Introduction
Cerebrovascular ischemia is challenging to treat due
to its rapid progression, and there are a limited number of
effective treatments. The resulting cerebral infarction can cause
severe sequelae in patients and markedly affects quality of life
(1). The penumbral areas around
the main cerebral lesions contain numerous dormant or semi-dormant
brain cells that can only maintain their integrity, rather than
perform their normal function, as a result of reduced energy and
oxygen supply (2). The protection
of these cells is important for clinical treatment of infarction
(3). Bone marrow stromal cells
(BMSCs) are skeletal progenitor cells that can differentiate into
bone, cartilage, fat and nerve cells (4,5).
BMSCs are easy to acquire, expand, genetically manipulate and
transplant in vivo, and are becoming increasingly used in
the field of neural regeneration and transplantation (6).
Moderate hypothermia is well recognized to protect
the brain from hypoxic-ischemic injury, potentially via reducing
the cerebral metabolic rate of oxygen and lowering the synthesis
and release of excitotoxic neurotransmitters and inflammatory
mediators (7,8). The penumbral region of the brain is
an ischemic, hypoxic, inflammatory and toxic environment, which
limits stem cell transplantation into the penumbra due to their
reduced survival and proliferation, in addition to premature aging
(9–11). The combination of neural stem cell
transplantation into the penumbra and moderate hypothermia may
provide an improved treatment regime for ischemic stroke (12). However, the molecular mechanisms of
protection by the combination neural stem cells and hypothermia
remain unclear. Understanding these mechanisms is important to
provide more effective and rational clinical treatments involving
moderate hypothermia.
Small ubiquitin-related modifiers (SUMOs) are an
important class of post-translational modification protein factors
and involved in maintaining genome stability (13), protein-protein interactions,
translocation between the cytoplasm and nucleus, and limiting
ubiquitination by combining with target proteins (14,15).
SUMO modification is a dynamic and reversible event in cells,
suggesting that certain SUMOylation-associated pathological events
may be reversible. Notably, cerebral ischemia has been reported to
induce SUMOylation in neuronal cells with increased binding of
SUMOs to target proteins, thereby performing a protective role
(16,17). Thus, it was hypothesized that the
neuroprotective actions of moderate hypothermia following cerebral
infarction may be associated with increased SUMOylation of multiple
proteins in neurons.
Materials and methods
Experimental animals
A total of 40 12-week-old (307±28.3 g) and 2 newborn
(5.1±0.62 g) Sprague-Dawley rats were purchased from the Animal
Center of the Cancer Institute of the Chinese Academy of Medical
Science (Beijing, China). Animals were housed in the Animal
Experimental Center of the Fifth Central Hospital of Tianjin
(Tianjin, China) at 20–25°C with 50±5% humidity. All experiments
were performed according to the Principles of Laboratory Animal
Care (18) and was approved by the
Ethics Committee of The Fifth Central Hospital of Tianjin.
Cell culture
Methods for the extraction and culture of BMSCs have
been described previously (4,19).
Briefly, the long bones of the hind legs were dissected from the
two newborn male rats, and bone marrow plugs were extracted from
the bones by flushing the bone marrow cavity with complete culture
medium (RASMX-90011, Cyagen Biosciences Inc. Santa Clara, CA, USA).
About 5×107 cells were seeded in a 75-cm2
culture flask and incubated at 37°C in a humidified atmosphere with
5% CO2. Non-adherent cells were removed after 24 h, and
the culture medium was replaced every 3 days. Adherent cells
reached 90–95% confluence within 10–15 days and were passaged with
0.25% trypsin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at a ratio of 1:3. Cells at passage 3 were characterized
by flow cytometry (Abcam, Shanghai, China).
Oxygen-glucose deprivation model and
moderate hypothermic therapy
To simulate cerebral ischemia, BMSCs were cultured
in an anoxic chamber (Forma Scientific Anaerobic System; Thermo
Fisher Scientific, Inc.) (20).
Glucose-, L-aspartic acid-, L-glutamic acid-, and sodium
pyruvate-free neurobasal medium (Gibco; Thermo Fisher Scientific,
Inc.) was equilibrated overnight in the anoxic chamber with the
anoxic gas mixture (85% N2, 10% H2 and 5%
CO2). Cultured cells were washed three times with the
anoxic medium and then transferred to the anoxic chamber. Following
60 min of oxygen-glucose deprivation (OGD), the anoxic medium was
replaced with neurobasal/B27 medium, and the cells were transferred
to incubators set at 33°C or 37°C with a gas mixture of 95% air and
5% CO2 for an additional 24 h. The neurobasal/B27 medium
was pre-warmed to 33°C or 37°C for induction of moderate
hypothermia.
Small interfering RNA
transfection
A small interfering RNA (siRNA) duplex targeted to
the Ubc9 gene was purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China) (sense, 5′GGG AAG GAC CCT TGT TTA A3′;
anti-sense, 5′CTT AAA GGC GTT CGT TAG G3′). Lipofectamine RNAiMAX
transfection reagent (Thermo Fisher Scientific, Inc.) was used
according to the manufacturer's instructions. SiUBC9 and a
universal negative control (SiNC) were purchased from Shanghai
GenePharma Co., Ltd. SiUBC9 and SiNC were applied at 10 nM
dissolved in Opti-MEM (Thermo Fisher Scientific, Inc.).
