Introduction
Liver transplantation is considered to be the most
effective solution to various end-stage liver pathologies. The
persistent shortage of suitable donors that have had brain death
confirmed has prompted a reexamination of donors after circulatory
death (DCD) to extend the pool of organs for transplantation.
However, DCD is considered as a primary risk factor to inferior
liver transplantation outcomes and complications, including primary
non-function and early allograft dysfunction, due to the higher
vulnerability to ischemia-reperfusion injury (IRI) (1). Therefore, novel strategies that
improve the preservation conditions of DCD organs and retain their
vitality are required (2,3). Various experimental and clinical
reports have indicated the effectiveness of hypothermic machine
perfusion (HMP) in improving DCD liver preservation when compared
with cold storage (CS), as HMP attenuates IRI compared with CS
(4–6). HMP is a dynamic hypothermic technique
for preserving transplantation organs. It simulates the
physiological conditions of organs by supplying them with oxygen
and/or key nutrients, and removing waste products (4,7).
However, the molecular mechanisms underlying the attenuation of IRI
by HMP remain unclear.
Post-translational modification has important
functions in various cellular processes (8). Acetylation, one type of
post-translational modification, is essential for the activation of
the transcription factor nuclear factor-κB (NF-κB) (9–11),
which is one of the critical contributors to IRI (12–15).
Acetylation of RelA/p65 at lysine 310, a subunit of NF-κB, was
reported to modulate various transcriptional activities of NF-κB
(11). Activated NF-κB is
controlled by a feedback mechanism via deacetylation. Sirtuin-1
(SIRT-1), a member of the sirtuin family, is a deacetylase that has
been reported to reduce IRI in a nicotinamide adenine dinucleotide
(NAD)+-dependent manner (16–18).
Furthermore, SIRT-1 has been demonstrated to inhibit NF-κB by
deacetylating RelA/p65 at lysine 310 in non-small-cell lung cancer
cells (19). Therefore, given the
protective role of SIRT-1 in IRI, the present study aimed to
investigate the modulatory effects of HMP on SIRT-1 in regulating
NF-κB p65 activity, which in turn protects DCD livers from IRI.
Materials and methods
Animals
A total of 6 adult male Sprague-Dawley rats (age,
8–10 weeks; weight, 250–300 g) were obtained from the Experimental
Animal Culture Center of Hubei Centers for Disease Control (Wuhan,
China). All animals were housed under standard laboratory
conditions (temperature, 25±2°C; relative humidity, 55±5%; a 12-h
light/dark cycle) with free access to food and water. All animal
procedures were approved by the Animal Ethics Committee of Wuhan
University and handled in accordance with the Experimental Animal
Regulations of the People's Republic of China and the Guide for the
Care and Use of Laboratory Animals of the USA (20).
HMP and isolated perfused rat liver
(IPRL) system
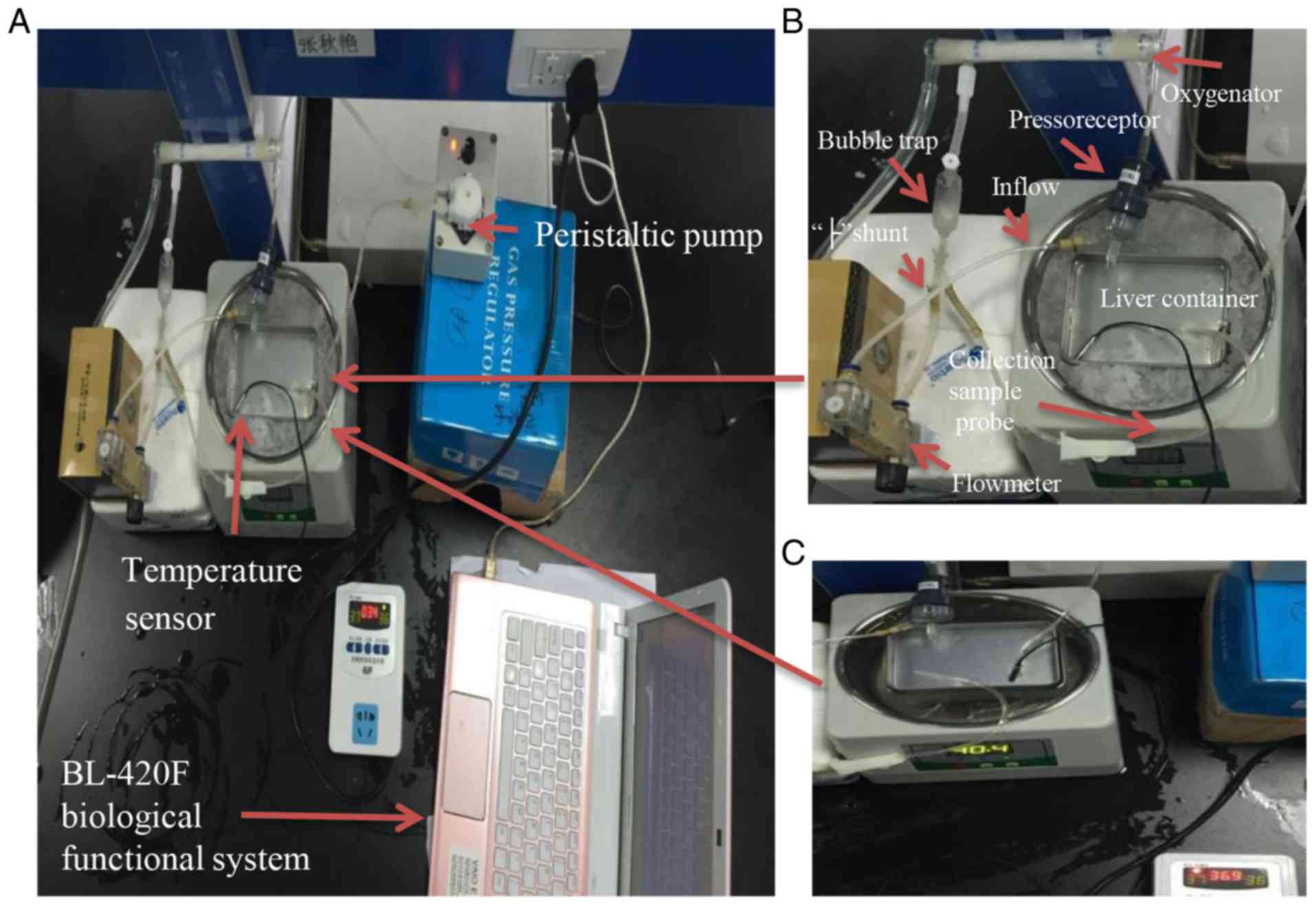
A photograph of the recirculating perfusion system
is presented in Fig. 1. It
consisted of a thermostat water bath (Jiaxing Zhongxin Medical
Instruments Co., Ltd., Jiaxing, China) containing water or ice
water mixture, a liver container for perfusion installed in the
water bath, a temperature sensor (Jiangsu Singhe Electronic
Technology Co., Ltd., Suqian, China) inserted into the container to
measure the fluid temperature, a peristaltic pump (Shanghai Jingke
Industrial Co., Ltd., Shanghai, China), a hollow-fiber membrane
oxygenator (Dongguan Kewei Medical Instrument Co., Ltd., Dongguan,
China), a self-made bubble trap used to communicate between the ‘├’
shunt tube and the oxygenator to deliver the bubble, a flow meter
(Nanjing Careermen Instrument Co., Ltd., Nanjing, China) connected
to one side of the ‘├’ shunt tube to measure the hepatic inflow,
and the other side of the ‘├’ shunt tube was used as a probe for
liver inflow sample collection. To measure the perfusion portal
pressure, a portal vein cannula was connected to the baroreceptor
of a BL-420F biological functional system (Chengdu Techman Software
Co., Ltd., Chengdu, China).
