Introduction
Insulin resistance (IR) is known to be an important
factor, which can lead to the onset of type 2 diabetes (1). The mechanism by which IR develops is
complex; during the insulin signal transduction process,
alterations to the structure or activity of certain molecules,
including insulin receptor, insulin receptor substrate,
phosphoinositide 3-kinase, glucose transporter 4 (GLUT-4), and
glucokinase, can result in IR. Additionally, the abnormal
expression of nuclear factor κB (NF-κB) and its associated genes in
different regions of an organism can affect the occurrence of IR
directly or indirectly (2).
Autophagy is a cellular process, which sequesters
senescent or damaged proteins in autophagosomes for recycling of
their products (3). Autophagy is
also involved in the removal of cells, which have undergone
classical type 1 programmed cell death or apoptosis. Therefore,
autophagy can be generally considered as a protector of cells
against various stressors and a cellular response to stress
(4). Paradoxically, autophagy can
also lead to a form of non-apoptotic cell death, which is termed
type 2 programmed cell death. Thus, autophagy can either protect
cells or promote cell death, depending on the cellular and
environmental context.
Dysfunctional autophagy may be involved in the
pathophysiology of several diseases, including neurodegenerative
disorders, cardiomyopathy and cancer (5). This may involve the accumulation of
damaged molecules and organelles. Autophagy also appears to be
involved in the cellular changes associated with aging (6). Insulin and intracellular molecules,
including mammalian target of rapamycin (mTOR) are well-known
inhibitors of autophagy, whereas glucagon, a counter-regulatory
hormone of insulin, induces autophagy (7,8).
These observations support the hypothesis that autophagy is
involved in the natural history of diabetes via its involvement in
hormone activity and organelle function (9).
As a key element in extracellular glucose transport
to insulin-sensitive cells, the translocation of GLUT-4 to the cell
membrane is regulated by insulin (10). In patients with type 2 diabetes,
the expression levels of GLUT-4 in skeletal muscles are
significantly decreased, which indicates decreased
glucose-processing ability (11).
Autophagy is a cellular degrading process, which can promote cell
survival and cell death. Microtubule-associated protein 1 light
chain 3 (LC3) and p62/SQSTM1 (P62) are associated with
autophagosomal membranes, which engulf cytoplasmic content for
subsequent degradation (12).
Hyperglycemia stimulates the p62/protein kinase Cζ interaction,
which mediates the activation of NF-κB, increases the expression of
NADPH oxidase 4, and activates inflammatory cytokines in the
vascular smooth muscle (13).
At present, there are no effective treatments
specific for IR. Geniposide is an iridoid compound isolated from
Gardenia jasminoides Elli, which has been previously
reported to significantly promote glucose uptake (14). In the present study, a HepG2 cell
model of IR was constructed to determine whether geniposide can
promote autophagy to inhibit IR in HepG2 cells via the
P62/NF-κB/GLUT-4 pathway.
Materials and methods
Reagents
RPMI-1640 medium and foetal bovine serum (FBS) were
from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Geniposide was from Xi'an Hao-xuan Bio-tech Co., Ltd. (Xi'an,
China). Tetrazolium (MTT), recombinant human insulin and trypsin
were from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Rabbit
anti-human NF-κB (P65), phosphorylated (p-)NF-κB (p-P65), GLUT-4,
LC3, P62 and β-actin were from Cell Signalling Technology, Inc.
(Danvers, MA, USA). Rapamycin and 3-methyladenine (3-MA) were
purchased from Selleck Chemicals (Houston, TX, USA). The
streptavidin-peroxidase immunostaining kit was from Zymed; Thermo
Fisher Scientific, Inc. Diaminobenzidine was from Beijing Dingguo
Changsheng Biotechnology Co., Ltd. (Beijing, China) and the glucose
detection kit was from Shanghai Rongsheng Biotechnology Co., Ltd.
(Shanghai, China).
Cell culture
The HepG2 cells were obtained from the Shanghai
Institute of Cell Biology, Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in RPMI-1640 medium containing 10%
FBS at 37°C in 5% CO2. The medium was replaced every 2
days and passaged every 3–4 days following trypsinisation.
IR cell model construction
The IR cell model was constructed as previously
described (15). Briefly,
log-phase HepG2 cells were harvested following trypsinisation. The
cells (5×104 /ml) were seeded into 96-well plates and
cultured for 4 h. Following attachment of the cells to the bottom
of the plate, the medium was replaced with RPMI-1640 medium
supplemented with 1% FBS and containing 0, 50, 100, 200 or 500
nmol/l insulin. The control cells were treated with RPMI-1640
medium supplemented with 1% FBS. The cells were cultured at 37°C in
5% CO2 for 12, 24 and 36 h. The glucose levels in the
supernatants were detected using a glucose detection kit according
to the manufacturer's protocol, and were used as an index for
determining IR. In the rapamycin group, the cells were treated with
40 nmol/l rapamycin for 2 h, followed by IR model establishment,
and (7) in the 3-MA group, the
cells were treated with 60 µM 3-MA for 2 h, followed by IR model
establishment.
MTT assay
The present study evaluated cell survival using an
MTT assay. The cells (~5,000 cells/well) were plated in a 96-well
plate and incubated overnight in 100 µl culture medium. The cells
were then treated with 15.63–125 mg/l geniposide. Following
treatment for 20 h at 37°C, 20 µl MTT (5 mg/ml) was added to each
well. Following incubation for 4 h at 37°C in 5% CO2,
the MTT was removed, replaced with 200 µl dimethyl sulfoxide, and
incubated for 20 min at 37°C until the crystals had dissolved. The
optical density (OD) of each well was measured using an ELx800
microculture plate reader (BioTek Instruments, Winooski, VT, USA)
with a test wavelength of 490 nm. The cell survival rate (SR) was
determined as follows: SR = [(OD drug well - OD treated cell
well)/(OD control well - OD cell well)] × 100%. Each experiment was
repeated at least five times.
