Introduction
Vascular dementia (VD) is a syndrome that frequently
occurs following brain atherosclerosis or stroke, and is an
intellectual disorder resulting from various cerebrovascular
diseases (1,2). VD primarily manifests as dysfunction
of memory and cognition, accompanied with motor, language, visual
or personality disorders (3,4). The
pathogenesis of VD is associated with neuronal degeneration,
apoptosis or necrosis resulting from ischemic brain injury
(5,6). Currently, no effective method has
been developed to treat VD. Butylphthalide (NBP) is a lipid-soluble
drug that has previously been demonstrated to target multiple areas
of the brain to induce protective effects. Various pharmacological
functions that NBP exhibits includes shrinking focal ischemic
lesions, improving microcirculation, elevating blood flow at the
ischemic region and decreasing effects of neurological damage
(7,8). L- and D-type isomers of NBP have
differing effects, as D-NBP may antagonize the L-NBP function in
treating neuron degeneration, whereas L-NBP has potent
anti-ischemic and anti-VD effects in the brain, however the
underlying functional mechanism remains to be fully elucidated
(9,10).
Brain-derived neurotrophic factor (BDNF)) is
distributed in the central nervous system. During neural
development, it improves the pathological status of neurons,
facilitates their survival and differentiation, and protects
against injury. It may additionally modulate neuronal functions via
multiple signal transduction pathways. BDNF may affect learning and
memory functions in a synergistic manner with the
N-methyl-D-aspartate receptor (NMDAR) (11,12).
Tyrosine kinase receptor B (TrkB) is important during neuronal
differentiation, growth and development and may alleviate neuronal
injury. Following brain ischemia, cell apoptosis is associated with
signal pathways including phosphatidyl inositol-3/serine-threonine
protein kinase (PI3K/Akt) and mitogen activated protein kinase,
exerting a protective role (13,14).
The present study established a rat VD model via bilateral ligation
of common carotid arteries, and observed the effect of L-NBP on
neurological functions and hippocampal BDNF and TrkB expression
levels, in an attempt to investigate the underlying mechanism of
the protective effects of L-NBP against VD.
Materials and methods
Experimental animals
A total of 90 healthy male Sprague-Dawley rats (6
months old, body weight 240–260 g) were purchased from Laboratory
Animal Center, Chinese Medicine Academy (Beijing, China)
(certificate no. SYXK-2013-0025), and were housed separately in a
specific-pathogen-free grade facility at 23±1°C with 50±20%
relative humidity. All rats had free access to food and water ad
libitum and kept on a 12:12 h light:dark cycle. Animals were
randomly divided into sham, model and L-NBP groups (n=30 each).
Following establishment of the VD model, the L-NBP group received
drugs via gastric perfusion (10 mg/kd/day) for 3 weeks at 1 ml/100
g volume. An equal volume of vegetable oil was administered to sham
and model groups. The present study was approved by the ethics
committee of Jilin University (Changchun, China).
Drugs and reagents
L-NBP (99% purity; Shijiazhuang Pharmaceutical Group
Co., Ltd., Hebei, China) was diluted in vegetable oil at 10%
working concentration. Chloral hydrate and paraformaldehyde were
purchased from Kemiou Chem (http://public.company.lookchem.cn/) (Tianjin, China).
Rabbit anti-BDNF, rabbit anti-Akt1/2 and rabbit anti-NMDAR
antibodies were purchased from Boster Biological Technology
(Pleasanton, CA, USA). TRIzol® reagent and reverse
transcription kit were provided by Invitrogen; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody was
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Primers were provided by Invitrogen; Thermo Fisher Scientific, Inc.
Morris water maze apparatus (Model DMS-2) was provided by
Pharmacological Institute, Chinese Medicine Academy (Beijing,
China).
Animal model preparation
A Morris water maze test was firstly performed to
screen out 110 rats with normal learning and memory functions. A
total of 90 were randomly selected to generate a VD model using
bilateral ligation of common carotid artery, as previously
described (15). In brief,
bilateral common carotid artery (2-VO) was permeant ligated in two
surgeries with a 72 h time interval. Prior to each surgery, rats
fasted for 8 h. Following anesthesia with 10% chloral hydrate, rats
were laid in the supine position, with a middle incision made in
the neck. The bilateral common carotid artery was separated
carefully to leave the nerves intact. Double ligations were
performed using surgical silks. A total of 30 rats in the sham
group received only vessel separation and not ligation. During
surgery, rectal temperature of rats was kept at 36.5–37.5°C.