Immunofluorescence
BMSCs were seeded onto glass slides and cultured at
33°C for 2 or 24 h. The cells were then fixed in 4%
paraformaldehyde/phosphate-buffered saline (PBS), blocked with 20%
goat serum (Gibco; Thermo Fisher Scientific, Inc.), and incubated
with anti-SUMO1 (ab11672; 1:1,000; Abcam, Cambridge, MA, USA) and
anti-SUMO2/3 (ab3742; 1:500; Abcam) antibodies at 4°C overnight.
Then, the cells were incubated in Fluorescein (FITC) or Texas
Red-labeled secondary antibodies (sc-2012 or sc-3917; 1:500; Santa
Cruz Biotechnology, Santa Cruz, CA, USA), with PBST for 3 min and
washed 3 times; followed by 4,6-diamidino-2-phenylindole to stain
cell nuclei for 5 min, darkening the specimen, and washed 4 times
with PBST. Image Pro Plus 6.0 software (Media Cybernetics,
Rockville, MD, USA) was used for semi quantitative analysis. The
light densities of the nucleus and cytoplasm were measured. A
result was considered as positive when the ratio was >1, and
then the positive rate was calculated.
Western blot analysis
To avoid de-SUMOylation of proteins during sample
preparation, BMSCs were homogenized in ice-cold RIPA lysis buffer
(EMD Millipore, Billerica, MA, USA). The cell lysates were
centrifuged at 17,970 × g for 15 min at 4°C. The supernatant was
collected, and protein concentrations were measured using a
bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.).
Samples were mixed with sample buffer (Invitrogen; Thermo Fisher
Scientific, Inc.), incubated at 70°C for 10 min, and then applied
to sodium dodecyl sulfate polyacrylamide gel electrophoresis (4–15%
gels; Invitrogen; Thermo Fisher Scientific, Inc.) with 30 µg total
protein added per well. Subsequent to electrophoresis, the proteins
were transferred to polyvinylidene fluoride membranes (EMD
Millipore). The membranes were blocked in a Tris-HCl-buffered salt
solution containing 0.1% Tween-20 (TBST) and 5% skimmed milk
powder, and then incubated with anti-SUMO1 (1:1,000), anti-SUMO2/3
(1:1,000), or anti-UBC9 (cat. no. 4786; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) antibodies overnight at 4°C.
Then, the membranes were washed five times with TBST, followed by
incubation with horseradish peroxidase-conjugated goat anti-rabbit
IgG (111–035-003; 1:2,000; Jackson Immuno Research Laboratories,
Inc., West Grove, PA, USA) as the secondary antibody for 1 h at
room temperature. Following five washes with 0.1% TBST, proteins
were detected using a C-Digit Blot Scanner (LI-COR Biosciences,
Lincoln, NE, USA). The membrane was then stripped and re-incubated
with an anti-β-actin primary antibody (cat. no. 3700; 1:1,000; Cell
Signaling Technology, Inc.). Data were evaluated by image analysis
software (ImageJ version 1.48; National Institutes of Health,
Bethesda, MD, USA).
Apoptosis detection
BMSCs were harvested, washed with PBS, immersed in
permeabilization solution for 5 min, and then incubated with 25 µl
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) reaction mixture (Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) in a humidified chamber at 37°C for 60 min.
Subsequent to washing with PBS, nuclei were counterstained with
Hoechst 33258 (Sigma-Aldrich; Merck Millipore). The samples were
then washed with PBS and deionized water, mounted and observed
under a fluorescence microscope (Olympus Corporation, Tokyo,
Japan). The total number of cells (Hoechst-positive) and the number
of apoptotic cells (TUNEL-positive) were quantified. The apoptotic
rate (%) was calculated by the number of apoptotic cells/the number
of total cells ×100%.
Lactate dehydrogenase (LDH) activity
detection
After harvesting the BMSCs, LDH content in the
culture media was measured by an enzyme-linked immunosorbent assay
(LDH Activity Assay kit; BioVision, Inc., San Francisco, CA, USA)
according to the manufacturer's protocols.
Rat middle cerebral artery occlusion
model and moderate hypothermic treatment
A total of 40 adult male Sprague-Dawley rats were
randomly divided into four groups. For surgery, animals were
maintained by a small animal ventilator (Shanghai Yuyan Instruments
Co., Ltd., Shanghai, China), and rectal temperature was monitored
to control body temperature. A 1 cm longitudinal incision was made
between the sternum and mandible, the left common carotid artery
was isolated, external carotid and internal carotid arteries were
visualized under a microscope (Olympus Corporation), the proximal
heart end of the carotid artery and distal heart end of the
external carotid artery were ligated, and a nylon suture (head-end
diameter, 0.23 mm; trunk diameter, 0.18 mm) was inserted from the
carotid artery into the middle cerebral artery (~12.0 mm deep) and
fixed with a suture.
BMSCs or SiUBC9 BMSCs were transplanted into the
ischemic penumbra after establishment of the middle cerebral artery
occlusion (MCAO) models as reported previously (12,21,22).
To induce moderate hypothermia, rats transplanted with BMSCs were
placed on a hypothermia blanket (Shanghai Yuyan Instruments Co.,
Ltd.), and a rectal temperature monitor was used to control body
temperature at 32–34°C for 12 h. The animals were then removed from
anesthesia and gradually returned to normal body temperature.