Fluorescence microscopy for detection
of autophagy
The cells (~10,000 cells/well) were cultured in
6-well plates to 60–70% confluence. Lipofectamine 3000®
was mixed with Opti-minimum essential media containing 2 µg/l green
fluorescent protein (GFP)-LC3 plasmid (Hanbio Biotechnology Co.,
Ltd., Shanghai, China) according to the manufacturer's protocol.
The medium was then replaced with RPMI-1640 containing 10% FBS for
12 h, following which the cells were treated as described above.
The cells then were washed with PBS and mounted using Vectashield
containing 1 µg/ml DAPI (Vector Laboratories, Inc., Burlingame, CA,
USA). Images of the cells were captured using a fluorescence
microscope.
Transmission electron microscopy
(TEM)
The cell samples were placed in 1% glutaraldehyde
and post-fixed with 2% osmium tetroxide. The cells were centrifuged
at 1,000 × g for 15 min at 4°C and then the cell pellets were
embedded in epon resin. The data were quantified by counting the
number of autophagosomes per cross-sectioned cell by transmission
electron microscopy.
Immunostaining
The log-phase HepG2 cells were harvested and
5×104/ml cells were seeded on glass coverslips in
24-well plates. When the cells had attached to the coverslips, the
medium was replaced with RPMI-1640 medium supplemented with 1% FBS
containing 100 nmol/l insulin. The cells were divided into three
groups comprising a control group of untreated HepG2 cells, an
untreated group of IR cells without geniposide treatment, and a
treated group of IR cells treated with 62.5 mg/l geniposide. All
groups were cultured for 4, 8, 12 and 24 h prior to fixing of the
cells 4% paraformaldehyde for 30 min at room temperature.
Immunostaining was performed using GLUT-4, P62 and p-P65 detection
kits according to the manufacturer's protocol. Staining was
visualised in five visual fields and the differences in staining
intensity were measured using ImageJ software v6.0 (National
Institutes of Health, Bethesda, MD, USA).
Semi-quantitative polymerase chain
reaction (sqPCR) analysis
The mRNA expression levels of NF-κB
and GLUT-4 in the cells were detected using sqPCR analysis.
The RNA was isolated using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) Briefly, cDNA was synthesized from 1 µg RNA in
the presence of a ribonuclease inhibitor (Sigma-Aldrich; Merck
KGaA), dNTPs, Oligo(dT) 18 primers, and RevertAid™ M-MuLV reverse
transcriptase (Fermentas; Thermo Fisher Scientific, Inc.) in a
total volume of 25 µl. The primers used were as follows: NF-κB,
sense 5′-TAGCCCAGCATCGCCTCT-3′ and antisense
5′-TTTGACACGGCAGTCCTCCATGGGA-3′ (target product of 641 bp); GLUT-4,
sense 5′-CCCCGCTACCTCTACATCATCCA-3′ and antisense
5′-CCACCAACAACACCGAGACCAA-3′ (355 bp); internal control β-actin,
sense 5′-TGGCATCCACGAAACTAC-3′ and antisense 5′-GCATCCTGTCGGCAAT-3′
(125 bp).
sqPCR was performed using a Takara mRNA Selective
PCR kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's instructions, with a total reaction volume of 25 µl,
under the following thermocycling conditions: Initial denaturation
at 94°C for 10 min, denaturation at 94°C for 1 min, annealing for
1.5 min (NF-κB and GLUT-4 at 65°C, β-actin at 51°C), and extension
at 72°C for 2 min for 35 cycles, followed by a final extension at
72°C for 10 min. The PCR products were separated by 1.5% agarose
gel electrophoresis and ethidium bromide staining was used for
visualization. The target bands were analysed densitometrically
using a GS-800 calibrated densitometer (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and with Gel-Pro Analyser 4.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). The results were calculated
as the ratio of the OD value relative to that of β-actin.
Western blot analysis
The cells were lysed in radioimmunoprecipitation
assay buffer containing phosphatase inhibitor cocktail I
(Sigma-Aldrich; Merck KGaA) and a protease inhibitor cocktail
mini-tablet (Roche Diagnostics GmbH, Mannheim, Germany), followed
by centrifugation at 8,000 × g at 4°C for 15 min. Protein
concentration was determined by performing a bicinchoninic acid
assay. An equal quantity of protein (50 µg) from each sample was
separated on a 10, 12 or 15% SDS-polyacrylamide gel and then
transferred onto nitrocellulose membranes. The membranes were
probed with antibodies against P65 (rabbit IgG; cat. no. 4767;
1:1,000), p-P65 (rabbit IgG; cat. no. 8214; 1:1,000), GLUT-4
(rabbit IgG; cat. no. 2213; 1:1,000), LC3 (rabbit IgG; cat. no.
12513; 1:1,000), P62 (rabbit IgG; cat. no. 5114; 1:1,000) and
β-actin (rabbit IgG, cat. no. 4970, 1:1,000; all Cell Signaling
Technology, Inc.) at 4°C overnight. Following washing with PBS, a
horseradish peroxidase-conjugated secondary antibody (anti-rabbit
IgG; cat. no. 14708S; 1:2,000; Cell Signaling Technology, Inc.) was
added to the membranes for 2 h at room temperature. The bound
antibody was visualized using an enhanced chemiluminescence system
(EMD Millipore, Billerica, MA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). All values are presented as the
mean ± standard deviation. Data were analyzed by one-way analysis
of variance followed by Tukey's post hoc test (equal variances) or
Dunnett's T3 post hoc test (unequal variances). P<0.05 was
considered to indicate a statistically significant difference.
Results
Geniposide decreases supernatant
glucose levels
As shown in Table
I, the glucose levels in the supernatant of all groups treated
with 100 nmol/l recombinant human insulin were significantly
higher, compared with those in the blank groups at the indicated
time points (P<0.01). However, when the IR HepG2 cells were
treated with 62.5 mg/l geniposide, the glucose levels in the
supernatant decreased in a time-dependent manner (P<0.01). These
results indicated that geniposide was able to ameliorate IR in
HepG2 cells following insulin treatment, improve the utilization of
insulin, and decrease the glucose levels in the supernatant.
 | Table I.Glucose content in culture supernatant
at different insulin concentrations and treatment times. |
Table I.
