Introduction
Ovarian cancer (OC) is a common female gynecological
cancer. Although the incidence of OC is lower than those of
cervical and endometrial cancer, the case-fatality ratio of OC is
the highest among all gynecological tumor types (1,2).
Owing to the lack of effective methods for early diagnosis and the
nonspecific symptoms, OC is typically detected at the advanced
stage (3). Furthermore, the
incidence of OC is projected to increase (4) and the 12-year case-fatality rate has
only slightly declined in the past few decades (5). Accordingly, novel methods clarifying
the mechanisms of OC development are urgently needed in order to
improve the early detection and to identify novel therapeutic
targets improving the patient survival rate.
Integrin-linked kinase (ILK) has multiple roles in
the pathological procession of OC (6,7). As
an important integrin-proximal kinase, the over-activation of ILK
is implicated in cancer cell growth and tumor angiogenesis
(8,9). In contrast, ILK gene silencing
induces OC cell apoptosis in vitro (10) and suppresses tumorigenesis of OC
cells in vivo (11). These
observations indicate that ILK may be a useful biomarker and a
therapeutic target for OC treatment. Functional interactions
between ILK and other biological factors have also been identified
to enhance its anti-apoptotic role in tumor cells. For example,
synergistic interactions between ILK and the wnt signaling pathway
contribute to the acceleration of breast tumor progression
(12). Additionally, ILK
upregulation is significantly correlated with the downregulation of
miR-542-3p in human colon cancer tissues (13). The activated wnt signaling pathway
promotes epithelial-mesenchymal transition via microRNAs
(miRNAs/miRs) (14), whereas its
inhibition results in apoptosis of OC cells (15–18).
In the present study, interactions among ILK, the
wnt signaling pathway and miRNAs were investigated in OC cells. The
hypothesis that ILK inhibition leads to the inactivation of the wnt
signaling pathway via the upregulation of miRNAs was examined. A
microarray analysis was performed to reveal global alterations in
the miRNA expression profile in OC cells when the ILK gene
was silenced. The present results provide novel insight into the
mechanisms underlying the functional interactions between ILK and
other biological factors in OC.
Materials and methods
ILK short hairpin (sh)RNA expression
lentivirus
An ILK shRNA expression lentivirus that was
described and applied previously was used in the present study
(10). The primer sequences of the
shRNA targeting human ILK and the scrambled shRNA (negative
control, NC) were as follows: 5′-TCAACCAGGGGACGATCAT-3′ and
5′-TTCTCCGAACGTGTCACGT-3′, respectively (Shanghai GenePharma Co.,
Ltd., Shanghai, China).
Cell culture and transfection
The A2780 human OC cell line was purchased from
Shanghai BoQuaner Biological Science and Technology Ltd., Co.
(Shanghai, China), and the cells were cultured in 10% fetal bovine
serum (FBS)-RPMI-1640 (Thermo Fisher Scientific Inc., Waltham, MA,
USA) in a 5% CO2 humidified (95%) incubator at 37°C.
Cells were seeded into 6-well plates (5×105 cells/well)
and allowed to adhere for 24 h at 37°C. Subsequently, the cells
were transduced with the ILK lentivirus or NC lentivirus
(1×108 TU/ml) and continuously cultured for another 12 h
at 37°C. Fresh medium was then replaced with RPMI-1640 supplemented
with 10% FBS and lysed for 72 h. The number of green fluorescent
protein-positive cells was determined by an inverted microscope
(Axio VertA1; Carl Zeiss, Oberkochen, Germany).
Cell viability assay
Cells were cultured in 96-well microtitration plates
at a density of 5×104 cells/well. Cell viability was
assessed by measuring mitochondrial dehydrogenase activity. After
lentiviral transfection, 10 µl
3-[4,5-dimeth-ylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide
(MTT; 5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
added to each well for 4 h at 37°C in the dark. Subsequently, the
purple formazan crystals were dissolved in 200 µl dimethyl
sulfoxide. Absorbance was read at 540 nm using a
spectrophotometer.
Western blot assay
Cells in 6-well culture clusters were
growth-arrested with RPMI-1640 supplemented with 10% FBS for 24 h
at 37°C, prior to ILK lentivirus (cat no. 34314–1) or NC lentivirus
(cat no. LVCONO77) (both from Shanghai Genechem Co., Ltd.,
Shanghai, China) infection. After the lentiviral transfection for
72 h at 37°C, cells were lysed with lysis buffer and incubated for
30 min on ice. The lysates were subsequently sonicated and
centrifuged for 10 min at 4°C (14,800 × g), and the insoluble
fraction was discarded. Protein samples (50 µg) were subjected to
10% SDS-PAGE and blotted on a nitrocellulose membrane. The blots
were blocked with 5% nonfat milk for 2 h at room temperature and
probed with primary antibodies, including ILK (cat no. ab52480;
1:1,000 dilution), β-catenin (cat no. ab32572; 1:1,000),
phosphorylated (p)-focal adhesion kinase (FAK; Tyr397; cat no.
ab81298; 1:1,000), p-RAC-α serine/threonine protein kinase (Akt;
Ser473; cat no. ab81283; 1:1,000) and β-actin (cat no. ab8226;
1:1,000) (all from Abcam, Cambridge, MA, USA), in
phosphate-buffered saline (PBS) and incubated at 4°C overnight. The
membranes were washed with PBS with Tween-20 (0.05%) and were
incubated with a fluorochrome-conjugated secondary antibody
(1:5,000 dilution; cat no. 150077; Thermo Fisher Scientific, Inc.)
for 1 h at room temperature. Finally, bands were analyzed using the
Imaging System and quantified using the Odyssey software (version
1.2) (both from LI-COR Biosciences, Lincoln, NE, USA) based on
intensity (area × optical density) in each group with β-actin as an
internal control. Results are expressed as the fold change by
normalizing the data to the control values.
Measurement of caspase-3 activity
The Caspase-3 Activity kit was obtained from
Beyotime Institute of Biotechnology (Haimen, China). Caspase-3
activity was measured based on the cleavage of a chromogenic
caspase substrate, Ac-DEVD-pNA (acetyl-Asp-Glu-Val-Asp
p-nitroanilide). The protein samples were prepared as indicated in
the previous section (western blotting assay). Total protein (50
µg) was added to the reaction buffer containing Ac-DEVD-pNA (2 mM)
and incubated for 4 h at 37°C. The absorbance of yellow pNA
cleaved from its corresponding precursors was subsequently
determined using an ultraviolet spectrometer at 405 nm. The
specific caspase-3 activity, normalized with the total proteins
from cell lysates, was expressed as the fold change of the baseline
caspase activity which was measured from normal cells cultured in
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS.
