Introduction
The primary aim of treating peripheral nerve
defects, which is also one of the major challenges in regenerating
medicine, is reconstructing continuity of the nerve stumps to
regain nerve conduction and functional recovery. End-to-end
suturing is usually applied for nerve lesions without defect or
with a short gap; however, nerve grafts are essential to
reconstruct long nerve defects. Autologous nerve graft remains the
most effective choice, but it is has some limitations, including
secondary surgery, painful neuroma formation, scarring and limited
donor sites (1,2). In the last few years, cell
transplantation, especially for Schwann cells (SCs), has indicated
its benefits. SCs serve an important role in peripheral nerve
regeneration, and are essential for peripheral nerve development.
It has been reported that transplanted cultured SCs could enhance
axonal regeneration across nerve gaps (3). Nevertheless, clinical SC therapy is
also limited because of secondary surgery, donor site complication
and the difficulties of separation, culture and proliferation in
vitro. Therefore, it is desirable for alternative cells.
Multipotent stem cells, such as neural stem cells and bone marrow
stromal cells, which self-renew and multi-potently differentiate,
may be the ideal alternative cells. They proliferate rapidly in
vitro and differentiate into SC-like cells within specific
substrates (4). However, because
of several disadvantages, including the morbidity of the donor
site, painful procedures and low content, the applications of
mulipotent stem cells are restricted.
Recently, adipose derived stem cells (ADSCs), are
isolated from adipose tissue, have been identified as having
similar phenotype and gene expression profiles to bone marrow
stromal cells (5,6). They are multipotent cells that
differentiate along several mesenchymal tissue lineages, including
adipocytes, osteoblasts, chondrocytes, endothelial cells and
cariomyocytes, and express many cytokines and chemokines. ADSCs
also have many clinical advantages, including anabundant source,
easy and safe accessibility, rapid proliferation and immunological
tolerance. Thus, ADSCs may represent alternative cells to SCs.
However, the interaction of ADSCs with SCs which exist in nerve
stumps remains unknown. It was hypothesized that ADSCs may exert
their efficacy by promoting peripheral nerve regeneration not only
via differentiation into SC-like cells and direct release of growth
factors, chemokines and cytokines, but via indirect modulation of
cellular behavior of SCs. To test this hypothesis, investigated the
influences of ADSCs on proliferation and neurotrophic function of
SCs were investigated using co-culture models in vitro.
Materials and methods
Cell culture
All animal experiments protocols were approved by
the Ethics Committee of China Medical University (Shenyang, China)
and performed according to China Medical University guidelines. A
total of 5male Sprague-Dawley (SD) rats, (age, 8–12 weeks; weight
200–250 g), which were obtained by the Experimental Animal
Department of China Medical University (Shenyang, China) under room
temperature with free access to food and water, were pre-medicated
with Ketalar (50 mg/ml, 0.2 ml/100 g body weight; Jiangsu Hengrui
Medicine Co. Ltd., Lianyungang, China), and sacrificed to harvest
the inguinal fat pad and dissect the adipose tissue (5). The adipose tissue was digested using
0.1% collagenase type I at 37°C for 30 min. Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) (both from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) was added for neutralization, and the stromal vascular
fraction was separated from the floating adipocytes by
centrifugation at 300–500 × g at room temperature for 10 min.
Subsequently, the cells in the stromal vascular fraction were
resuspended and cultured in DMEM supplemented with 10% FBS. A total
of 24 h later, the adherent cells were reserved by changing the
culture medium, and were passaged three times before used
insubsequent experiments.
A total of 6 male SD rats (age, 6 days; weight,
10–14 g), which were obtained by the Experimental Animal Department
of China Medical University under room temperature with free access
to food and water, were pre-medicated with Ketalar (50 mg/ml, 0.2
ml/100 g body weight; Jiangsu Hengrui Medicine Co. Ltd.), and
sacrificed to obtain the sciatic nerves. Briefly, the skin of the
hind legs of the rats were cut, the thigh muscles were slit with
tweezers to expose the sciatic nerves, which were subsequently
removed. The sciatic nerves were sectioned into 2-mm segments and
digested using 0.25% trypsin and 1% type I collagenase at 37°C for
60 min. The suspension was centrifuged at 300–500 × g at room
temperature for 10 min and resuspended in DMEM supplemented with
10% FBS. After cell adherence, Cytosine Arabinoside (C1768;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) solution (10 µM) was
added to the culture medium and cells incubated for 48 h to remove
fibroblasts. DMEM supplemented with forskolin (2 µM) and 10% FBS
was then added for 48 h to promote proliferation. Following this,
purified cells were cultured and passaged at 37°C in an atmosphere
containing 5% CO2, once the cells reached 80%
confluence.
Adipogenic and osteogenic
differentiation of rat ADSCs
To verify the cell multipotent differentiation, rat
ADSCs of three passages were used. After they were grown to 80%
confluence, the cells were cultured for 14 days in adipogenic
induction medium, consisting of DMEM supplemented with 10% FBS,
isobutyl-methylxanthine (0.5 mM), dexamethasone (1 µM), insulin (10
µM) and indomethacin (200 µM), and cultured for 21 days in
osteogenic induction medium, consisting of DMEM supplemented with
10% FBS, dexamethasone (0.1 µM), ascorbate-2-phosphate (50 µM) and
beta-glycerophosphate (10 mM). Following this, Oil-Red O and
Alizarin Red S staining were used to confirm adipogenic and
osteogenic differentiation of the rat ADSCs at room temperature for
14 and 21 days, respectively. Cells were imaged under light
microscopes (Nikon Corporation, Toyko, Japan).