Glucose content in culture supernatant
at different insulin concentrations and treatment times.
|
| Glucose content
(mmol/l) |
|---|
|
|
|
|---|
| Duration (h) | Control | IR (100 nmol/l
insulin) | Geniposide (62.5
mg/l) |
|---|
| 12 |
17.93±0.83b | 22.95±0.47 |
21.17±0.65a |
| 24 |
16.16±0.46b | 20.91±1.04 |
17.38±0.82b |
| 36 |
13.97±0.87b | 18.21±0.64 |
14.95±0.93b |
Geniposide has no significant effect
on IR cell survival
As shown in Table
II, treatment with 15.63–125 µg/ml geniposide had no
significant effect on the proliferation of the IR cells
(P>0.05), compared with cells in the blank groups. Therefore,
62.50 µg/ml was selected as an effective concentration of
geniposide in the present study.
 | Table II.Effects of geniposide on the survival
rate of insulin-resistant HepG2 cells. |
Table II.
Effects of geniposide on the survival
rate of insulin-resistant HepG2 cells.
| Geniposide
(µg/ml) | Cell survival rate
(100%) | P-value |
|---|
| 15.63 | 0.99±0.14 | >0.05 |
| 31.25 | 1.12±0.13 | >0.05 |
| 62.50 | 1.08±0.21 | >0.05 |
| 125.0 | 1.03±0.19 | >0.05 |
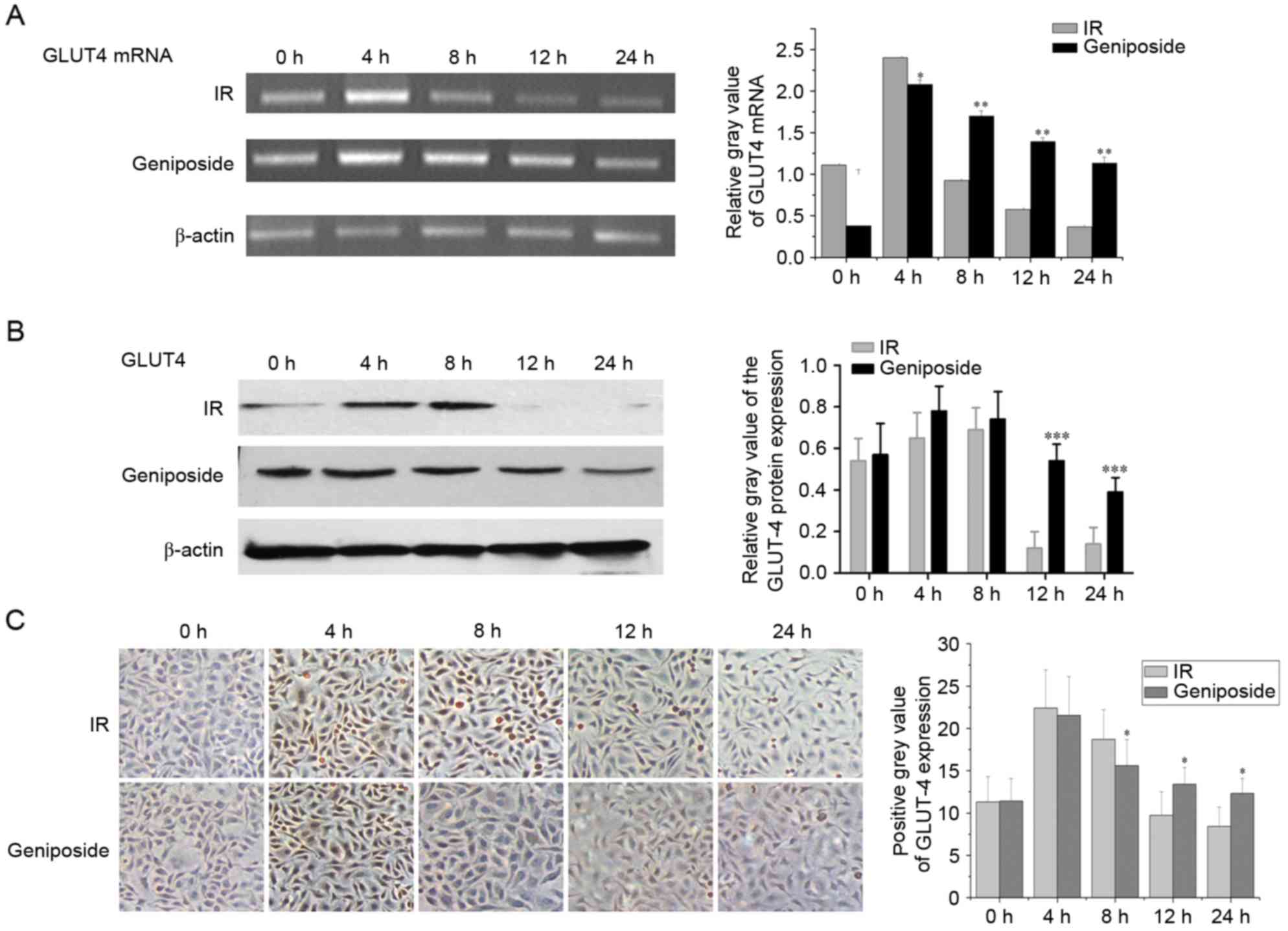
Effect of geniposide on the mRNA
expression of GLUT-4
As shown in Fig.
1A, in the IR cells, the mRNA expression of GLUT-4
peaked at 4 h (P<0.01) and began to decline at 8 h, but remained
higher than that at 0 h (P<0.05). The mRNA expression of
GLUT-4 decreased at 12 h to below the level at 0 h, reaching
a nadir at 24 h (P<0.05). In the geniposide-treated cells, the
mRNA expression of GLUT-4 peaked at 4 h (P<0.01),
although the increase was less than that observed in the control
group. Subsequently, the mRNA expression of GLUT-4 began to
decline at 8 and 12 h, but remained higher than that at 0 h
(P<0.01), reaching a nadir at 24 h. The mRNA expression of
GLUT-4 was higher, compared with that in the control group
(P<0.05).
