Introduction
Glucocorticoid-induced osteoporosis (GIOP) is the
most common form of secondary osteoporosis due to the wide use of
glucocorticoid therapy in clinical practice (1,2).
Long-time glucocorticoid treatment leads to early and rapid bone
loss, and increased fracture risk. GIOP predominantly affects the
skeletal regions containing abundant cancellous bone, including the
lumbar spine and proximal femur (3). The bone loss caused by
glucocorticoids results from their direct effects on osteoblasts,
osteoclasts and osteocytes. Previous studies have shown that excess
glucocorticoids inhibit the proliferation and increase the
apoptosis of osteoblasts and osteocytes (4), and prolong osteoclast survival
(5).
It is well known that bone tissue in adult mammalian
animals undergoes continuous remodeling through a strictly
controlled balance between bone formation and resorption. This
dynamic balance of physiological bone mass is maintained constantly
by osteoblasts and osteoclasts. Osteoblasts, which differentiate
from mesenchymal stem cells in the bone marrow and give rise to
osteocytes, are responsible for bone formation (6,7). By
contrast, osteoclasts are derived from mononucleated hematopoietic
progenitor cells and are specialized bone resorptive cells
(8). Osteoblasts also secrete the
receptor activator of nuclear factor-κB ligand (RANKL), which is
essential for osteoclast differentiation and regulates bone
resorption in this manner (9,10).
Bone marrow-derived mesenchymal stem cells (BMSCs)
are important in maintaining the homeostasis of bone mass and are
vital in the pathogenesis of osteoporosis (11,12).
The defective proliferation and osteogenic differentiation ability
of BMSCs reduce bone mass in the process of osteoporosis. Several
studies have shown that the osteogenic differentiation of BMSCs may
be suppressed under stressful conditions, and finally lead to
osteoporosis (13,14). Osteoclast precursors differentiate
into osteoclasts in the presence of monocyte colony stimulating
factor and RANKL (15,16). The formation and activity of
osteoclasts are increased in osteoporosis (17). Shi et al reported that
glucocorticoids exerted dose-dependent effects, which promote
osteoclastogenesis in vivo and stimulate RANKL-induced
osteoclast formation and function in vitro (18).
Ligusticum wallichii Franchat, also known as
Chuanxiong, is considered one of the most widely used traditional
Chinese medicines. Tetramethylpyrazine (TMP), the pharmacologically
active component extracted from Chuanxiong, has been found to have
anti-inflammatory, anticancer, anti-oxidative and anti-apoptotic
effects in several types of cell (19–21).
In our previous study, it was shown that TMP promoted the viability
and inhibited the glucocorticoid-induced apoptosis of BMSCs
(22). However, whether TMP can
ameliorate the defective osteogenic differentiation of BMSCs in
GIOP, and how TMP affects the formation and function of osteoclasts
in GIOP remain to be elucidated.
In the present study, the protective effects of TMP
on BMSC differentiation and osteoclast formation in GIOP were
investigated in vivo and in vitro. The resulting data
demonstrated that TMP promoted osteogenesis and inhibited
osteoclastogenesis in GIOP rats, and indicated the inhibition of
RANKL and IL-6 in BMSCs as a possible mechanism for the protective
effects of TMP against glucocorticoid-induced
osteoclastogenesis.
Materials and methods
Animals
A total of 20 4-month-old female Sprague-Dawley
rats, weighing 217±15.5 g, were obtained from the Experimental
Animal Center at The Fourth Military Medical University (Xi'an,
China), and were housed under specific pathogen-free conditions
(20°C; 12-h light/dark cycles; 50–55% humidity) with free access to
food and water. The rats were administered intraperitoneally with
either distilled water as the control group (n=5) or 2.5 mg/kg
prednisolone (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
as the GIOP group (n=15) daily for 12 weeks. At 1 week following
the first administration, the 15 rats in the GIOP group were
randomly divided into three experimental groups, each containing
five rats. The rats were injected intraperitoneally with either
sesame oil (as a vehicle control), 5 mg/kg body weight of TMP or 20
mg/kg body weight of TMP (Sigma-Aldrich; Merck Millipore) daily for
12 weeks. The doses of TMP used were selected based on previous
in vivo studies (19,23).
Subsequently, BMSCs were isolated from the rats, and the fourth
lumbar vertebrae, distal femurs and blood samples were collected
from the rats in the control and GIOP groups. No significant
differences in total body weights were found among the groups prior
to the rats being sacrificed. All experimental procedures involving
animals were approved by the Ethics in Animal Research Committee of
The Fourth Military Medical University (permission no.
20110405-5).
Micro-computed tomography (CT)
analysis
The fourth lumbar vertebrae were scanned using an
Explore Locus SP Pre-Clinical Specimen micro-CT (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) with 8 mm resolution, a 50 kV
tube voltage and a 0.1 mA tube current. Reconstruction and 3D
quantitative analyses were performed using GEHC MicroView software,
version 2.1 (GE Healthcare Bio-Sciences). Similar settings for
scans and analyses were used for all samples. The trabecular bone
region from the vertebral body was outlined for each micro-CT
slice, which excluded the cranial and caudal endplate regions. The
following 3D indices in the defined region of interest were
analyzed: Bone mineral density (BMD), relative bone volume/total
volume (BV/TV), trabecular number (Tb.N), trabecular thickness
(Tb.Th), structure model index (SMI) and trabecular separation
(Tb.Sp). The operator performing the scan analyses was blinded to
the treatment procedures involving the specimens.
Histochemistry and
immunohistochemistry
Following fixation for 2 days using 4%
paraformaldehyde, the left femurs were transferred to 80% formic
acid for decalcification for 14 days, embedded in paraffin and then
cut into horizontal sections of 5-µm thickness. For
tartrate-resistant acid phosphatase (TRAP) staining, the sections
were reactivated in 0.2 M Tris buffer and then stained using Acid
Phosphatase Kit-387-A (Sigma-Aldrich; Merck Millipore) for 2 h at
room temperature according to the manufacturer's protocol. For
staining of osterix (OSX), the sections were incubated with
anti-OSX primary antibody (ab22552, 1:100; Abcam, Cambridge, MA,
USA) at 4°C overnight. All sections were observed and images were
captured using a florescence microscope (Olympus BX-60; Olympus
Corporation, Tokyo, Japan). For each sample, values represent
five-stained sections of equivalent depth.
