Introduction
Colorectal cancer (CRC) is one of the most prevalent
malignant tumors of the digestive tract and >1.2 million
individuals have been diagnosed with CRC, with 600,000 mortalities
reported annually, which severely impairs human survival and health
worldwide (1). Although surgical
resection remains the primary treatment option for CRC,
chemotherapy has become an optimal and unique approach for patients
with advanced-stage CRC who are not surgical candidates,
particularly patients with metastases and those who require
adjuvant treatment to prevent relapse (2–5). As
a frequently used chemotherapeutic drug for CRC (6), 5-fluorouracil (5-FU) can yield
multidrug resistance (MDR) during chemotherapy, which is the
primary cause of chemotherapy failure, and the recurrence and
metastasis of CRC (7,8).
Following acquisition of MDR, the migratory and
adhesive potential of tumor cells is enhanced, which is the leading
cause of metastasis, recurrence and invasion in malignant tumors
(5,9). Epithelial-mesenchymal transition
(EMT) is one of the fundamental modes of metastasis, and is defined
as the biological process through which epithelial cells
differentiate into mesenchymal cells under the stimulation of
specific factors (10,11).
Transforming growth factor-β (TGF-β) is a vital
factor that is responsible for regulating the EMT process (12). TGF-β serves a dual role in
inhibiting and promoting the incidence and progression of malignant
tumors. During the onset of malignant tumors, TGF-β is capable of
inhibiting cancer progression by suppressing cancer cell
proliferation, accelerating cancer cell apoptosis and preventing
the incidence of oncogenic inflammation. In advanced stages, TGF-β
is overexpressed, and instead can accelerate the progression and
metastasis of malignant tumors, by promoting cell metastasis,
immune evasion and angiogenesis through the regulation of EMT
(13–17). With respect to the TGF-β signaling
pathway as a target, inhibiting the TGF-β pathway within tumor
cells can decrease the incidence of EMT, thereby reducing the
production of mesenchymal-like cells and decreasing the incidence
of tumor metastasis (17–19).
Hedyotis diffusa Willd (HDW) belongs to the
Rubiaceae family, and is a traditional Chinese herbal medicine that
can dissipate heat and toxicity, alleviate abscesses and masses,
promote blood flow, and ease pain (20). It has been applied in the treatment
of various inflammation-associated diseases and malignant tumors,
and is proven to possess anticancer effects against CRC and other
malignant tumors, without evident adverse events (21,22).
The authors previously demonstrated that HDW can inhibit
proliferation and angiogenesis, induce apoptosis, and reverse MDR
in CRC cells (23–27). However, the underlying mechanism,
particularly in MDR-associated metastasis, remains to be
elucidated.
To further study the anti-CRC effects and underlying
molecular mechanism of HDW, particularly in terms of MDR-associated
metastasis, the present study used the 5-FU resistant CRC cell line
HCT-8/5-FU as a high-metastasis model (9) to analyze the effect of HDW on the
viability, and migratory and invasive potential of HCT-8/5-FU
cells, and on the regulation of the TGF-β signaling pathway.
Materials and methods
Materials and reagents
RPMI-1640 medium (cat. no. C11875500BT), fetal
bovine serum (FBS; cat. no. 10099-141), penicillin-streptomycin
(cat. no. SV30010), 0.25% trypsin-EDTA (cat. no. 25200-072), Pierce
radioimmunoprecipitation assay buffer (cat. no. 89901), Pierce BCA
Protein Assay kit (cat. no. 23227) and SuperSignal™ West Pico
Chemiluminescent Substrate (cat. no. 34080) were all purchased from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). MTT was obtained
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The BD BioCoat
Matrigel Invasion Chamber was purchased from BD Biosciences (San
Jose, CA, USA). The PrimeScript RT Reagent kit was provided by
Takara Biotechnology Co., Ltd. (Dalian, China). TRIzol reagent was
obtained from Thermo Fisher Scientific, Inc. Anti-neural
(N)-cadherin (cat. no. ab98952) and epithelial (E)-cadherin (cat.
no. ab128804) antibodies were purchased from Abcam (Cambridge, UK).
Anti-TGF-β (cat. no. 3711), Mothers against decapentaplegic homolog
4 (SMAD4; cat. no. 3716) and β-actin (cat. no. 4967) antibodies
were provided by Cell Signaling Technology, Inc. (Danvers, MA,
USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit
secondary antibody (cat. no. E030120) was purchased from EarthOx
Life Science (Millbrae, CA, USA).
Preparation of ethanol extract of HDW
(EEHDW)
EEHDW was prepared as described previously (25). Stock solutions of EEHDW were
prepared by dissolving the EEHDW powder in 100% dimethyl sulfoxide
(DMSO) to a final concentration of 500 mg/ml and stored at −20°C.
The working concentrations of EEHDW were made by diluting the stock
solution in the culture medium. The final concentrations of DMSO in
the medium were <0.5%.
Cell culture
The human colorectal 5-FU resistant cell line
HCT-8/5-FU and its parental cell line HCT-8 were obtained from
Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Cells were
maintained in RPMI-1640 medium containing 10% (v/v) FBS, 100 U/ml
penicillin and 100 g/ml streptomycin, while the HCT/5-FU cells were
cultured with an additional 15 g/ml 5-FU, at 37°C in a humidified
atmosphere containing 5% CO2.
Cell viability evaluation
Cell viability was assessed by MTT assay. HCT-8,
HCT-8/5-FU or HCT-8 cells were seeded into 96-well plates at a
density of 1×104 cells/well in 0.1 ml media and were
treated with various concentrations of 5-FU (0, 25, 50, 100, 200,
400, 800 and 1600 mM) for 48 h. HCT-8/5-FU cells were seeded into
96-well plates at a density of 8×103 cells/well in 0.1
ml medium. Cells were treated with various concentrations (0, 0.5,
1 and 2 mg/ml) of EEHDW for different periods of time. A total of
100 ml MTT (0.5 mg/ml in PBS) was added to each well and the
samples were incubated for an additional 4 h at 37°C. The
purple-blue MTT formazan precipitate was dissolved in 100 µl DMSO.