Penicillin sodium was applied at the focal region to prevent
infection, via intramuscular injection (200,000 units) daily for 3
days. A total of 6 weeks following surgery, the Morris water maze
was used to select 60 rats for the VD model group. The judgement
criteria were: (averaged escape latency-escape latency in control
group)/escape latency of target group >20%. These animals were
randomly divided into model and L-NBP groups (n=30), the latter of
which received L-NBP (10 mg/kg) via gastric perfusion for three
weeks (1 ml/100 g volume). Sham and model groups received equal
volumes of vegetable oil.
Behavioral test
The water-maze test included navigation and
exploration sessions. The water temperature was maintained at
20±2°C and water depth was 30 cm. During the navigation test, rats
were trained for 5 consecutive days and the escape latency from
entering the pool to climbing onto the platform was recorded. In
the navigation task, swimming path and times of crossing the
original platform within 2 min were recorded.
Hematoxylin and eosin (H&E)
staining for brain tissue pathology
Rats from all groups were anesthetized with 10%
chloral hydrate and brain tissue was fixed in 4% paraformaldehyde
at 4°C overnight. Rats were then rapidly decapitated followed by
removal of the cerebellum, olfactory bulb and lower brain stem.
Brain sections from 4 mm posterior of the chiasm toward the
cerebellum were embedded in paraffin by RM2126 microtome and were
stained using the H&E method with staining in hematoxylin
solution for 8 min and counterstaining in eosin solution for 1 min
at 23°C. A light field microscope was used to observe tissue
morphology.
Semi-quantitative polymerase chain
reaction (semi-qPCR) for BDNF and TrkB mRNA level in brain
tissues
Total RNA was extracted from brain tissues, and
reverse transcription used to obtain cDNA (High-Capacity cDNA
Reverse Transcription kit; Thermo Fisher Scientific, Inc.). UV
spectrometry was used to quantify DNA concentrations. Using
specific primers (Table I), PCR
was performed, amplified products were analyzed in 1.5% agarose gel
electrophoresis in triplicate and visualized by staining with
ethidium bromide. Gene expression level was expressed as relative
level against β-actin.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Target gene | Sequence (5′→3′) | Fragment length
(bp) |
|---|
| BDNF | Forward:
GTGACAGTAGTAGCGAGTGGG | 278 |
|
| Reverse:
TATCCTTATGAACCGGCA |
|
| TrkB | Forward:
CCAAGAGGCTAAATCCAGTCC | 248 |
|
| Reverse:
CCAGGTTACCAACATGCTAATA |
|
| β-actin | Forward:
GAGACCTACAAGACCCCAGCC | 445 |
|
| Reverse:
TCGGCGCATCGGTACCGCTCA |
|
Western blotting for protein levels of
BDNF, TrkB and Akt
Rat brain tissues were lysed in RIPA lysis buffer
(Thermo Fisher Scientific, Inc.). Proteins were collected by
centrifugation at 13,000 × g for 5 min at 4°C, and were quantified
using a BCA protein assay kit. Protein samples were separated by
12% SDS-PAGE with loading of 20 mg protein per lane, and were
transferred to a polyvinylidene membrane. Following blocking with
5% bovine serum albumin (Thermo Fisher Scientific, Inc.). at room
temperature for 1 h, the membrane was incubated with primary
antibodies against BDNF (1:1,000; catalog no. ab108319; Abcam),
TrkB (1:1,000; catalog no. ab33655; Abcam), Akt (1:2,000; catalog
no. ab18785; Abcam) or β-actin (1:2,000; catalog no. ab8227; Abcam)
at 4°C overnight. Following washing 3 times with Tris buffered
saline-Tween-20, a secondary antibody (1:1,000; catalog no.
65–6120; Thermo Fisher Scientific, Inc.) was added for a 1 h
incubation at room temperature. A chromogenic substrate (1-Step
Ultra TMB-Blotting solution; catalog no. 37574; Thermo Fisher
Scientific, Inc.) was then added for development, followed by
exposure to a dark room. Quantity One software version 4.6.5
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to analyze
protein bands, which were expressed as a relative level against the
internal reference.
Immunohistochemistry for NMDAR protein
expression
Rats were sacrificed and brain tissue extracted.