ELISA
The levels of bone degradation markers, serum
C-telopeptide of type I collagen (CTX-1) and TRAP were measured in
the blood samples of the rats using ELISA assay kits
(Immunodiagnostic Systems, Ltd., Tyne & Wear, UK) according to
the manufacturer's protocol. BMSCs were seeded in 6-well plates at
a density of 1×10−6 cells/well. Following osteogenic
induction with or without 10−6 M dexamethasone (Dex;
Sigma-Aldrich; Merck Millipore) at 37°C for 14 days, the BMSCs were
incubated in serum-free medium with or without TMP (50, 100 or 200
µM) at 37°C for 48 h. The expression levels of RANKL and IL-6 in
the culture medium were measured using ELISA assay kits (R&D
Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol. Total protein concentrations were measured
using a Bradford protein assay.
Osteogenic differentiation assay in
BMSCs
The isolation and primary culture of BMSCs were
performed as previously described (24), and the cells were characterized
using MSC minimal criteria (25).
Following osteogenic induction with 10−6 M Dex for 14
days, the BMSCs were incubated with serum-free medium with or
without TMP (50, 100, or 200 µM) for 48 h. Then the cells were
stained using a BCIP/NBT Alkaline Phosphatase Color Development kit
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
activity of alkaline phosphatase (ALP) was detected as previously
described (26) and calculated
using absorbance measurements at 405 nm. All sections were observed
using an Olympus BX-60 microscope (Olympus Corporation). Following
a 21-day period of osteogenic induction, Alizarin Red S staining
was performed to detect calcium deposition, as previously described
(27). The absorbance of the
released Alizarin Red S was measured using a Thermo Labsystems
Multiscan MK-3 enzyme-linked microplate reader (Thermo Fisher
Scientific, Inc.) at a wavelength of 562 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA
synthesis was performed using the Prime Script RT reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China). The RT-qPCR
analysis was performed using a CFX96 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) instrument. Individual reactions were conducted
in 96-well optical reaction plates using SYBR Premix Ex Taq II (Tli
RNaseH Plus) (Takara Biotechnology Co., Ltd.) as previously
described (18). Amplifications
were performed as follows: Initial denaturation at 95°C for 30 sec,
followed by 40 cycles of denaturation at 95°C for 5 sec and
annealing at 58°C for 15 sec. The expression levels of target genes
were normalized to the reference gene GAPDH. The 2−ΔΔCq
method was applied to calculate the relative gene expression
(28). The 5′-3′ sequences of the
forward and reverse primers were as follows: ALP, forward
5′-GTCCCACAAGAGCCCACAAT-3′ and reverse 5′-CAACGGCAGAGCCAGGAAT-3′;
collagen, type I, α1 (COL1A1), forward
5′-GACATGTTCAGCTTTGTGGACCTC-3′ and reverse
5′-AGGGACCCTTAGGCCATTGTGTA; osteocalcin (OCN),
5′-CAGTAAGGTGGTGAATAGACTCCG-3′ and reverse
5′-GGTGCCATAGATGCGCTTG-3′; osterix (OSX), forward
5′-CACCCATTGCCAGTAATCTTCGT-3′ and reverse
5′-GGACTGGAGCCATAGTGAGCTTCT-3′; TRAP, forward
5′-GCCTCTTGCGTCCTCTATGA-3′ and reverse 5′-AGCACCATCCACGTATCCA-3′;
nuclear factor of activated T-cells, cytoplasmic 1 (NFATC1),
forward 5′-GCTCGCCTTTTCAACTTTCT-3′ and reverse
5′-GCCTGGGACACACCTTTCTA-3; cathepsin K (CTSK), forward
5′-CGACTATCGAAAGAAAGGCTATG-3′ and reverse
5′-AAAGCCCAACAGGAACCAC-3′; GAP DH, forward
5′-CCTGCACCACCAACTGCTTA-3′ and reverse
5′-GGCCATCCACAGTCTTCTGAG-3′.
Detection of osteoclast formation in
vitro
The hematopoietic mononucleated precursors of
osteoclasts were isolated from the bone marrow and seeded in 6-well
plates at a density of 1×10−6 cells/well. Cells were
cultured in the presence of 100 ng/ml RANKL and 10−6 M
Dex at 37°C for 7 days. Osteoclast formation was measured by
quantifying the TRAP-positive stained cells. Briefly, the cells
were fixed with 10% formalin for 10 min and ethanol/acetone (1:1)
for 1 min, and then stained using the Acid Phosphatase Kit-387-A
(Sigma-Aldrich; Merck Millipore). The osteoclasts in each well were
counted using a light microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Statistical analysis
Statistical analyses were performed using SPSS
software, version 15.0 (SPSS, Inc., Chicago, IL, USA). Quantitative
data are presented as the mean ± standard deviation and were
compared using a one-way analysis of variance followed by a
Bonferroni post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
TMP improves osteoblast
differentiation and osteoclast maturation in GIOP rats
To confirm the effects of TMP on bone mass and
micro-architecture, a GIOP rat model was established. The fourth
lumbar vertebrae were collected and scanned via micro-CT
assessment. The analyses of the trabecular bone of the lumbar
vertebrae indicated that excess glucocorticoids significantly
reduced bone mass and deteriorated bone micro-architecture, as
indicated by decreases in the BMD, BV/TV, Tb.N and Tb.Th, and
increases in the SMI and Tb.Sp of the GIOP rats (Fig. 1A and B). Treatment of the GIOP rats
with TMP partially ameliorated these bone parameters and improved
the micro-architecture of the trabecular bone in the lumbar
vertebrae. These results demonstrated that TMP protected the
trabecular bone mass from excess exposure to glucocorticoids.