The absorbance was measured at 570 nm using an ELISA reader
(ELX800; BioTek Instruments, Inc., Winooski, VT, USA). The
resistance index (RI) of the HCT-8/5-FU cells to 5-FU was
calculated by dividing the drug concentration required to inhibit
growth by 50% (IC50) for HCT-8/5-FU cells by the
IC50 value for the parental cells (HCT-8).
IC50 values were determined using nonlinear regression
analysis.
Wound healing assay
HCT-8/5-FU cells were seeded into 6-well plates at a
density of 5×105 cells/well in 2 ml medium. After 24 h
of incubation, cells were scratched vertically in each well using a
P200 pipette tip. A phase-contrast inverted microscope at a
magnification of ×100 was used to observe three randomly-selected
fields of view along the scraped line and images of each well were
captured. Cells were then treated with indicated concentrations (0,
0.5, 1 and 2 mg/ml) of EEHDW for 24 h, and another set of images
were captured by the same method. A reduction in the width of the
scratch indicates a sign of migration.
Measurement of cell migration and
invasion by Transwell assay
The migration assay assay was performed using
Transwell cell culture chambers, and the invasion assay was
performed using Transwell cell culture chambers coated with
Matrigel (BD Biosciences). The inserts were placed within a 24-well
chamber containing 0.7 ml RPMI-1640 with 10% FBS as a
chemoattractant. A total of 2.5×105 cells were seeded
into 6-well plates per well and were treated with different
concentrations (0, 0.5, 1 and 2 mg/ml) of EEHDW for 24 h. Cells
(5×104 cells) were seeded into the inserts suspended in
0.2 ml serum-free RPMI-1640 medium. Cells were incubated at 37°C
with 5% CO2 for 12 or 24 h for the migration and
invasion assays, respectively. The upper surface of the filter was
scraped to remove non-migratory cells. Migratory and invasive cells
were fixed with ice-cold 4% paraformaldehyde for 10 min and stained
with crystal violet at room temperature for 15 min. For
quantification, the average number of migratory or invasive
cells/field was assessed by counting five random fields under a
phase-contrast microscope (FMIL/DFC295; Leica Microsystems GmbH,
Wetzlar, Germany) at a magnification of ×200.
Adhesion assay
HCT-8/5-FU cells were seeded into 6-well plates at a
density of 2×105 cells/well in 2 ml medium and were
treated with different concentrations (0, 0.5, 1 and 2 mg/ml) of
EEHDW for 24 h. Cells were digested and suspended in RPMI-1640
medium. Cells were seeded in 6-well plates at a density of
2×104 cells/well and incubated for 2 h. The supernatant
was discarded, and the cells were washed two times with PBS.
Adhered cells were stained with 0.1% crystal violet at room
temperature for 15 min. The adhered cells were counted under a
phase-contrast microscope at a magnification of ×200.
RNA extraction and reverse
transcription-semi-quantitative polymerase chain reaction
(RT-sqPCR) analysis
HCT-8/5-FU cells were seeded into 6-well plates in 2
ml medium and were treated with indicated concentrations of EEHDW
for 24 h. Total RNA was isolated with TRIzol reagent. Oligo
(dT)-primed RNA (1 µg) was reverse-transcribed using the
PrimeScript RT Reagent kit (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocol. The cDNA was used to
determine the mRNA levels of TGF-β, SMAD4, E-cadherin and
N-cadherin using sqPCR with PCR kit (Master mix; Applied
Biosystems, Thermo Fisher Scientific, Inc.). GAPDH was used as an
internal control. The RT-sqPCR conditions were performed for 30
cycles as follows: Denaturation at 94°C for 40 sec, annealing at
60°C for 40 sec and extension at 72°C for 45 sec. The following
primers were used for the amplification of transcripts: TGF-β
forward, 5′-ACCCACAACGAAATCTATGACA-3′ and reverse,
5′-CTAAGGCGAAAGCCCTCAAT-3′; SMAD4 forward,
5′-GATTTGCGTCAGTGTCATCG-3′ and reverse,
5′-AGTCTAAAGGTTGTGGGTCTG-3′; E-cadherin forward,
5′-CTACAATGCCGCATCGCTT-3′ and reverse,
5′-GTATACGTAGGGAAACTCTCTCGGTC-3′; N-cadherin forward,
5′-AAGAACGCCAGGCCAAACAAC-3′ and reverse,
5′-CTGGCTCAAGTCATAGTCCTGGTCT-3′; and GAPDH forward,
5′-GTCATCCATGACAACTTTGG-3′ and reverse, 5′-GAGCTTGACAAAGTGGTCGT-3′.
The PCR was repeated in 3 independent times. A Thermal Cycler
(Bio-Rad S1000; Hercules, CA, USA) was used to perform the
experiment. Samples were analyzed by 1.5% agarose gel
electrophoresis and the DNA bands were examined using a gel
documentation system (Gel Doc XR+; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Western blot analysis
HCT-8/5-FU cells were seeded into 25 cm2
flasks at a density of 2.5×105 cells/ml in 5 ml medium.