Flow cytometry
Rat ADSCs of three passages were washed three times
with phosphate-buffed saline (PBS) after trypsinization. The cells
were incubated with fluorescein isothiocyanate (FITC)-conjugated
primary antibodies, CD29, CD44, CD45 and CD11, away from light at
room temperature for 20 min. After the cells were washed with PBS,
flow cytometry (FACS Asia; BD Biosciences, Franklin Lakes, NJ, USA)
was used to analyze the samples, the results were analyzed with
WinMDI2.9 (Purdue University Cytometry Laboratories, West
Lafayette, IN, USA).
Cell viability assay of ADSCs
Rat ADSCs of three passages were seeded into 96-well
plates at a cell density of 2×104/ml, and cultured in
DMEM supplemented with 10% FBS. After 1, 2, 3, 4, 5, 6 or 7 days'
culture, cells were incubated with 10 µl Cell Counting kit-8
solution (Beyotime Institute of Biotechnology, Haimen, China) at
37°C for 3 h. The samples were measured spectrophotometrically at a
wavelength of 450 nm with a Microelisa reader (Infinite M200PRO,
Tecan Group Ltd., Männedorf, Switzerland).
Immunocytochemistry
Rat ADSCs of three passages were used and three
experiments were performed for each sample. ADSCs were fixed in 4%
paraformaldehyde and blocked with 5%normal goat serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C for 20
min. Following this, cells were incubated with the following rabbit
monoclonal primary antibodies, all purchased from Abcam (Cambridge,
UK): Anti-nerve growth factor (NGF; 1:100; cat no. ab52918),
anti-glial cell line-derived neurotrophic factor (GDNF; 1:100; cat.
no. ab176564), anti-fibronectin (FN; 1:100; cat. no. ab45688) and
anti-laminin (LN; 1:100; cat. no. ab133645) overnight at 4°C. Cells
were then incubated with FITC-conjugated goat anti-rabbit secondary
antibodies (Origene Technologies, Inc., Beijing, China) at room
temperature for 30 min. DAPI was used to label the cell nuclei, and
then the cells were examined undera fluorescence microscope (IX
71/DP 70, Olympus Corporation, Tokyo, Japan).
ELISA
According to the manufacturer's protocol, ELISA kits
all purchased from Abcam were used to examine the concentrations of
NGF (cat. no. ab193736), GDNF (cat. no. ab213901), FN (cat. no.
ab108850) and LN (cat. no. ab11973) in the supernatants of cultured
medium.
Induction of rat ADSCs into SC-like
cells
Rat ADSCs of three passages were cultured in DMEM
supplemented with β-mercaptoethanol (1 mM) for 24 h, and then
treated with DMEM supplemented with 10% FBS and all-trans-retinoic
acid (35 ng/ml) for 72 h. Cells were washed with PBS and incubated
at 37°C in SC-like cell induction medium, which was DMEM
supplemented with 10% FBS, forskolin (14 µM), platelet-derived
growth factor-AA (5 ng/ml), basic fibroblast growth factor (10
ng/ml) and recombinant human heregulin-β1 (200 ng/ml), for 14
days.
Co-culture of ADSCs and SCs
Rat ADSCs of three passages were seeded into plates
at a density of 2×103/ml. Cells were cultured into
6-well plates for RT-PCR, western blotting and ELISA, into 24-well
plates for immunocytochemistry, and into 96-well plates for cell
viability assays. SCs were seeded into Transwell cell inserts (pore
size, 0.4 µm) at a density of 2×103/ml, and then
incubated at humidified 37°C environment with 5% CO2 for
72 h in DMEM supplemented with 10% FBS. The plate with cell inserts
not seeded with ADSCs or SCs served as control.
Immunocytochemistry for ADSCs of the
co-culture system
ADSCs of the co-culture system which were seeded on
the bottom at a density of 2×103/ml were used for the
experiments. Three experiments were performed for each sample.
Primary antibodies against S100 (rabbit monoclonal, 1:100, cat. no.
ab52642, Abcam) and glial filament acidic protein (GFAP; rabbit
monoclonal, 1:100, cat. no. ab68428, Abcam) were added after cell
fixing in 4% paraformaldehyde and blocking with normal goat serum,
and incubated overnight at 4°C. Cells were then incubated at room
temperature for 30 min with FITC-conjugated goat anti-rabbit
secondary antibodies (Origene Technologies, Inc.). DAPI was used to
label the cell nuclei at room temperature for 3 min, and then the
cells were examined with a fluorescence microscope (IX 71/DP 70,
Olympus Corporation).
Western blotting
ADSCs of the co-culture system which were seeded on
the bottom were used for the experiments. Proteins were extracted
using a Nuclear and Cytoplasmic Protein Extraction Kit (P0028,
Beyotime Institute of Biotechnology, Haimen, China) and detected by
BCA Protein Assay Kit (P0009, Beyotime Institute of Biotechnology).
Protein extracts (50 µg per lane) were resolved using 10% SDS-PAGE
and electrophoretically transferred onto polyvinylidene difluoride
(PVDF) membranes. Then PVDF membranes were incubated with PBS
containing 5% nonfat milk and 0.1% Tween 20 at room temperature for
1 h, and blocked with 2.5% normal bovine serum at room temperature
for 1 h. Following this, the membranes were probed with primary
antibodies againstanti-S-100 (rabbit monoclonal; 1:1,000; cat. no.
ab52642; Abcam), GFAP (rabbit monoclonal; 1:1,000; cat. no.
ab68428; Abcam) and GAPDH (rabbit polyclonal; 1:2,500; cat. no.
ab9485; Abcam) at 4°C overnight. The membranes were then incubated
with a horseradish peroxidase-conjugated secondary antibody (cat.
no. A0208; 1:5,000; Beyotime Institute of Biotechnology) at room
temperature for 1 h, following three washes with TBS and 0.1%
Tween-20. The immuno blots were detected using Enhanced
Chemiluminescence western blotting reagents (Beyotime Institute of
Biotechnology) after washing, and the image was scanned with a
GS800 Densitometer Scanner.