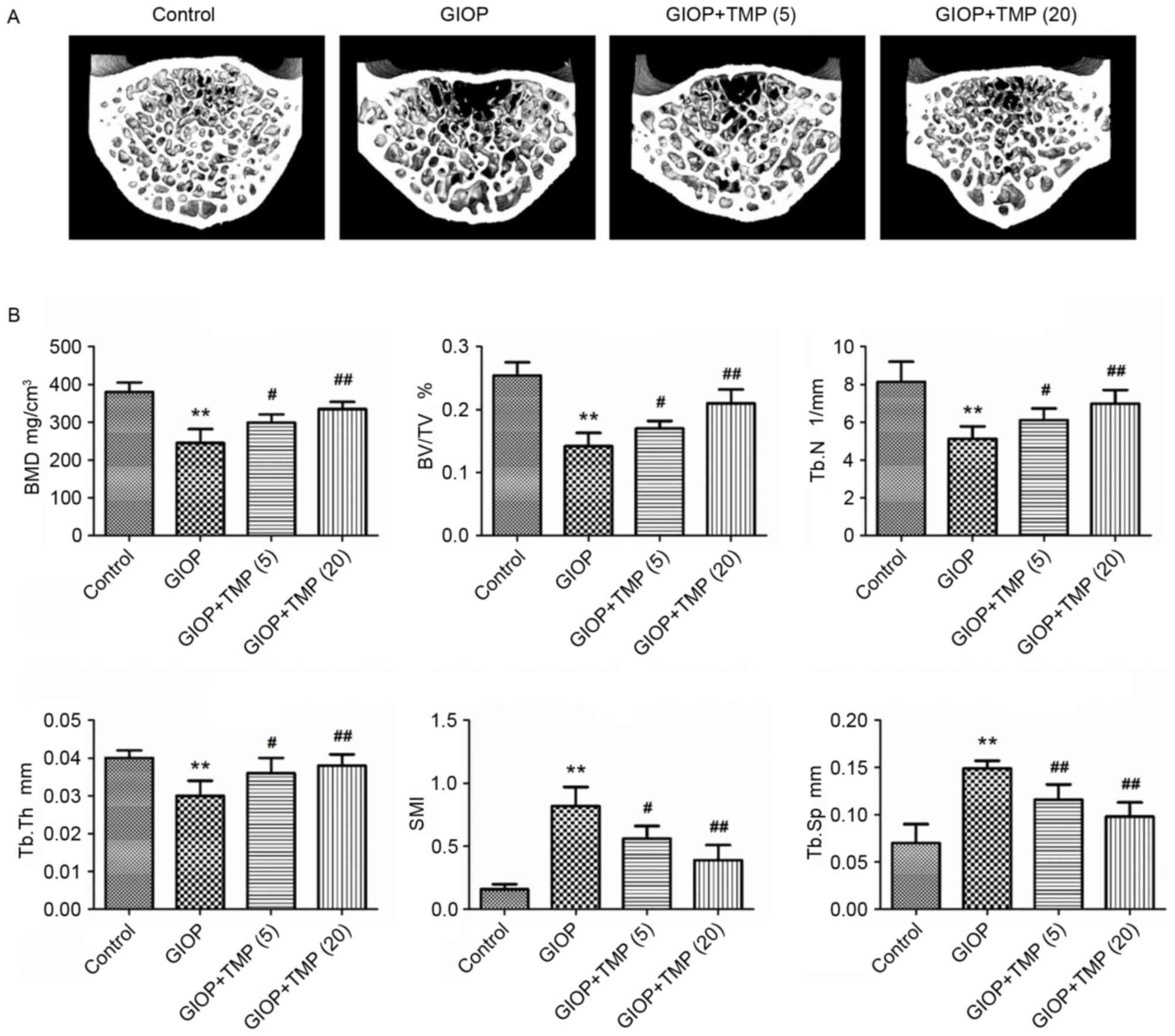 | Figure 1.TMP improves trabecular bone mass in
GIOP rats. (A) Micro-CT images within the fourth lumbar vertebrae
region (magnification, ×40). (B) Micro-CT analysis quantification
within the fourth lumbar vertebrae region. The 3D indices in the
defined region of interest were analyzed. **P<0.01, vs. control
group; #P<0.05 and ##P<0.01, vs. GIOP
group. (5) and (20) represent 5 and 20 mg/kg body weight
of TMP, respectively. GIOP, glucocorticoid-induced osteoporosis;
TMP, tetramethylpyrazine; BMD, bone mineral density; BV/TV,
relative bone volume over the total volume; Tb.N, trabecular
number; Tb.Th, trabecular thickness; SMI, structure model index;
Tb.Sp, trabecular separation. |
TMP promotes osteogenesis and inhibits
osteoclastogenesis in GIOP rats in vivo
To investigate whether TMP affects osteogenesis and
osteoclastogenesis in vivo, immunostaining and TRAP staining
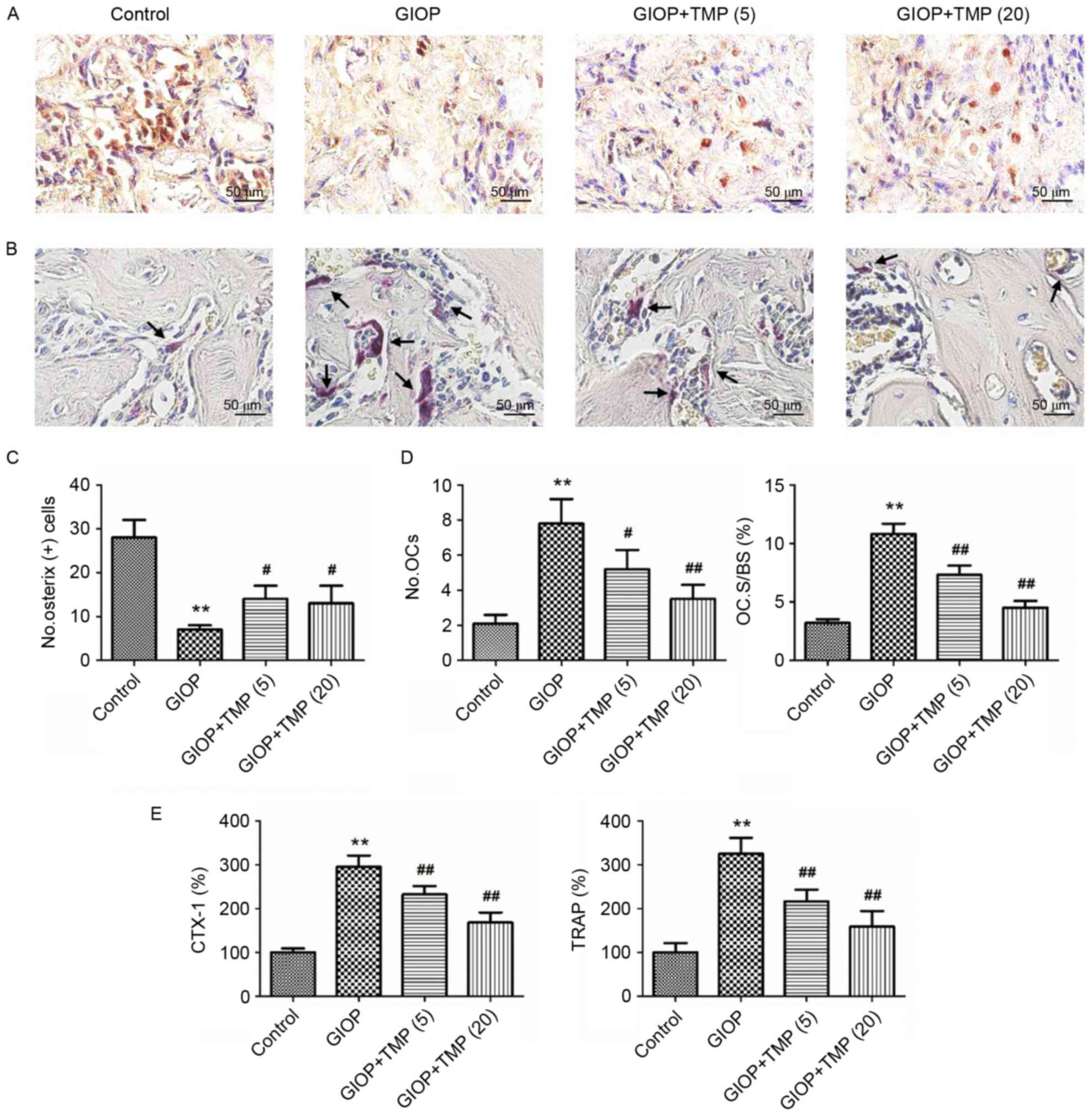
of distal femurs were performed. As shown in Fig. 2, glucocorticoids significantly
reduced osteogenesis and enhanced osteoclastogenesis, compared with
the control group. However, TMP treatment significantly increased
the number of OSX-positive cells and decreased the number and
spread of TRAP-positive cells (Fig.