Animals were recovered for 2, 7, 14 or 21 days, and neurological
functions were assessed by neurological severity scores (NSSs)
(23,24).
Statistics analysis
All experiments were repeated at least three times.
Data are expressed as the mean ± standard error. Statistical
software (GraphPad Prism 6; GraphPad Software, Inc., La Jolla, CA,
USA) was used for all statistical tests. Comparisons between
multiple groups were evaluated by one-way analysis of variance, and
comparisons between two groups were evaluated by Student's unpaired
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Moderate hypothermia promotes SUMO1
and SUMO2/3 binding to target proteins and induces SUMO
translocation from the cytoplasm to the nucleus in BMSCs
BMSCs extracted from rats were cultured in
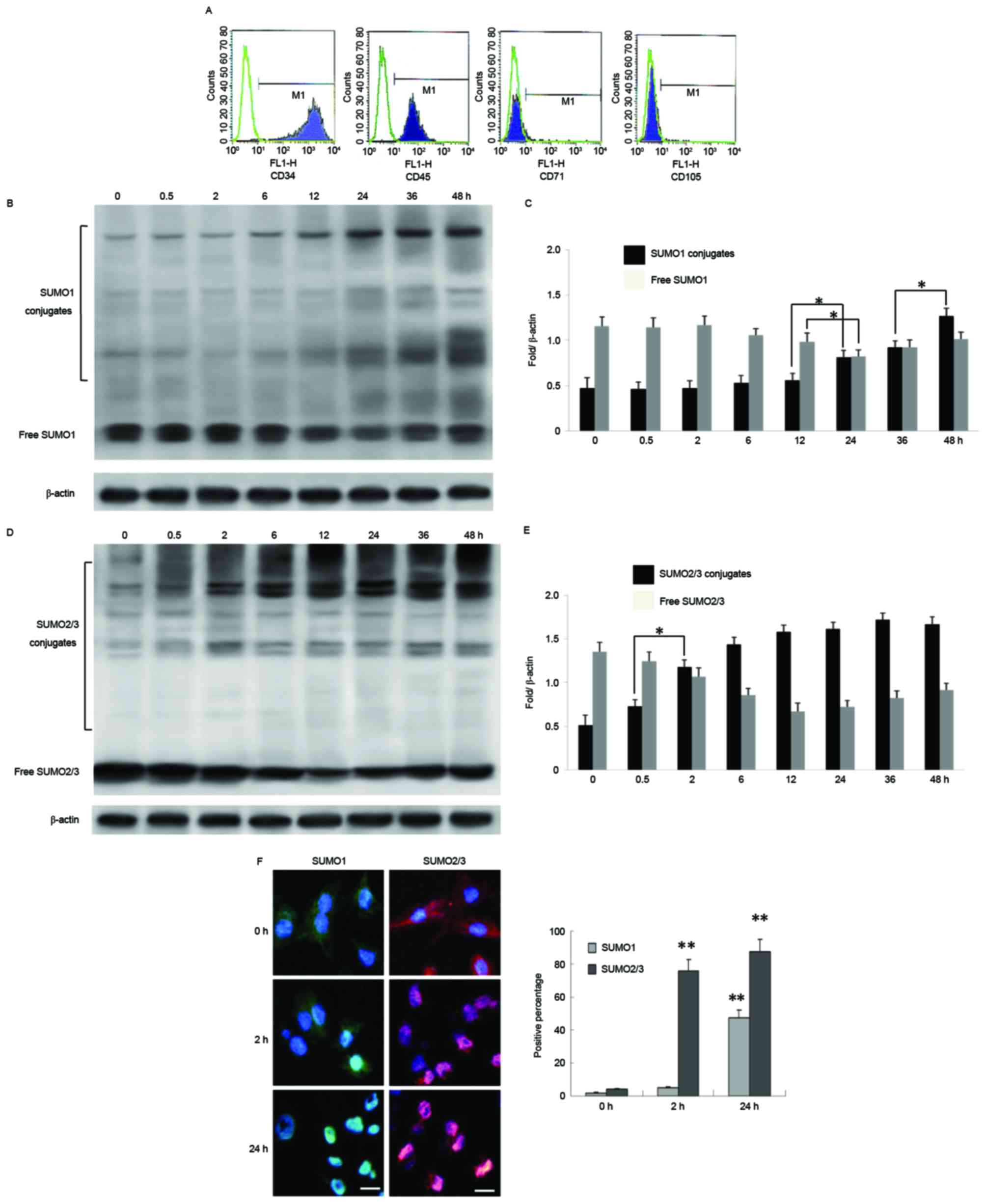
conditioned medium and identified by flow cytometry. The results
indicated high levels of expression of BMSC-specific proteins CD71
and CD105, however no expression of hematopoietic stem
cell-specific proteins CD34 and CD45 (Fig. 1A). BMSCs were then cultured at 33°C
for 0–48 h and used to detect expression of SUMO1 and SUMO2/3.
There was a trend towards a slow increase in expression of free
SUMO1 conjugates up to 24 h after moderate hypothermia (33°C), a
significant decrease after 24 h, and a marginal increase at 48 h.