The HMP system (Fig. 1A
and B) consisted of the above-mentioned recirculating perfusion
system without an oxygen supply. The water bath was powered off and
filled with ice water mixture to maintain the temperature of
perfusate at 0–4°C.
The IPRL system (Fig.
1A and C) consisted of all of the above-mentioned parts of the
recirculating perfusion system. In the present study, the top of
the water in the water bath, where a container for the rat liver
was installed, had a lower temperature compared with the bottom.
According to the results of a preliminary experiment, the water
bath was set at 40°C to maintain the temperature of perfusate in
the liver container at 36–37°C. Krebs-Henseleit buffer (Macgene
Biotechnology, Ltd., Beijing, China) with 4% dextran was added into
the liver container for reperfusion (21). The perfusate was oxygenated (95%
O2 and 5% CO2; pO2 >500 mm Hg)
and recirculated for 2 h at 36–37°C under a portal venous perfusion
pressure of 10.3 mm Hg, as described previously (22).
Surgery and experimental design
Rats were anesthetized with chloral hydrate (10%; 3
ml/kg; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) by
intraperitoneal injection. A midline incision was used to expose
the abdomen, the liver was freed from all ligamentous attachments
and the common bile duct was cannulated with an epidural guiding
tube (Jiangsu Changfeng Medical Industry Co., Ltd., Yangzhou,
China) to collect the total bile outflow during reperfusion.
Cardiac death was induced by hypoxia following
incision of the diaphragm without portal clamping or prior
heparinization (23). The warm
ischemia time in situ began from the point of cardiac
arrest. After 30 min, an intravenous injection of 2 ml saline
containing 100 IU heparin (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) via the right iliac vein was performed
(24). Subsequently, the hepatic
artery was ligated, the portal vein was cannulated by a self-made
polyethylene (PE) catheter (outer diameter, 2.1 mm; inner diameter,
1.8 mm) and flushed in situ with 20 ml 4°C
histidine-tryptophan-ketoglutarate (HTK) solution (Dr. Franz Köhler
Chemie GmbH, Bensheim, Germany). The liver was then removed and a
PE catheter (internal diameter, 3 mm) was cannulated into the
suprahepatic inferior vena cava for the collection of hepatic
effluent (25). Finally, the liver
was randomly assigned either to the CS or HMP group.
In the CS group (n=3), livers underwent static CS in
150 ml HTK solution at 0–4°C for 3 h. In the HMP group (n=3),
livers were connected to the HMP system (Fig. 1B). HMP was performed via the portal
vein with 0–4°C HTK solution (150 ml) for 3 h at a rate of 0.5
ml/g/min without oxygenation, as detailed previously (25).
Investigating IRI using an IPRL
system
IPRL is an in vitro system that permits the
investigation of physiological and pathophysiological conditions in
rat livers, including IRI in DCD livers after transplantation
(26). After 3 h of CS or HMP
preservation, the viability of all livers was evaluated using IPRL,
as detailed previously (23). To
simulate the slow rewarming period in liver transplantation, livers
were left untouched on a petri dish at room temperature for ~15 min
prior to reperfusion (23). During
the normothermic reperfusion (NR), which was performed for 2 h, the
portal pressure and portal flow were measured for the calculation
of the intrahepatic resistance (IHR) using the following formula:
IHR (mmHg/ml/min)=portal pressure (mmHg)/portal flow (ml/min).
Hepatic effluent was collected through the PE
catheter cannulated in the suprahepatic vena cava every hour and
analyzed for the enzyme activities of alanine transaminase (ALT),
aspartate transaminase (AST) and lactate dehydrogenase (LDH), and
oxygen (O2) consumption rate. The enzyme activities were
assessed using ALT (cat. no. C009-2), AST (cat. no. C010-2) and LDH
assay kits (cat. no. A020-2; all Nanjing Jiancheng Bioengineering
Institute), according to the manufacturer's protocol.
Hepatic O2 consumption was used to assess
the metabolic activity of the livers. Perfusate samples were
respectively collected from the portal inflow and the PE catheter
placed in the suprahepatic vena cava. Subsequently, the
O2 content in perfusate samples was measured immediately
by a pH-blood gas analyzer (i-STAT; Abbott Point of Care, Inc.,
Princeton, NJ, USA). O2 consumption by livers was
calculated according to standard formulas, as detailed previously
(22).
Bile was collected through the epidural guiding tube
placed in the common bile duct every hour. As the density of bile
was equal to the water, the bile flow was gravimetrically estimated
and expressed as µl/h/g liver.
At the end of the 2 h in vitro isolated
perfusion period (the end of the IPRL assay), livers were weighed
and a portion was fixed in 10% formaldehyde for 24 h at room
temperature for histological analysis. The rest of the liver was
immersed in liquid nitrogen and stored at −80°C until subsequent
experiments.
Measurement of adenosine triphosphate
(ATP) content in livers
Liver samples (20 mg) stored at −80°C were
homogenized using 4.2% perchloric acid and 1 mM diethylenetriamine
pentaacetic acid. Subsequently, samples were centrifuged at 14,000
× g and 4°C for 5–10 min, and the supernatants were collected and
brought to pH 6 by 69% K2CO3. Finally, ATP
content was measured using an ATP assay kit (Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
protocol. Protein concentration was determined using the
bicinchoninic acid (BCA) method; ATP levels were expressed as
µmol/g protein.
Measurement of malondialdehyde (MDA)
content and superoxide dismutase (SOD) activity in livers
Frozen liver samples were prepared and centrifuged
as described for determination of ATP levels, subsequently, the
supernatants were collected and used immediately for MDA content
and SOD activity assays, according to the standard protocol of the
MDA assay (cat. no. A003-1; Nanjing Jiancheng Bioengineering
Institute) and SOD assay kits (cat. no. A001-1-1; Nanjing Jiancheng
Bioengineering Institute).
Histology
The formaldehyde (10%)-fixed liver samples were
paraffin-embedded and serially cut to 5-µm sections, which was
followed by staining with hematoxylin and eosin (HE; hematoxylin
staining for 5–15 min and eosin staining for 1–3 min; all performed
at room temperature) for histological analysis at ×200
magnification using a light microscope.