Cell viability assay of SCs of the
co-culture system
SCs of co-culture system were seeded into 96-well
plates and cultured in DMEM supplemented with 10% FBS. After 1–7
days of culture, cells were incubated with 10 µl CCK-8 at 37°C for
3 h. The samples were measured spectrophotometrically at 450 nm
with an Infinite M200PRO Microelisa reader (Infinite M200PRO).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total cellular RNA was isolated from ADSCs using RN
easy Kit (cat. no. 15596026; Invitrogen; Thermo Fisher Scientific,
Inc.), then a reverse transcription reaction was performed using a
Reverse Transcription Kit (cat. no. K1691; Invitrogen; Thermo
Fisher Scientific, Inc.). The PCR reaction was performed with the
following thermocycling conditions: 94°C for 45 sec, 58°C for 35
sec and 72°C for 40 sec for a total 30 cycles. The primer sequences
for NGF, GDNF, FN and LN and β-actin used in this study are
presented in Table I. Image Pro
Plus 7.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used for
densitometry.
 | Table I.Primer sequencesin reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequencesin reverse
transcription-polymerase chain reaction.
| Gene | Sequence (5′-3′) |
|---|
| NGF-F |
CCTCTTCGGACACTCTGGATTT |
| NGF-R |
TCCGTGGCTGTGGTCTTATCT |
| GDNF-F |
CAGAGGGAAAGGTCGCAGAG |
| GDNF-R |
ATCAGTTCCTCCTTGGTTTCGTAG |
| FN-F |
AAGCTACCATTCCAGGCCAC |
| FN-R |
GCTCATCTCCTTCCTCGCTC |
| LN-F |
ACAGATTGGCTAAGACCGCA |
| LN-R |
ACATCTCAGGCCCCTTCTCT |
| β-actin-F |
TCCTGTGGCATCCACGAAACT |
| β-actin-R |
GGAGCAATGATCTTGATCTTC |
Statistical analysis
All data are expressed as the mean ± standard