Effect of geniposide on the expression
of NF-κB and GLUT-4
GLUT-4 is present on the cell membrane and in the
cytoplasm; positive staining signals are brown in colour. In the
control group, the protein expression levels of GLUT-4 were highest
at 4 and 8 h, and staining intensity was higher, compared with that
at 0 h (P<0.05; Fig. 1B). The
staining intensity began to decline at 12 h, with levels below that
at 0 h and reaching a nadir at 24 h. Following geniposide
treatment, the expression levels of GLUT-4 peaked at 4 h, although
the increase was less than that observed in the control group. The
expression levels began to decline at 8 and 12 h, but remained
higher than the level at 0 h. The expression of GLUT-4 reached a
nadir at 24 h and was lower than that in the control group at the
same time point (Fig. 1B and
C).
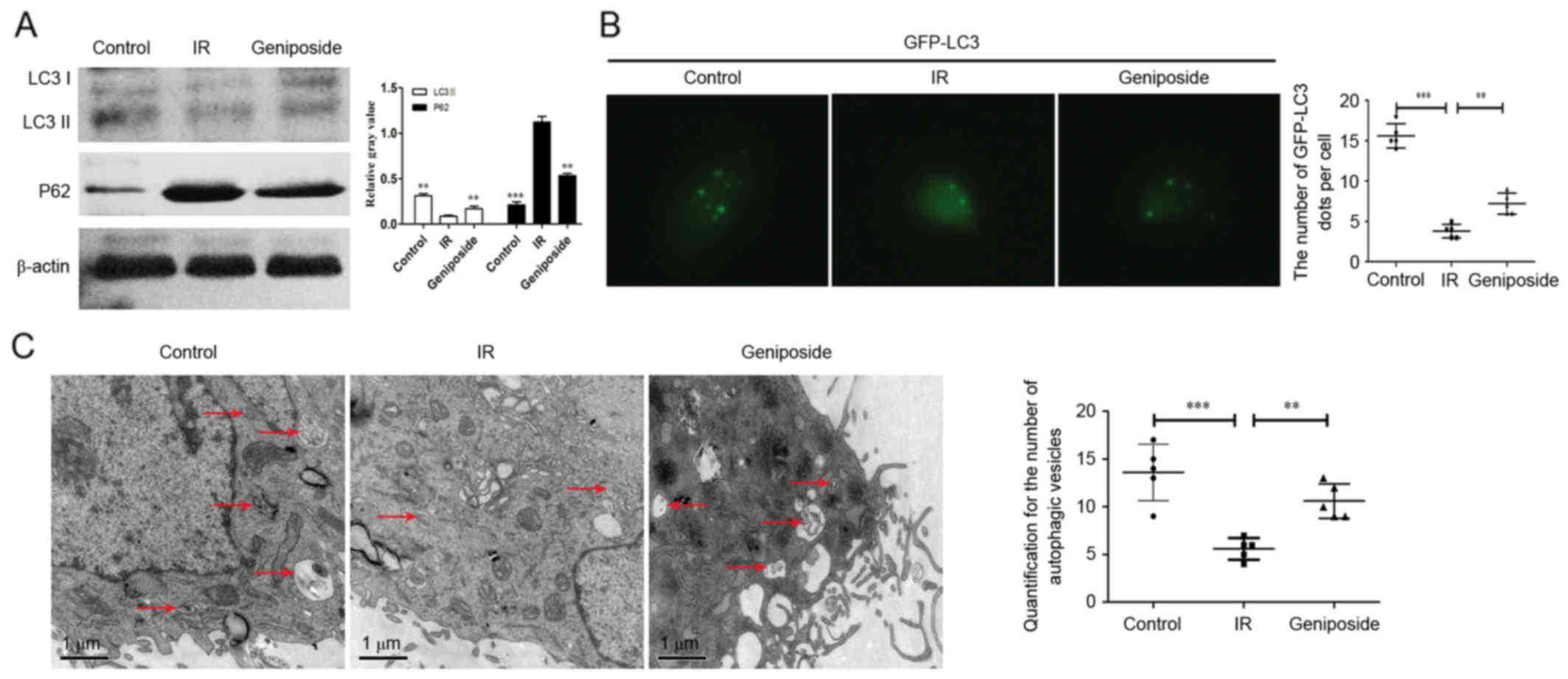
Geniposide promotes autophagy in IR
HepG2 cells
As shown in Fig.
2A, compared with the control group, the protein expression
levels of LC3II were downregulated in the IR HepG2 cells. There was
also a significant increase in the expression of LC3II in the
geniposide-treated HepG2 cells, compared with the cells in the IR
group (P<0.01). By contrast, the expression levels of P62 were
increased in the IR HepG2 cells, whereas geniposide decreased the
expression of P62 in the IR cells.
The appearance of GFP-LC3 within the cytoplasm
reflects the recruitment of LC3 protein to autophagosomes. As shown
in Fig. 2B, IR significantly
decreased the number of GFP-LC3 dots, compared with those in the
control group (P<0.001). There was also a significant increase
in the number of GFP-LC3 dots in the geniposide-treated HepG2
cells, compared with the number in the IR group (P<0.01). In
addition, autophagosomes, containing partially degraded cytoplasmic
material, were observed using TEM (Fig. 2C). The number of autophagosomes
within the IR group was significantly decreased, compared with that
in the control group (P<0.001), however, geniposide decreased
the number of autophagosomes (P<0.01).