2A-D). In addition, the activities of CTX-1 and TRAP were
detected to measure the serum levels of osteoclastic markers.
Compared with the control group, the activities of CTX-1 and TRAP
were markedly elevated in the GIOP group. However, treatment with
TMP significantly decreased the serum activities of CTX-1 and TRAP
(Fig. 2E). These data suggested
that treatment with TMP partially promoted osteogenesis and
inhibited osteoclastogenesis in the GIOP rats.
Protection by TMP on osteogenic
differentiation of BMSCs
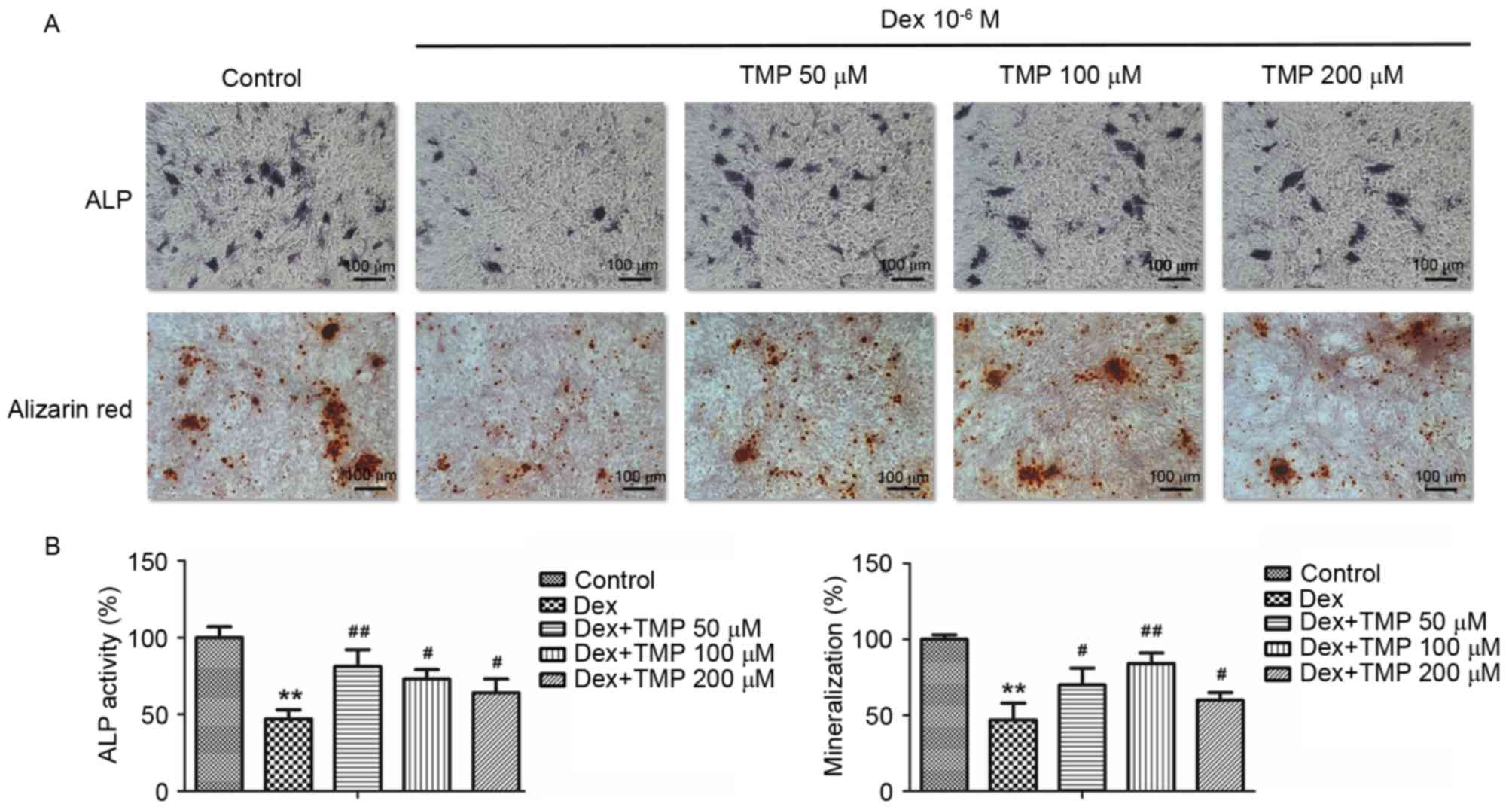
To examine whether TMP promoted the osteogenic
differentiation of BMSCs against excess glucocorticoids in
vitro, the activity of ALP and calcium mineralization were
examined, in addition to the mRNA expression levels of osteogenic
genes, including ALP, COL1A1, OCN and OSX. A 10−6 MA
concentration of Dex was used a high dose of glucocorticoids. As
shown in Fig. 3A and B,
10−6 M Dex significantly decreased the expression of ALP
and the level of calcium mineralization, compared with the control
group. However, the groups treated with TMP treatment exhibited
significant increases in the activity of ALP and mineralization. In
addition, TMP significantly elevated the expression levels of the
osteogenic genes, compared with those in the Dex-only treated group
(Fig. 4). Taken together, these
data revealed that TMP improved the osteogenic differentiation of
the BMSCs against excess glucocorticoids.