Following removal of the cerebellum and the olfactory bulb, the
cerebral cortex and hippocampus were collected, fixed with 2%
paraformaldehyde overnight at room temperature, dehydrated through
70, 80, 95% alcohol (5 min each) followed by 3 incubations in 100%
alcohol (5 min each). Subsequently, the tissue was embedded in
paraffin and sliced to 4 µm thickness using a RM2126 microtome. The
slides were deparaffinized and transferred to 100% alcohol 2 times
(3 min each), and then transfered once through 95, 70 and 50%
alcohols respectively for 3 min each, followed by blocking of
endogenous peroxidase activity by incubating sections in 3%
hydrogen peroxide solution in methanol at room temperature for 10
min. Immunohistochemistry staining (Ultra Vision Detection System)
was used to detect NMDAR protein levels using rabbit anti-mouse
NMDAR polyclonal antibody (catalogue number PA1059) (1:1,500)
(incubation at room temperature for 1 h) and biotinylated secondary
antibody (1:200) (catalog no. ab64256, Abcam) (incubation for 10–15
min at room temperature). Following DAB development and
counter-staining in Hematoxylin for 1–2 min at room temperature.
The color of the antibody staining in the tissue sections was
observed under a microscopy (CX31; Olympus, Corporation, Tokyo,
Japan). Image-pro plus version 7.0 software was used to analyze the
images.
Statistical analysis
SPSS software, version 20.0 (IBM Corp., Armonk, NY,
USA) was used to analyze all data, in which measurement data were
firstly tested for normal distribution. Those fitting the normal
distribution were presented as the mean ± standard deviation.
One-way analysis of variance was employed to compare means across
multiple groups, followed by the least significant difference test
in paired comparison. P<0.05 was considered to indicate a
statistically significant difference.
Results
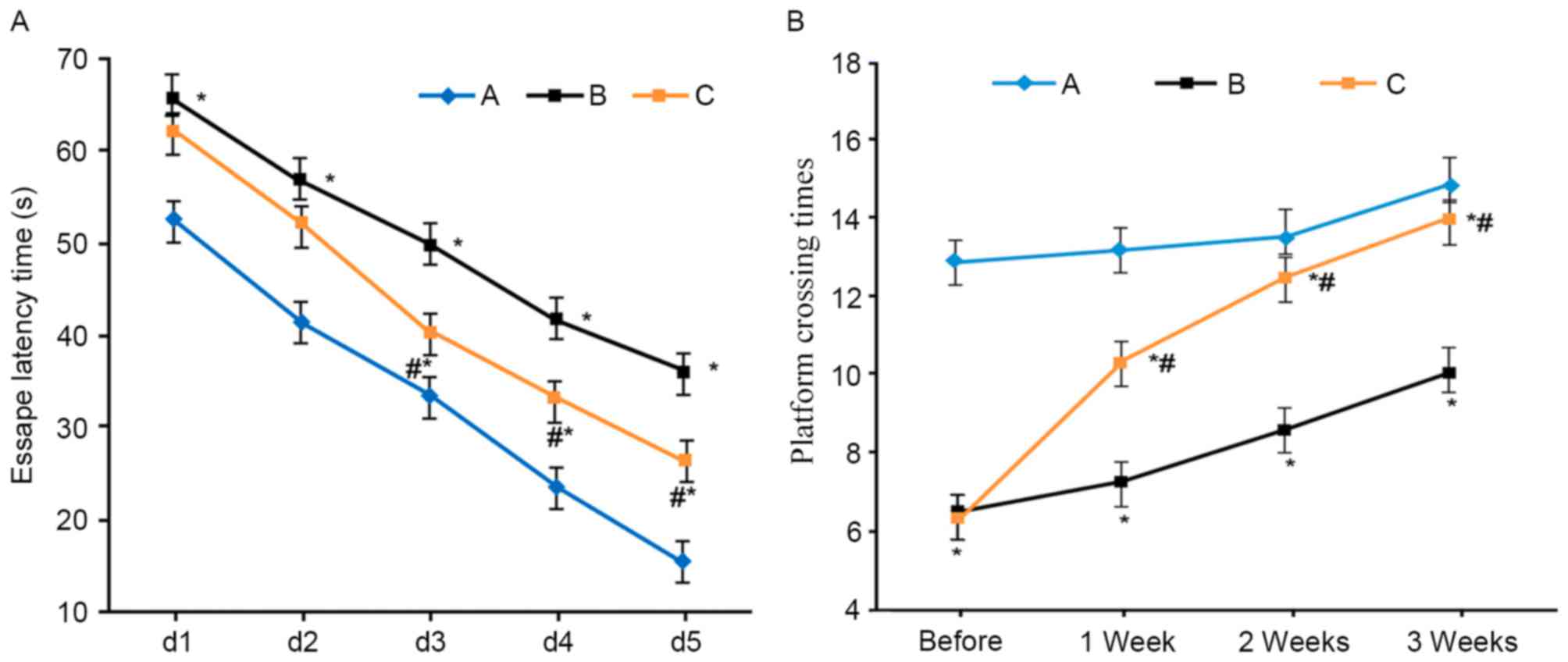
Learning and memory functions of VD
rats
Compared with the sham group, VD model rats
exhibited elongated escape latency and lower platform crossing
times (P<0.05). L-NBP treatment shortened escape latency and
increased platform crossing times (P<0.05 compared with model
group; Fig. 1).