Cell migration and invasion
assays
To analyze wound healing, SKOV3 cells were seeded in
6-well plates at a density of 5×105 cells/well with
RPMI. When the cells reached 80% confluency, they were infected
with the ILK or NC lentivirus. After 48 h, the cell monolayer was
wounded using a plastic pipette tip. Cells were subsequently rinsed
with PBS and cultured with serum-free RPMI for 24 h at 37°C. The
wound closure was observed and imaged under a phase-contrast
microscope (Olympus Corporation, Tokyo, Japan). For the Transwell
assays, 8-µm pore size chambers (Corning, Inc., Corning, NY, USA)
were used with or without an insert coated with Matrigel (BD
Biosciences, San Jose, CA, USA). A total of 48 h after infection
with the ILK or NC lentivirus at 37°C, 1×105 cells in
serum-free medium were added to the upper chamber. The lower
chamber was filled with 10% FBS RPMI. After 24 h of incubation at
37°C, the cells remaining on the upper surface of the membrane were
removed, whereas the cells that had invaded through the membrane
were fixed with 100% methanol for 15 min at room temperature and
stained with 0.1% crystal violet for 20 min at room temperature.
Images of SKOV3 cells were obtained under a phase-contrast
microscope (Olympus Corporation).
Cell adhesion assay
Cultures of SKOV3 cell lines were transduced with
the ILK or NC lentivirus and harvested. Following a 48-h
incubation, cells (1×105/well) were added to 96-well
plates coated with fibronectin (5 µg/ml; BD Biosciences), followed
by a 2-h incubation at 37°C. Cells were washed by PBS three times.
Subsequently, cells were incubated for 1.5 h in 10% Cell Counting
Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) in
normal culture medium at 37°C for color conversion. Relative cell
adhesion to fibronectin was calculated as the fold change in cell
adhesion of normal cells to fibronectin, which was measured as the
absorbance by using an ultraviolet spectrometer at 450 nm.
Immunofluorescence analysis
Cells plated in 12-well chamber slides coated with
fibronectin (5 µg/ml; BD Biosciences) were transduced with the ILK
or NC lentivirus for 48 h at 37°C. Following this, cells were fixed
in 100% methanol, and blocking solution (1% bovine serum albumin
and 0.1% Triton X-100 in PBS) was added. Cells were incubated at
room temperature for 2 h and were exposed to the primary antibody
(4°C, overnight). The following specific antibodies were used:
ILK-1 (dilution, 1:500; cat no. ab76468) and Talin (dilution,
1:100; cat no. ab157808) (both from Abcam). Following this, cells
were incubated with two fluorochrome-conjugated secondary
antibodies (goat anti-rabbit IgG, Alexa Fluor 594, cat no. A11037;
and goat anti-mouse IgG, Alexa Fluor Plus 488, cat no. A32723)
(both from Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. The nuclei were stained using DAPI (Beyotime Institute
of Biotechnology, Shanghai, China) for 20 min at room temperature.
Immunofluorescence was examined under a fluorescence microscope
(Nikon 80i; Nikon Corporation, Tokyo, Japan).
Total RNA extraction and microarray
analysis
The lysed cells were centrifuged for 10 min at 4°C
(450 × g). TRIzol reagent (Thermo Fisher Scientific Inc.) was used
to extract RNA from cells transduced with ILK or NC lentivirus.
Three independent samples were examined for each group. The RNA
eluate was stored at −80°C for the microarray analysis. In the
present study, the lentivirus-infected cell samples underwent
global scanning of miRNA expression using the Agilent Human miRNA
Microarray (Release 19.0, 8×60K). Briefly, sample labeling,
microarray hybridization and washing were performed following the
manufacturer's protocol (Agilent Technologies Inc., Santa Clara,
CA, USA), as described previously (18). Differentially expressed miRNAs were
identified based on the following criteria: fold change >1.5 and
P<0.05. Only the probes flagged as ‘Detected’ in all samples
were included in the analysis. ‘Detected’ indicates a positive
normalized expression value detected for a probe. The miRNA
microarray analysis was performed by Shanghai Oe Biotech Co., Ltd.
(Shanghai, China). The microarray data were submitted to the Gene
Expression Omnibus (accession no. GSE83721).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To evaluate differentially expressed miRNAs, RNAs
from normal A2780 cells and cells transduced with ILK or NC
lentivirus were extracted using TRIzol reagent and the
concentration was determined by ultraviolet spectrophotometry.
After the RNA concentration and purity were quantified, RNA was
used as a template for cDNA synthesis by RT. A quantitative
analysis of genes was performed using the ABI 7500 Fast Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The PCR conditions used to detect the genes were as follows: 95°C
for 10 min, followed by 35 cycles of 95°C for 15 sec and 60°C for 1
min. Intracellular mRNA levels of ILK, wnt3a,
wnt4, wnt5a and catenin β1 (CTNNβ1)
were also detected in the three groups (the normal, NC lentivirus
infection and the ILK lentivirus infection groups). GAPDH
was used as an internal reference. Intracellular expression
alterations in various miRNAs, including miR-15a-5p, miR-29c-3p,
miR-30a-5p and miR-200a-3p, were also investigated in the three
groups. Quantitation threshold (Cq) values for each gene were
normalized to those of GAPDH, and Cq values for miRNAs were
normalized to U6. Relative expression levels were calculated using
the following equation: 2−ΔΔCq (19). Table
I presents the primer sequences for the genes.
 | Table I.Primer sequences for the genes used
in the present study. |
Table I.
Primer sequences for the genes used
in the present study.
| Gene name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| miR-15a-5p |
GCGGCGGTAGCAGCACATAATG |
ATCCAGTGCAGGGTCCGAGG |
| miR-29c-3p |
GCGGCGGTAGCACCATTTGAAATC |
ATCCAGTGCAGGGTCCGAGG |
| miR-30a-5p |
GCGGCGGTGTAAACATCCTCGAC |
ATCCAGTGCAGGGTCCGAGG |
| miR-200a-3p |
GCGGCGGTAACACTGTCTGGTAAC |
ATCCAGTGCAGGGTCCTCGAC |
| CTNNB1 |
ACTAAACAGGAAGGGATGGAAGG |
AGATGACGAAGAGCACAGATGG |
| ILK |
GTCTCCACCTGCTCCTCATC |
TCCTCATCAATCATTACACTACGG |
| wnt3a |
TGGCCCCACTCGGATACTT |
TGGGCATGATCTCCACGTAGT |
| wnt4 |
CCTTCGTGTACGCCATCTCTTC |
GCGATGTTGTCAGAGCATCCT |
| wnt5a |
ATCGACTATGGCTACCGCTTTG |
CCACATCAGCCAGGTTGTACA |
| U6 |
GCTTCGGCACATATACTAAAAT |
CGCTTCACGAATTTGCGTGTCAT |
| GAPDH |
CATGTTCGTCATGGGTGTGAA |
GGCATGGACTGTGGTCATGAG |
Pathway analysis
To explore the pathways affected by significantly
dysregulated miRNAs in response to ILK silencing, a Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway analysis was
performed using the Database Annotation, Visualization and
Integrated Discovery (DAVID) bioinformatics online tool (20). The miRTarBase database was used to
obtain the set of experimentally supported miRNA-target
interactions (MTIs) for the significantly dysregulated miRNAs in
the microarray analysis and verified by RT-qPCR (21). Only the MTIs supported by at least
one piece of strong evidence or by at least ≥2 pieces of less
strong evidence were retained for further KEGG pathway analyses.
