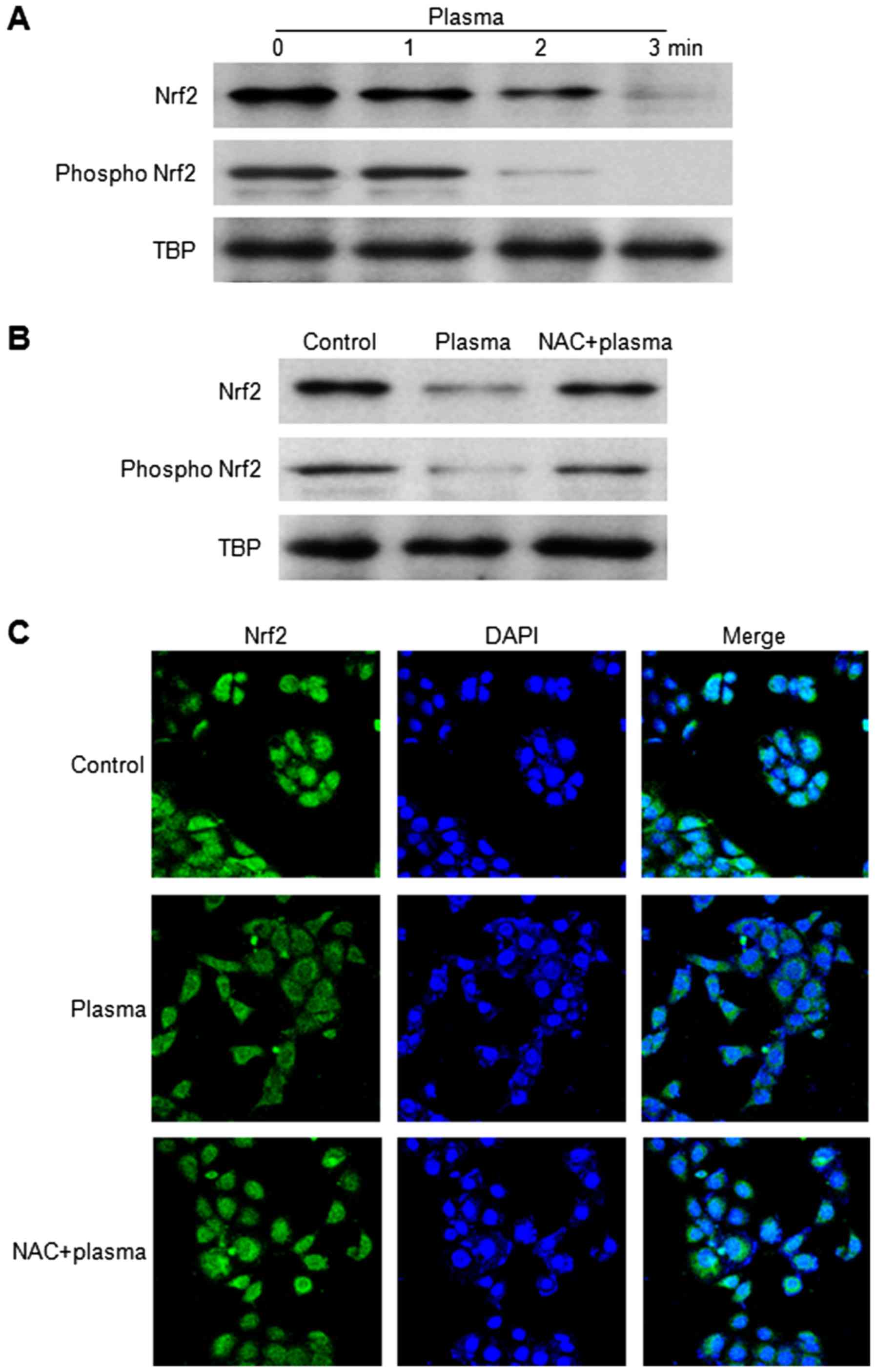
(9,24). Western blot analysis revealed that
the expression levels of nuclear Nrf2 and phospho-Nrf2 in HaCaT
cells were reduced by DBD plasma in a time-dependent manner
(Fig. 3A); however, the expression
levels of the two proteins were restored in cells that were
pretreated with NAC prior to plasma exposure (Fig. 3B). To identify the effect of DBD
plasma on the localization of the Nrf2 protein, immunocytochemical
analysis was performed (Fig. 3C).