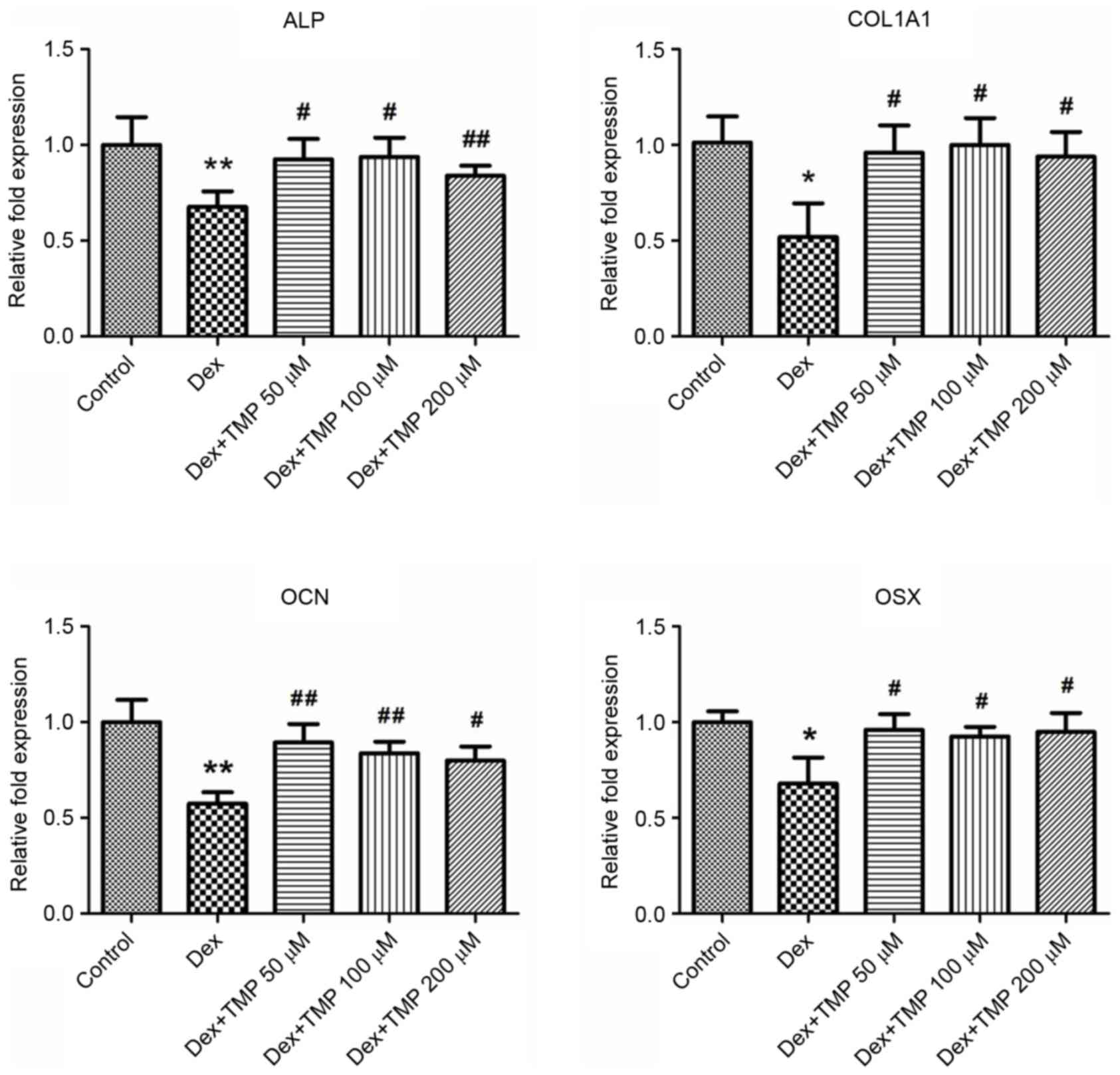 | Figure 4.TMP elevates the mRNA expression
levels of osteogenic genes. Expression of GAPDH served as a
control. *P<0.05 and **P<0.01, vs. control group;
#P<0.05 and ##P<0.01, vs. Dex group
(n=5). ALP, alkaline phosphatase; COL1A1, collagen, type I, α1;
OCN, osteocalcin; OSX, osterix; Dex, dexamethasone; TMP,
tetramethylpyrazine. |
TMP inhibits osteoclastic
differentiation in vitro
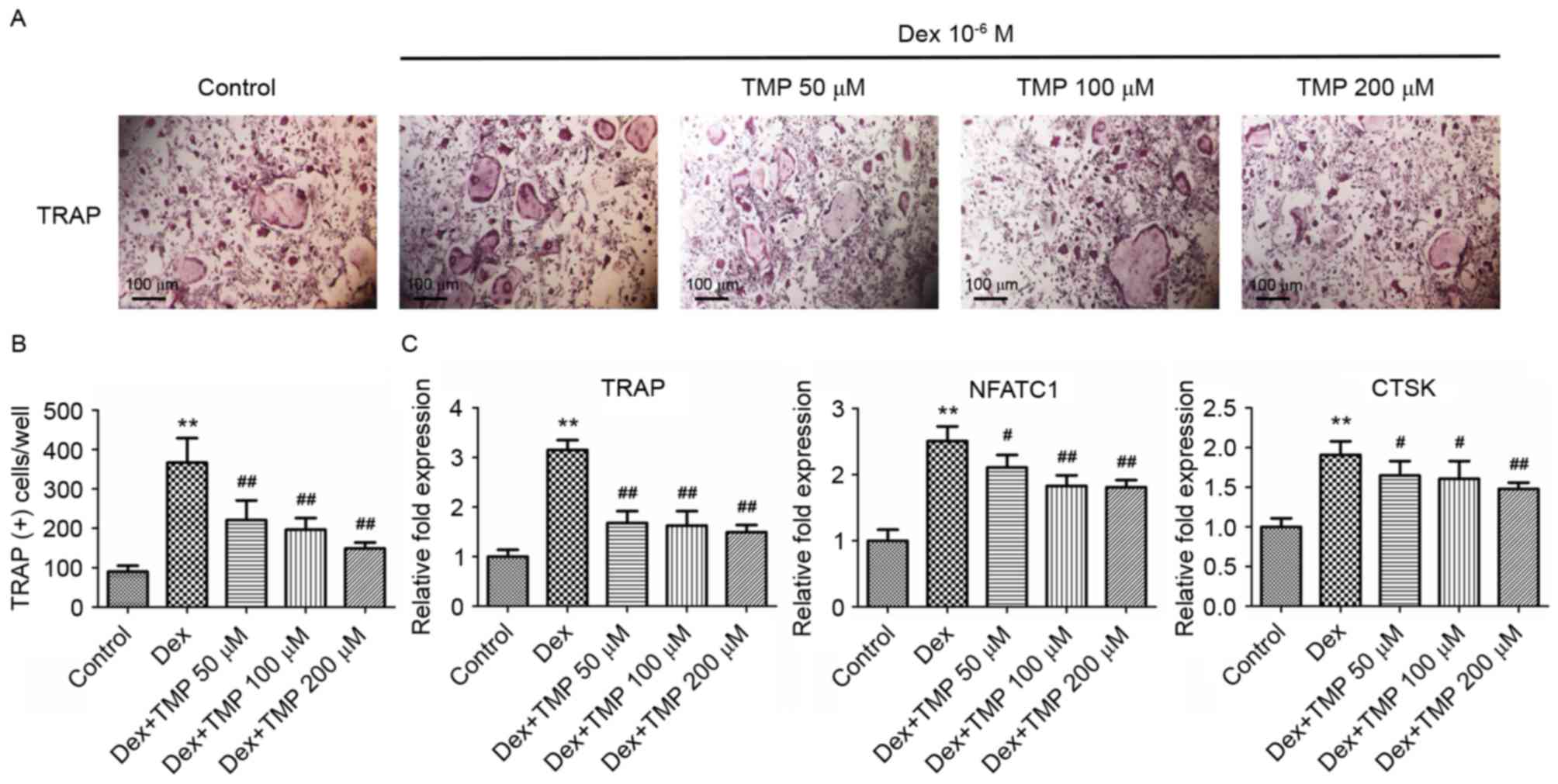
To examine whether TMP can protect osteoclast
precursors against glucocorticoids in vitro, the present
study examined osteoclast differentiation in the presence of excess
glucocorticoids using TRAP staining. A previous study showed that
high doses of Dex stimulated osteoclast formation in vitro
(18), therefore, the osteoclasts
in the present study were also exposed to 10−6 M Dex. It
was found that exposure to 10−6 M Dex alone
significantly increased the number of TRAP-positive cells (Fig. 5A and B). Treatment with different
concentrations of TMP (50, 100 or 200 µM) significantly reduced the