It was suggested that, following 36–48 h of moderate hypothermia,
production of new free SUMO1 may exert cytoprotective effects
(Fig. 1B and C). In contrast,
short term treatment of BMSCs with moderate hypothermia (<2 h)
induced a trend towards an increase in combined SUMO2/3 expression,
which peaked at 24–36 h, and then remained elevated after 36 h
(Fig. 1D and E). BMSCs cultured
under moderate hypothermia for 2 or 24 h also presented with marked
translocation of SUMO1 and SUMO2/3 from the cytoplasm to the
nucleus (SUMO1 at 24 h and SUMO2/3 at 2 h; Fig. 1E and F).
SiUBC9 inhibits SUMO binding to target
proteins in BMSCs under hypoxia
UBC9 is the SUMO-conjugating enzyme E2 that acts on
inactive SUMOs to induce SUMOylation of substrates together with
the SUMO-activating enzyme E1, SUMO ligase E3, and adenosine
triphosphate (25). In the present
study, BMSCs were cultured under OGD to simulate hypoxia-ischemia.
Using RNA interference in BMSCs, expression of UBC9 was
significantly reduced compared with wild-type (WT) and SiNC BMSCs
(Fig. 2A and B). Following OGD,
expression of SUMO conjugates and free SUMOs was significantly
stimulated in WT and SiNC BMSCs. Conversely, in UBC9 knockdown
BMSCs, SUMO1 and SUMO2/3 conjugates were reduced significantly,
while the level of free SUMOs was significantly increased under
normal and OGD culture conditions (Fig. 2C-F).
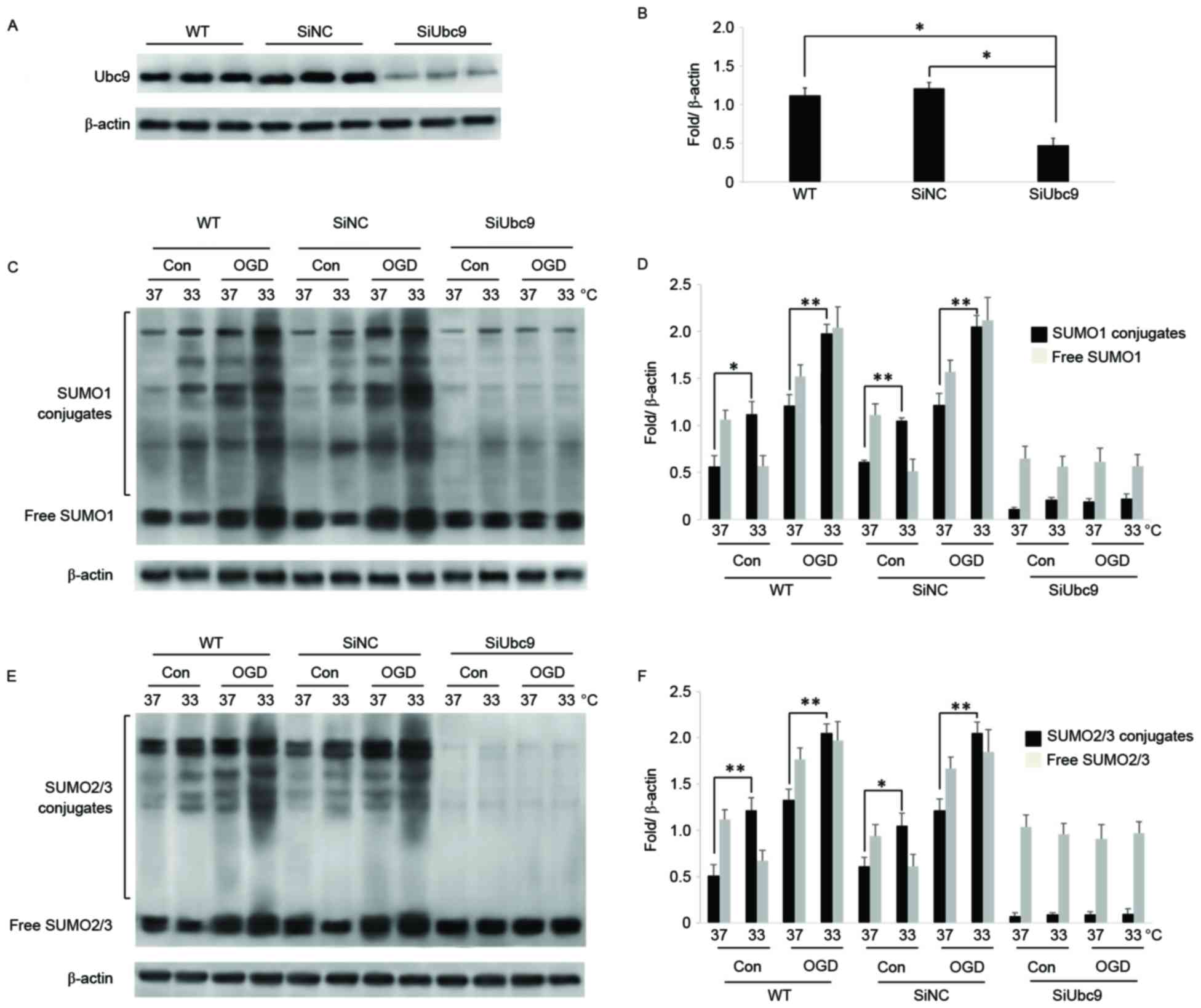 | Figure 2.SiUBC9 blocks binding of SUMOs to
target proteins following OGD in BMSCs. (A) UBC9 expression
measured by western blotting. β-actin was used for normalization
(n=3). (B) Ratios of target proteins to β-actin (n=3). Data are
expressed as the mean ± standard error; *P<0.05. (C) Expression
of SUMO1 conjugates and free SUMO1 in WT, SiNC, and SiUBC9 BMSCs
following OGD. β-actin was used for normalization (n=3). (D) Ratios
of SUMO1 conjugates and free SUMO1 to β-actin. Data are expressed
as the mean ± standard error (n=3). (E) Expression of SUMO2/3
conjugates and free SUMO2/3 in WT, SiNC, and SiUBC9 BMSCs following