The nuclear localization of Nrf2 was reduced by exposure of HaCaT
cells to DBD plasma, as demonstrated by a decrease in Nrf2 and DAPI
co-staining. However, this effect was attenuated by pretreatment
with NAC. These results indicated that nuclear expression of Nrf2
was reduced by exposure to DBD plasma, and that this reduction was
partly abrogated by pretreatment with NAC.
Non-thermal DBD plasma attenuates
phosphorylation of Akt in HaCaT cells
Nrf2 is sequestered in the cytoplasm by Kelch-like
ECH-associated protein 1 (Keap1). However, Nrf2 may translocate to
the nucleus following phosphorylation by activated Akt, which
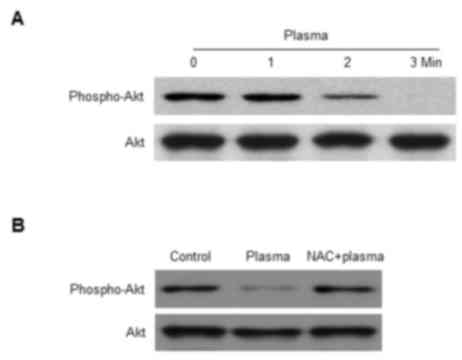
serves a role in a cell survival pathway (25). The expression level of the active
phospho-Akt protein was reduced by exposure of HaCaT cells to DBD
plasma in a time-dependent manner (Fig. 4A). Furthermore, the reduced
phosphorylation of Akt was blocked by pretreatment of cells with
NAC (Fig. 4B). These results
suggested that exposure to DBD plasma reduces the phosphorylation
of Akt, and that this modification may be restored by pretreatment
with NAC.
Discussion
Exposure to non-thermal plasma generates ROS and
induces oxidative stress in living cells and tissues (16–18).
Our previous study revealed that exposure of HaCaT cells to
non-thermal DBD plasma generated ROS and resulted in DNA damage
(19). The present study focused
on the mechanism underlying DNA damage induced by DBD plasma by
investigating the generation of the modified DNA base, 8-oxoG, the
most stable product of oxidative DNA damage caused by ROS. The
8-oxoG base is considered highly mutagenic as it may pair with
adenine as well as cytosine, and accumulates in nuclear and
mitochondrial DNA (26). As
guanine is more vulnerable to oxidation than other nitrogen bases,
ROS may readily oxidize it to 8-oxoG. However, OGG1 identifies
8-oxoG bases in DNA and initiates BER to remove them (27). The DNA glycosylase activity of OGG1
preferentially excises 8-oxoG bases that are paired with cytosine.
A DNA glycosylase encoded by the MUTYH gene, a homolog of
Escherichia coli mutY, excises adenines located opposite
8-oxoG in the template strand (28). Once cytosine is inserted and
adenine is removed, 8-oxoG can be removed by OGG1 via the BER
mechanism (29).
In the present study, exposure of HaCaT cells to DBD
plasma enhanced the level of 8-oxoG and reduced level of OGG1. By
contrast, pretreatment of cells with NAC, an established ROS
scavenger, suppressed these effects. The OGG1 gene is
regulated by binding of the Nrf2 transcription factor to
antioxidant response elements in its promoter region (9,27).
The transcriptional activity of Nrf2 requires its translocation to
the nucleus, which is regulated by Keap1-mediated anchoring in the
cytoplasm (30). The Nrf2-Keap1
complex dissociates to block the ubiquitination of Nrf2, and
translocation of this transcription factor is initiated by its
phosphorylation at serine 40 and specific threonine residues, in
addition to modification of cysteine residues in Keap1 (31,32).
Phosphorylation of Nrf2 is achieved by various kinases including
Akt (33). In the present study,
the expression levels of Nrf2 and phsopho-Nrf2 in the nuclear
fraction by DBD plasma exposure was reduced (Fig. 3A). This might be due to the
downregulation of transcription of Nrf2 and Akt pathway (upstream
to phosphorylate Nrf2) by DBD plasma exposure.
In conclusion, the results of the present study
revealed that DBD plasma exposure generates 8-oxoG and disrupts
OGG1 gene transcription by downregulation of the Akt-Nrf2
signaling pathway. However, pretreatment with NAC, a well-known
scavenger of various free radicals, including ROS, suppressed
8-oxoG level and activated the Akt-Nrf2-OGG1 signaling pathway.