numbers of TRAP-positive cells, compared with those in the group
treated with Dex alone. The expression levels of several
osteoclastogenesis-related genes were also investigated, including
TRAP, NFATC1 and CTSK, using RT-qPCR analysis on day 3. Compared
with the control group, all of the above genes were significantly
upregulated in the group treated with Dex alone. However, treatment
with all concentrations of TMP downregulated the expression of
these genes, compared with those in the Dex-only treatment group
(Fig. 5C). These data indicated
that TMP acts as a potent inhibitor of osteoclastic differentiation
under glucocorticoid exposure in vitro.
TMP inhibits the expression of RANKL
and IL-6 in BMSCs
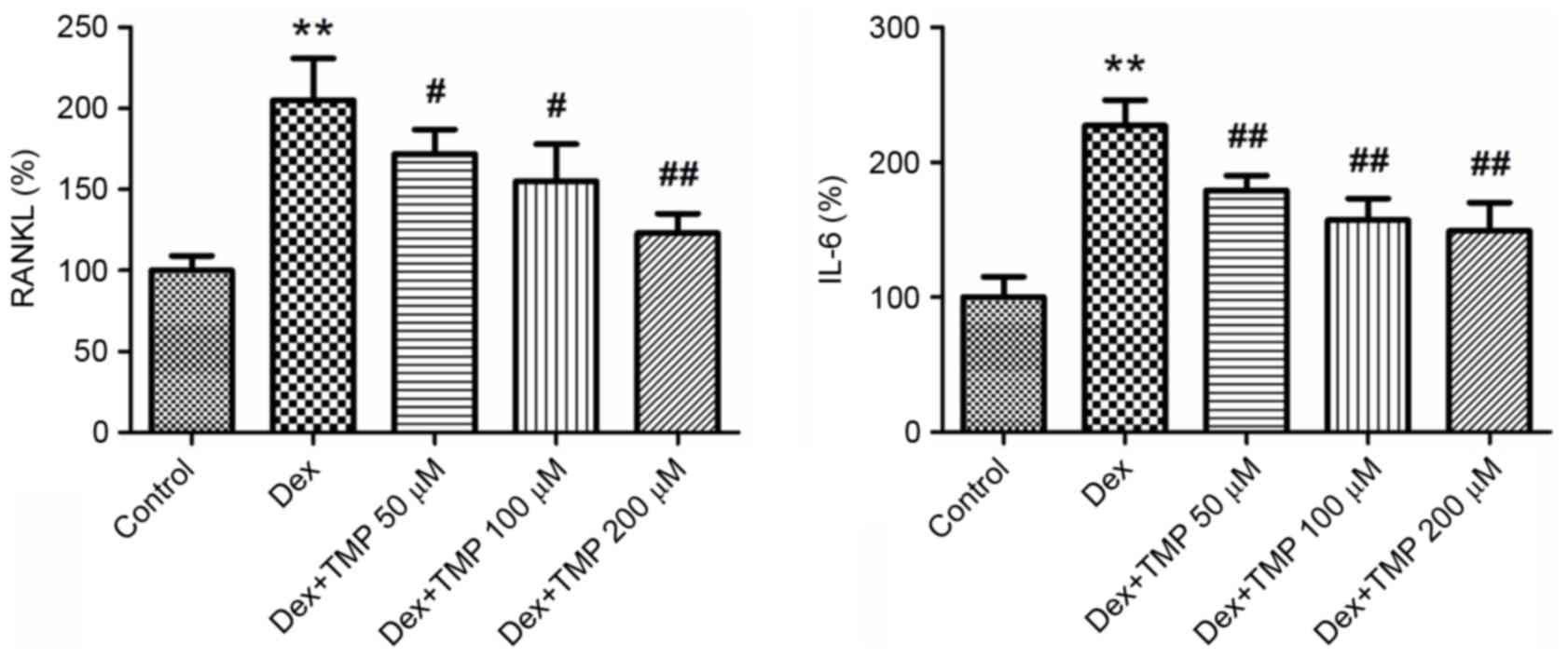
To investigate the possible reasons for the
protective effects of TMP under Dex exposure, the present study
measured the expression levels of RANKL and IL-6 in BMSCs using
ELISA analysis. RANKL, which is originally formed by osteoblasts,
binds to RANK on osteoclasts and results in osteoclast activation.