Western blotting
Rat liver tissues were homogenized and lysed with
lysis buffer (50 mM Tris-HCl, 137 mM NaCl, 10% glycerol, 100 mM
sodium orthovanadate, 1 mM PMSF, 10 mg/ml aprotinin, 10 mg/ml
leupeptin, 1% Nonidet P-40 and 5 mM protease inhibitor cocktail; pH
7.4; Beyotime Institute of Biotechnology, Jiangsu, China). Protein
concentration was determined by a BCA assay (Beyotime Institute of
Biotechnology). The proteins (30 µg/lane) were combined with
β-mercaptoethanol and bromophenol blue for electrophoresis, and
separated via 10% PAGE. Proteins were then transferred to
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), which were blocked with 5% skim milk for 2 h at
room temperature. Membranes were then incubated overnight at 4°C
with the following primary antibodies: Rabbit anti-SIRT-1 (cat. no.
bs-0921R; 1:400; Beijing Biosynthesis Biotechnology Co., Ltd,
Beijing, China), rabbit anti-NF-κB p65 (cat. no. bs-20355R; 1:400;
Beijing Biosynthesis Biotechnology Co., Ltd.), rabbit anti-acetyl
(K310) p65 (cat. no. ab19870; 1:300; Abcam, Cambridge, UK) and
rabbit anti-β-actin (cat. no. GB13001-3; 1:1,500; Wuhan Goodbio
Technology Co. Ltd., Wuhan, China). This was followed by incubation
with horseradish peroxidase-conjugated goat anti-rabbit IgG (cat.
no. GB23303; 1:5,000; Wuhan Goodbio Technology Co. Ltd.) for 1 h at
room temperature. The reactive bands were visualized using an
Electrochemiluminescence kit (cat. no. P0018; Beyotime Institute of
Biotechnology). Quantification of protein bands was performed using
ImageJ v1.42q software (National Institutes of Health, Bethesda,
MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for interleukin (IL)-6 and
tumor necrosis factor (TNF)-α mRNAs in liver tissues
RNA was extracted from the frozen liver tissue of
rats using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham,
MA, USA), according to the manufacturer's protocol, and quantified
using a NanoDrop instrument (Thermo Fisher Scientific, Inc.). RNA
was reverse-transcribed into cDNA using the Thermo Scientific
RevertAidä First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) with a total volume of 20 µl, containing total
RNA (1 µg), Oligo(dT)18 primer (1 µl), 5X Reaction
Buffer (4 µl), RiboLock RNase Inhibitor (20 U/µl; 1 µl), 10 mM dNTP
Mix (2 µl), RevertAid M-MuLV RT (200 U/µl; 1 µl) and water
(nuclease-free). The reverse transcription reactions were performed
with the following conditions: 42°C for 1 h and 75°C for 5 min.
qPCR was performed using the SYBR® Select Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) in a
StepOnePlus Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for
10 sec and 60°C for 30 sec. All of the reactions were performed 3
times in triplicate. The relative mRNA expression was analyzed by
the comparative threshold cycle (2−∆∆Cq) method
(27) and the results were
normalized to those of β-actin. The primer sequences used were as
follows: TNF-α, 5′-AGTCCGGGCAGGTCTACTTT-3′ (forward) and
5′-TTCAGCGTCTCGTGTGTTTC-3′ (reverse); IL-6,
5′-GGTCTTCTGGAGTTCCGTTTC-3′ (forward) and
5′-TCCATTAGGAGAGCATTGGAA-3′ (reverse). β-actin,
5′-TGCTATGTTGCCCTAGACTTCG-3′ (forward) and
5′-GTTGGCATAGAGGTCTTTACGG-3′ (reverse).
Assessment of NAD+/NADH in
the liver
The levels of NAD+ and NADH in liver
tissue homogenate were measured using the Coenzyme I NAD (H)
content test kit (Nanjing Jiancheng Bioengineering Institute).
Total NAD+ and NADH were quantified according to the
manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using SPSS v16.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard error of the mean. After the assumption of
normality and equal variance between groups was confirmed, the
differences between CS and HMP groups were analyzed using
independent samples t-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
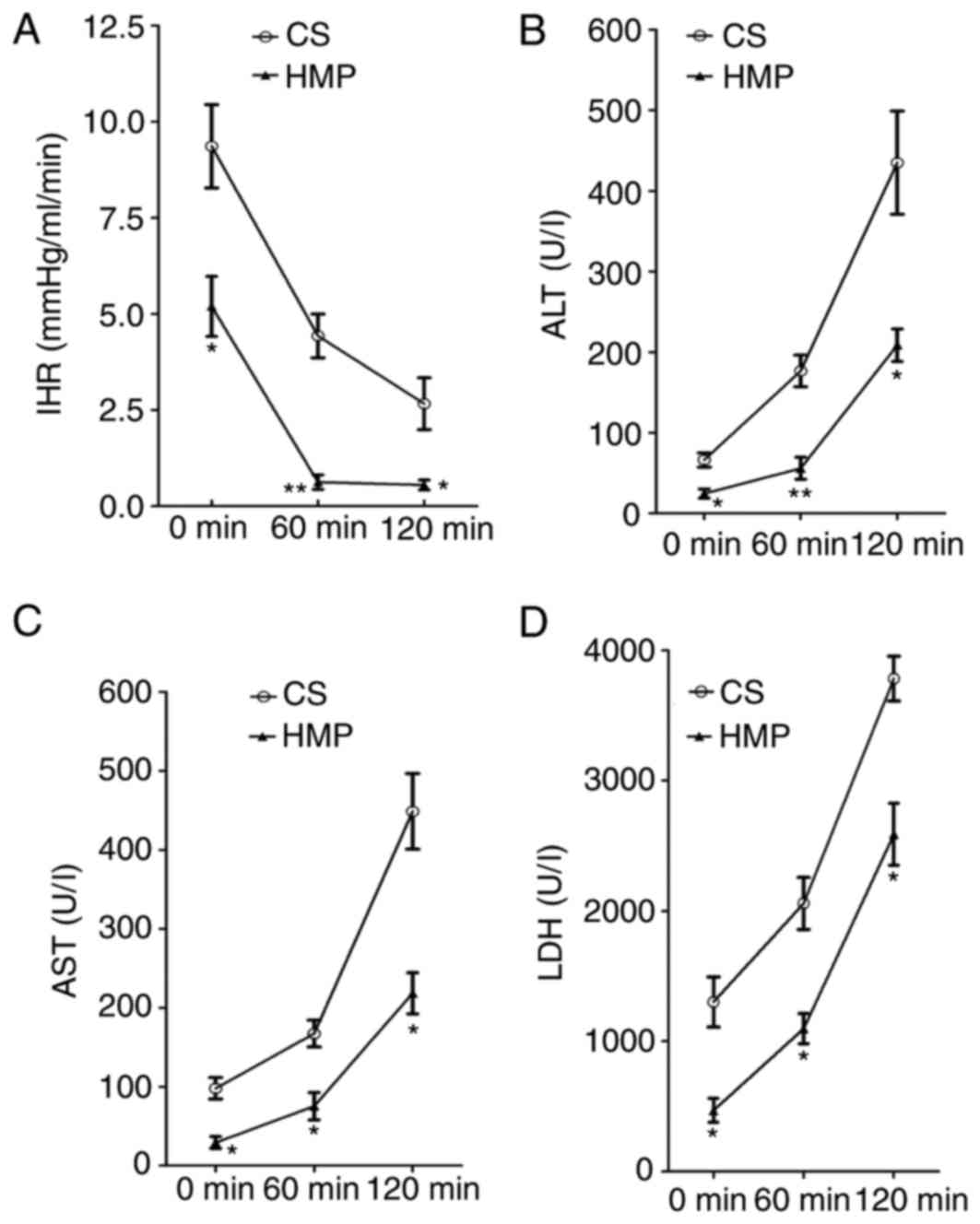
HMP decreases IHR during NR
The portal pressure and portal flow were used to
calculate the IHR of DCD livers preserved by static CS or HMP at
various time points during NR. A significant difference was
observed between the CS and HMP groups at each analyzed time point
during NR (Fig. 2A). IHR in the
livers in the CS group was significantly higher compared with the
HMP group at 0 (9.36±1.08 vs. 5.20±0.78 mmHg/ml/min; P=0.036), 60
(4.43±0.57 vs. 0.63±0.19 mmHg/ml/min; P=0.003) and 120 min
(2.67±0.67 vs. 0.56±0.13 mmHg/ml/min; P=0.037).