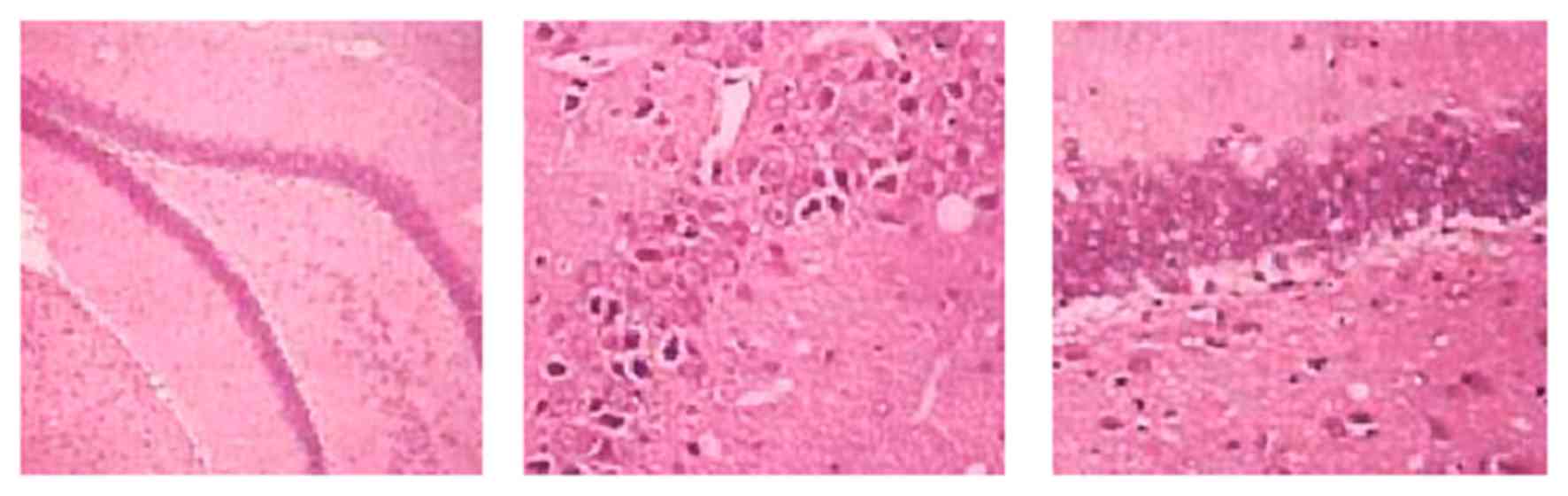
Pathology of VD rat brains
H&E staining results revealed a normal cortical
structure and morphology of sham rats, which had regular
arrangement of hippocampal tissues with intact morphology, high
cell number, normal structure and clear nucleus. VD model rats had
diffused injury in both cortical and hippocampal regions, the
latter of which had irregular distribution of cells, with
incomplete morphology, less cell number, abnormal structure,
condensed nucleus and glial hypertrophy. The L-NBP treatment group
had alleviated injury compared with the model group, as indicated
by less neuron denaturing or necrosis (Fig. 2).