IL-6 also acts as an activator of osteoclast formation. The
expression levels of these two cytokines were significantly
elevated by Dex treatment. However, when different concentrations
of TMP were administered to the BMSCs, the levels of RANKL and IL-6
were significantly downregulated (Fig.
6). These results suggested that the inhibition of RANKL and
IL-6 in BMSCs is a possible mechanism for the protective effects of
TMP against Dex-induced osteoclastogenesis.
Discussion
The survival and function of BMSCs are essential for
the maintenance of bone tissue homeostasis, as are osteoclasts. In
our previous study, it was found that the viability of BMSCs
derived from GIOP rats was decreased, and that TMP protected the
BMSCs by inhibiting apoptosis in the GIOP state (22,29).
The present study further investigated the effects of TMP on the
osteogenic function of BMSCs and osteoclast formation to provide
evidence supporting the potential application of TMP in the
prevention and treatment of GIOP.
Long-term glucocorticoid therapy results primarily
in trabecular bone loss in patients with GIOP. As GIOP rats share
similar features with patients with GIOP, the micro-architecture of
the fourth lumbar vertebrae in GIOP rats adequately reflects the
change of bone mass in GIOP. Previous static bone histomorphometric
analysis has shown that trabecular bone mass is markedly decreased
in GIOP rats (30), which is
consistent with the data obtained in the present study. It has also
been reported that TMP therapy exerts positive effects in a rat
model of Parkinson's disease and spinal cord injury (23,31).
In the present study, it was confirmed that TMP treatment
ameliorated the decreased trabecular bone mass of the lumbar
vertebrae in the GIOP rats, and it may be a suitable candidate for
the prevention and treatment of GIOP. Of note, it was found that
treatment with TMP significantly increased the number of
OSX-positive cells and decreased the number of TRAP-positive cells
in vivo, which suggested that TMP affected osteogenesis and
osteoclastogenesis in the GIOP rats.
Although Dex is commonly used in the cell culture
medium to differentiate BMSCs into different types of mature cells,
the effects of different concentrations of Dex on BMSCs have been
reported to vary. Gao et al and others reported that
10−6 M Dex had negative effects on BMSCs obtained from
mice or rats (29,32). In the present study,
10−6 M Dex was also used to generate an in vitro
model of excess glucocorticoid exposure stress. The resulting data
demonstrated that 10−6 M Dex caused a decline in
cellular ALP activity, calcium mineralization and the expression of
osteogenic genes, which was consistent with the in vivo
results showing decreased osteogenesis in the GIOP rats. However,
TMP partially reversed the inhibitory effect of Dex on BMSC
osteogenic differentiation. These results suggested that TMP not
only improved the viability of BMSCs, but also ameliorated the
defective osteogenic differentiation of BMSCs, contributing to the
promotion of osteogenesis in GIOP.
The effects of osteoclasts in GIOP, and the effects
of glucocorticoids on osteoclast formation and function remain to
be fully elucidated. Kim et al documented that
glucocorticoids inhibited the proliferation of osteoclast
precursors in vitro (33).
Shi et al found that high doses of glucocorticoids promoted
osteoclastogenesis, whereas low doses had no such effects (18). The data obtained in the present
study showed that 10−6 M Dex markedly promoted
osteoclast formation in vitro, and TMP decreased osteoclast
numbers and the expression of osteoclast-specific genes under Dex
exposure. The inhibition of osteoclastogenesis in the presence of
glucocorticoids may account for the anti-osteoporotic effects of
TMP.
The RANKL secreted by osteoblasts, which belongs to
the tumor necrosis factor superfamily, regulates osteoclast
differentiation and leads to bone resorptive activities.
Osteoblasts also secrete IL-6, which affects the expression of
RANKL, enhances osteoclastogenesis and is implicated in the
pathogenesis of osteoporosis (34). It has been reported that the
enhancement of osteoclastogenesis in GIOP may be attributed to the
upregulated expression of RANKL caused by glucocorticoids (35). The findings of the present study
are consistent with this, which showed that TMP inhibited the
generation of RANKL and IL-6 in BMSCs exposed to glucocorticoids
following osteogenic induction. This data suggested that TMP may
indirectly affect osteoclasts by decreasing the expression of RANKL
and IL-6 in BMSCs, and partly explains why TMP reduces
osteoclastogenesis in GIOP. There are additional mechanisms
accounting for the effects of TMP on BMSCs and osteoclasts. Several
studies have reported that TMP decreases the production of reactive
oxygen species, and protects cells against toxicity and oxidative
stress (20,23). In addition to regulating RANKL and
IL-6, the anti-oxidative property of TMP may be involved in its
anti-GIOP effects, of which further investigation is required.
In conclusion, the present study investigated
whether TMP had an effect on the osteogenic differentiation of
BMSCs and formation of osteoclasts following exposure to excess
glucocorticoids in vivo and in vitro. The results
showed that TMP downregulated RANKL and IL-6, promoted osteogenesis
and inhibited osteoclastogenesis to ameliorate the change in bone
mass in the GIOP state. These results suggested that TMP may be a
promising drug for the prevention and treatment of GIOP.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81572192 and 81472043) and
the Program for Changjiang Scholars and Innovative Research Team in
University (grant no. IRT13051).
References
|
1
|
Hofbauer LC, Hamann C and Ebeling PR:
Approach to the patient with secondary osteoporosis. Eur J
Endocrinol. 162:1009–1020. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rizzoli R and Biver E:
Glucocorticoid-induced osteoporosis: Who to treat with what agent?
Nat Rev Rheumatol. 11:98–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Canalis E, Mazziotti G, Giustina A and
Bilezikian JP: Glucocorticoid-induced osteoporosis: Pathophysiology
and therapy. Osteoporos Int. 18:1319–1328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Brien CA, Jia D, Plotkin LI, Bellido T,
Powers CC, Stewart SA, Manolagas SC and Weinstein RS:
Glucocorticoids act directly on osteoblasts and osteocytes to
induce their apoptosis and reduce bone formation and strength.
Endocrinology. 145:1835–1841. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia D, O'Brien CA, Stewart SA, Manolagas
SC and Weinstein RS: Glucocorticoids act directly on osteoclasts to
increase their life span and reduce bone density. Endocrinology.