deviation. One-way analysis of variance followed by Tukey's post
hoc test was used to analyse the differences between groups.
Statistical analyses were conducted with SPSS13.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
significant difference.
Results
Identification of rat ADSCs
Rat ADSCs isolated from adipose tissue were adhered
after 3,4 h, and the suspended cells were removed after 24 h.
Spindle cells presented proliferation from 3 days and colony
formation from 5 days, and were passaged after 7 days when they
reached 80–90% confluence. Rat ADSCs of 3 passages were used for
further experiments. Rat ADSCs presented large, flat and mono-layer
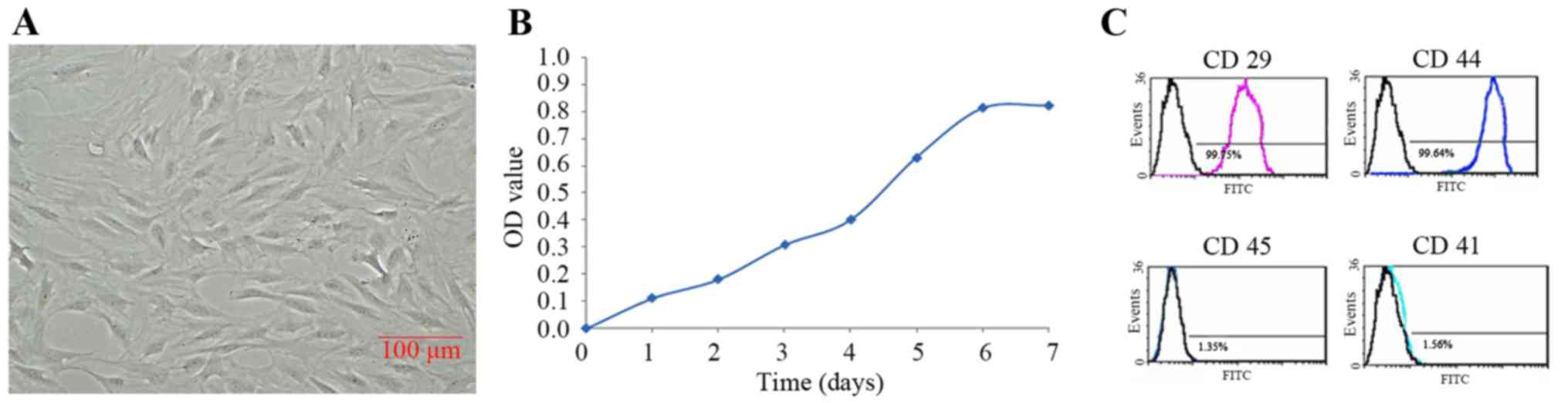
cells, arranged in bundles or whorls (Fig. 1A). Cell viability assay indicated
that there was a lag phase of cell proliferation within 48 h, but
expanded rapidly from 3 days and appeared peak at 6 days (Fig. 1B). Flow cytometry demonstrated that
rat ADSCs of passage 3 were CD29 and CD44 positive, but CD45 and
CD11 negative (Fig. 1C). To verify
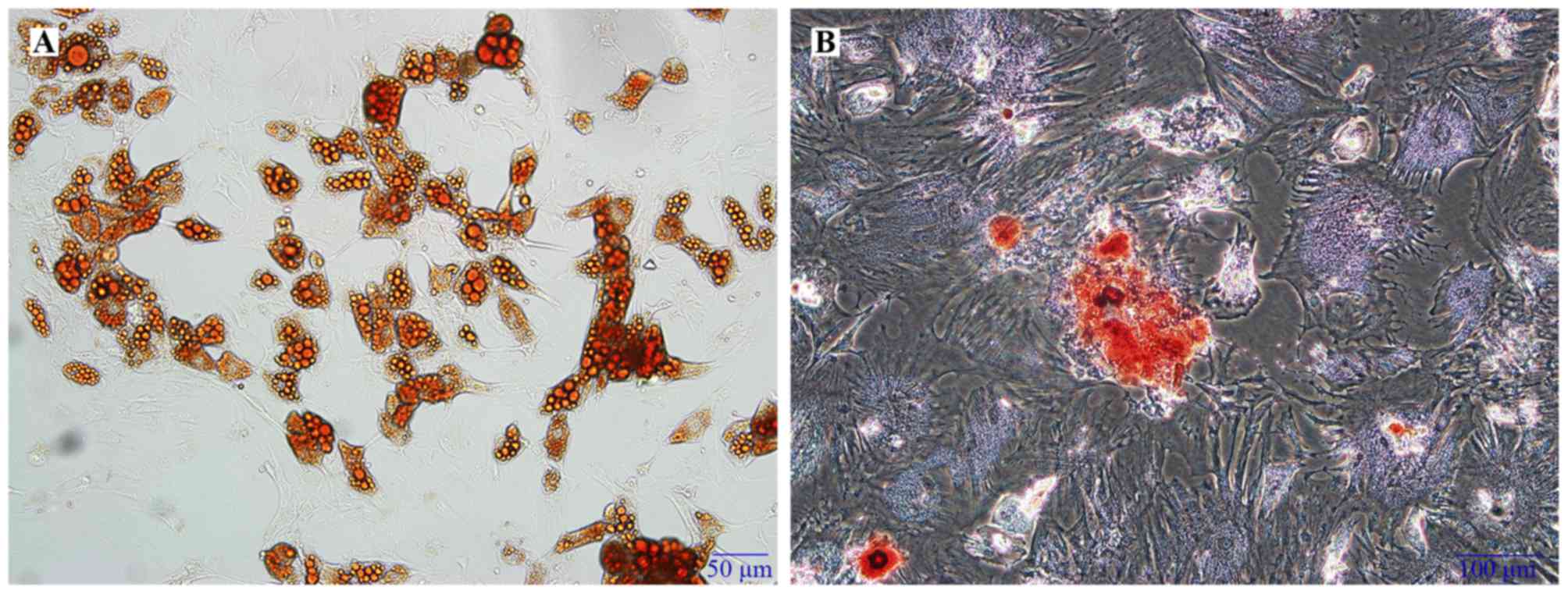
the cell multipotent differentiation, adipogenic differentiation
was verified by Oil-Red O staining which was demonstrated
intracellular lipid droplets (Fig.
2A), while osteogenic differentiation was confirmed by Alizarin
Red S staining, which demonstrated calcium deposits (Fig. 2B).
Secretion function of ADSCs
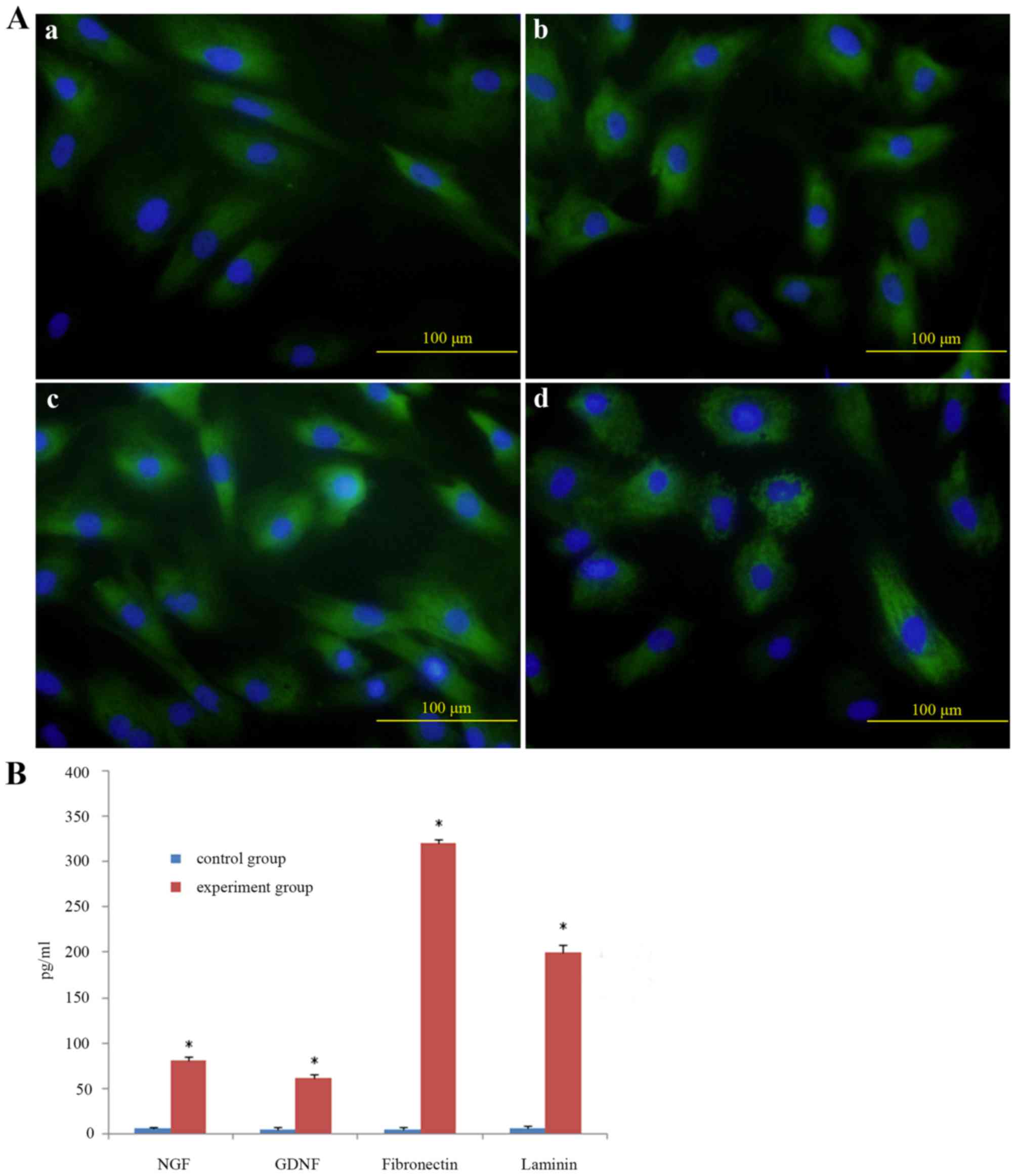
To determine the secretion function of rat ADSCs,
neurotrophic factors such as NGF, GDNF, FN and LN were detected by
immunocytochemistry. It demonstrated that ADSCs were positive for
NGF, GDNF, FN and LN (Fig. 3A).