Cells were treated with the indicated concentrations of EEHDW for
24 h. The treated cells were lyzed with radioimmunoprecipitation
assay buffer containing protease and phosphatase inhibitor
cocktails. Total protein concentrations were determined by BCA
assay. Equal amounts of total protein (50 µg) were resolved via
SDS-PAGE on a 10% gel and electroblotted onto polyvinylidene
difluoride membranes. The membranes were blocked with 5% nonfat dry
milk at room temperature for 2 h, and probed with primary
antibodies TGF-β (1:1,000 dilution), SMAD4 (1:1,000 dilution),
E-cadherin (1:1,000 dilution), N-cadherin (1:1,000 dilution), and
β-actin (1:1,000 dilution) overnight at 4°C. Membranes were
subsequently incubated with the HRP-conjugated secondary antibody
(1:2,000 dilution) at room temperature for 1 h and followed by
enhanced chemiluminescence detection using SuperSignal West Pico
Chemiluminescent Substrate. Image Lab™ software (version 3.0;
Bio-Rad Laboratories, Inc.) was used for densitometric analysis and
quantification of western blots.
Statistical analysis
All data are presented as the mean of three repeats
and were analyzed using the SPSS package for Windows (version 22.0;
IBM Corp., Armonk, NY, USA). Statistical analysis of the data was
performed using the Student's t-test and one-way analysis of
variance, followed by Dunnett's and the Least Significant
Difference post hoc tests, as appropriate. Differences with
P<0.05 was considered to indicate a statistically significant
difference.
Results
HCT-8/5-FU cells are resistant to
treatment with 5-FU
To verify the 5-FU resistance profiles of the CRC
cell lines, MTT assays were used to detect the cell viability and
the resistance index (RI) was used to evaluate the degree of
resistance. HCT-8 and HCT-8/5-FU cells were exposed to different
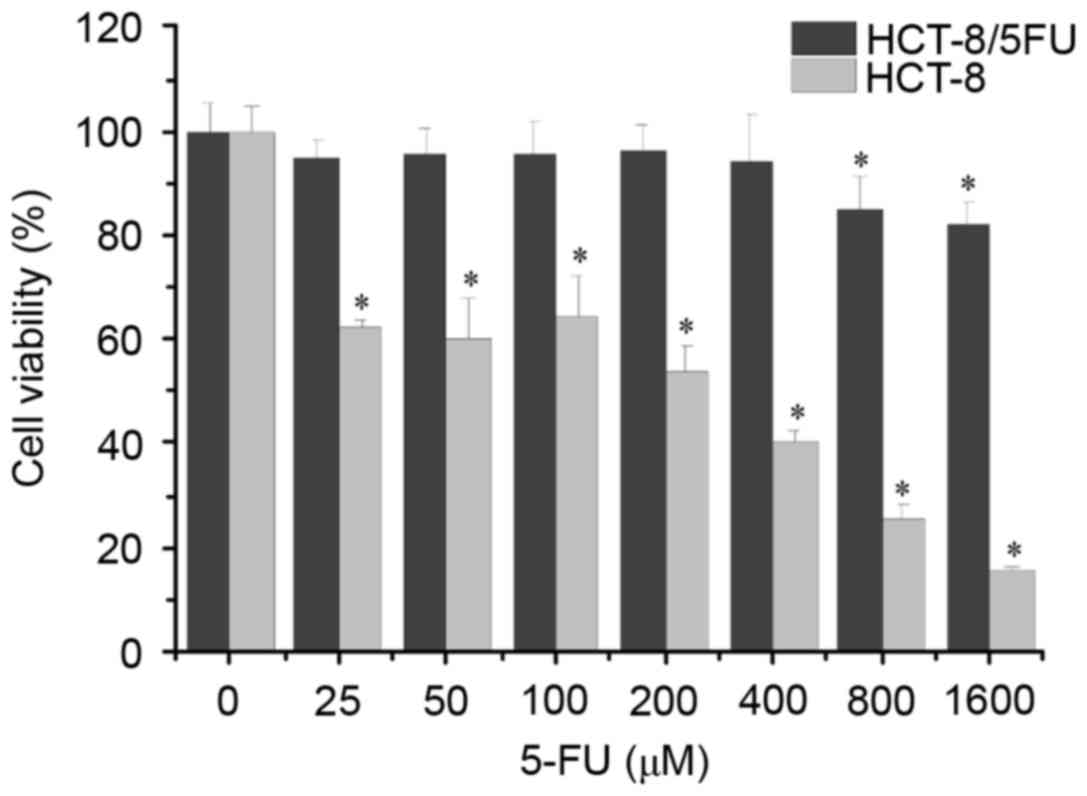
concentrations of 5-FU for 48 h. As shown in Fig. 1, the results demonstrated that the
viability of the HCT-8 cells was significantly decreased following
treatment with ≥25 µM 5-FU compared with the untreated cells,
whereas the viability of the HCT-8/5-FU cells was significantly
decreased following treatment with ≥800 µM 5-FU. The half-maximal
inhibitory concentration of 5-FU was 119.48 mM in HCT-8 cells and
2.803 mM in HCT-8/5-FU cells, and the RI for 5-FU was 23.45
(>1.5) (data not shown). These results indicated that the
HCT-8/5-FU cells used in the present study can be used as a 5-FU
resistance model.