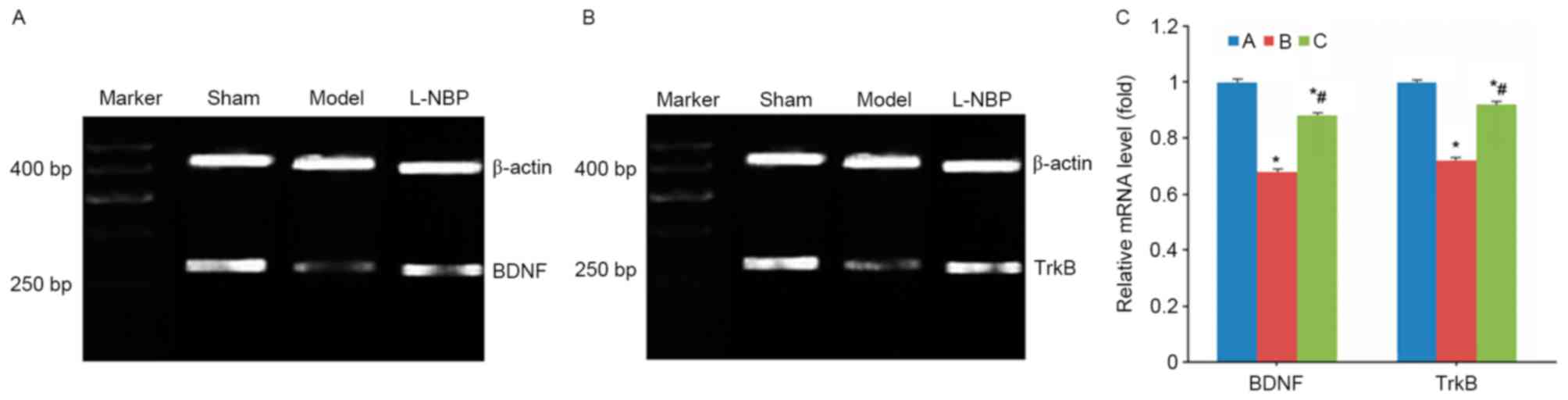
L-NBP increases BDNF and TrkB mRNA
levels in VD rats
VD model rats demonstrated decreased mRNA levels of
BDNF and TrkB (P<0.05 compared with sham group), whereas the
L-NBP group exhibited increased mRNA expression levels of BDNF and
TrkB (P<0.05 compared to model group, Fig. 3).
L-NBP increases BDNF, TrkB and Akt
protein levels in VD rats
Compared with the sham group, model rats
demonstrated decreased protein levels of BDNF, TrkB and Akt
proteins (P<0.05). However, the L-NBP treated group had elevated
expression levels of these proteins in brain tissues (P<0.05
compared with model group, Fig.
4).
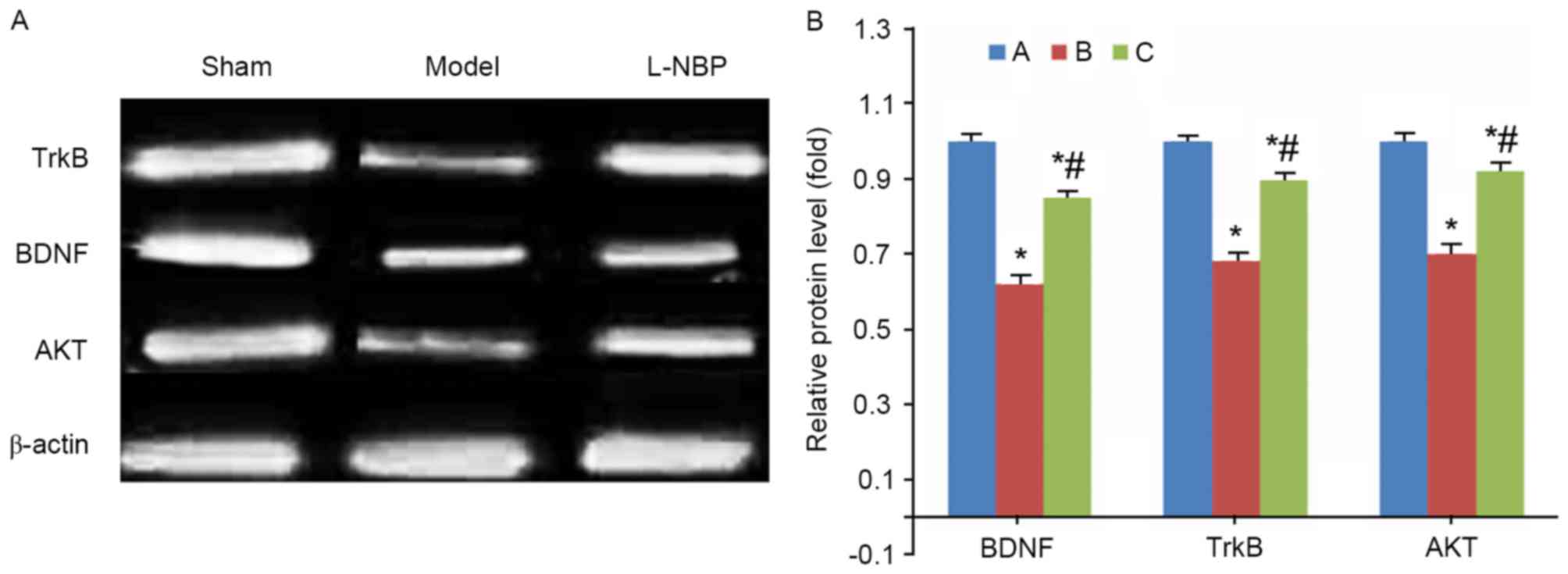 | Figure 4.BDNF, TrkB and Akt protein expression
in rat brain tissues. (A) Representative image and (B) quantitative
analysis revealing fold-increase of BDNF, TrkB and Akt protein
expression levels, detected by western blotting. *P<0.05 vs.