Strong evidence is the evidence that was validated by the reporter
assay, western blot or qPCR; whereas, less strong evidence is the
evidence that was validated by microarray, pulsed stable-isotope
labeling by amino acids in cultured cells or next-generation
sequencing. The official gene symbols of all targets of the miRNAs
were uploaded to the database for the KEGG pathway analysis.
Significantly over-represented pathways were identified when genes
involved in a given pathway accounted for >5% of the whole set
of uploaded genes and the false discovery rate (FDR) was
<0.05.
Statistical analysis
Data are expressed as the mean ± standard error.
Statistical analyses were performed with Student's t-test or
one-way analysis of variance followed by Dunnett's test, where
appropriate, using GraphPad Prism v6.0 (GraphPad software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
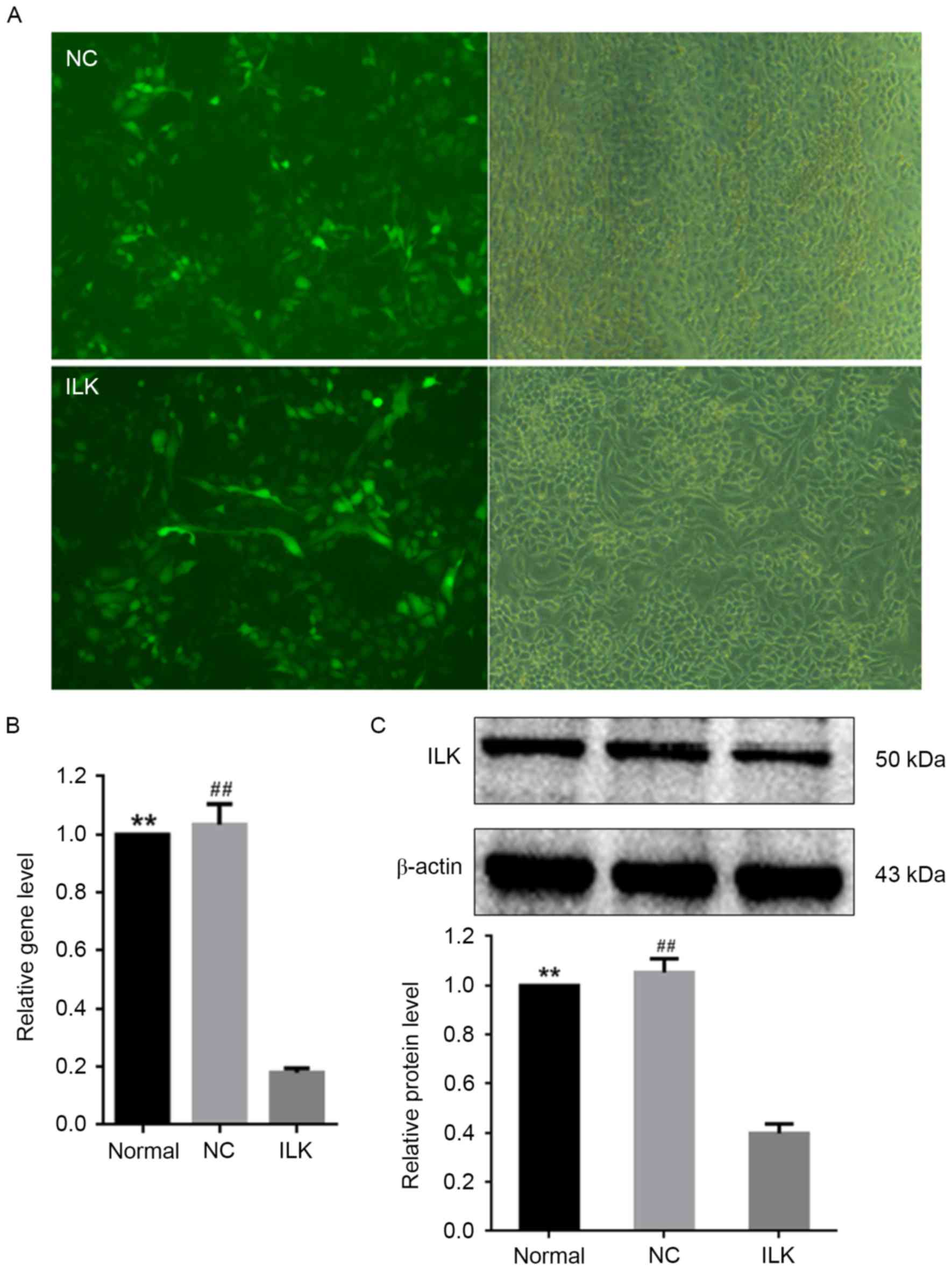
Silencing of ILK by lentiviral
transfection induces apoptosis in OC cells
The shRNA lentiviral transfection was used for
silencing the ILK gene in OC cells. Lentiviral transfection
markedly downregulated the intracellular expression of ILK
(Fig. 1A) and ILK mRNA
expression (Fig. 1B). The protein
expression of ILK was ~60% lower in cells infected with the
lentivirus vector expressing ILK shRNA than in normal cells
(Fig. 1C). These results
demonstrated the efficiency of ILK shRNA transfection via
the lentivirus vector. A strong reduction in ILK expression
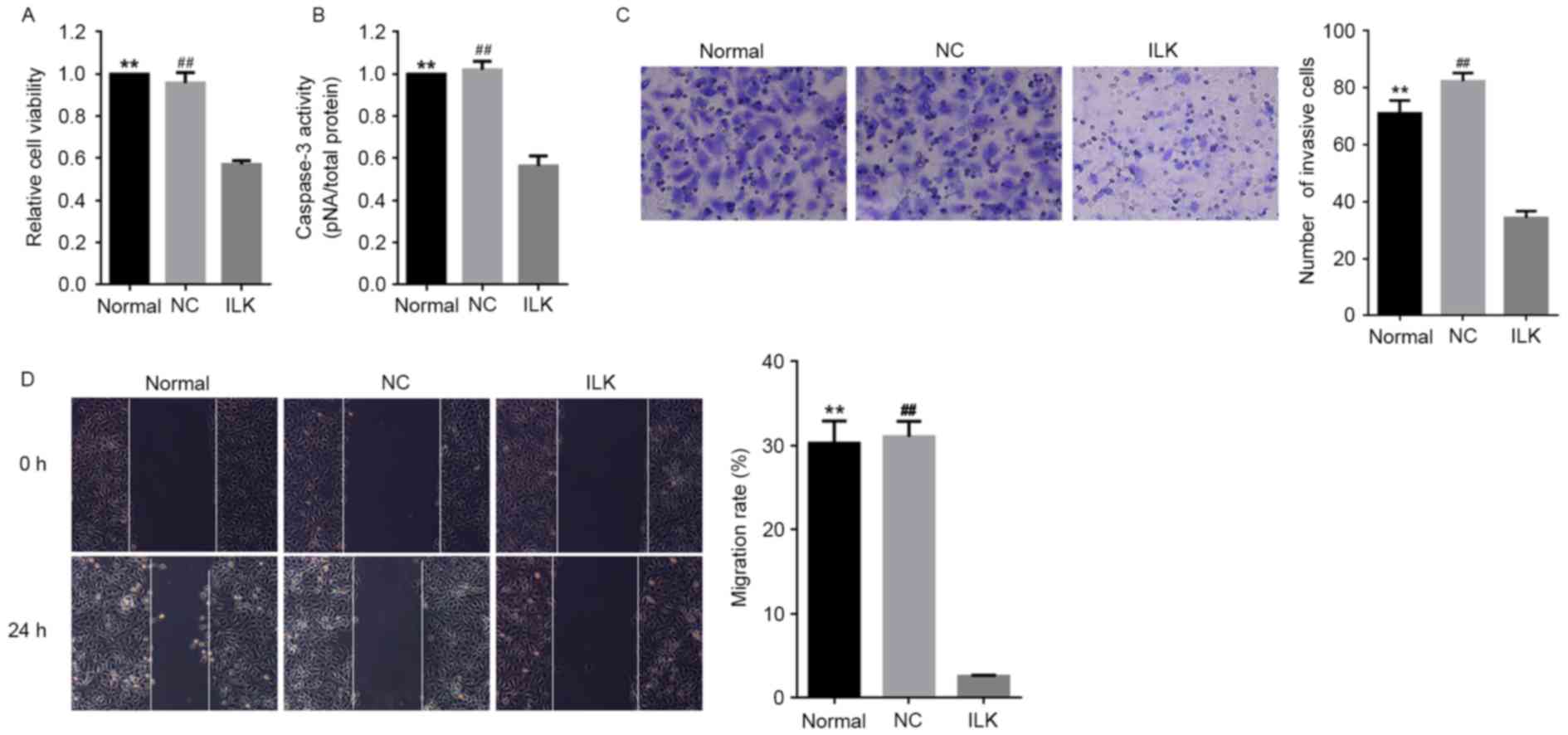