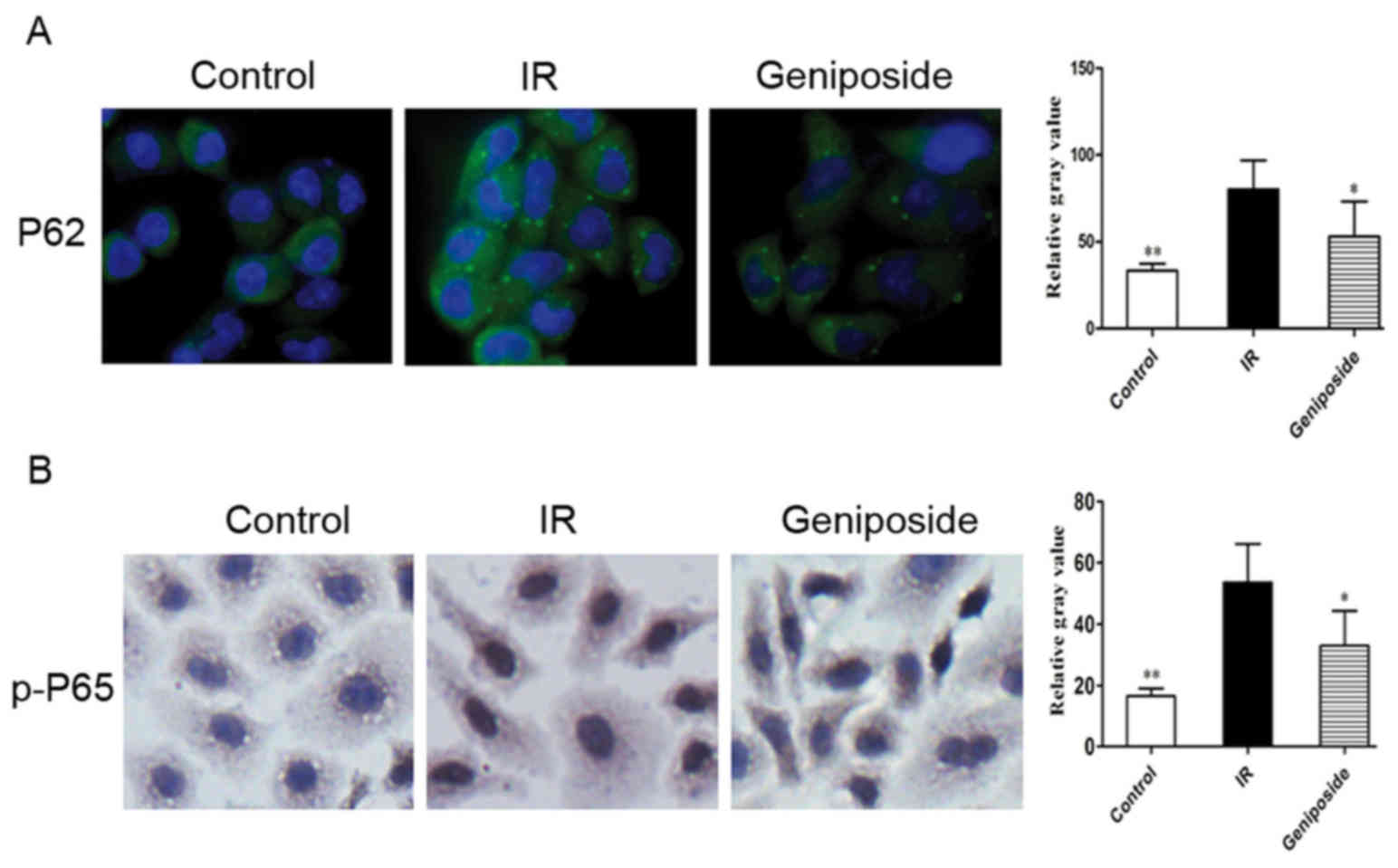
Effect of geniposide on the expression
of P62 and p-P65
P62 is present in the cytoplasm; positive staining
signals are green. In the control group, marked positive staining
of P62 was apparent in the IR cells, with a higher intensity,
compared with that in control (P<0.05). Following geniposide
treatment, the P62 staining intensity decreased, compared with that
in the control group (Fig.
3A).
As shown in Fig.
3B, p-P65 was present in the nucleus and cytoplasm. p-P65 is
translocated from the cytoplasm into the nucleus upon cell
activation, increasing its presence in the nucleus; positive
staining signals are brown in colour. Compared with the control
group, the relative gray value of positive cytoplasmic p-P65
staining was the highest, and there was an increase in positive
nuclear staining in the IR cells (P<0.05). Following geniposide
treatment, the relative gray value of p-P65 positive staining
decreased, compared with that in IR group.