EEHDW inhibits the viability of
HCT-8/5-FU cells
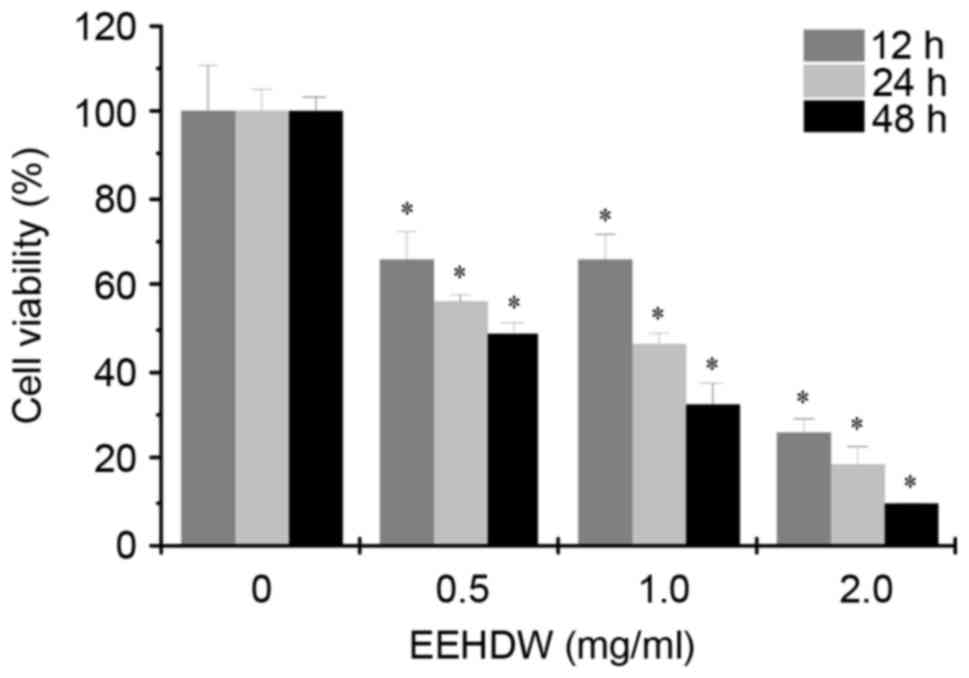
The effect of EEHDW on the viability of HCT-8/5-FU
cells was determined by MTT assay. As demonstrated in Fig. 2, the cell viability was decreased
in response to different concentrations (0.5, 1.0 and 2.0 mg/ml) of
EEHDW for 12, 24 and 48 h. The results demonstrated that treatment
with EEHDW resulted in a time- and dose-dependent inhibitory effect
in HCT-8/5-FU cells.
EEHDW inhibits the migration and
invasion of HCT-8/5-FU cells
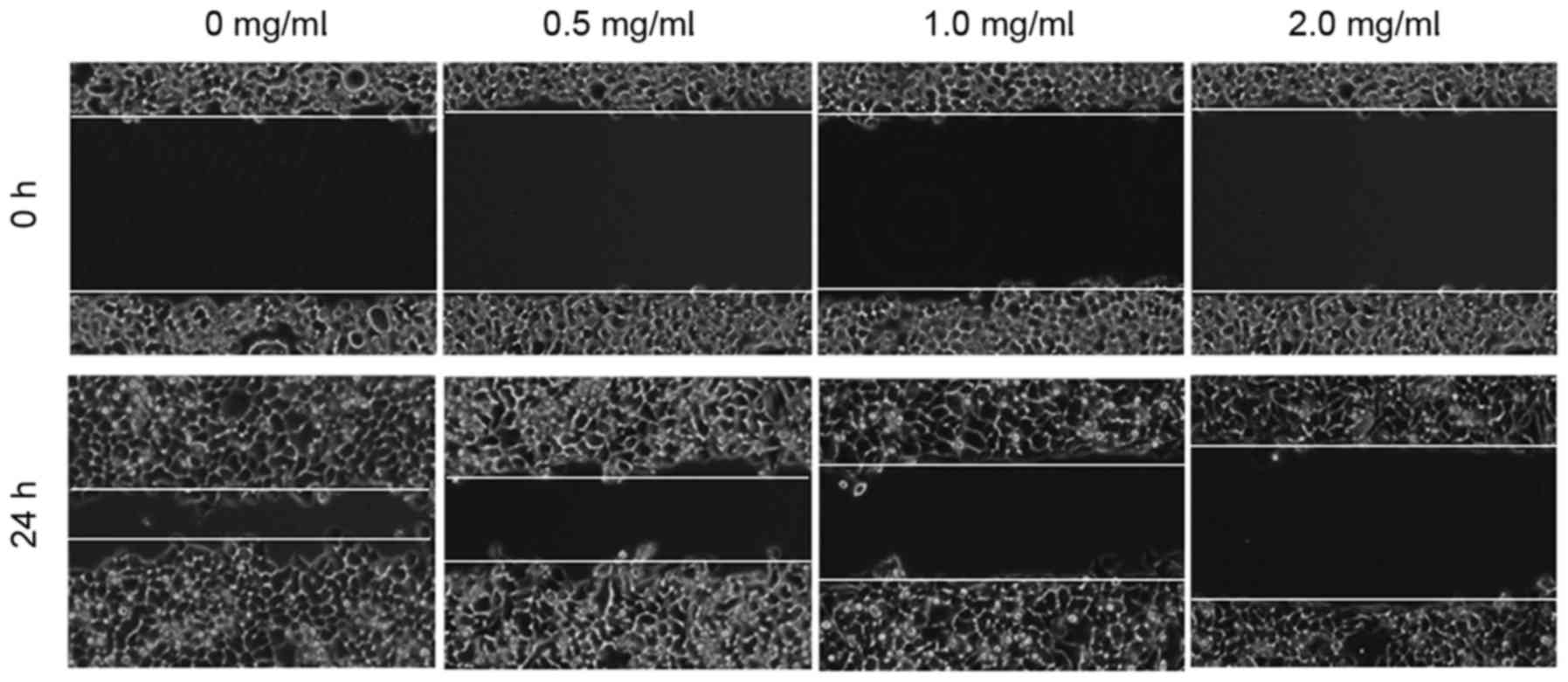
The effect of EEHDW on the migration of HCT-8/5-FU
cells was determined using a wound healing assay. As demonstrated
in Fig. 3, 24 h following the
introduction of a wound, the untreated HCT-8/5-FU cells migrated
into the clear area, whereas treatment with EEHDW inhibited the