significantly decreased cell viability and induced apoptosis in OC
cells compared with normal conditions (P<0.01, Fig. 2A and B respectively). This finding
strongly suggested that ILK is a potent anti-apoptotic gene
in OC cells and promotes the pathological development of OC,
consistent with our previous study (10). ILK silencing led to a
remarkable reduction in the migratory ability of OC cells (Fig. 2C and D). Consistently, ILK
silencing led to the reduced migratory ability of OC cells
(Fig. 2C and D). No significant
influence of lentiviral transfection alone was detected on ILK
expression and the overall state of OC cells.
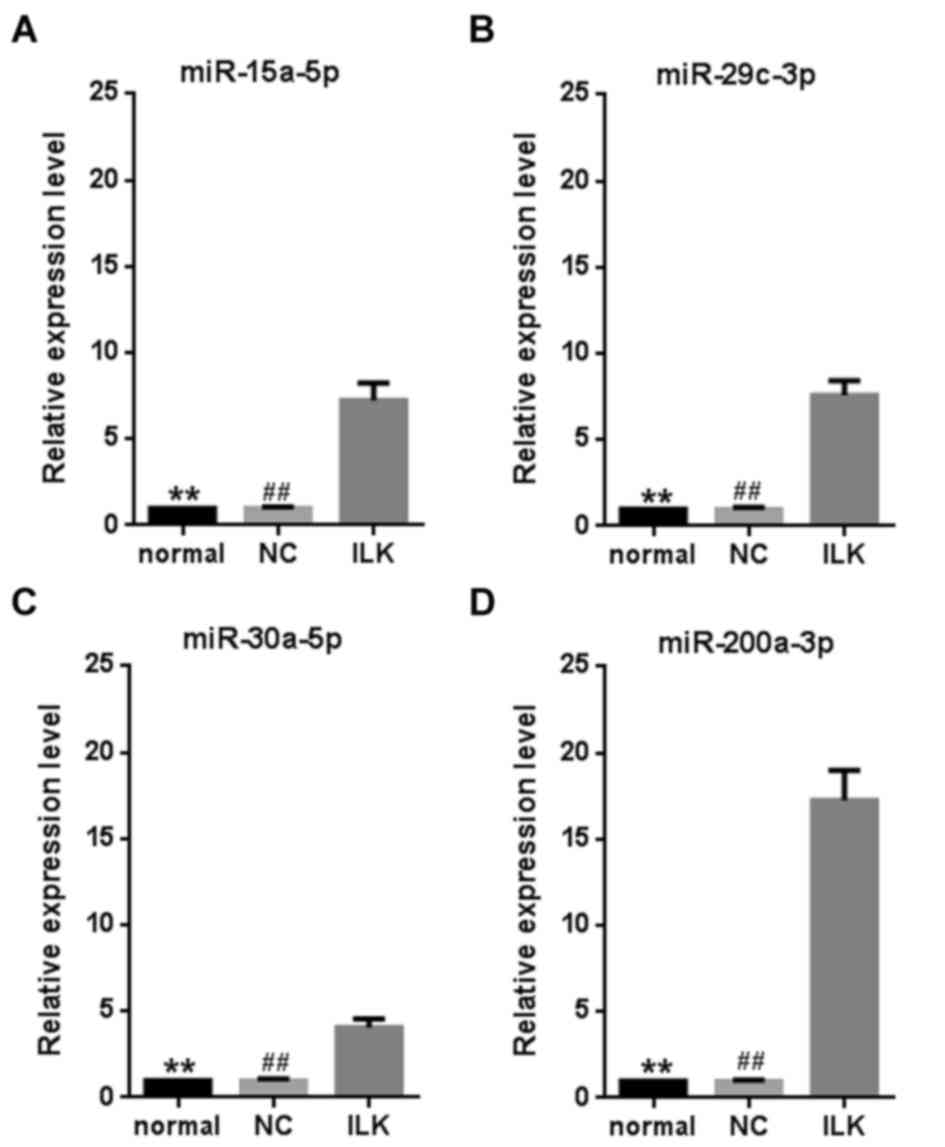
Multiple miRNAs are significantly
upregulated when ILK is silenced
To explore whether miRNAs mediate the anti-apoptotic
functions of ILK, global miRNA expression in OC cells was examined
after ILK shRNA lentivirus transfection. The microarray assay
revealed 294 miRNAs that were expressed in the cells (accession
number: GSE83721). Among them, 14 were significantly upregulated by
ILK silencing (>1.5-fold change and P<0.05, Table II). However, no miRNA was
significantly downregulated by ILK silencing. Four miRNAs
(miR-15a-5p, miR-29c-3p, miR-30a-5p and miR-200a-3p) were selected
for further validation using a RT-qPCR assay (P<0.01, Fig. 3). The miRNA miR-200a-3p exhibited
the greatest fold change (~17.2 fold) compared with the control
cells. As ILK is a vital anti-apoptotic gene in OC (10), the strong change in miR-200a-3p
expression suggests that it has a vital role in OC.