OGD. β-actin was used for normalization (n=3). (F) Ratios of
SUMO2/3 conjugates and free SUMO2/3 to β-actin. Data are expressed
as the mean ± standard error (n=3). *P<0.05, **P<0.01. Si,
small interfering; SUMO, small ubiquitin-related modifier; BMSCs,
bone marrow stromal cells; OGD, oxygen-glucose deprivation; WT,
wild-type; NC, negative control; Con, control. |
Moderate hypothermia causes
SUMOylation-dependent reductions in BMSC apoptosis and LDH
secretion following hypoxia
WT and SiNC BMSCs cultured at 33°C following OGD
exhibited significant decreases in apoptosis and LDH secretion
compared with those cultured at 37°C. However, UBC9 knockdown BMSCs
exhibited no resistance against moderate hypothermia (Fig. 3A and B). Thus, a lack of UBC9
hinders SUMO binding to target proteins, it is speculated that the
protective effect of moderate hypothermia may be associated with
intracellular protein SUMOylation. Notably, down-regulation of UBC9
expression was additionally observed in BMSCs under normal culture
conditions, which was associated with an increase in BMSC apoptosis
and LDH release, suggesting a role of protein SUMOylation in the
maintenance of cellular functions and resistance. These results
indicated that knockdown of UMBC9 reduced the binding ability of
SUMOs to target proteins and suppressed the cytoprotective
effect.
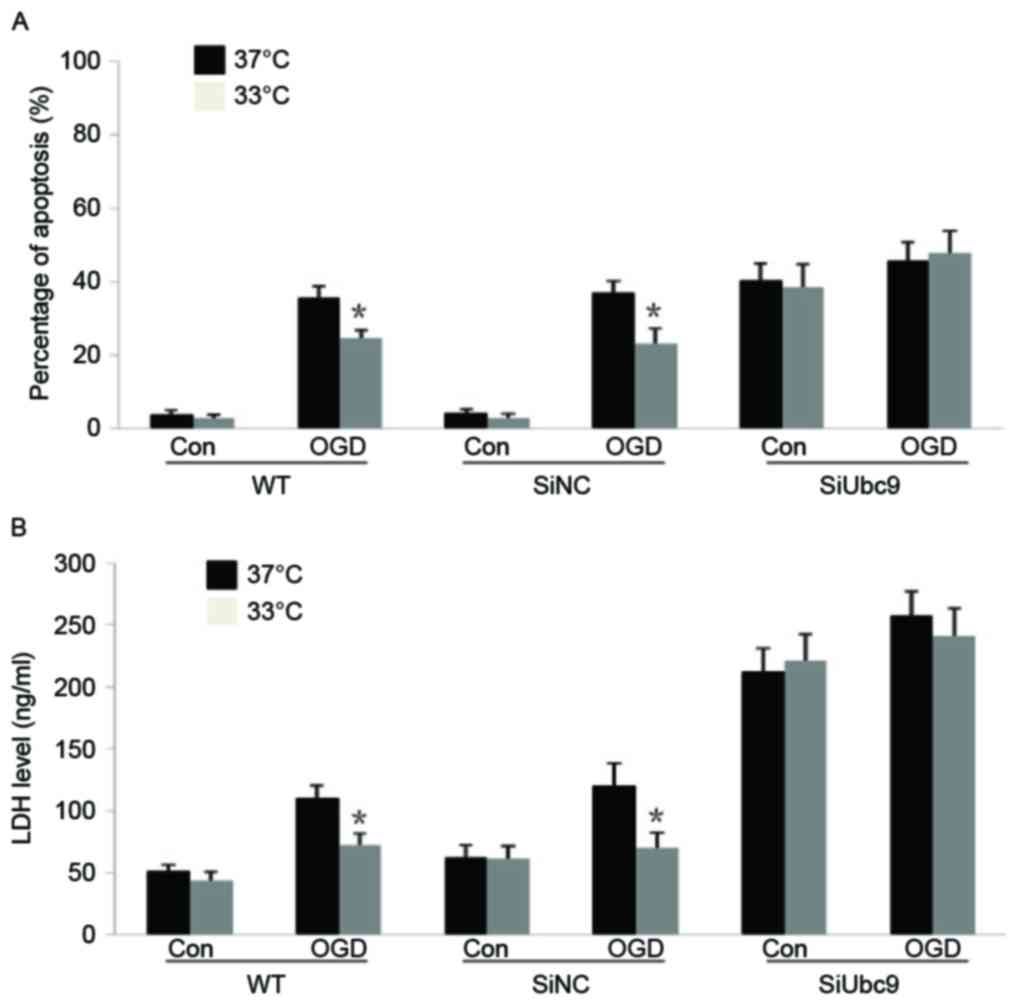 | Figure 3.SiUBC9 inhibits the protective effect
of moderate hypothermia on BMSCs after OGD. (A) WT, SiNC, and
SiUBC9 BMSCs were cultured for 24 h after OGD treatment and then
cultured at 33 or 37°C for 24 h. The percentage of apoptotic BMSCs
is presented. Data are expressed as the mean ± standard error
(n=4). *P<0.05, treated at 33°C vs. 37°C within one group. (B)
WT, SiNC and SiUBC9 BMSCs were cultured for 24 h after OGD and then
cultured at 33 or 37°C for 24 h. The LDH level in the culture
medium is presented. Data are expressed as the mean ± standard
error (n=4). *P<0.05, treated at 33°C vs. 37°C within one group.