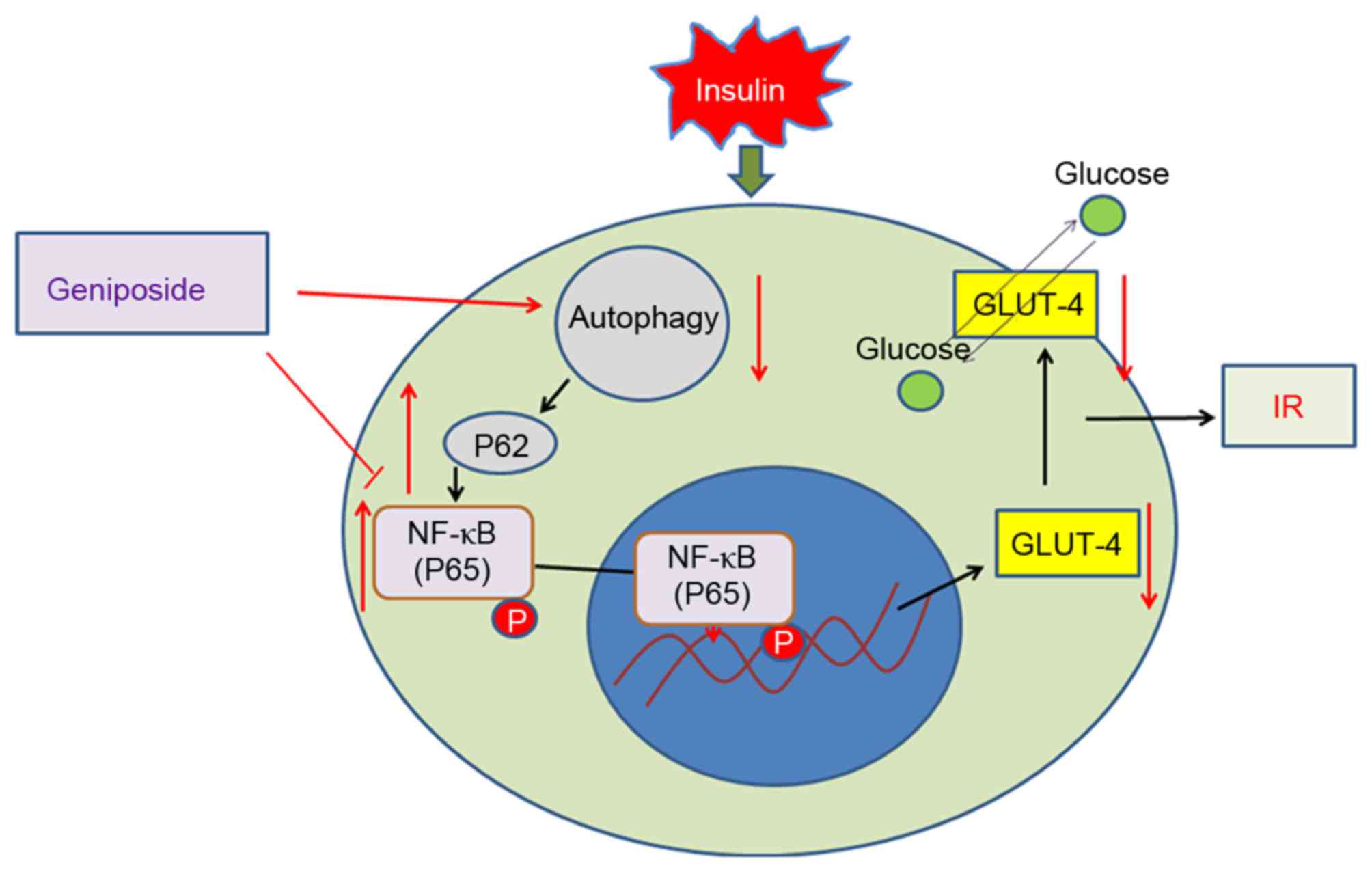
Activating autophagy can inhibit IR in
HepG2 cells via P62/NF-κB/GLUT-4
To assess whether activating autophagy can inhibit
IR in the cell model, the effects of activating or inhibiting
autophagy were examined using rapamycin and 3-MA. As shown in
Fig. 4A, compared with the IR
group, the protein expression levels of LC3II and GLUT-4 were
upregulated in the rapamycin treated HepG2 cells, whereas the
protein expression levels of P62 were decreased. However, 3-MA
decreased the expression levels of LC3II and GLUT-4, and increased
the expression of P62 in the IR HepG2 cells, whereas the protein
expression levels of P62 decreased. Rapamycin also promoted the
mRNA expression of GLUT-4 (P<0.01; Fig. 4B). These histopathological changes
were accompanied by increased and decreased glucose content in the
culture supernatant. As shown in Fig.
4C, rapamycin decreased glucose content, whereas 3-MA increased
glucose content in the culture supernatant, respectively
(P<0.001, rapamycin vs. IR; P<0.01, 3-MA vs. IR).
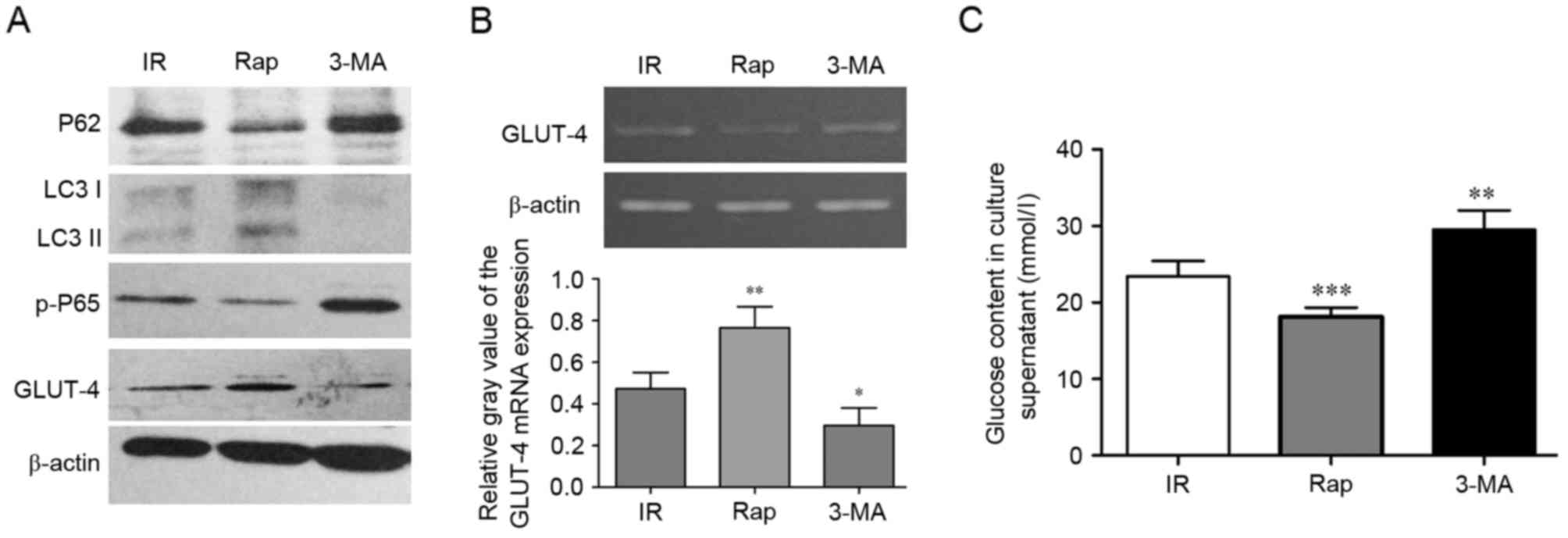 | Figure 4.Activating autophagy can inhibit IR in
HepG2 cells via P62/NF-κB/GLUT-4. (A) Western blot analysis for
P62, LC3, p-P65 and GLUT-4 in HepG2 cells. (B) mRNA expression of
GLUT-4 in HepG2 cells treated with IR, Rap and 3-MA were
detected using semi-quantitative polymerase chain reaction
analysis. (C) Measurements of glucose content in culture
supernatants of HepG2 cells treated with IR, Rap and 3-MA.