 | Table II.Significantly upregulated miRNAs by
ILK silencing. |
Table II.
Significantly upregulated miRNAs by
ILK silencing.
| miRNAs | Fold change | P-value |
|---|
| miR-29b-3p | 2.87 |
2.67×10−2 |
| miR-551b-3p | 2.80 |
8.01×10−3 |
| miR-30e-5p | 2.20 |
1.25×10−2 |
| miR-135b-5p | 2.09 |
1.21×10−2 |
| miR-301a-3p | 1.90 |
9.08×10−3 |
| miR-96-5p | 1.84 |
1.48×10−2 |
| miR-27a-3p | 1.83 |
4.67×10−3 |
| miR-30a-5p | 1.77 |
7.78×10−4 |
| miR-200a-3p | 1.76 |
1.68×10−2 |
| miR-29c-3p | 1.68 |
5.48×10−3 |
| miR-15a-5p | 1.63 |
9.97×10−3 |
| miR-210-3p | 1.56 |
3.36×10−2 |
| miR-106b-5p | 1.56 |
7.61×10−3 |
| miR-181a-5p | 1.54 |
9.50×10−3 |
miRNAs mediate functional interactions
between ILK and multiple cancer-associated signaling pathways
Using the miRTarBase database, 366 experimentally
validated MTIs were identified for 13 of the 14 dysregulated miRNAs
(Table II) and 331 mRNA genes.
The analysis did not identify any gene as a target of miR-551b-3p.
By searching the 331 genes against the DAVID website, nine
significantly over-represented KEGG pathways were obtained,
including focal adhesion, cell cycle, wnt signaling and six
cancer-associated pathways (Table
III). These results supported the anti-apoptotic role of ILK in
OC cells. It is possible that the remarkable upregulation of the
miRNAs suppresses cancer-associated signaling pathways and leads to
apoptosis.
 | Table III.Significantly overrepresented KEGG
pathways (FDR <0.05). |
Table III.
Significantly overrepresented KEGG
pathways (FDR <0.05).
| KEGG pathway | Gene (no) | % (331 genes in
total) | FDR |
|---|
| Pathways in
cancer | 44 | 13.7 |
7.60×10−18 |
| Focal adhesion | 25 | 7.8 |
1.00×10−7 |
| Prostate
cancer | 22 | 6.8 |
1.80×10−12 |
| Chronic myeloid
leukemia | 20 | 6.2 |
1.10×10−11 |
| Small cell lung
cancer | 20 | 6.2 |
1.00×10−10 |
| Cell cycle | 20 | 6.2 |
1.80×10−7 |
| Pancreatic
cancer | 19 | 5.9 |
7.40×10−11 |
| Colorectal
cancer | 17 | 5.3 |
1.70×10−7 |
| Wnt signaling
pathway | 17 | 5.3 |
1.00×10−3 |
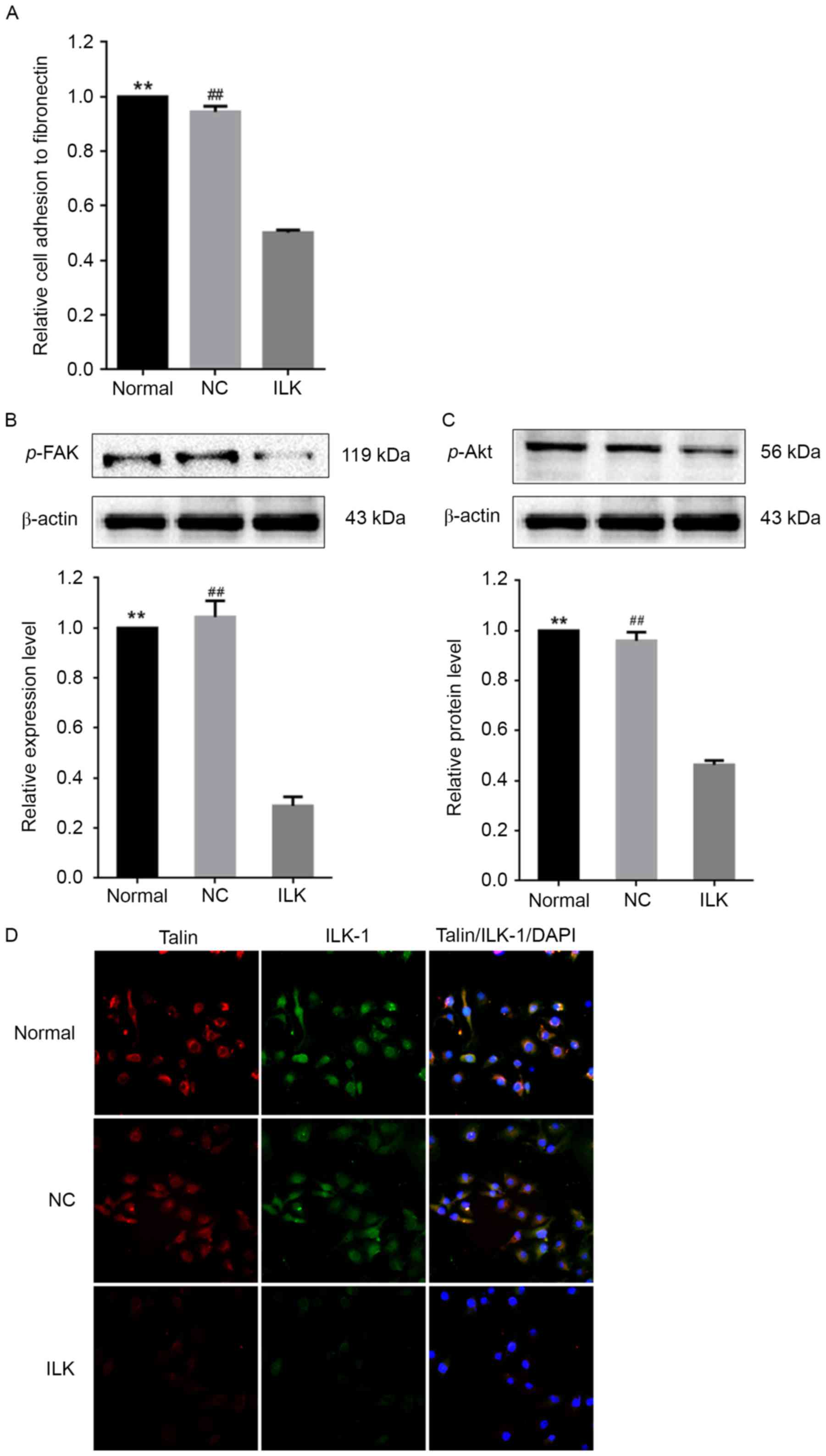
ILK silencing down-regulates
phosphorylation of FAK and Akt and reduces cell adhesion
The pathway analysis revealed an overrepresentation
of target genes of dysregulated miRNAs involved in focal adhesion
(FDR=1×10−7). Consistent with these findings, ILK
silencing markedly reduced the ability of OC cells to adhere to
fibronectin (Fig. 4A). The FAK/Akt
signaling pathway serves an important role in pressure-stimulated
cancer cell adhesion (22).