Si, small interfering; BMSCs, bone marrow stromal cells; OGD,
oxygen-glucose deprivation; WT, wild-type; NC, negative control;
Con, control; LDH, lactate dehydrogenase. |
Moderate hypothermia causes
SUMOylation-dependent improvements in survival of BMSCs
transplanted into the ischemic penumbra
WT and SiUBC9 BMSCs were transplanted into the
penumbra of MCAO model rats that were then subjected to moderate
hypothermia. There was a progressive reduction in neurological
function as assessed by the NSSs of MCAO animals without BMSC
transplantation or moderate hypothermia. In contrast, MCAO rats
with BMSC transplantation had improved neurological functions,
whereas animals with BMSC transplantation combined with moderate
hypothermia showed further improvements. However, the improvement
in neurological functions of MCAO rats that received
transplantations of BMSCs with UBC9 knockdown following moderate
hypothermia was less than that in animals transplanted with BMSCs
at 33°C (Table I). These data
suggest that transplantation of BMSCs into the penumbra improves
neurological function following MCAO, and that moderate hypothermia
increases the activity and survival of transplanted BMSCs and
promotes functional recovery, potentially via increased
intracellular protein SUMOylation.
 | Table I.Comparison of neurological severity
scores at different times between groups. |
Table I.
Comparison of neurological severity
scores at different times between groups.
| Neurological severity
scores | n | 2 days | 7 days | 14 days | 21 days |
|---|
| MCAO | 10 | 34.14±3.47 | 28.19±4.02 | 20.41±3.78 | 17.43±3.55 |
| 37°C BMSCs | 10 |
28.49±3.45a |
20.58±3.09a |
15.45±2.38a |
14.67±2.11a |
| 33°C BMSCs | 10 |
27.15±2.47a,b |
17.49±2.19a,b |
12.58±2.16a,b |
10.43±1.93a |
| 33°C BMSCs
SiUBC9 | 10 |
28.11±2.72b |
19.14±3.01b | 14.09±2.33 | 12.45±2.96 |
Discussion
There has been a progressive increase in the
incidence of cerebral infarction due to the increasing rates of
diabetes, hypertension, and high cholesterol in the general
population. Early interventional therapy is an effective treatment
for cerebral ischemia. However, the majority of patients are not
treated within this time window, resulting in serious sequelae.
Thus, new treatments are urgently required, which are effective
outside of this time window. BMSCs are skeletal progenitor cells
that are easy to obtain, expand in vitro, and transplant
in vivo. Previous studies have demonstrated that BMSCs can
be differentiated into bone, cartilage, fat and nerve cells, and
that BMSCs may be neuroprotective (26–28).
Thus, transplantation of BMSCs into the infarction area and
subsequent differentiation into neuronal cells may provide a novel
treatment for cerebral infarction. However, the hypoxic-ischemic
environment in brain tissue following infarction can markedly
reduce the survival of transplanted cells (29).
SUMOs are a class of important ubiquitin-like
proteins. There are four SUMO family members that have been
identified in humans (SUMO1-4), by contrast, the SUMO-conjugating
enzyme E2 has only one form (UBC9), and RNA interference knockdown
of UBC9 expression can effectively block SUMOylation of
intracellular proteins (30).
SUMOs have both free and conjugate forms that maintain the balance
of cellular SUMOylation. Protein SUMOylation serves an important
role in maintenance of genomic stability, regulation of protein
interactions, nuclear/cytoplasmic translocation, transcription
factor activity, and inhibition of protein biotinylation. Notably,
proteomic studies have identified that, following cerebrovascular
ischemia, numerous proteins closely associated with physiological
and pathological processes, including oxidative stress,
inflammation, DNA synthesis, energy transfer and metabolism, are
SUMOylated, which may aid in the removal of foreign material,
reduce inflammatory responses, and regulate cellular proliferation
and apoptosis (31,32). In addition, it was observed that
SUMO2/3 are neuroprotective and improve behavioral outcomes
following experimental cerebral ischemia (17,33).
Moderate hypothermic treatment is an effective
therapy for treatment of high intracranial pressure and brain
injury caused by severe hypoxia-ischemia. Hypothermia reduces the
cellular metabolic rate, increases energy reserves to allow
cellular recovery, reduces the inflammatory response and improves
the brain microenvironment (34).