Acknowledgements
The present study was supported by the R&D
Program of Plasma Advanced Technology for Agriculture and Food
(Plasma Farming) via the National Fusion Research Institute of
Korea (NFRI), funded by the Government.
References
|
1
|
Fortini P, Pascucci B, Parlanti E,
D'Errico M, Simonelli V and Dogliotti E: 8-Oxoguanine DNA damage:
At the crossroad of alternative repair pathways. Mutat Res.
531:127–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruner SD, Norman DP and Verdine GL:
Structural basis for recognition and repair of the endogenous
mutagen 8-oxoguanine in DNA. Nature. 403:859–866. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakabeppu Y: Cellular levels of
8-oxoguanine in either DNA or the nucleotide pool play pivotal
roles in carcinogenesis and survival of cancer cells. Int J Mol
Sci. 15:12543–12557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Görgens H, Müller A, Krüger S, Kuhlisch E,
König IR, Ziegler A, Schackert HK and Eckelt U: Analysis of the
base excision repair genes MTH1, OGG1 and MUTYH in patients with
squamous oral carcinomas. Oral Oncol. 43:791–795. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klungland A, Rosewell I, Hollenbach S,
Larsen E, Daly G, Epe B, Seeberg E, Lindahl T and Barnes DE:
Accumulation of premutagenic DNA lesions in mice defective in
removal of oxidative base damage. Proc Natl Acad Sci USA.
96:13300–13305. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boiteux S and Radicella JP: The human OGG1
gene: Structure, functions, and its implication in the process of
carcinogenesis. Arch Biochem Biophys. 377:1–8. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De S, ouza-Pinto NC, Eide L, Hogue BA,
Thybo T, Stevnsner T, Seeberg E, Klungland A and Bohr VA: Repair of
8-oxodeoxyguanosine lesions in mitochondrial dna depends on the
oxoguanine dna glycosylase (OGG1) gene and 8-oxoguanine accumulates
in the mitochondrial dna of OGG1-defective mice. Cancer Res.
61:5378–5381. 2001.PubMed/NCBI
|
|
8
|
Sampath H, McCullough AK and Lloyd RS:
Regulation of DNA glycosylases and their role in limiting disease.
Free Radic Res. 46:460–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh B, Chatterjee A, Ronghe AM, Bhat NK
and Bhat HK: Antioxidant-mediated up-regulation of OGG1 via NRF2
induction is associated with inhibition of oxidative DNA damage in
estrogen-induced breast cancer. BMC Cancer. 13:2532013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo QZ, D'Angelo N and Merlino RL: Shock
formation in a negative ion plasma. Phys Plasmas. 5:28681998.
View Article : Google Scholar
|
|
11
|
Goree J: Charging of particles in a
plasma. Plasma Sources Sci Technol. 3:4001994. View Article : Google Scholar
|
|
12
|
Leduc M, Guay D, Leask RL and Coulombe S:
Cell permeabilization using a non-thermal plasma. New J Phys.
11:1150212009. View Article : Google Scholar
|
|
13
|
Kalghatgi S, Friedman G, Fridman A and
Clyne AM: Endothelial cell proliferation is enhanced by low dose
non-thermal plasma through fibroblast growth factor-2 release. Ann
Biomed Eng. 38:748–757. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ermolaeva SA, Varfolomeev AF, Chernukha
MY, Yurov DS, Vasiliev MM, Kaminskaya AA, Moisenovich MM, Romanova
JM, Murashev AN, Selezneva II, et al: Bactericidal effects of
non-thermal argon plasma in vitro, in biofilms and in the animal
model of infected wounds. J Med Microbiol. 60:75–83. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vandamme M, Robert E, Lerondel S, Sarron
V, Ries D, Dozias S, Sobilo J, Gosset D, Kieda C, Legrain B, et al:
ROS implication in a new antitumor strategy based on non-thermal
plasma. Int J Cancer. 130:2185–2194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahn HJ, Kim KI, Kim G, Moon E, Yang SS and
Lee JS: Atmospheric-pressure plasma jet induces apoptosis involving
mitochondria via generation of free radicals. PLoS One.