ILK silencing led to decreases in the phosphorylation levels
of FAK and Akt by ~70% and ~50%, respectively (Fig. 4B and C). This result suggests that
the FAK/Akt signaling pathway was markedly inactivated by
ILK silencing in OC cells. It was observed that ILK
silencing led to the concomitant downregulation of talin, another
important constituent element of the focal adhesion complex that
stabilizes the cytoskeleton, and kinases, such as ILK-1 (Fig. 4D). This result implies that
ILK silencing might result in the formation of unstable
focal contact.
ILK silencing reduced the activity of
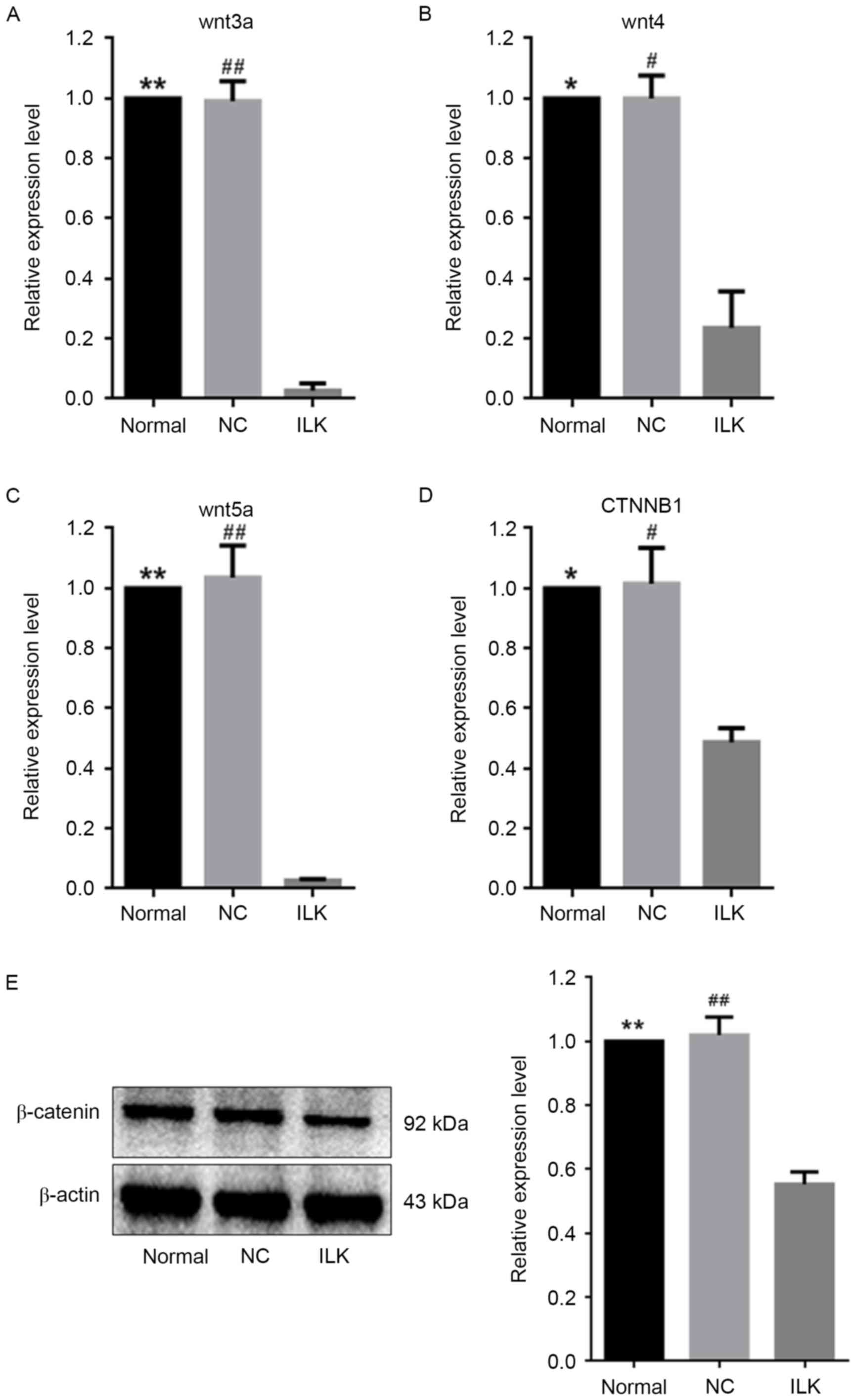
the wnt signaling pathway via multiple miRNAs
Multiple studies have demonstrated that
overactivation of the wnt signaling pathway is involved in the
pathological development of OC (17,23,24).
Four of the 14 dysregulated miRNAs that are illustrated in Table II regulate genes involved in the
wnt signaling pathway, i.e. miR-15a-5p, miR-29c-3p, miR-30a-5p and
miR-200a-3p and are involved in the post-transcriptional regulation
of wnt3a, wnt4, wnt5a, and CTNNB1,
respectively (25–28). CTNNB1 encodes β-catenin, a
crucial signaling factor in the wnt signaling pathway. In the
present study, these four miRNAs were upregulated when ILK
was specifically silenced in OC cells (Fig. 3). Consistent with this, reduced
expression of their target genes in the wnt signaling pathway was
observed, suggesting weakened wnt activity (Fig. 5A-D). The protein expression of
β-catenin decreased by ~45% when ILK was silenced (Fig. 5E).