HMP reduces the release of ALT, AST
and LDH during NR
To characterize the hepatocyte viability, the
release of ALT, AST and LDH enzymes was determined during
reperfusion. Increasing levels of ALT, AST and LDH enzymes were
observed during the course of NR in CS and HMP groups. However, a
significant reduction of ALT enzyme leakage was observed in the HMP
group compared with the CS group at 0 (24.67±5.61 vs. 66.33±8.67
U/l; P=0.016), 60 (56.00±13.58 vs. 176.67±19.68 U/l; P=0.007) and
120 min (208.67±20.28 vs. 435.00±63.89 U/l; P=0.028), as presented
in Fig. 2B. Similar patterns were
observed for AST and LDH levels in the HMP and CS groups. AST
levels were significantly reduced in the HMP group at 0 (29.33±7.54
vs. 98.00±13.58 U/l; P=0.011), 60 (75.33±17.42 vs. 167.33±16.95
U/l; P=0.019) and 120 min (218.33±25.99 vs. 448.67±47.91 U/l;
P=0.013; Fig. 2C). Similarly, LDH
levels were significantly decreased in the HMP group compared with
the CS group at 0 (469.77±92.05 vs. 1,301.97±193.53 U/l; P=0.018),
60 (1,096.50±115.13 vs. 2,058.33±199.25 U/l; P=0.014) and 120 min
(2,588.50±237.96 vs. 3,784.07±172.23 U/l; P=0.015; Fig. 2D).
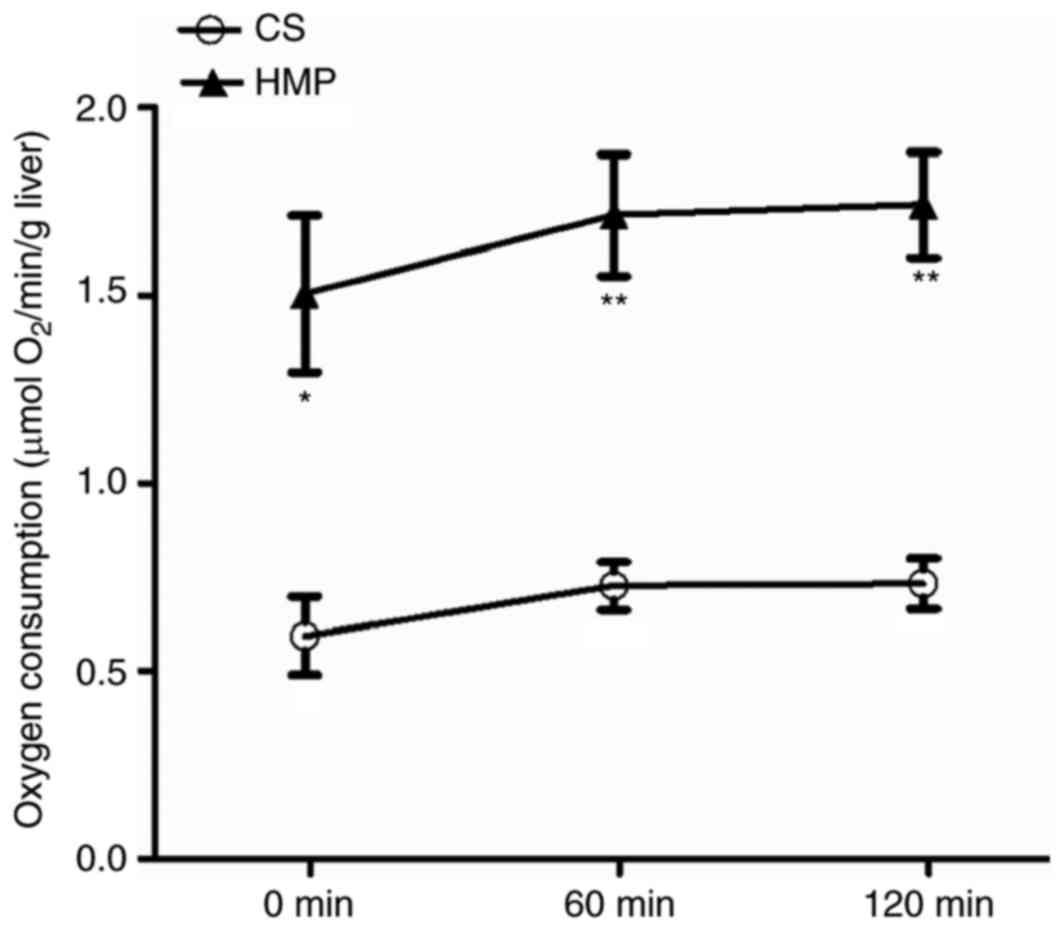
HMP improves O2 consumption
rate, ATP levels and bile production in DCD rat livers
Efficient O2 consumption is crucial for
ATP production, which maintains the energy status of cells. The
influence of CS and HMP on the O2 consumption of DCD rat
livers was analyzed during reperfusion (Fig. 3). DCD livers in the HMP group
exhibited significantly higher rates of O2 consumption
when compared with the CS group at 0 (1.50±0.21 vs. 0.59±0.10 µmol
O2/min/g liver; P=0.017), 60 (1.71±0.16 vs. 0.73±0.06
µmol O2/min/g liver; P=0.005) and 120 min (1.74±0.14 vs.