migration of HCT-8/5-FU cells in a dose-dependent manner. In order
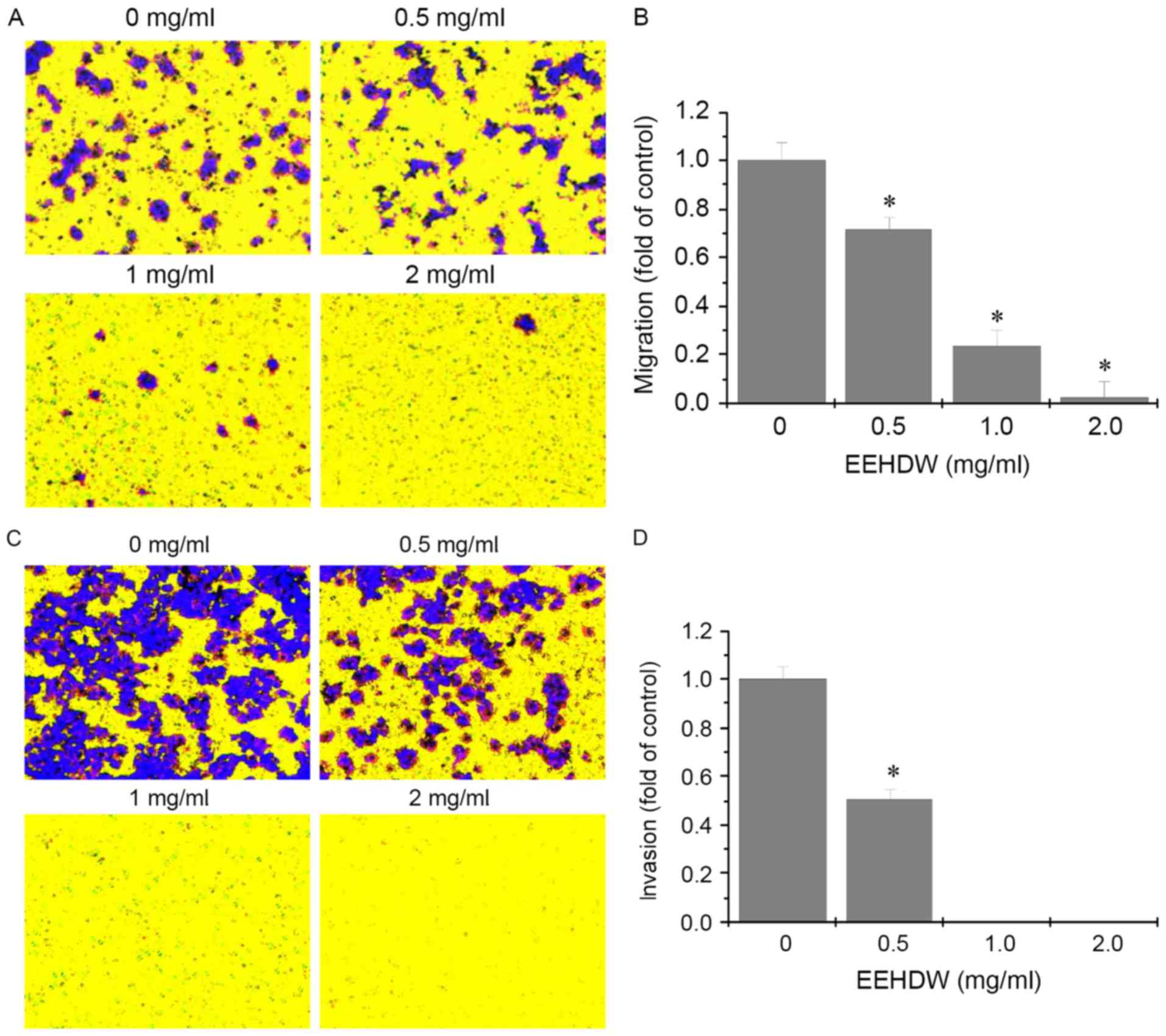
to investigate further, Transwell assays were performed to
determine the effects of EEHDW on the migration and invasion of
HCT-8/5-FU cells. As demonstrated in Fig. 4, following treatment with different
concentrations of EEHDW, the number of migratory and invasive cells
decreased in a dose-dependent manner. These results suggested that
EEHDW can inhibit metastasis in HCT-8/5-FU cells.
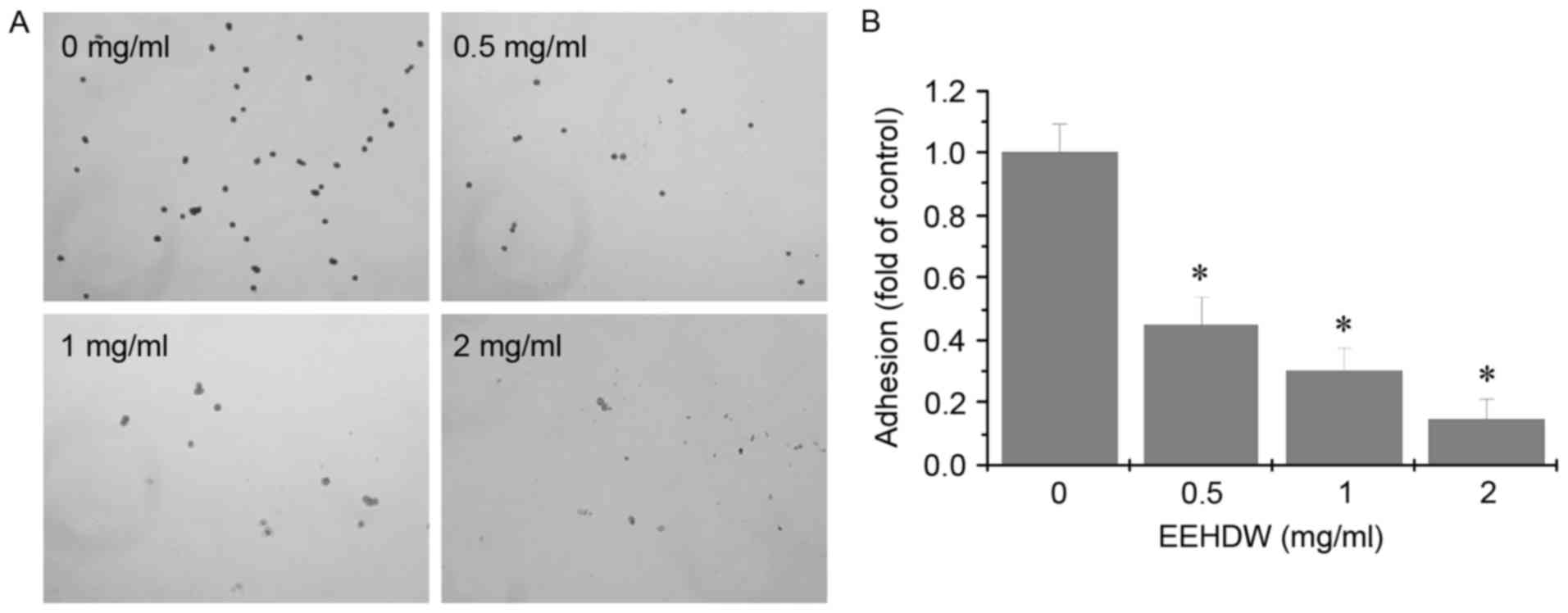
EEHDW inhibits adhesion in HCT-8/5-FU
cells
The effect of EEHDW on adhesion in HCT-8/5-FU cells
was determined using the adhesion assay. As demonstrated in
Fig. 5, following treatment with
different concentration of EEHDW, compared with the control, the
adhesive ability of the HCT-8/5-FU cells was attenuated.