Discussion
After activation by hormones, growth factors and
cytokines, the multidomain focal adhesion protein serine/threonine
kinase ILK is overexpressed, and this overexpression is causally
associated with changes in cell survival, migration, and
angiogenesis (8,9). Accordingly, ILK has a well-known role
in the progression of hormonal cancers, such as OC (29). It has previously been suggested
that silencing of ILK leads to apoptosis in OC cells
(10), but the mechanisms
mediating this effect are unclear. Accordingly, extensive
investigations of the roles of ILK in OC are necessary (1,2).
Many recent studies have suggested that miRNAs are involved in the
progression of OC and serve important roles in various cellular
functions, including cell survival and migration (30–35).
In the present study, the influence of ILK on global miRNA
expression was explored by applying microarray techniques.
Lentivirus-mediated silencing of ILK caused the marked
upregulation of 14 miRNAs in OC cells. A functional pathway
analysis revealed the crucial involvement of ILK in multiple
cancer-associated signaling pathways.
In the progression of OC, the overexpression of ILK
in cancer cells could activate multiple downstream singling
pathways, such as the vascular endothelial growth factor and Akt
signaling pathways, and eventually stimulate cancer cell
phenotypes, such as survival, migration and angiogenesis (29). Consistent with the results of a
previous study (10), the present
study validated that the transfection of OC cells with the
ILK shRNA lentivirus resulted in decreased ILK expression,
at both the mRNA and protein expression levels. When ILK was
silenced, reduced cell viability and lower capase-3 and cell
migration ability were observed, indicating the tumor-promoting
effect of ILK in OC cells. These results suggested that the
specific inhibition of ILK is a feasible strategy for OC treatment
(11,36).
It was also observed that ILK silencing
caused global alterations in miRNA expression. A signature composed
of 14 upregulated miRNAs was associated with ILK silencing
in OC cells. The KEGG pathway analysis of their targets revealed
significant enrichment for genes in cancer-associated pathways.
This finding was consistent with the changes in cell phenotypes
after ILK silencing (10,11).
Overrepresentation of target genes involved in focal adhesion
(FDR=1×10−7) was consistent with previous observations
by Wang and Basson (37), who
demonstrated that ILK silencing causes reduced
phosphorylation of FAK and Akt in cancer cells. The phosphorylation
of FAK and Akt is necessary for pressure-stimulated cancer cell
adhesion. In the present study, it was observed that ILK silencing
down-regulated the activity of the FAK/Akt signaling pathway. By
regulating the expression of multiple miRNAs that target genes in
the focal adhesion pathway, ILK may serve a vital role in cell
adhesion, an important biological process leading to cancer
metastasis. Cell adhesion was impaired when ILK was silenced in OC
cells. Further experiments are needed to validate this finding
obtained by our pathway analysis.
A miRNA-mediated functional association between ILK
and the wnt signaling pathway was also detected. Inhibition of the
wnt signaling pathway leads to apoptosis in OC cells (15–18).
In the present study, ILK silencing substantially increased
the levels of four miRNAs (miR-15a-5p, miR-29c-3p, miR-30a-5p and
miR-200a-3p) that regulate genes in the wnt signaling pathway and
caused significant downregulation of target genes, including
wnt3a, wnt4, wnt5a and CTNNB1 (25–28).
Decreased expression of β-catenin suggests reduced activity of the
wnt signaling pathway (38).
Oloumi et al (39) have
demonstrated that the pharmacological inhibition of ILK results in
the suppression of the nuclear stabilization of β-catenin in
wnt3a-expressing cells. This result was consistent with the finding
that ILK silencing inhibited the activity of the wnt
signaling pathway via miRNA-mediated target gene regulation.
In conclusion, the present study investigated the
roles of ILK in OC based on global miRNA expression analysis.
Specific ILK silencing induced significant alterations in
the expression of many miRNAs, suggesting multi-faceted roles of
ILK in OC. Furthermore, ILK-mediated modulation of the wnt
signaling pathway via multiple miRNAs was identified in the present
study. This finding implies indirect functional interactions
between ILK and the wnt signaling pathway, both of which have roles
in OC progression.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81401502 and
31301095) and the Research Foundation of the First Affiliated
Hospital of Harbin Medical University (grant nos. 2015Y006 and
2015B014).
Glossary
Abbreviations
Abbreviations:
|
ILK
|
integrin-linked kinase
|
|
FDR
|
false discovery rate
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
miRNA
|
microRNA
|
|
MTI
|
miRNA-target interaction
|
|
OC
|
ovarian cancer
|
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marchetti C, Pisano C, Facchini G, Bruni
GS, Magazzino FP, Losito S and Pignata S: First-line treatment of
advanced ovarian cancer: Current research and perspectives. Expert
Rev Anticancer Ther. 10:47–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen JG, White M, Cruz A and
Farias-Eisner R: In 2014, can we do better than CA125 in the early
detection of ovarian cancer? World J Biol Chem. 5:286–300. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sopik V, Iqbal J, Rosen B and Narod SA:
Why have ovarian cancer mortality rates declined? Part I.
Incidence. Gynecol Oncol. 138:741–749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sopik V, Iqbal J, Rosen B and Narod SA:
Why have ovarian cancer mortality rates declined? Part II.
Case-fatality. Gynecol Oncol. 138:750–756. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahmed N, Oliva K, Rice GE and Quinn MA:
Cell-free 59 kDa immunoreactive integrin-linked kinase: A novel
marker for ovarian carcinoma. Clin Cancer Res. 10:2415–2420. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bagnato A and Rosanò L:
Epithelial-mesenchymal transition in ovarian cancer progression: A
crucial role for the endothelin axis. Cells Tissues Organs.
185:85–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmed N, Riley C, Oliva K, Stutt E, Rice
GE and Quinn MA: Integrin-linked kinase expression increases with
ovarian tumour grade and is sustained by peritoneal tumour fluid. J
Pathol. 201:229–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lössner D, Abou-Ajram C, Benge A,
Aumercier M, Schmitt M and Reuning U: Integrin alphavbeta3
upregulates integrin-linked kinase expression in human ovarian
cancer cells via enhancement of ILK gene transcription. J Cell
Physiol. 220:367–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Q, Xiao L, Yuan D, Shi X and Li P:
Silencing of the integrin-linked kinase gene induces the apoptosis
in ovarian carcinoma. J Recept Signal Transduct Res. 32:120–127.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Q, Li C, Zhang YY, Chen W, Lv JL, Sun J
and You QS: Silencing of integrin-linked kinase suppresses in vivo
tumorigenesis of human ovarian carcinoma cells. Mol Med Rep.
7:1050–1054. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oloumi A, Maidan M, Lock FE, Tearle H,
McKinney S, Muller WJ, Aparicio SA and Dedhar S: Cooperative
signaling between Wnt1 and integrin-linked kinase induces
accelerated breast tumor development. Breast Cancer Res.