*P<0.05, **P<0.01 and ***P<0.001 vs. IR group. IR, insulin
resistance; LC3, GLUT-4, glucose transporter 4; Rap, rapamycin;
3-MA, 3-methyladenine; p-, phosphorylated. |
Discussion
IR is a condition, which can lead to type 2 diabetes
(1), however, no specific
treatment has been identified. The present study investigated the
effect of geniposide, an iridoid compound derived from the fruit of
G. jasminoides Ellis, which can be hepatotoxic, in a HepG2
cell model of IR. Geniposide significantly improved IR, which may
have been associated with its dynamic regulation of the expression
of NF-κB and GLUT-4. The peroxisome proliferator-activated
receptor-γ (PPARγ) receptor is an important element responsible for
regulating blood sugar by mediating the effects of insulin on
glucose uptake (16). The
downregulation of PPARγ contributes to IR (17). Using PPARγ to investigate the
inducible expression of signalling pathway reporter genes, the
hypoglycaemic effect of geniposide in vivo and in
vitro has been suggested to be associated with PPARγ receptor
activation (18). However,
Sriwijitkamol et al (19)
reported that the NF-κB pathway was overactivated in the muscular
tissue of subjects with type 2 diabetes. In animal studies,
inhibition of NF-κB β/NF-κB pathway inhibition significantly
improved insulin sensitivity (20). In the present study, the expression
of NF-κB was downregulated following geniposide treatment. It has
been reported that NF-κB downregulates the expression of GLUT-4
(21) and that genipin, a
geniposide metabolite, inhibits NF-κB (22). Therefore, it is possible that
geniposide treatment reversed the inhibitory effect of NF-κB on the
expression of GLUT-4. Noguchi et al (23) reported that IR is caused partly by
the downregulation of GLUT-4, although it is also associated with
impaired GLUT-4 translocation to the plasma membrane under insulin
stimulation. The results of the present study suggested that
geniposide improved the expression of GLUT-4, an important
downstream insulin receptor site.
A product of the G. jasminoides Ellis fruit,
geniposide, has been reported to cause hepatotoxicity in rats
(24). However, in the present
study, geniposide did not affect HepG2 cell viability, indicating
that the concentration used (62.50 µg/ml) may be safe for use in
animals and subsequently in humans. Liu et al (25) also reported that geniposide had a
protective effect on insulin secretion in rat INS-1 cells, which
indicated that it may also have a protective effect against IR in
humans.
In the present study, the expression levels of
GLUT-4 decreased over time following a peak at 4 h. At its lowest
point, 24 h following geniposide treatment, the mRNA expression of
GLUT-4 remained higher than that in the control at the same time
point. Of note, the peak protein expression of GLUT-4 did not
exceed that of the control at the same time point, whereas the
nadir of the protein expression of GLUT-4 was lower, compared with
that of the control. Similarly, Leguisamo et al (17) reported lower protein levels of
GLUT-4 in induced IR in spontaneously hypertensive neonate rats,
although the mRNA levels of GLUT-4 were not investigated.
Autophagy is a highly conserved intracellular
lysosomal catabolic process, which degrades aged, damaged or
dysfunctional proteins, intracellular organelles and cytoplasmic
components to maintain cellular homeostasis (26). The basal level of autophagy has a
unique housekeeping role in the regulation of cardiac geometry and
function (27). Impaired autophagy
may contribute to various end organ complications in IR and
diabetes, including cardiomyopathy and nephropathy (28).
In the present study, it was shown that the protein
expression levels of LC3II were downregulated in IR HepG2 cells. IR
decreased autophagic activity, as evidenced by the decrease in the
expression of LC3 and the accumulation of P62 protein. These
results suggested that the level of autophagy can be altered by the
duration of exposure to high insulin, which also indicates the
putative role of autophagy under IR conditions. There was also a
significant increase in the expression of LC3II in the
geniposide-treated HepG2 cells, compared with that in the IR group.
By contrast, the expression levels of P62 increased in the IR HepG2
cells, whereas geniposide decreased the expression of P62 in the IR
cells. Geniposide promoted autophagy in the IR HepG2 cells, and
activating autophagy inhibited IR in HepG2 cells via
P62/NF-κB/GLUT-4 (Fig. 5).
Taken together, the findings of the present study
indicated that geniposide may be a viable method for treating IR, a
condition, which can escalate to type 2 diabetes but for which no
specific treatment has been identified. This requires further
attention, and future investigations are required with a focus on
characterising geniposide.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81141059 and U1404805).
References
|
1
|
Lillioja S, Mott DM, Spraul M, Ferraro R,
Foley JE, Ravussin E, Knowler WC, Bennett PH and Bogardus C:
Insulin resistance and insulin secretory dysfunction as precursors
of non-insulin-dependent diabetes mellitus. Prospective studies of
pima indians. N Engl J Med. 329:1988–1992. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kadowaki T: Insights into insulin
resistance and type 2 diabetes from knockout mouse models. J Clin
Invest. 106:459–465. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin XM, Ding WX and Gao W: Autophagy in
the liver. Hepatology. 47:1773–1785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thapalia BA, Zhou Z and Lin X: Autophagy,
a process within reperfusion injury: An update. Int J Clin Exp
Pathol. 7:8322–8341. 2014.PubMed/NCBI
|
|
5
|
Hashimoto S: Autophagy in the respiratory
diseases. Respir Investig. 54:383–384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vucicevic L, Misirkic-Marjanovic M,
Paunovic V, Kravic-Stevovic T, Martinovic T, Ciric D, Maric N,
Petricevic S, Harhaji-Trajkovic L, Bumbasirevic V and Trajkovic V:
Autophagy inhibition uncovers the neurotoxic action of the
antipsychotic drug olanzapine. Autophagy. 10:2362–2378. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rovira J, Ramirez-Bajo MJ, Banon-Maneus E,
Moya-Rull D, Ventura-Aguiar P, Hierro-Garcia N, Lazo-Rodriguez M,
Revuelta I, Torres A, Oppenheimer F, et al: mTOR inhibition:
Reduced insulin secretion and sensitivity in a rat model of
metabolic syndrome. Transplant Direct. 2:e652016.PubMed/NCBI
|
|
8
|
Wang Q and Ren J: mTOR-Independent
autophagy inducer trehalose rescues against insulin
resistance-induced myocardial contractile anomalies: Role of p38
MAPK and Foxo1. Pharmacol Res. 111:357–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarparanta J, Garcia-Macia M and Singh R:
Autophagy and mitochondria in obesity and type 2 diabetes. Curr
Diabetes Rev. 2016.