EEHDW regulates the TGF-β pathway in
HCT-8/5-FU cells
To further study the mechanism of EEHDW's
antimetastatic effect, the mRNA and protein expression of TGF-β
pathway-associated factors in HCT-8/5-FU cells was determined using
RT-sqPCR and western blotting, respectively. As demonstrated in
Fig. 6, treatment with EEHDW
downregulated the expression of mRNA and protein levels of TGF-β,
SMAD4 and N-cadherin, and upregulated the mRNA and protein levels
of E-cadherin, in a dose-dependent manner, suggesting that EEHDW
may inhibit the metastasis of HCT-8/5-FU cells through the
suppression of the TGF-β signaling pathway.
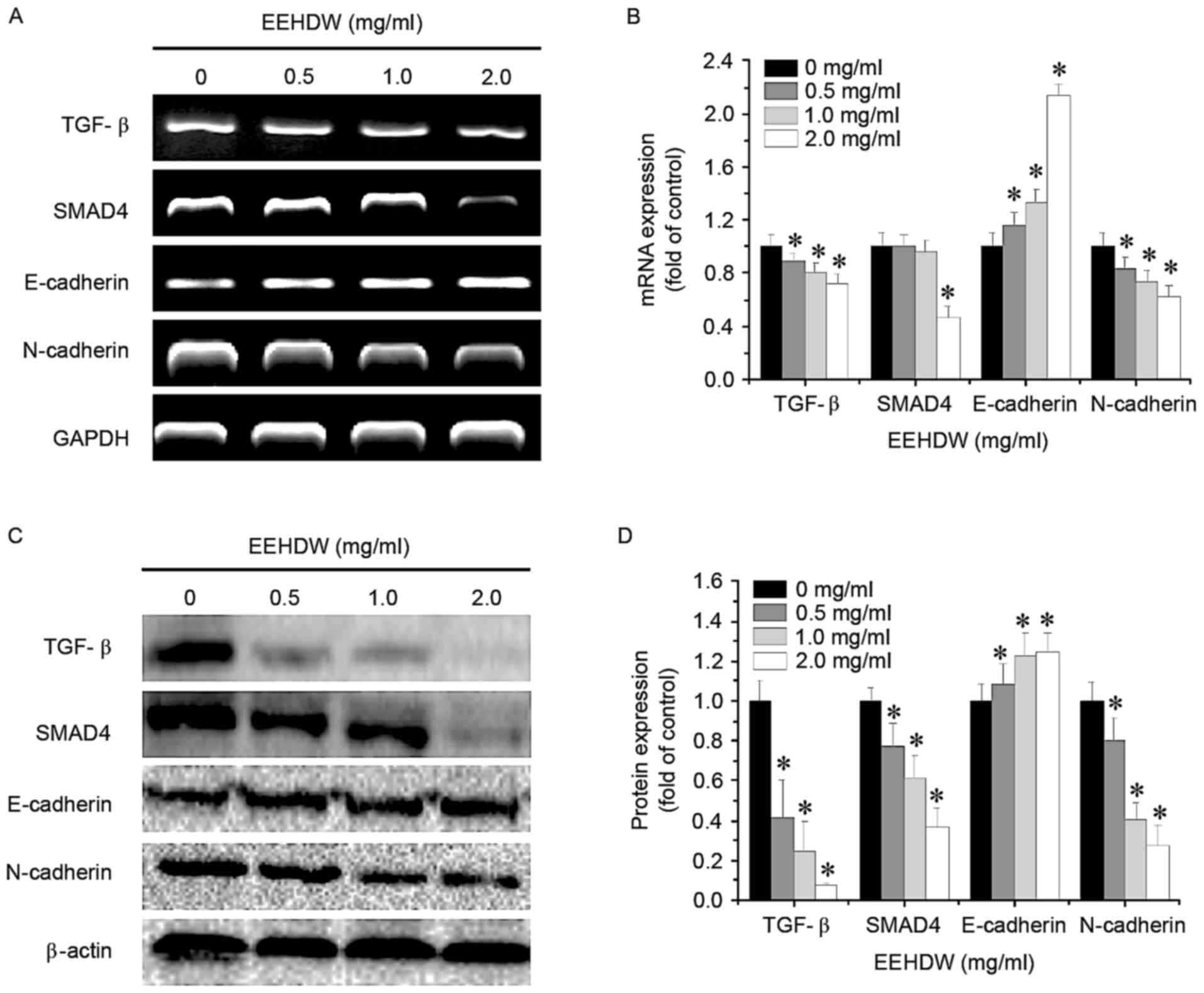 | Figure 6.Effect of EEHDW on the activation of
the TGF-β signaling pathway in HCT-8/5-FU cells. HCT-8/5-FU cells
were treated with the indicated concentrations of EEHDW for 24 h.