In the present study, the association between moderate hypothermic
treatment and protein SUMOylation was observed in BMSCs following
hypoxia-ischemia. It was identified that conjugated SUMO2/3 were
rapidly and highly induced in BMSCs subsequent to moderate
hypothermic treatment, while free SUMO2/3 were decreased with
marked SUMO2/3 nuclear translocation. These data suggest that the
protective actions of moderate hypothermia are associated with the
binding of SUMO2/3 to target proteins, rather than the initial
expression of SUMO2/3 (35). In
contrast, there was slow and low level SUMOylation of target
proteins by SUMO1 with only moderate nuclear translocation. Further
studies are required to determine the specific target proteins that
combine with SUMOs and their biological functions in the
SUMOylation pathway.
It was observed that the levels of conjugated SUMO1
and SUMO2/3, in addition to their nuclear translocation, were
progressively increased in BMSCs following OGD treatment (SUMO2/3
within 2 h and SUMO1 from 24–48 h). Similar results have been
reported previously (33). The
rate of BMSC apoptosis and LDH secretion induced by OGD treatment
were also reduced by moderate hypothermic treatment. Furthermore,
these effects disappeared following UBC9 knockdown in BMSCs, which
blocks the binding of SUMOs to their target proteins (36). Finally, the effects of BMSCs,
moderate hypothermia and combination therapy on the recovery of
neurological function in MCAO rats were investigated. Results
indicated that transplantation of BMSCs into the ischemic penumbra
in MCAO rats combined with moderate hypothermia was optimal for
restoring neurological function. Furthermore, this protection was
SUMOylation-dependent, explained by the fact that the improvement
in neurological function in MCAO rats receiving transplantation of
BMSCs with UBC9 knockdown following moderate hypothermia had a
reduced quality of neurological function than animals transfected
with BMSCs at the same temperature.
In summary, moderate hypothermia significantly
enhances the ability of SUMOs to bind to their target proteins in
BMSCs, suggesting an important role in regulating cellular
apoptosis and functions following hypoxia-ischemia. Thus, moderate
hypothermia promotes neural stem cell survival in the ischemic
penumbra by increasing SUMOylation of multiple proteins. The
results provide a further understanding of the molecular mechanisms
underlying the protective actions of moderate hypothermia, and
provide a theoretical basis for the use of bone marrow stem cell
transplantation combined with moderate hypothermia for treatment of
cerebral infarction.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant no. 81471175), Tianjin
Health Bureau Science and Technology Projects (grant no. 2014KY23)
and Tanggu Science and Technology Promotion Projects (grant no. 201
3KJXQ03).
References
|
1
|
van Rooy MJ and Pretorius E: Obesity,
hypertension and hypercholesterolemia as risk factors for
atherosclerosis leading to ischemic events. Curr Med Chem.
21:2121–2129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
An H, Ford AL, Chen Y, Zhu H, Ponisio R,
Kumar G, Shanechi AM, Khoury N, Vo KD, Williams J, et al: Defining
the ischemic penumbra using magnetic resonance oxygen metabolic
index. Stroke. 46:982–988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kirton A and Deveber G: Life after
perinatal stroke. Stroke. 44:3265–3271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baksh D, Song L and Tuan RS: Adult
mesenchymal stem cells: Characterization, differentiation, and
application in cell and gene therapy. J Cell Mol Med. 8:301–316.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Black IB and Woodbury D: Adult rat and
human bone marrow stromal stem cells differentiate into neurons.
Blood Cells Mol Dis. 27:632–636. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen JR, Cheng GY, Sheu CC, Tseng GF, Wang
TJ and Huang YS: Transplanted bone marrow stromal cells migrate,
differentiate and improve motor function in rats with
experimentally induced cerebral stroke. J Anat. 213:249–258. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Campos F, Blanco M, Barral D, Agulla J,
Ramos-Cabrer P and Castillo J: Influence of temperature on ischemic
brain: Basic and clinical principles. Neurochemistry Int.
60:495–505. 2012. View Article : Google Scholar
|
|
8
|
Froehler MT and Ovbiagele B: Therapeutic
hypothermia for acute ischemic stroke. Expert Rev Cardiovasc Ther.
8:593–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eckert MA, Vu Q, Xie K, Yu J, Liao W,
Cramer SC and Zhao W: Evidence for high translational potential of
mesenchymal stromal cell therapy to improve recovery from ischemic
stroke. J Cereb Blood Flow Metab. 33:1322–1334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu SP, Wei Z and Wei L: Preconditioning
strategy in stem cell transplantation therapy. Transl Stroke Res.
4:76–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shinozuka K, Dailey T, Tajiri N, Ishikawa
H, Kim DW, Pabon M, Acosta S, Kaneko Y and Borlongan CV: Stem Cells
for Neurovascular Repair in Stroke. J Stem Cell Res Ther.
4:129122013.PubMed/NCBI
|
|
12
|
Tu Y, Chen C, Sun HT, Cheng SX, Liu XZ, Qu
Y, Li XH and Zhang S: Combination of temperature-sensitive stem
cells and mild hypothermia: A new potential therapy for severe
traumatic brain injury. J Neurotrauma. 29:2393–2403. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yurchenko V, Xue Z and Sadofsky MJ: SUMO
modification of human XRCC4 regulates its localization and function
in DNA double-strand break repair. Mol Cell Biol. 26:1786–1794.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cubenas-Potts C and Matunis MJ: SUMO: A
multifaceted modifier of chromatin structure and function. Dev
Cell. 24:1–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bossis G and Melchior F: Regulation of
SUMOylation by reversible oxidation of SUMO conjugating enzymes.