ELISA revealed that NGF, GDNF, FN and LN in the supernatants of
cultured medium were significantly higher than those cultured alone
(Fig. 3B), which was consistent
with immunocytochemistry results.
Induction of rat ADSCs into SC-like
cells
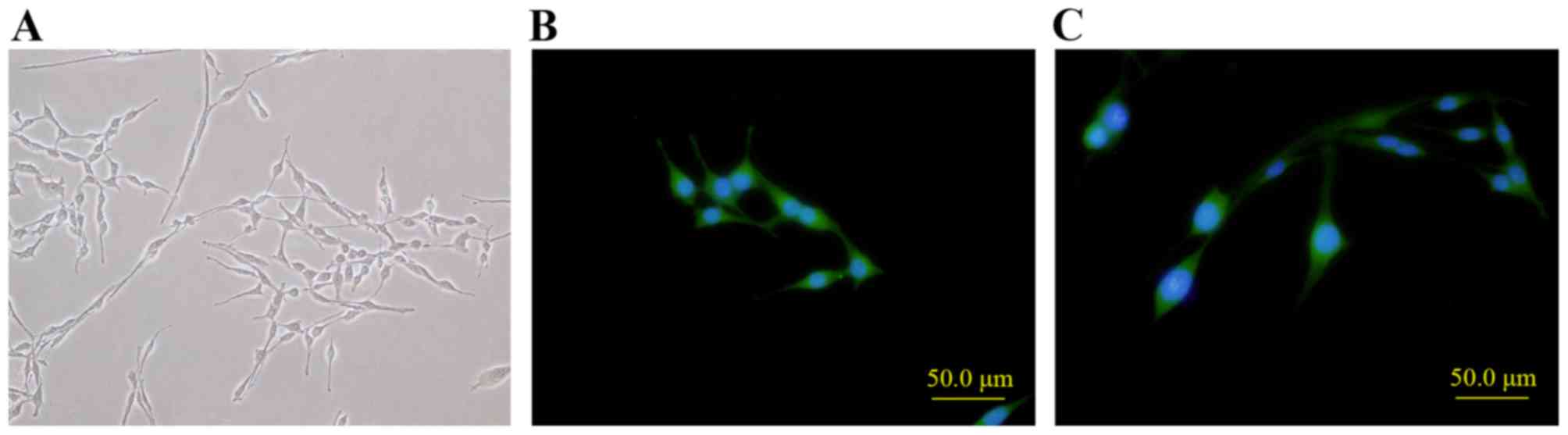
After incubation in SC-like cell induction medium
for 14 days, rat ADSCs of 3 passages which were large and flat were
observed as smaller, spindle, bipolar and with long protrusions on
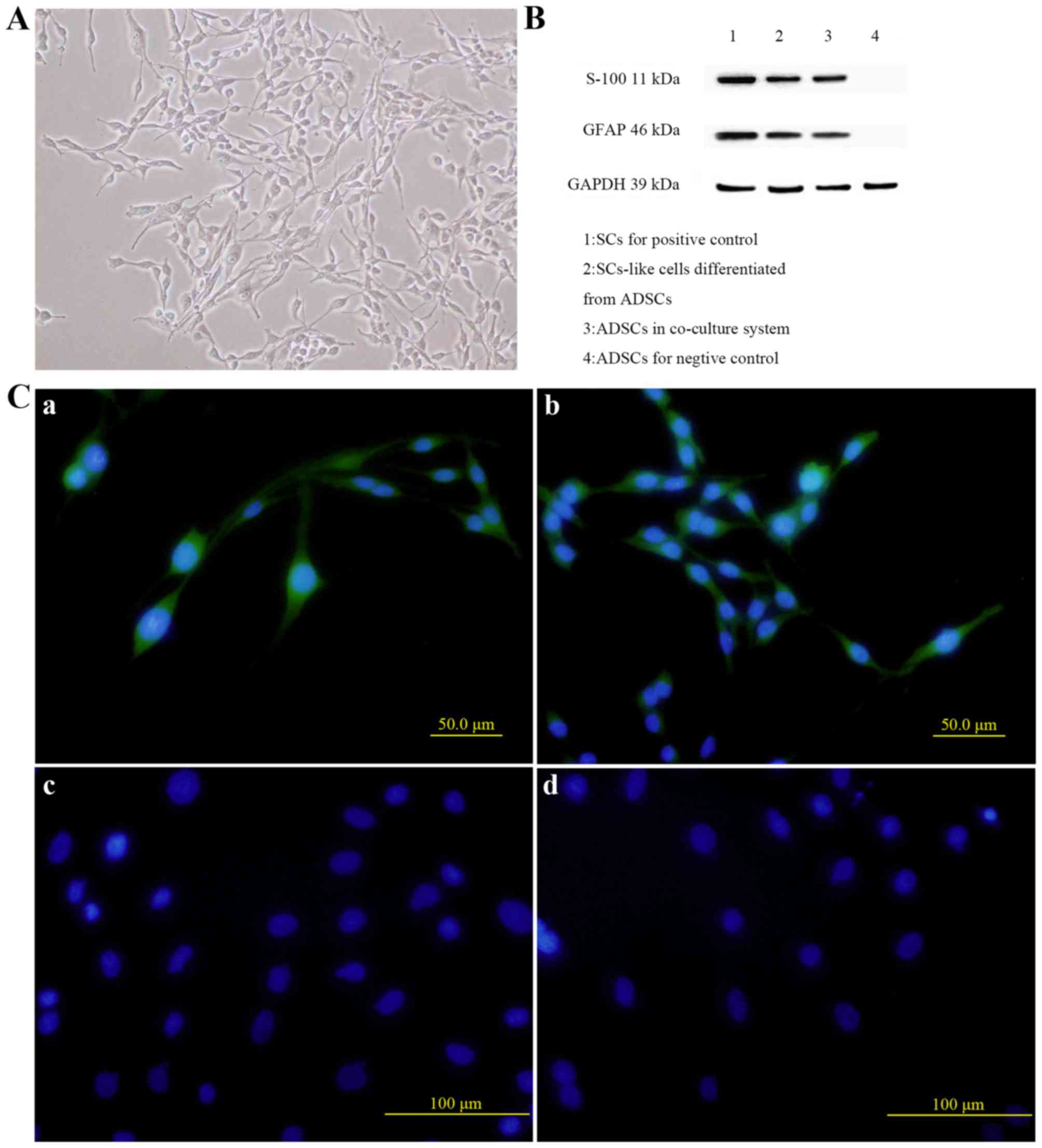
two ends, which is similar to SC morphology (Fig. 4A). Also, the SCs-like cells could
proceed to proliferation. To determine the SCs-like property, SC
markers S100 and GFAP were detected with immunocytochemistry. It
demonstrated that S100 and GFAP were positive in SC-like cells
(Fig. 4B and C, respectively), but
negative in rat ADSCs of 3 passages which were served as the
control (data not shown).
Co-culture of ADSCs and SCs
To investigate the influences of ADSCs and SCs on
each other, a co-culture model in vitro was applied. Rat
ADSCs changed to SCs-like morphology which were smaller, spindle,
bipolar and with long protrusions on two ends after 14 days of
co-culture (Fig. 5A). Western blot
analysis demonstrated that protein expression levels of GFAP and
S100 in ADSCs co-cultured with SCs for 14 days were significantly
higher than those in ADSCs cultured alone (Fig. 5B). Immunocytochemistry shared
consistent results with western blot analysis (Fig. 5C), confirming that rat ADSCs could
be induced into SC-like cells in vitro in a co-culture
system, compared with the control group.
Cell viability assay indicated that the cell
viability of SCs co-cultured with ADSCs for 3, 4, 5, 6 and 7 days
was significantly higher than those cultured alone (Fig. 6A). NGF, GDNF, FN and LN levels in
the supernatants of cells in the co-culture system were
significantly higher compared with cells cultured alone (Fig. 6B), as ELISA revealed. RT-PCR
demonstrated that mRNA expression levels of neurotrophic factors
(NGF, GDNF) and extracellular matrix components (FN, LN) in SCs
co-cultured with ADSCs for 14 days were significantly higher than
those in SCs cultured alone (Fig.
6C). These findings suggested that a higher level of