0.73±0.07 µmol O2/min/g liver; P=0.003). Anaerobic
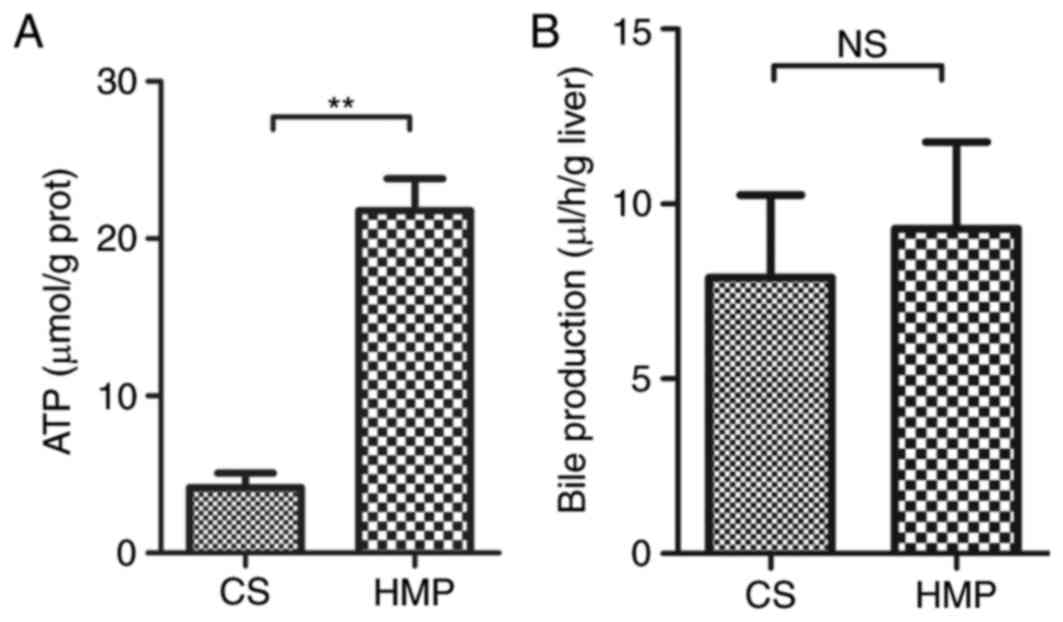
metabolism leads to rapid depletion of ATP, which in turn leads to
the disruption of homeostasis. Therefore, the present study also
determined ATP levels in HMP or CS preserved DCD livers. In the HMP
group, ATP levels in liver tissues obtained after 2 h reperfusion
were significantly higher compared with the CS group (21.80±2.01
vs. 4.16±0.93 µmol/g protein; P=0.001; Fig. 4A). However, no significant
difference in the production of bile flow was observed between
livers preserved by HMP or CS (Fig.
4B). Notably, the bile flow production was marginally higher in
the HMP group compared with the CS group, however, this did not
reach statistical significance after 120 min reperfusion (9.29±2.48
vs. 7.90±2.36 µl/h/g liver; P=0.704).
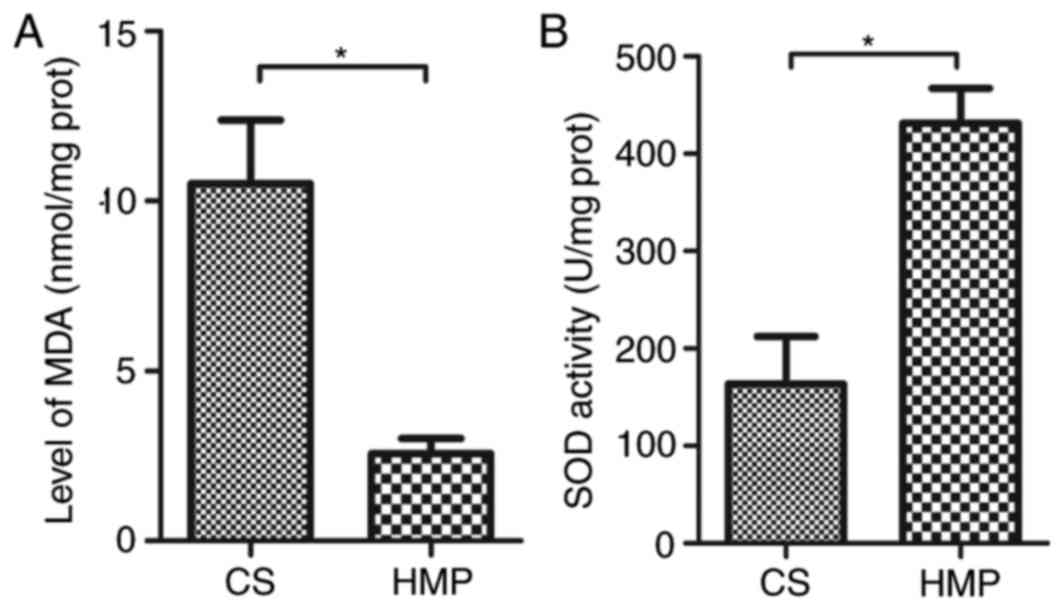
HMP attenuates oxidative damage
following NR
MDA, an end-product of lipid oxidation, serves as an
important indicator for the assessment of oxidative damage. There
was a significant reduction in MDA levels in livers preserved by
HMP compared with those preserved by CS (2.57±0.45 vs. 10.52±1.86
nmol/mg protein; P=0.014; Fig.
5A), which indicates lower levels of oxidative stress following
HMP. In addition, an increase in the activity of SOD was observed
in livers of the HMP group compared with the CS group (431.41±35.99
vs. 163.34±48.97 U/mg protein; P=0.012; Fig. 5B), indicating a higher antioxidant
activity in the cells preserved by HMP.