147:5592–5599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ash P, Loutit JF and Townsend KM:
Osteoclasts derived from haematopoietic stem cells. Nature.
283:669–670. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lagasse E and Weissman IL: Enforced
expression of Bcl-2 in monocytes rescues macrophages and partially
reverses osteopetrosis in op/op mice. Cell. 89:1021–1031. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bonyadi M, Waldman SD, Liu D, Aubin JE,
Grynpas MD and Stanford WL: Mesenchymal progenitor self-renewal
deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null
mice. Proc Natl Acad Sci USA. 100:pp. 5840–5845. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miura M, Chen XD, Allen MR, Bi Y, Gronthos
S, Seo BM, Lakhani S, Flavell RA, Feng XH, Robey PG, et al: A
crucial role of caspase-3 in osteogenic differentiation of bone
marrow stromal stem cells. J Clin Invest. 114:1704–1713. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen TL: Inhibition of growth and
differentiation of osteoprogenitors in mouse bone marrow stromal
cell cultures by increased donor age and glucocorticoid treatment.
Bone. 35:83–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Q, Shi J, Gao B, Zhang HY, Fan J, Li
XJ, Fan JZ, Han YH, Zhang JK, Yang L, et al: Gastrodin: An ancient
Chinese herbal medicine as a source for anti-osteoporosis agents
via reducing reactive oxygen species. Bone. 73:132–144. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boyce BF: Advances in osteoclast biology
reveal potential new drug targets and new roles for osteoclasts. J
Bone Miner Res. 28:711–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang K, Niu J, Kim H and Kolattukudy PE:
Osteoclast precursor differentiation by MCPIP via oxidative stress,
endoplasmic reticulum stress, and autophagy. J Mol Cell Biol.
3:360–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiu Y, Xu H, Zhao C, Li J, Morita Y, Yao
Z, Xing L and Boyce BF: Chloroquine reduces osteoclastogenesis in
murine osteoporosis by preventing TRAF3 degradation. J Clin Invest.
124:297–310. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi J, Wang L, Zhang H, Jie Q, Li X, Shi
Q, Huang Q, Gao B, Han Y, Guo K, et al: Glucocorticoids:
Dose-related effects on osteoclast formation and function via
reactive oxygen species and autophagy. Bone. 79:222–232. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L, Wei X, Hou Y, Liu X, Li S, Sun B,
Liu X and Liu H: Tetramethylpyrazine analogue CXC195 protects
against cerebral ischemia/reperfusion-induced apoptosis through
PI3K/Akt/GSK3β pathway in rats. Neurochem Int. 66:27–32. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong X, Ivanov VN, Davidson MM and Hei TK:
Tetramethylpyrazine (TMP) protects against sodium arsenite-induced
nephrotoxicity by suppressing ROS production, mitochondrial
dysfunction, pro-inflammatory signaling pathways and programed cell
death. Arch Toxicol. 89:1057–1070. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu N, Zhang Z, Chen P, Zhong Y, Cai X, Hu
H, Yang Y, Zhang J, Li K, Ge J, et al: Tetramethylpyrazine (TMP),
an active ingredient of Chinese herb medicine Chuanxiong,
attenuates the degeneration of trabecular meshwork through
SDF-1/CXCR4 axis. PLoS One. 10:e01330552015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Zhang HY, Gao B, Shi J, Huang Q,
Han YH, Hu YQ, Lu WG, Zhao ZJ, Liu BH, et al: Tetramethylpyrazine
protects against glucocorticoid-induced apoptosis by promoting
autophagy in mesenchymal stem cells and improves bone mass in
glucocorticoid-induced osteoporosis rats. Stem Cells Dev.
26:419–430. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu C, Zhang J, Shi X, Miao S, Bi L, Zhang
S, Yang Q, Zhou X, Zhang M, Xie Y, et al: Neuroprotective effects
of tetramethylpyrazine against dopaminergic neuron injury in a rat
model of Parkinson's disease induced by MPTP. Int J Biol Sci.
10:350–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bouffi C, Bony C, Courties G, Jorgensen C
and Noël D: IL-6-dependent PGE2 secretion by mesenchymal stem cells
inhibits local inflammation in experimental arthritis. PLoS One.
5:e142472010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Harimoto K, Liu J, Guo J, Hinshaw
S, Chang Z and Wang Z: Spata4 promotes osteoblast differentiation
through Erk-activated Runx2 pathway. J Bone Miner Res.
26:1964–1973. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen JJ, Zhang NF, Mao GX, He XB, Zhan YC,
Deng HB, Song DQ, Li DD, Li ZR, Si SY, et al: Salidroside
stimulates osteoblast differentiation through BMP signaling
pathway. Food Chem Toxicol. 62:499–505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Fan J, Lin YS, Guo YS, Gao B, Shi
QY, Wei BY, Chen L, Yang L, Liu J and Luo ZJ: Glucocorticoids
induce autophagy in rat bone marrow mesenchymal stem cells. Mol Med
Rep. 11:2711–2716. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cui L, Li T, Liu Y, Zhou L, Li P, Xu B,
Huang L, Chen Y, Liu Y, Tian X, et al: Salvianolic acid B prevents
bone loss in prednisone-treated rats through stimulation of
osteogenesis and bone marrow angiogenesis. PLoS One. 7:e346472012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu J, Cao Y, Wu T, Li D and Lu H: Micro-CT
as a tool to investigate the efficacy of tetramethylpyrazine in a
rat spinal cord injury model. Spine (Phila Pa 1976). 41:1272–1278.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao B, Huang Q, Jie Q, Zhang HY, Wang L,
Guo YS, Sun Z, Wei BY, Han YH, Liu J, et al: Ginsenoside-Rb2
inhibits dexamethasone-induced apoptosis through promotion of
GPR120 induction in bone marrow-derived mesenchymal stem cells.
Stem Cells Dev. 24:781–790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim HJ, Zhao H, Kitaura H, Bhattacharyya
S, Brewer JA, Muglia LJ, Ross FP and Teitelbaum SL: Glucocorticoids
suppress bone formation via the osteoclast. J Clin Invest.
116:2152–2160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Papanicolaou DA and Vgontzas AN:
Interleukin-6: The endocrine cytokine. J Clin Endocrinol Metab.
85:1331–1333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka Y: Glucocorticoid and bone
metabolism and disease. Clin Calcium. 23:229–235. 2013.(In
Japanese). PubMed/NCBI
|