neurotrophic factors and expression levels of extracellular matrix
components were present in the co-culture system.
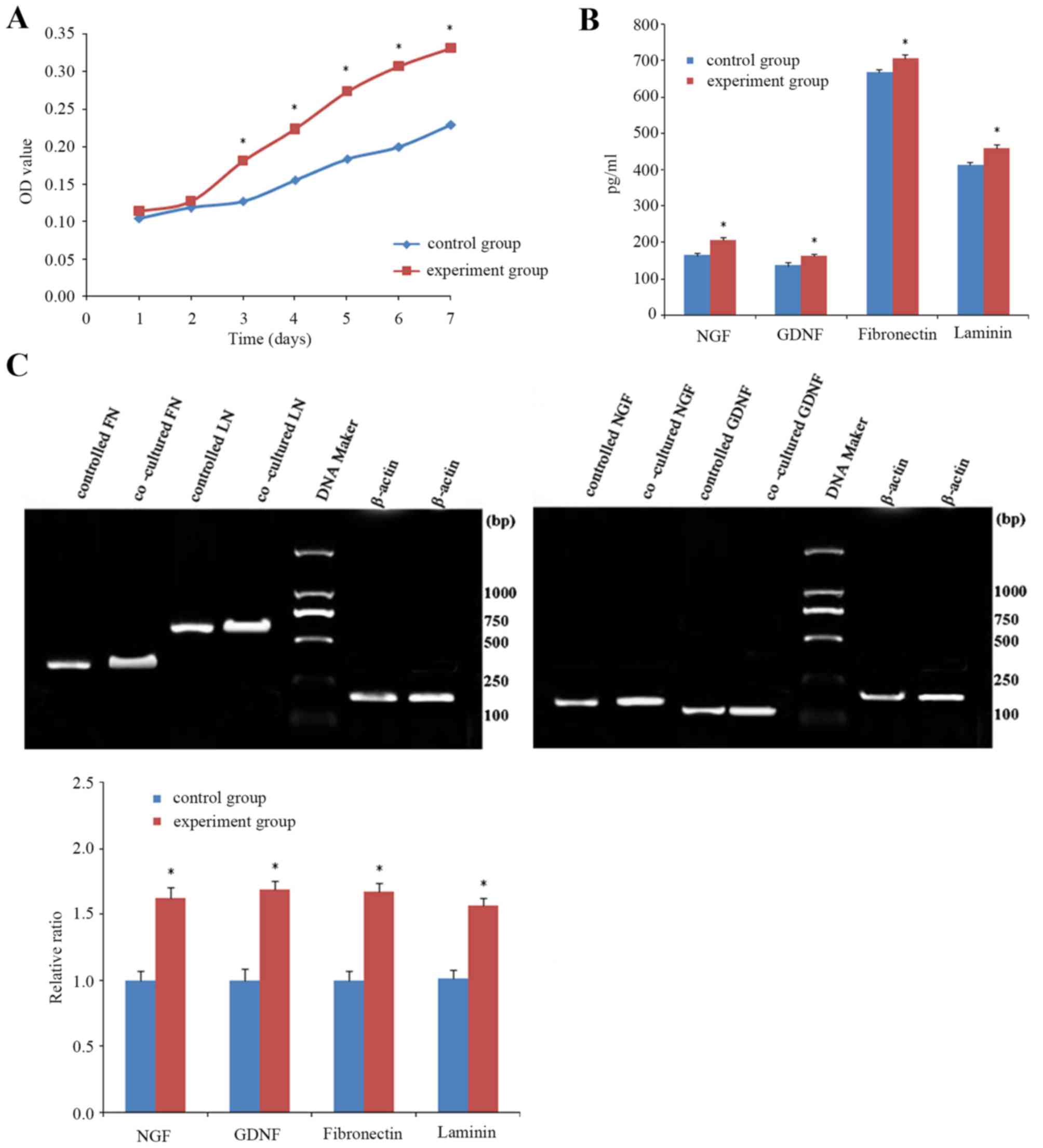 | Figure 6.SCs in the co-culture system. (A) Cell
viability assay indicated that the cell viability of SCs
co-cultured with ADSCs for 3, 4, 5, 6 and 7 days was significantly
higher than those cultured alone. (B) Secretion of neurotrophic
factors in the co-culture system. NGF, GDNF, FN and LN in the
supernatants of SCs in the co-culture system were significantly
higher than in SCs cultured alone, as ELISA revealed. (C) Reverse
transcription-polymerase chain reaction demonstrated that mRNA
expression levels of neurotrophic factors (NGF, GDNF) and
extracellular matrix components (FN, LN) in SCs co-cultured with
ADSCs for 14 days were significantly higher than those in SCs
cultured alone. Data are expressed as the mean ± standard
deviation. *P<0.01 vs. control group. FN, fibronectin; LN,
laminin; NGF, nerve growth factor; GDNF, glial cell line-derived
neurotrophic factor; SC, Schwann cell; ADSCs, adipose derived stem
cells; OD, optical density. |
Discussion
For long nerve defects, the autologous nerve graft
is currently the best treatment option due to intrinsic SCs and
extracellular matrix proteins. They could provide essential
neurotrophic factors and structural support for peripheral nerve
regeneration. Various studies have demonstrated that SCs could
enhance axonal regeneration across nerve gaps by means of clearing
the degenerated axon, proliferating greatly, secreting neurotrophic
factors and providing ideal microenvironment (7–9).
However, clinical SC therapy has its limitations because of the
need for secondary surgery, sacrifice of another nerve and the
donor site complication. Furthermore, it is complextoisolate,
culture and SCs. An attractive alternative option of SCs may be
multipotent stem cells, as many studies have demonstrated (10–12),
such as neural stem cells, hair follicular stem cells and bone
marrow stromal cells, which could self-renew and multi-potently
differentiate. Although they canproliferate in vitro rapidly
and differentiate into SC-like cells within specific substrates,
their applications are also restricted because of their
disadvantages: The morbidity of donor site, painful procedures and