(A) The mRNA expression levels of TGF-β, SMAD4, E-cadherin and
N-cadherin in HCT-8/5-FU cells were determined and (B) quantified
by RT-sqPCR analysis. (C) The protein expression levels of TGF-β,
SMAD4, E-cadherin and N-cadherin in HCT-8/5-FU cells were
determined and (D) quantified by western blotting. β-actin or GAPDH
was used as the internal control for western blotting or RT-sqPCR,
respectively. Images are representatives of three independent
experiments. Data were normalized to the expression of untreated
controls (100%) and shown as the mean ± standard deviation from
three independent experiments. *P<0.05 vs. the control cells.
EEHDW, ethanol extract of Hedyotis diffusa Willd; TGF-β,
transforming growth factor-β; SMAD4, Mothers against
decapentaplegic homolog 4; E, epithelial; N, neural; RT-sqPCR,
reverse transcription-semi-quantitative polymerase chain
reaction. |
Discussion
The MDR of tumor cells refers to the phenomenon
through which tumor cells demonstrate resistance to multiple drugs
with varying mechanisms and chemical structures. The incidence of
tumor cell MDR is a leading cause of chemotherapy failure in
clinical treatment. Following the acquisition of drug resistance,
the metastasis of tumor cells is enhanced, which is the primary
factor leading to tumor recurrence, invasion and metastasis
(10). Therefore, it is necessary
to identify novel drugs that can reverse MDR and inhibit the
metastasis of tumor cells. HDW is a traditional Chinese medicine
and exhibits anticancer effects. The authors previously
demonstrated that HDW can reverse MDR in CRC (28). The results of the present study
demonstrated that HCT-8/5-FU cells exhibit drug resistance to 5-FU.
The EEHDW was able to inhibit cell proliferation, and suppress the
migratory, invasive and adhesive potential of HCT-8/5-FU cells,
suggesting that EEHDW exerts an in vitro effect by
inhibiting the metastasis of CRC cells with MDR.
Previous investigations have demonstrated that
metastatic tumor cells undergo EMT, which includes the loss of
cell-cell adhesion, destruction of the tumor basement membrane and
extracellular matrix, and reconstruction of the cytoskeleton,
enhancing cell mobility and inducing metastasis (29,30).
As a part of reversible cell reorganization, EMT is regulated by
multiple circuits at the transcriptional, post-transcriptional, and
translational levels (31,32). Following EMT, tumor cells may
invade, and also secrete an array of growth factors and chemokines,
which can stimulate and recruit stromal cells, thereby indirectly
accelerating tumor cell migration and permeating into the
circulation system to form metastatic lesions (15). Through these processes, epithelioid
malignant cells acquire migratory and invasive activity.
Human TGF-β is a 25-kDa disulfide-linked dimeric
protein. EMT mediated by TGF-β is proven to serve a pivotal role in
the infiltration and metastasis of malignant tumors (33). Consequently, TGF-β is necessary to
evaluate the effect of TGF-β-mediated EMT upon the infiltration and
metastasis of tumors, which provides strategies for reducing the
metastatic rate of malignant tumors. Targeting the TGF-β signaling
pathway can decrease the incidence of EMT, thereby decreasing the
production of mesenchymal-like cells and lowering the incidence of
tumor metastasis (34,35). As a transcription factor, SMAD4
serves a crucial role in the transduction of the TGF-β signal
(36). Epithelial and mesenchymal
cells display distinct phenotypes and functions. Epithelial cells
exhibit basal polarity and express high levels of
epithelium-labeled E-cadherin to form intimate epithelial cell
adhesion (37). E-cadherin is
considered to be a main regulator of EMT, and the downregulated
expression of E-cadherin is a rate-limiting step in EMT. In the
presence of downregulated expression of E-cadherin, non-invasive
tumors can be transformed into highly-invasive tumors (38). Mesenchymal cells lack cell polarity
and highly express mesenchyme-labeled N-cadherin (39). Alterations in the expression levels
of E-cadherin and N-cadherin are a key mechanism underlying the EMT
of tumor cells, and are regulated by the TGF-β signaling
transduction pathway. The results of the present study demonstrated
that EEHDW can downregulate the expression of TGF-β, SMAD4 and
N-cadherin, and upregulate the expression of E-cadherin, in
HCT-8/5-FU cells. Therefore, EEHDW can inhibit the incidence of EMT
by suppressing the activation of the TGF-β signaling pathway,
thereby inhibiting the metastasis of CRC cells.
In conclusion, EEHDW exerts its antimetastatic
activity through suppression of TGF-β/SMAD4 signaling
pathway-mediated EMT. The results of the present study may provide
a foundation for the development of a multi-potent anticancer agent
for the clinical treatment of CRC.
Acknowledgements
The present study was sponsored by the Research Fund
for the Doctoral Program of Higher Education of China (grant no.
20133519110003), Project Funding for the Training of Young and
Middle-aged Backbone Personnel of Fujian Provincial Health and
Family Planning Commission (grant no. 2016-ZQN-67), and the
Developmental Fund of Chen Keji Integrative Medicine (grant nos.