12:R382010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oneyama C, Morii E, Okuzaki D, Takahashi
Y, Ikeda J, Wakabayashi N, Akamatsu H, Tsujimoto M, Nishida T,
Aozasa K and Okada M: MicroRNA-mediated upregulation of
integrin-linked kinase promotes Src-induced tumor progression.
Oncogene. 31:1623–1635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghahhari NM and Babashah S: Interplay
between microRNAs and WNT/β-catenin signalling pathway regulates
epithelial-mesenchymal transition in cancer. Eur J Cancer.
51:1638–1649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Fan L, Xia X, Rao Y, Ma Q, Yang J,
Lu Y, Wang C, Ma D and Huang X: Silencing Wnt2B by siRNA
interference inhibits metastasis and enhances chemotherapy
sensitivity in ovarian cancer. Int J Gynecol Cancer. 22:755–761.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sanchez AM, Giorgione V, Viganò P, Papaleo
E, Candiani M, Mangili G and Panina-Bordignon P: Treatment with
anticancer agents induces dysregulation of specific Wnt signaling
pathways in human ovarian luteinized granulosa cells in vitro.
Toxicol Sci. 136:183–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Yan ZQ, Li B, Yin SY, Sun Q, Kou
JJ, Ye D, Ferns K, Liu HY and Liu SL: Reduced expression of SOX7 in
ovarian cancer: A novel tumor suppressor through the Wnt/β-catenin
signaling pathway. J Ovarian Res. 7:872014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng J, Zhang D, Pan N, Sun N, Wang Q, Fan
J, Zhou P, Zhu W and Jiang L: Identification of miR-194-5p as a
potential biomarker for postmenopausal osteoporosis. Peer J.
3:e9712015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
da W Huang, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009.PubMed/NCBI
|
|
21
|
Hsu SD, Tseng YT, Shrestha S, Lin YL,
Khaleel A, Chou CH, Chu CF, Huang HY, Lin CM, Ho SY, et al:
miRTarBase update 2014: An information resource for experimentally
validated miRNA-target interactions. Nucleic Acids Res. 42(Database
Issue): D78–D85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thamilselvan V, Craig DH and Basson MD:
FAK association with multiple signal proteins mediates
pressure-induced colon cancer cell adhesion via a Src-dependent
PI3K/Akt pathway. FASEB J. 21:1730–1741. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosanò L, Cianfrocca R, Tocci P, Spinella
F, Di Castro V, Caprara V, Semprucci E, Ferrandina G, Natali PG and
Bagnato A: Endothelin A receptor/β-arrestin signaling to the Wnt
pathway renders ovarian cancer cells resistant to chemotherapy.
Cancer Res. 74:7453–7464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mostowska A, Pawlik P, Sajdak S, Markowska
J, Pawałowska M, Lianeri M and Jagodzinski PP: An analysis of
polymorphisms within the Wnt signaling pathway in relation to
ovarian cancer risk in a Polish population. Mol Diagn Ther.
18:85–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bonci D, Coppola V, Musumeci M, Addario A,
Giuffrida R, Memeo L, D'Urso L, Pagliuca A, Biffoni M, Labbaye C,
et al: The miR-15a-miR-16-1 cluster controls prostate cancer by
targeting multiple oncogenic activities. Nat Med. 14:1271–1277.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hawkins SM, Creighton CJ, Han DY, Zariff
A, Anderson ML, Gunaratne PH and Matzuk MM: Functional microRNA
involved in endometriosis. Mol Endocrinol. 25:821–832. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Selbach M, Schwanhäusser B, Thierfelder N,
Fang Z, Khanin R and Rajewsky N: Widespread changes in protein
synthesis induced by microRNAs. Nature. 455:58–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia H, Ng SS, Jiang S, Cheung WK, Sze J,
Bian XW, Kung HF and Lin MC: miR-200a-mediated downregulation of
ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma
cell growth, migration and invasion. Biochem Biophys Res Commun.
391:535–541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cortez V, Nair BC, Chakravarty D and
Vadlamudi RK: Integrin-linked kinase 1: Role in hormonal cancer
progression. Front Biosci (Schol Ed). 3:788–796. 2011.PubMed/NCBI
|
|
30
|
Luo P, Fei J, Zhou J and Zhang W:
microRNA-126 suppresses PAK4 expression in ovarian cancer SKOV3
cells. Oncol Lett. 9:2225–2229. 2015.PubMed/NCBI
|
|
31
|
Guo T, Yu W, Lv S, Zhang C and Tian Y:
miR-302a inhibits the tumorigenicity of ovarian cancer cells by
suppression of SDC1. Int J Clin Exp Pathol. 8:4869–4880.
2015.PubMed/NCBI
|
|
32
|
Fan Y, Fan J, Huang L, Ye M, Huang Z, Wang
Y, Li Q and Huang J: Increased expression of microRNA-196a predicts
poor prognosis in human ovarian carcinoma. Int J Clin Exp Pathol.
8:4132–4137. 2015.PubMed/NCBI
|
|
33
|
Muralidhar GG and Barbolina MV: The
miR-200 Family: Versatile players in epithelial ovarian cancer. Int
J Mol Sci. 16:16833–16847. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu R, Liu F, Li L, Sun M and Chen K:
miR-498 regulated FOXO3 expression and inhibited the proliferation
of human ovarian cancer cells. Biomed Pharmacother. 72:52–57. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wen Z, Zhao S, Liu S, Liu Y, Li X and Li
S: MicroRNA-148a inhibits migration and invasion of ovarian cancer
cells via targeting sphingosine-1-phosphate receptor 1. Mol Med
Rep. 12:3775–3780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi YP, Kim BG, Gao MQ, Kang S and Cho
NH: Targeting ILK and β4 integrin abrogates the invasive potential
of ovarian cancer. Biochem Biophys Res Commun. 427:642–648. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang S and Basson MD: Integrin-linked
kinase: A multi-functional regulator modulating extracellular
pressure-stimulated cancer cell adhesion through focal adhesion
kinase and AKT. Cell Oncol. 31:273–289. 2009.PubMed/NCBI
|
|
38
|
Yoshioka S, King ML, Ran S, Okuda H,
MacLean JA II, McAsey ME, Sugino N, Brard L, Watabe K and Hayashi
K: WNT7A regulates tumor growth and progression in ovarian cancer
through the WNT/β-catenin pathway. Mol Cancer Res. 10:469–482.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oloumi A, Syam S and Dedhar S: Modulation
of Wnt3a-mediated nuclear beta-catenin accumulation and activation
by integrin-linked kinase in mammalian cells. Oncogene.
25:7747–7757. 2006. View Article : Google Scholar : PubMed/NCBI
|