low content.
ADSCs have been used reconstructive medicine in
recent years, and they are a more attractive source for tissue
engineering compared with other adult stem cells. ADSCs have an
abundant source, are easy to isolate from fat tissue and inject
immediately post-isolation, are able to self-renew with a high
growth rate, leave limited donor site morbidity and possess
multi-potent differentiation properties when grown in
lineage-specific induction medium (13–15).
Previous reports, and the present study, indicated that ADSCs could
differentiate into SC-like cells in specific medium, as well as
when co-cultured with SCs, and could secrete neurotrophic factors
(NGF, GDNF) and extracellular matrix components (FN, LN), which are
essential factors for nerve regeneration (3,16–20).
The present study co-cultured ADSCs and SCs, and
compared the results with SCs cultured alone. The results
demonstrated that following co-culture, more neurotrophic factors
and extracellular matrix components were released, and ADSCs could
promote SC proliferation and survival and enhance neurotrophic
factor expression via released soluble molecules, implicating the
neurotrophic active effects and potential to promote peripheral
nerve regeneration of ADSCs.
An important factor to be considered is the
differentiation state of the applied stem cells. According to the
present study, ADSCs could be transplanted into the damaged
peripheral nerve directly to promote peripheral nerve regeneration,
and avoid the culture and differentiation in vitro.
Therefore, the clinical application could be simplified by limiting
treatment to one surgical procedure, saving time and costs.
Additionally, operating complications are reduced to a minimum.
This prediction should be verified by more additional experiments
in the future.
Neurotrophic factors and the extracellular matrix
serve as trophic and supporting factors that serve a vital role in
peripheral nerve development and regeneration, especially for long
nerve regeneration, which has been verified by previous studies
(21,22). They may stimulate neuritis
outgrowth and the proliferation and migration of SCs, and provide a
preferable microenvironment for peripheral nerve regeneration.