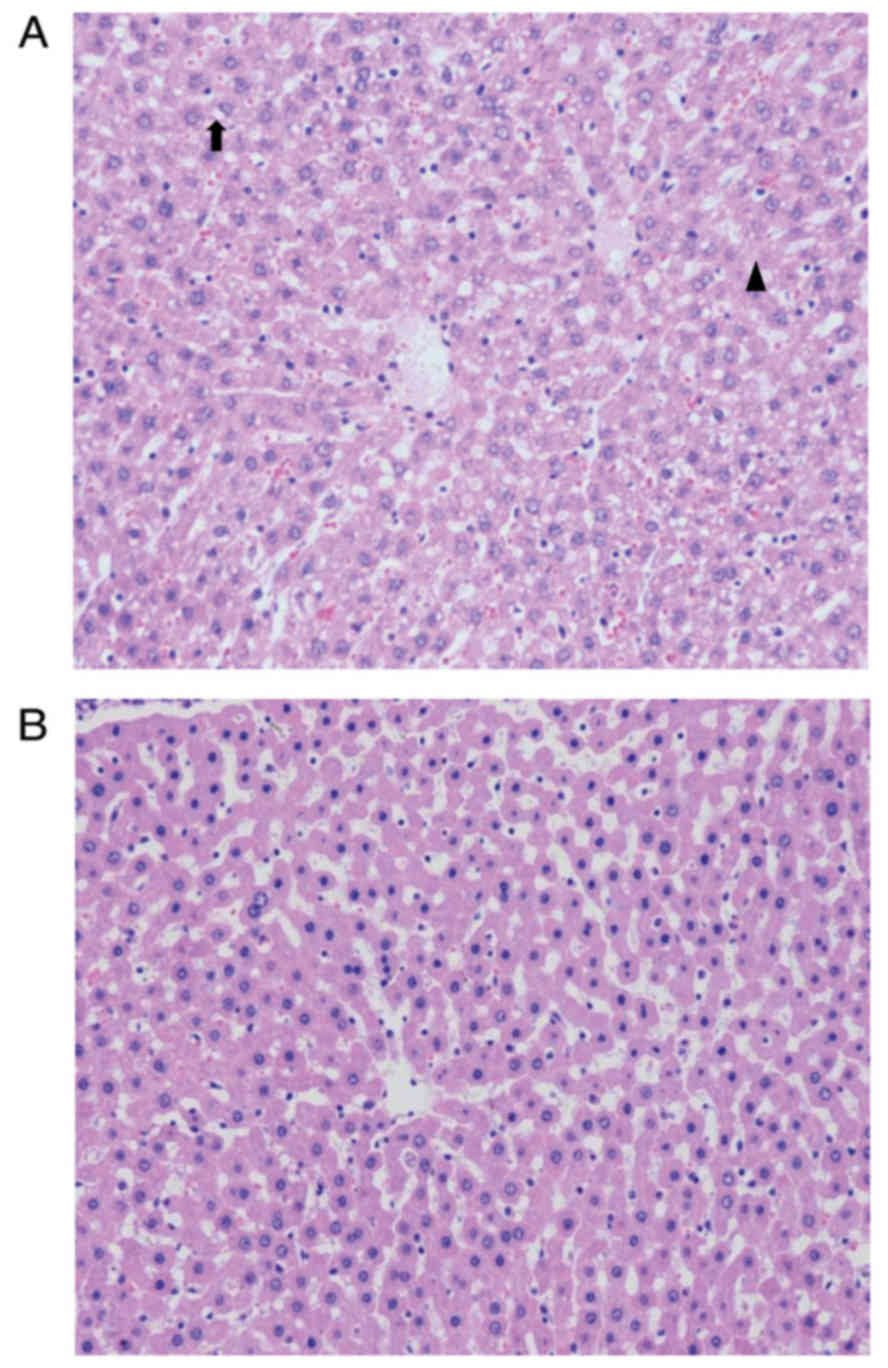
HMP improves the hepatic morphology of
DCD rats
HE staining demonstrated a more intact hepatic
cytoarchitecture in tissues preserved by HMP compared with those
preserved by CS. In the HMP group, hepatic sinusoids were dilated
due to the perfusion pressure, however, few sinusoidal endothelial
cells exhibited signs of shrinkage and detachment. In addition,
areas of hydropic changes and necrosis were limited in livers
preserved by HMP compared with those preserved by CS (Fig. 6).
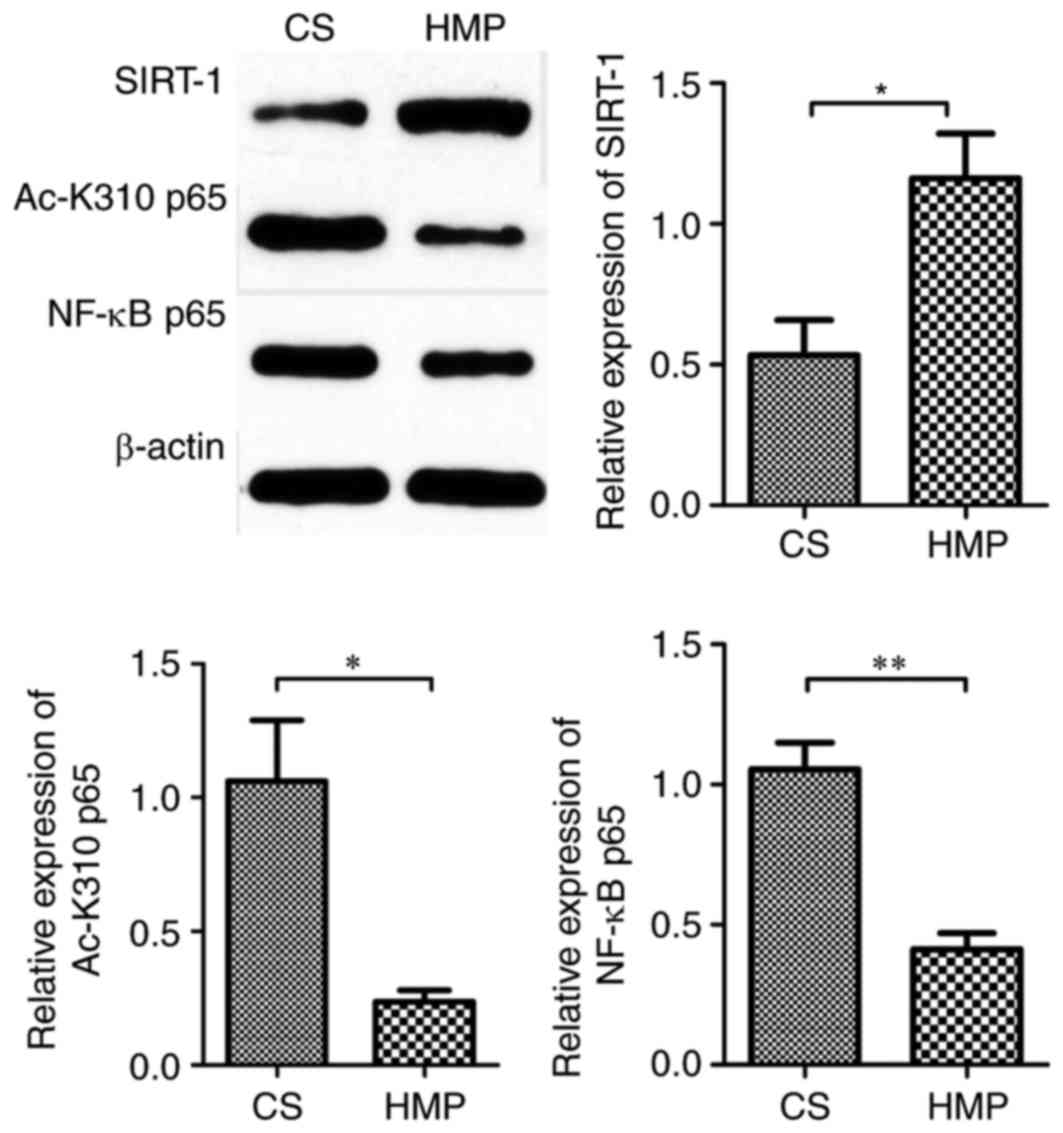
HMP attenuates hepatic NF-κB
p65-induced inflammatory response in DCD rats via SIRT-1-mediated
deacetylation of the acetylated-NF-κB p65
The present study subsequently determined the
protein expression of NF-κB p65 and observed a significant decrease
in the expression in livers preserved by HMP compared with the CS
group (0.41±0.06 vs. 1.05±0.09; P=0.004; Fig. 7). The activation of NF-κB p65 is
reversed by deacetylation. Therefore, to investigate whether HMP
attenuated hepatic NF-κB p65 activity via SIRT-1-mediated
deacetylation of NF-κB p65, the protein expression of SIRT-1 and
acetylated-NF-κB p65 (an active form of post-translationally
modified NF-κB p65) was also determined by western blotting. SIRT-1
expression levels were increased in the HMP group compared with the
CS group (1.16±0.16 vs. 0.53±0.12; P=0.035; Fig. 7). In addition, the protein
expression of acetylated-NF-κB p65 was significantly reduced by HMP
preservation when compared with CS preservation (0.24±0.04 vs.