6:e281542011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arjunan KP and Clyne AM: Non-thermal
dielectric barrier discharge plasma induces angiogenesis through
reactive oxygen species. Conf Proc IEEE Eng Med Biol Soc.
2011:2447–2450. 2011; PubMed/NCBI
|
|
18
|
Ishaq M, Kumar S, Varinli H, Han ZJ, Rider
AE, Evans MD, Murphy AB and Ostrikov K: Atmospheric gas
plasma-induced ROS production activates TNF-ASK1 pathway for the
induction of melanoma cancer cell apoptosis. Mol Biol Cell.
25:1523–1531. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim KC, Piao MJ, Hewage SR Madduma, Han X,
Kang KA, Jo JO, Mok YS, Shin JH, Park Y, Yoo SJ and Hyun JW:
Non-thermal dielectric-barrier discharge plasma damages human
keratinocytes by inducing oxidative stress. Int J Mol Med.
37:29–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Struthers L, Patel R, Clark J and Thomas
S: Direct detection of 8-oxodeoxyguanosine and 8-oxoguanine by
avidin and its analogues. Anal Biochem. 255:20–31. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heo HJ, Kim HK, Youm JB, Cho SW, Song IS,
Lee SY, Ko TH, Kim N, Ko KS, Rhee BD and Han J: Mitochondrial
pyruvate dehydrogenase phosphatase 1 regulates the early
differentiation of cardiomyocytes from mouse embryonic stem cells.
Exp Mol Med. 48:e2542016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hazra TK, Izumi T, Boldogh I, Imhoff B,
Kow YW, Jaruga P, Dizdaroglu M and Mitra S: Identification and
characterization of a human DNA glycosylase for repair of modified
bases in oxidatively damaged DNA. Proc Natl Acad Sci USA.
99:3523–3528. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang KA and Hyun JW: Oxidative stress,
Nrf2 and epigenetic modification contribute to anticancer drug
resistance. Toxicol Res. 33:1–5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piao MJ, Kim KC, Choi JY, Choi J and Hyun
JW: Silver nanoparticles down-regulate Nrf2-mediated 8-oxoguanine
DNA glycosylase 1 through inactivation of extracellular regulated
kinase and protein kinase B in human chang liver cells. Toxicol
Lett. 207:143–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Chen Y, Sternberg P and Cai J:
Essential roles of the PI3 kinase/Akt pathway in regulating
Nrf2-dependent antioxidant functions in the RPE. Invest Ophthalmol
Vis Sci. 49:1671–1678. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakabeppu Y, Sakumi K, Sakamoto K,
Tsuchimoto D, Tsuzuki T and Nakatsu Y: Mutagenesis and
carcinogenesis caused by the oxidation of nucleic acids. Biol Chem.
387:373–379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Radicella JP, Dherin C, Desmaze C, Fox MS
and Boiteux S: Cloning and characterization of hOGG1, a human
homolog of the OGG1 gene of Saccharomyces cerevisiae. Proc Natl
Acad Sci USA. 94:8010–8015. 1997; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Slupska MM, Luther WM, Chiang JH, Yang H
and Miller JH: Functional expression of hMYH, a human homolog of
the Escherichia coli MutY protein. J Bacteriol. 181:6210–6213.
1999.PubMed/NCBI
|
|
29
|
Ohtsubo T, Nishioka K, Imaiso Y, Iwai S,
Shimokawa H, Oda H, Fujiwara T and Nakabeppu Y: Identification of
human MutY homolog (hMYH) as a repair enzyme for 2-hydroxyadenine
in DNA and detection of multiple forms of hMYH located in nuclei
and mitochondria. Nucleic Acids Res. 28:1355–1364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung JS, Lee SY, Kim DH and Kim HS:
Protopanaxatriol ginsenoside Rh1 upregulates phase II antioxidant
enzyme gene expression in rat primary astrocytes: Involvement of
MAP kinases and Nrf2/are signaling. Biomol Ther (Seoul). 24:33–39.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-are pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sykiotis GP and Bohmann D:
Stress-activated cap'n'collar transcription factors in aging and
human disease. Sci Signal. 3:re32010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boutten A, Goven D, Artaud-Macari E,
Boczkowski J and Bonay M: NRF2 targeting: A promising therapeutic
strategy in chronic obstructive pulmonary disease. Trends Mol Med.
17:363–371. 2011. View Article : Google Scholar : PubMed/NCBI
|