sham group; #P<0.05 vs. model group. A, sham group;
B, model group; C, L-NPB group. BDNF, brain-derived neurotrophic
factor; TrkB, tyrosine kinase receptor B; Akt, serine-threonine
protein kinase; L-NBP, L-butylphthalide. |
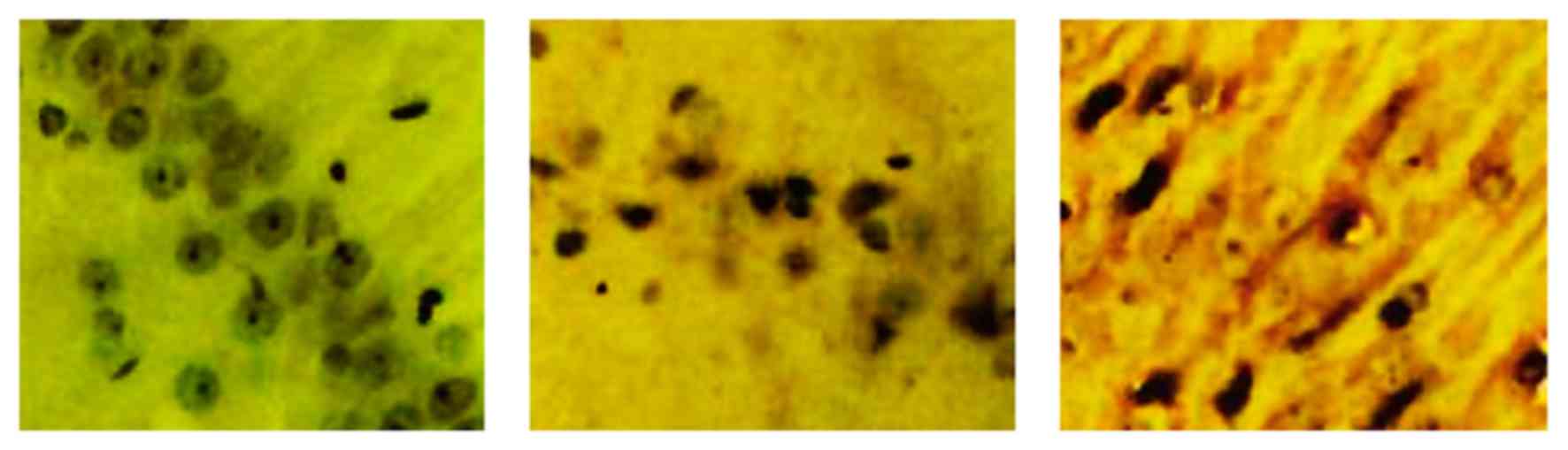
NMDAR protein expression analysis
Model group exhibited a lighter staining of NMDAR in
the hippocampal CA1 regions, compared with the sham group, whereas
the L-NBP group revealed a darker staining compared with the model
group (Fig. 5).
Discussion
The pathological mechanism of VD remains to be fully
elucidated. BDNF and NMDAR are closely associated with the learning
and memory process. Synaptic excitation may lead to BDNF release
from axons via N-type calcium ion channels and intracellular
calcium release (16).
Voltage-gated ion channels and NMDAR may regulate BDNF release; a
neurotrophic factor with a biological function dependant on
specific binding to TrkB. Various studies have demonstrated that
the co-activation of BDNF and its receptor TrkB elevates synaptic
plasticity, facilitates axonal and dendritic growth and increases
synaptic terminal density (17,18).
Exogenous BDNF may additionally decrease brain infarction volume.
BDNF-like immune reactive products are widely distributed in
ipsilateral neocortex neurons. Following brain ischemia, BDNF
expression is largely varied, with a significant suppression of
expression levels in the hippocampus detectable during the first
few days. A total of one-week following the ischemia, BDNF and TrkB
expression levels are elevated, thus protecting neurons, although
such enhanced self-protection does not have a long-term effect
(19,20). It has previously been demonstrated
that L-NBP may facilitate an increase of BDNF mRNA/protein
expression levels in hippocampal regions of VD rats. BDNF mRNA
levels are associated with the severity of ischemia-induced injury.