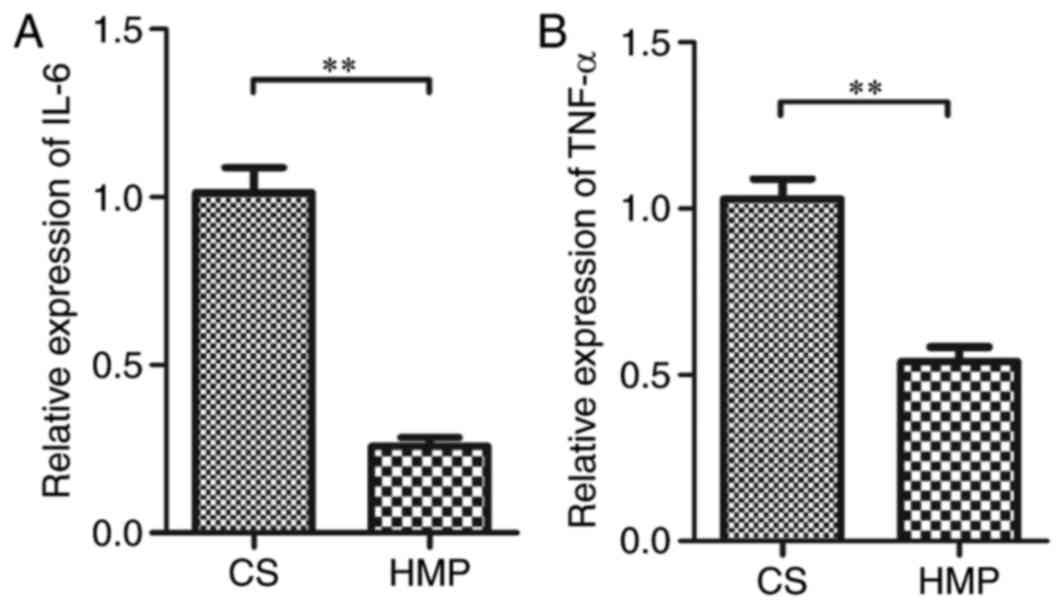
1.06±0.23; P=0.023; Fig. 7). IL-6
and TNF-α are NF-κB-mediated genes. Therefore, to assess the level
of the NF-κB p65-induced inflammatory response, the mRNA expression
of the inflammatory cytokines IL-6 and TNF-α was determined. The
mRNA expression of IL-6 was significantly reduced in the HMP group
compared with the CS group (0.26±0.03 vs. 1.01±0.08;
P=3.06×10−8; Fig. 8A).
Similar results were obtained for TNF-α expression in the HMP and
the CS groups (0.54±0.04 vs. 1.03±0.06; P=2.59×10−6;
Fig. 8B). These results indicated
that the inflammatory response induced by NF-κB p65 was attenuated
by HMP preservation.
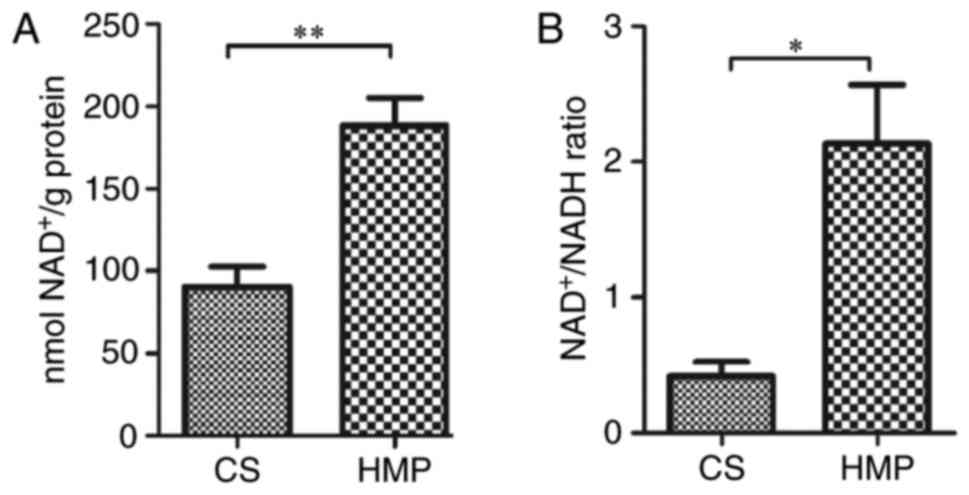
In addition to the protein expression of SIRT-1 and
acetylated-NF-κB p65, SIRT-1 activity indicators (hepatic
NAD+ levels and NAD+/NADH ratio) were also
measured. Consistent with results for SIRT-1 protein expression
(Fig. 7), SIRT-1 activity was
increased in the DCD livers preserved by HMP compared with the CS
group, as indicated by higher levels of NAD+
(188.14±16.89 vs. 90.61±12.03 nmol NAD+/g protein;
P=0.009; Fig. 9A) and
NAD+/NADH ratio (2.13±0.43 vs. 0.42±0.11; P=0.018;
Fig. 9B). These results indicate
that HMP preservation may attenuate the hepatic NF-κB p65-induced
inflammatory response in DCD rats via SIRT-1-mediated deacetylation
of NF-κB p65.
Discussion
The increasing demand for liver transplantation from
DCD donors means that enhanced organ preservation strategies
against IRI are required. IRI involves multiple complex mechanisms,
including direct ischemic damage to hepatocytes, indirect cellular
injury resulting from the accumulation of reactive oxygen species
(ROS) and inflammatory response (28–30).
The results of the present study indicate that HMP exhibits a
higher efficiency in preserving the functional and structural liver
integrity by attenuating IRI.
Cellular depletion of ATP due to O2
shortage is a major cause of IRI, which in turn induces serious
deleterious effects on hepatocytes (31). Hypothermia slows the cellular
metabolism and reduces O2 demands, however, the
mitochondrial functions are not terminated. It was previously
reported that HMP preservation solution had sufficient dissolved
O2 for cellular aerobic respiration, even in the absence
of active oxygenation (4). In the
current study, the compromised O2 consumption during
reperfusion in the CS group was significantly improved by HMP,
leading to higher ATP levels. This result is consistent with
previous reports indicating that HMP provided sufficient
O2 supply to meet the demands of cellular metabolism by
a continuous circulation of hypothermic solution (4,32,33).