CKJ2014013 and CKJ2015007).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
EEHDW
|
ethanol extract of Hedyotis
diffusa Willd
|
|
TGF-β
|
transforming growth factor-β
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang WQ, Fu FF, Li YX, Wang WB, Wang HH,
Jiang HP and Teng LS: Molecular biomarkers of colorectal cancer:
Prognostic and predictive tools for clinical practice. J Zhejiang
Univ Sci B. 13:663–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aakif M, Balfe P, Elfaedy O, Awan FN,
Pretorius F, Silvio L, Castinera C and Mustafa H: Study on
colorectal cancer presentation, treatment and follow-up. Int J
Colorectal Dis. 31:1361–1363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du B and Shim JS: Targeting
Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance
in Cancer. Molecules. 21(pii): E9652016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phillips TA, Howell A, Grieve RJ and
Welling PG: Pharmacokinetics of oral and intravenous fluorouracil
in humans. J Pharm Sci. 69:1428–1431. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Juchum M, Günther M and Laufer SA:
Fighting cancer drug resistance: Opportunities and challenges for
mutation-specific EGFR inhibitors. Drug Resist Update. 20:12–28.
2015. View Article : Google Scholar
|
|
8
|
Kim JK, Kang KA, Piao MJ, Ryu YS, Han X,
Fernando PM, Oh MC, Park JE, Shilnikova K, Boo SJ, et al:
Endoplasmic reticulum stress induces 5-fluorouracil resistance in
human colon cancer cells. Environ Toxicol Pharmacol. 44:128–133.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen A, Chen H, Chen Y, Lin J, Lin W, Liu
L, Sferra TJ and Peng J: Pien Tze Huang overcomes multidrug
resistance and epithelial-mesenchymal transition in human
colorectal carcinoma cells via suppression of TGF-β pathway. Evid
Based Complement Alternat Med. 2014:6794362014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pecina-Slaus N, Cicvara-Pecina T and Kafka
A: Epithelial-to-mesenchymal transition: Possible role in
meningiomas. Front Biosci (Elite Ed). 4:889–896. 2012.PubMed/NCBI
|
|
11
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberts AB: Molecular and cell biology of
TGF-beta. Miner Electrolyte Metab. 24:111–119. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trapani JA: The dual adverse effects of
TGF-beta secretion on tumor progression. Cancer Cell. 8:349–350.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heldin CH, Vanlandewijck M and Moustakas
A: Regulation of EMT by TGFβ in cancer. FEBS Lett. 586:1959–1970.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Massagué J: TGFbeta in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brunen D, Willems SM, Kellner U, Midgley
R, Simon I and Bernards R: TGF-β: An emerging player in drug
resistance. Cell Cycle. 12:2960–2968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moustakas A and Heldin P: TGFβ and
matrix-regulated epithelial to mesenchymal transition. Biochim
Biophys Acta. 1840:2621–2634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song LR: Zhonghuabencao. Shanghai Science
and Technology Press; Shanghai: pp. 4331999
|
|
21
|
Yang JJ, Hsu HY, Ho YH and Lin CC:
Comparative study on the immunocompetent activity of three
different kinds of Peh-Hue-Juwa-Chi-Cao, Hedyotis diffusa, H.
corymbosa and Mollugo pentaphylla after sublethal whole body
x-irradiation. Phytother Res. 11:428–432. 1997. View Article : Google Scholar
|
|
22
|
Li R, Zhao HR and Lin YM: Anti-tumor
effect and protective effect on chemotherapeutic damage of water
soluble extracts from Hedyotis diffusa. J Chin Pharmaceu Sci.
11:54–58. 2002.
|
|
23
|
Cai Q, Lin J, Wei L, Zhang L, Wang L, Zhan
Y, Zeng J, Xu W, Shen A, Hong Z and Peng J: Hedyotis diffusa Willd
inhibits colorectal cancer growth in vivo via inhibition of STAT3
signaling pathway. Int J Mol Sci. 13:6117–6128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin J, Wei L, Shen A, Cai Q, Xu W, Li H,
Zhan Y, Hong Z and Peng J: Hedyotis diffusa Willd extract
suppresses Sonic hedgehog signaling leading to the inhibition of
colorectal cancer angiogenesis. Int J Oncol. 42:651–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis diffusa Willd extract induces
apoptosis via activation of the mitochondrion-dependent pathway in
human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.PubMed/NCBI
|
|
26
|
Lin J, Wei L, Xu W, Hong Z, Liu X and Peng
J: Effect of Hedyotis diffusa Willd extract on tumor angiogenesis.
Mol Med Rep. 4:1283–1288. 2011.PubMed/NCBI
|
|
27
|
Lin M, Lin J, Wei L, Xu W, Hong Z, Cai Q,
Peng J and Zhu D: Hedyotis diffusa Willd extract inhibits HT-29
cell proliferation via cell cycle arrest. Exp Ther Med. 4:307–310.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Wang X, Shen A, Zhang Y, Chen Y,
Sferra T, Lin J and Peng J: Hedyotis diffusa Willd overcomes
5-fluorouracil resistance in human colorectal cancer HCT-8/5-FU
cells by downregulating the expression of P-glycoprotein and
ATP-binding casette subfamily G member 2. Exp Ther Med.
10:1845–1850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moustakas A and Heldin CH: The regulation
of TGFbeta signal transduction. Development. 136:3699–3714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nieto MA: Epithelial plasticity: A common
theme in embryonic and cancer cells. Science. 342:12348502013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zi Z, Chapnick DA and Liu X: Dynamics of
TGF-β/SMAD signaling. FEBS Lett. 586:1921–1928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Massagué J: How cells read TGF-beta
signals. Nat Rev Mol Cell Biol. 1:169–178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Massagué J and Wotton D: Transcriptional
control by the TGF-beta/Smad signaling system. EMBO J.
19:1745–1754. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor Snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tanaka T, Goto K and Iino M: Sec8
modulates TGF-β induced EMT by controlling N-cadherin via
regulation of Smad3/4. Cell Signal. 29:115–126. 2017. View Article : Google Scholar : PubMed/NCBI
|