|
|
10
|
Roccisana J, Sadler JB, Bryant NJ and
Gould GW: Sorting of GLUT4 into its insulin-sensitive store
requires the Sec1/Munc18 protein mVps45. Mol Biol Cell.
24:2389–2397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gaster M, Staehr P, Beck-Nielsen H,
Schrøder HD and Handberg A: GLUT4 is reduced in slow muscle fibers
of type 2 diabetic patients: Is insulin resistance in type 2
diabetes a slow, type 1 fiber disease? Diabetes. 50:1324–1329.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schläfli AM, Adams O, Galván JA, Gugger M,
Savic S, Bubendorf L, Schmid RA, Becker KF, Tschan MP, Langer R and
Berezowska S: Prognostic value of the autophagy markers LC3 and
p62/SQSTM1 in early-stage non-small cell lung cancer. Oncotarget.
7:39544–39555. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xi G, Shen X, Wai C, Vilas CK and Clemmons
DR: Hyperglycemia stimulates p62/PKCζ interaction, which mediates
NF-κB activation, increased Nox4 expression, and inflammatory
cytokine activation in vascular smooth muscle. FASEB J.
29:4772–4782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo LX, Xia ZN, Gao X, Yin F and Liu JH:
Glucagon-like peptide 1 receptor plays a critical role in
geniposide-regulated insulin secretion in INS-1 cells. Acta
Pharmacol Sin. 33:237–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang WY, Lee JJ, Kim Y, Kim IS, Park JS
and Myung CS: Amelioration of insulin resistance by scopoletin in
high-glucose-induced, insulin-resistant HepG2 cells. Horm Metab
Res. 42:930–935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HI and Ahn YH: Role of peroxisome
proliferator-activated receptor-gamma in the glucose-sensing
apparatus of liver and beta-cells. Diabetes. 53 Suppl 1:S60–S65.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leguisamo NM, Lehnen AM, Machado UF,
Okamoto MM, Markoski MM, Pinto GH and Schaan BD: GLUT4 content
decreases along with insulin resistance and high levels of
inflammatory markers in rats with metabolic syndrome. Cardiovasc
Diabetol. 11:1002012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Xia Z, Liu J and Yin F: Cell
signaling mechanisms by which geniposide regulates
insulin-degrading enzyme expression in primary cortical neurons.
CNS Neurol Disord Drug Targets. 14:370–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sriwijitkamol A, Christ-Roberts C, Berria
R, Eagan P, Pratipanawatr T, DeFronzo RA, Mandarino LJ and Musi N:
Reduced skeletal muscle inhibitor of kappaB beta content is
associated with insulin resistance in subjects with type 2
diabetes: Reversal by exercise training. Diabetes. 55:760–767.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai D, Yuan M, Frantz DF, Melendez PA,
Hansen L, Lee J and Shoelson SE: Local and systemic insulin
resistance resulting from hepatic activation of IKK-beta and
NF-kappaB. Nat Med. 11:183–190. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hommelberg PP, Plat J, Remels AH, van
Essen AL, Kelders MC, Mensink RP, Schols AM and Langen RC:
Trans-10, cis-12 conjugated linoleic acid inhibits skeletal muscle
differentiation and GLUT4 expression independently from NF-κB
activation. Mol Nutr Food Res. 54:1763–1772. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM,
Kim SJ, Kim BC, Jin C, Lim CJ and Park EH: Antiinflammatory effects
of genipin, an active principle of gardenia. Eur J Pharmacol.
495:201–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noguchi Y, Yoshikawa T, Marat D, Doi C,
Makino T, Fukuzawa K, Tsuburaya A, Satoh S, Ito T and Mitsuse S:
Insulin resistance in cancer patients is associated with enhanced
tumor necrosis factor-alpha expression in skeletal muscle. Biochem
Biophys Res Commun. 253:887–892. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding Y, Zhang T, Tao JS, Zhang LY, Shi JR
and Ji G: Potential hepatotoxicity of geniposide, the major iridoid
glycoside in dried ripe fruits of Gardenia jasminoides (Zhi-zi).
Nat Prod Res. 27:929–933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Guo L, Yin F, Zhang Y, Liu Z and
Wang Y: Geniposide regulates glucose-stimulated insulin secretion
possibly through controlling glucose metabolism in INS-1 cells.
PLoS One. 8:e783152013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cicchini M, Chakrabarti R, Kongara S,
Price S, Nahar R, Lozy F, Zhong H, Vazquez A, Kang Y and Karantza
V: Autophagy regulator BECN1 suppresses mammary tumorigenesis
driven by WNT1 activation and following parity. Autophagy.
10:2036–2052. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakai A, Yamaguchi O, Takeda T, Higuchi Y,
Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et
al: The role of autophagy in cardiomyocytes in the basal state and
in response to hemodynamic stress. Nat Med. 13:619–624. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nemchenko A, Chiong M, Turer A, Lavandero
S and Hill JA: Autophagy as a therapeutic target in cardiovascular
disease. J Mol Cell Cardiol. 51:584–593. 2011. View Article : Google Scholar : PubMed/NCBI
|