Under conditions of severe ischemia, BDNF mRNA transcription is
suppressed. Endogenous BDNF may significantly improve ischemia
injury of brain tissues. However, BDNF is unable to easily
penetrate the blood-brain barrier. L-NBP has multiple target sites,
thus elevating its endogenous protective effects (21,22).
L-NBP exhibits an increased potency in its therapeutic effect in
treating acute ischemia brain stroke, compared with D-LBP, which
may antagonize the anti-apoptotic effect of L-NBP. In the present
study, VD model rats demonstrated an elongated escape latency and
lower platform crossing times. L-NBP treatment shortened escape
latency and increased platform crossing times. Pathological
examination of rat brain tissues revealed diffused injury in
cortical and hippocampal regions of model rats, which had abnormal
structure of cortical and hippocampal neural tissues, with
condensed nucleus and glial hypertrophy. L-NBP rats had alleviated
neuron injury, with less necrosis of neurons, indicating that L-NBP
alleviated neurological functions and improved memory/learning
functions. L-NBP therefore resulted in certain neuroprotective
effects on VD, which was consistent with previous studies that
demonstrated that L-NBP may improve the cognitive function in
diabetic rats (23), as well as
the cognitive deficits and neuronal loss in the hippocampus of
cerebral repetitive ischemia/reperfusion mice (24).
NMDAR primarily consists of NR1 and NR2, which are
associated with synaptic plasticity. BDNF participates in long-term
memory formation, and is important in maintaining learning/memory
and regulating synaptic plasticity. The present study revealed
significantly elevated BDNF, TrkB, Akt and NMDAR expression levels
in L-NBP-treated VD rats, compared with model rats, suggesting that
L-NBP facilitated regeneration of neurons and prevented their late
onset apoptosis, via the BDNF-induced phosphatidyl inositol-3/Akt
signaling pathway, thus protecting cognitive functions and synaptic
transmission and protects against neuronal apoptosis. Endogenous
neuroprotective effects have important roles in ischemic brain
tissues. Their initiation, however, is relatively slow without
exogenous stimuli. Administration of exogenous L-NBP may protect
the integrity of neurons, facilitate synaptic plasticity and
improve rat memory and learning. However, the main limitation of
the present study was that a BDNF inhibitor was not administered
under the treatment of L-NBP to confirm whether L-NBP exerts a
protective role in cognitive function in VD rats. This will be the
focus of future investigation.
In conclusion, L-NBP upregulated hippocampal
expression of BDNF, TrkB, Akt and NMDAR in VD rats, thus
alleviating ischemia-induced brain injury and improving learning
and memory functions.
References
|
1
|
Lantz M, Sjöstrand C and Kostulas K:
Ischemic stroke and patent foramen ovale: Risk factors and genetic
profile. J Stroke Cerebrovasc Dis. 22:841–845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Brien JT and Thomas A: Vascular
dementia. Lancet. 386:1698–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yakushiji Y: Cerebral microbleeds:
Detection, associations and clinical implications. Front Neurol
Neurosci. 37:78–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueno M, Chiba Y, Matsumoto K, Murakami R,
Fujihara R, Kawauchi M, Miyanaka H and Nakagawa T: Blood-brain
barrier damage in vascular dementia. Neuropathology. 36:115–124.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Hai S, Zhou Y and Dong BR:
Cholinesterase inhibitors for rarer dementias associated with
neurological conditions. Cochrane Database Syst Rev: CD009444.
2015.
|
|
6
|
Kwon KJ, Kim MK, Lee EJ, Kim JN, Choi BR,
Kim SY, Cho KS, Han JS, Kim HY, Shin CY and Han SH: Effects of
donepezil, an acetylcholinesterase inhibitor, on neurogenesis in a
rat model of vascular dementia. J Neurol Sci. 347:66–77. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin W, Lan L, Huang Z, Ji J, Fang J, Wang
X, Ji H, Peng S, Xu J and Zhang Y: Discovery of a ring-opened
derivative of 3-n-butylphthalide bearing NO/H2S-donating moieties
as a potential anti-ischemic stroke agent. Eur J Med Chem.