Mol Cell. 21:349–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Silveirinha V, Stephens GJ and Cimarosti
H: Molecular targets underlying SUMO-mediated neuroprotection in
brain ischemia. J Neurochem. 127:580–591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang W, Sheng H, Homi HM, Warner DS and
Paschen W: Cerebral ischemia/stroke and small ubiquitin-like
modifier (SUMO) conjugation-a new target for therapeutic
intervention? J Neurochem. 106:989–999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trappe K, Thomas D, Bikou O, Kelemen K,
Lugenbiel P, Voss F, Becker R, Katus HA and Bauer A: Suppression of
persistent atrial fibrillation by genetic knockdown of caspase 3: A
pre-clinical pilot study. Eur Heart J. 34:147–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng J, Petersen BE, Steindler DA,
Jorgensen ML and Laywell ED: Mesenchymal stem cells spontaneously
express neural proteins in culture and are neurogenic after
transplantation. Stem Cells. 24:1054–1064. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang W, Thompson JW, Wang Z, Wang L, Sheng
H, Foster MW, Moseley MA and Paschen W: Analysis of
oxygen/glucose-deprivation-induced changes in SUMO3 conjugation
using SILAC-based quantitative proteomics. J Proteome Res.
11:1108–1117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bleilevens C, Roehl AB, Goetzenich A,
Zoremba N, Kipp M, Dang J, Tolba R, Rossaint R and Hein M: Effect
of anesthesia and cerebral blood flow on neuronal injury in a rat
middle cerebral artery occlusion (MCAO) model. Exp Brain Res.
224:155–164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui L, Zhang X, Yang R, Liu L, Wang L, Li
M and Du W: Baicalein is neuroprotective in rat MCAO model: Role of
12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic
phospholipase A2. Pharmacol Biochem Behav. 96:469–475. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Yang Y, Zhang GZ, Gao M, Ge GZ,
Wang QQ, Ji XC, Sun YL, Zhang HT and Xu RX: Stereotactic
Administration of Edaravone ameliorates collagenase-induced
intracerebral hemorrhage in rat. CNS Neurosci Ther. 22:824–835.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Titova EM, Ghosh N, Valadez ZG, Zhang JH,
Bellinger DL and Obenaus A: The late phase of post-stroke
neurorepair in aged rats is reflected by MRI-based measures.
Neuroscience. 283:231–244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johnson ES: Protein modification by SUMO.
Annu Rev Biochem. 73:355–382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sindberg GM, Lindborg BA, Wang Q, Clarkson
C, Graham M, Donahue R, Hering BJ, Verfaillie CM, Bansal-Pakala P
and O'Brien TD: Comparisons of phenotype and immunomodulatory
capacity among rhesus bone-marrow-derived mesenchymal stem/stromal
cells, multipotent adult progenitor cells, and dermal fibroblasts.
J Med Primatol. 43:231–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maltman DJ, Hardy SA and Przyborski SA:
Role of mesenchymal stem cells in neurogenesis and nervous system
repair. Neurochem Int. 59:347–356. 2011.PubMed/NCBI
|
|
28
|
Kurwale NS, Suri V, Srivastava A, Suri A,
Mohanti S, Yadav P, Sharma MC and Sarkar C: Role of bone marrow
derived pluripotent stem cells in peripheral nerve repair in adult
rats: A morphometric evaluation. J Neurosci Rural Pract. 6:152–159.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shinozuka K, Dailey T, Tajiri N, Ishikawa
H, Kaneko Y and Borlongan CV: Stem cell transplantation for
neuroprotection in stroke. Brain Sci. 3:239–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sommer S, Ritterhoff T, Melchior F and
Mootz HD: A stable chemical SUMO1-Ubc9 conjugate specifically binds
as a thioester mimic to the RanBP2-E3 ligase complex. Chembiochem.
16:1183–1189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Keusekotten K and Praefcke GJ:
Reconstitution of SUMO-dependent ubiquitylation in vitro. Methods
Mol Biol. 832:111–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang W, Wang L and Paschen W: Development
of a high-throughput screening assay for inhibitors of small
ubiquitin-like modifier proteases. J Biomol Screen. 18:621–628.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwabuchi M, Sheng H, Thompson JW, Wang L,
Dubois LG, Gooden D, Moseley M, Paschen W and Yang W:
Characterization of the ubiquitin-modified proteome regulated by
transient forebrain ischemia. J Cereb Blood Flow Metab. 34:425–432.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sahuquillo J and Vilalta A: Cooling the
injured brain: How does moderate hypothermia influence the
pathophysiology of traumatic brain injury. Curr Pharm Des.
13:2310–2322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L, Ma Q, Yang W, Mackensen GB and
Paschen W: Moderate hypothermia induces marked increase in levels
and nuclear accumulation of SUMO2/3-conjugated proteins in neurons.
J Neurochem. 123:349–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee YJ, Mou Y, Maric D, Klimanis D, Auh S
and Hallenbeck JM: Elevated global SUMOylation in Ubc9 transgenic
mice protects their brains against focal cerebral ischemic damage.
PLoS One. 6:e258522011. View Article : Google Scholar : PubMed/NCBI
|