Thus, in addition to SC-like cell differentiation, an alternative
possibility for their beneficial effect is the production of
neurotrophic factors and extracellular matrix components by
co-cultured ADSCs. In the present study, cultured ADSCs were
identified to be able to synthesize and release neurotrophic
factors such as NGF and GDNF, as well as to produce etxacellular
matrix proteins such as FN and LN in vitro, which might
partly explain the promoting effects of ADSCs to peripheral nerve
regeneration.
Furthermore, ADSCs and SCs have positive effects on
each other; ADSCs promote resident SCs proliferating and releasing
more neurotrophic factors, while SCs promote ADSCs to differentiate
into SC-like cells. The present study demonstrated that even if the
transplanted ADSCs couldn't differentiate into SC-like cells, they
could promote peripheral nerve regeneration by providing a suitable
microenvironment for resident SCs of nerve stumps. However, the
function of ADSC-differentiated SC-like cells remains
controversial.
In conclusion, the results of the present study
demonstrated that following co-culture, more neurotrophic factors
and extracellular matrix components were released, and ADSCs could
promote SC proliferation and survival and enhance neurotrophic
factor expression via released soluble molecules. These results
suggested that transplantation of undifferentiated ADSCs may have a
potential positive effect on peripheral nerve regeneration.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 51272286).
References
|
1
|
Yoshii S, Oka M, Shima M, Taniguchi A and
Akagi M: 30 mm regeneration of rat sciatic nerve along collagen
filaments. Brain Res. 949:202–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu J, Zhu QT, Liu XL, Xu YB and Zhu JK:
Repair of extended peripheral nerve lesions in rhesus monkeys using
acellular allogenic nerve grafts implanted with autologous
mesenchymal stem cells. Exp Neurol. 204:658–666. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mantovani C, Terenghi G and Shawcross SG:
Isolation of adult stem cells and their differentiation to Schwann
cells. Methods Mol Biol. 916:47–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hou SY, Zhang HY, Quan DP, Liu XL and Zhu
JK: Tissue-engineered peripheral nerve grafting by differentiated
bone marrow stromal cells. Neuroscience. 140:101–110. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heo JS, Choi Y, Kim HS and Kim HO:
Comparison of molecular profiles of human mesenchymal stem cells
derived from bone marrow, umbilical cord blood, placenta and
adipose tissue. Int J Mol Med. 37:115–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fansa H and Keilhoff G: Comparison of
different biogenic matrices seeded with cultured Schwann cells for
bridging peripheral nerve defects. Neurol Res. 26:167–173. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mosahebi A, Woodward B, Wiberg M, Martin R
and Terenghi G: Retroviral labeling of Schwann cells: In vitro
characterization and in vivo transplantation to improve peripheral
nerve regeneration. Glia. 34:8–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rutkowski GE, Miller CA, Jeftinija S and
Mallapragada SK: Synergistic effects of micropatterned
biodegradable conduits and Schwann cells on sciatic nerve
regeneration. J Neural Eng. 1:151–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keilhoff G, Goihl A, Langnäse K, Fansa H
and Wolf G: Transdifferentiation of mesenchymal stem cells into
Schwann cell-like myelinating cells. Eur J Cell Biol. 85:11–24.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY,
Wu CW, Wang CC, Wang WY, Huang YS and Hsu SH: Transplantation of
bone marrow stromal cells for peripheral nerve repair. Exp Neurol.
204:443–453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caddick J, Kingham PJ, Gardiner NJ, Wiberg
M and Terenghi G: Phenotypic and functional characteristics of
mesenchymal stem cells differentiated along a Schwann cell lineage.
Glia. 54:840–849. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Strem BM, Hicok KC, Zhu M, Wulur I,
Alfonso Z, Schreiber RE, Fraser JK and Hedrick MH: Multipotential
differentiation of adipose tissue-derived stem cells. Keio J Med.
54:132–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guilak F, Lott KE, Awad HA, Cao Q, Hicok
KC, Fermor B and Gimble JM: Clonal analysis of the differentiation
potential of human adipose-derived adult stem cells. J Cell
Physiol. 206:229–237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schäffler A and Büchler C: Concise review:
Adipose tissue-derived stromal cells-basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomita K, Madura T, Sakai Y, Yano K,
Terenghi G and Hosokawa K: Glial differentiation of human
adipose-derived stem cells: Implications for cell-based
transplantation therapy. Neuroscience. 236:55–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu JH, Ji YH, Dhong ES, Kim DH and Yoon
ES: Transplantation of adipose derived stem cells for peripheral
nerve regeneration in sciatic nerve defects of the rat. Curr Stem
Cell Res Ther. 7:347–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tomita K, Madura T, Mantovani C and
Terenghi G: Differentiated adipose-derived stem cells promote
myelination and enhance functional recovery in a rat model of
chronic denervation. J Neurosci Res. 90:1392–1402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun F, Zhou K, Mi WJ and Qiu JH: Combined
use of decellularized allogeneic artery conduits with autologous
transdifferentiated adipose-derived stem cells for facial nerve
regeneration in rats. Biomaterials. 32:8118–8128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kokai LE, Rubin JP and Marra KG: The
potential of adipose-derive dadult stem cells as a source of
neuronal progenitor cells. Plast Reconstr Surg. 116:1453–1460.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Midha R, Munro CA, Dalton PD, Tator CH and
Shoichet MS: Growth factor enhancement of peripheral nerve
regeneration through a novel synthetic hydrogel tube. J Neurosurg.
99:555–565. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mligiliche N, Endo K, Okamoto K, Fujimoto
E and Ide C: Extracellular matrix of human amnion manufactured into
tubes as conduits for peripheral nerve regeneration. J Biomed Mater
Res. 63:591–600. 2002. View Article : Google Scholar : PubMed/NCBI
|