115:369–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen XR, Tang M, Qi DS, Huang XJ, Liu HZ,
Zhang F, Wu J, Wang YW, Zhang XB, Guo JQ, et al: Butylphthalide
suppresses neuronal cells apoptosis and inhibits JNK-caspase3
signaling pathway after brain ischemia/reperfusion in rats. Cell
Mol Neurobiol. 36:1087–1095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niu H, Zhang Z, Wang H, Wang H, Zhang J,
Li C and Zhao L: The impact of butylphthalide on the
hypothalamus-pituitary-adrenal axis of patients suffering from
cerebral infarction in the basal ganglia. Electron Physician.
8:1759–1763. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang Y, Sun L, Xuan X and Wang J: Impacts
of N-Butylphthalide on expression of growth factors in rats with
focal cerebral ischemia. Bosn J Basic Med Sci. 16:102–107.
2016.PubMed/NCBI
|
|
11
|
Lee SH, Ko IG, Kim SE, Hwang L, Jin JJ,
Choi HH and Kim CJ: Aqueous extract of Cordyceps alleviates
cerebral ischemia-induced short-term memory impairment in gerbils.
J Exerc Rehabil. 12:69–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sawamoto A, Okuyama S, Yamamoto K, Amakura
Y, Yoshimura M, Nakajima M and Furukawa Y:
3,5,6,7,8,3′,4′-Heptamethoxyflavone, a citrus flavonoid,
ameliorates corticosterone-induced depression-like behavior and
restores brain-derived neurotrophic factor expression,
neurogenesis, and neuroplasticity in the Hippocampus. Molecules.
21:5412016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang M, Yuan Y, Lu J, Li HE, Zhao M, Ling
EA and Wu CY: Scutellarin promotes microglia-mediated astrogliosis
coupled with improved behavioral function in cerebral ischemia.
Neurochem Int. 97:154–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xing M, Sun Q, Wang Y, Cheng Y and Zhang
N: Hydroxysafflor yellow A increases BDNF and NMDARs in the
hippocampus in a vascular dementia rat model. Brain Res.
1642:419–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kato M, Iwata H, Katayama T, Asai H,
Narita H and Endo T: Possible therapeutic effect of T-794, a novel
reversible inhibitor of monoamine oxidase-A, on post-stroke
emotional disturbances, assessed in animal models of depression.
Biol Pharm Bull. 20:349–353. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du R, Teng JF, Wang Y, Zhao XY and Shi ZB:
Clinical study of Butylphthalide combined with Xue Shuan Tong on
serum inflammatory factors and prognosis effect of patients with
cerebral infarction. Pak J Pharm Sci. 28 5 Suppl:S1823–S1827.
2015.
|
|
17
|
Yang LC, Li J, Xu SF, Cai J, Lei H, Liu
DM, Zhang M, Rong XF, Cui DD, Wang L, et al: L-3-n-butylphthalide
promotes neurogenesis and neuroplasticity in cerebral ischemic
rats. CNS Neurosci Ther. 21:733–741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao W, Luo C, Wang J, Gong J, Li B, Gong
Y, Wang J and Wang H: 3-N-butylphthalide improves neuronal
morphology after chronic cerebral ischemia. Neural Regen Res.
9:719–726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao Y, Li J, Zhang P, Chen C and Li S:
Protective effects of dl-3n-butylphthalide against diffuse brain
injury. Neural Regen Res. 8:2615–2624. 2013.PubMed/NCBI
|
|
20
|
Hu J, Wen Q, Wu Y, Li B and Gao P: The
effect of butylphthalide on the brain edema, blood-brain barrier of
rats after focal cerebral infarction and the expression of Rho A.
Cell Biochem Biophys. 69:363–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Diao X, Pang X, Xie C, Guo Z, Zhong D and
Chen X: Bioactivation of 3-n-butylphthalide via sulfation of its
major metabolite 3-hydroxy-NBP: Mediated mainly by sulfotransferase
1A1. Drug Metab Dispos. 42:774–781. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghadernezhad N, Khalaj L, Pazoki-Toroudi
H, Mirmasoumi M and Ashabi G: Metformin pretreatment enhanced
learning and memory in cerebral forebrain ischaemia: The role of
the AMPK/BDNF/P70SK signalling pathway. Pharm Biol. 54:2211–2219.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Zhang S, Zhang L, Wang R and Wang M:
Effects of L-3-n-butylphthalide on cognitive dysfunction and NR2B
expression in hippocampus of streptozotocin (STZ)-induced diabetic
rats. Cell Biochem Biophys. 71:315–322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huai Y, Dong Y, Xu J, Meng N, Song C, Li W
and Lv P: L-3-n-butylphthalide protects against vascular dementia
via activation of the Akt kinase pathway. Neural Regen Res.
8:1733–1742. 2013.PubMed/NCBI
|