Furthermore, bile production during reperfusion was determined to
assess the functional recovery of the livers. In the present study,
although not statistically significant, bile production in the HMB
group was marginally higher compared with the CS group. This may be
attributed to the fact that the blood supply of bile duct came from
the hepatic artery, which was not perfused. The current study
performed perfusion via the portal vein, which may have led to
insufficient O2 and nutrient supply for the bile
duct.
Oxidative damage incurred by ROS generated under
hypothermia and hypoxia conditions is a crucial factor for
determining the severity of IRI. The present study observed a
significant reduction in the levels of the oxidative stress marker,
MDA, indicating that oxidative damage to the graft was attenuated
following HMP preservation compared with CS preservation.
Furthermore, an increase in the activity of SOD following
reperfusion was observed in HMP preservation compared with CS,
which indicates a cellular attempt to ameliorate the oxidative
damage.
The inflammatory response has a pivotal role in
liver IRI. In the present study, RT-qPCR demonstrated that the mRNA
expression of the inflammatory cytokines IL-6 and TNF α were
significantly decreased in HMP compared with the CS group.
Furthermore, the parenchymal integrity was examined by detecting
the release of ALT, AST and LDH enzymes. The results demonstrated a
significant reduction in the ALT, AST and LDH levels in samples
preserved by HMP compared with CS at the different time points.
Similar results were also observed in histological analysis. HMP
preservation improved the histological structure of DCD livers
compared with CS. These results verify that HMP improved the
preservation of the cellular integrity of hepatocytes compared with
the CS technique. The lower level of enzyme release and improved
histological results may be attributed to the improvement of
O2 consumption and ATP levels, and the attenuation of
oxidative damage and inflammatory response following HMP
preservation. These results were consistent with previous reports
(4–6).
NF-κB p65 has been reported to have a critical role
in the development of oxidative damage and inflammatory response in
IRI (12–15). Compared with the CS group, the
protein expression of NF-κB p65 was significantly reduced by HMP
preservation. Furthermore, the mRNA expression on the inflammatory
cytokines IL-6 and TNF-α, members of the NF-κB p65 transcript genes
(34), were significantly
decreased in the HMP livers. Therefore, we hypothesized that HMP
may inhibit the inflammatory response during IRI by reducing the
activation of NF-κB p65. However, the upstream molecular mechanism
required further investigation.
In the mitochondria, hypoxia causes the dysfunction
of respiratory chain, which subsequently leads to a decrease in the
levels of NAD+ and ratio of NAD+/NADH
(31). As a dynamic preservation
technique, it has been reported that HMP provides sufficient
O2 supply by the continuous circulation of the
hypothermic solution to maintain the cellular metabolism (4). Consistent with this report, the
results of the present study demonstrated that HMP livers exhibited
significantly increased O2 consumption, leading to
higher NAD+ levels and ratio of NAD+/NADH
compared with livers preserved by CS. SIRT-1, a class III histone
deacetylase, has been reported to reduce IRI through various
cellular processes. SIRT-1 activity requires the presence of
NAD+ as a substrate to deacetylate the downstream
proteins (16–18,35,36).
In addition, SIRT-1 was previously reported to protect the liver
from IRI (16–18). In the present study, DCD livers
preserved by HMP exhibited a significantly higher expression of
SIRT-1 compared with the CS group, indicating the superiority of
HMP preservation in protecting DCD rat livers from IRI. This
result, combined with the increase in NAD+ levels and
the ratio of NAD+/NADH, and the reduced expression of
NF-κB p65, IL-6 and TNF-α, led us to hypothesize that HMP
preservation may ameliorate the NF-κB p65-induced inflammatory
response during IRI in DCD rat livers via a SIRT-1-NAD+
dependent pathway.
Yeung et al (19) previously reported that SIRT-1
inhibited the transcriptional activity of NF-κB p65 by
deacetylating RelA/p65, a subunit of NF-κB, at lysine 310 in
non-small-cell lung cancer cells. In the present study, the
acetylation at lysine 310 (K310) of NF-κB p65 in the HMP group was
significantly decreased compared with the CS group, which indicates
that HMP may attenuate the inflammatory response during IRI via
SIRT-1 mediated deacetylation of NF-κB p65.
In conclusion, compared with CS, HMP preservation
provided a more sufficient O2 supply to maintain the
cellular metabolism, leading to increased levels of NAD+
and the ratio of NAD+/NADH, which subsequently enhanced
SIRT-1 activity. The activation of SIRT-1 by HMP led to the
inhibition of NF-κB p65 activity via the deacetylation of NF-κB p65
at K310 to attenuate the inflammatory response during IRI.
Therefore, the present study demonstrates that HMP may reduce IRI
by activation of a novel deacetylating pathway in DCD rat livers.
These findings may provide a theoretical basis for the clinical
application of HMP as an effective strategy to preserve DCD
livers.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. U1403222).
References
|
1
|
Blok JJ, Detry O, Putter H, Rogiers X,
Porte RJ, van Hoek B, Pirenne J, Metselaar HJ, Lerut JP, Ysebaert
DK, et al: Longterm results of liver transplantation from donation
after circulatory death. Liver Transpl. 22:1107–1114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perico N, Cattaneo D, Sayegh MH and
Remuzzi G: Delayed graft function in kidney transplantation.
Lancet. 364:1814–1827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brasile L, Stubenitsky BM, Booster MH,
Arenada D, Haisch C and Kootstra G: Hypothermia a limiting factor
in using warm ischemically damaged kidneys. Am J Transplant.
1:316–320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henry SD, Nachber E, Tulipan J, Stone J,
Bae C, Reznik L, Kato T, Samstein B, Emond JC and Guarrera JV:
Hypothermic machine preservation reduces molecular markers of
ischemia/reperfusion injury in human liver transplantation. Am J
Transplant. 12:2477–2486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carnevale ME, Balaban CL, Guibert EE,
Bottai H and Rodriguez JV: Hypothermic machine perfusion versus
cold storage in the rescuing of livers from non-heart-beating donor
rats. Artif Organs. 37:985–991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guarrera JV, Henry SD, Samstein B,
Odeh-Ramadan R, Kinkhabwala M, Goldstein MJ, Ratner LE, Renz JF,
Lee HT, Brown RS Jr and Emond JC: Hypothermic machine preservation
in human liver transplantation: The first clinical series. Am J
Transplant. 10:372–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taylor MJ and Baicu SC: Current state of
hypothermic machine perfusion preservation of organs: The clinical
perspective. Cryobiology. 60(3 Suppl): S20–S35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arnaudo AM and Garcia BA: Proteomic
characterization of novel histone post-translational modifications.
Epigenetics Chromatin. 6:242013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen LF and Greene WC: Assessing
acetylation of NF-kappaB. Methods. 36:368–375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang B, Yang XD, Lamb A and Chen LF:
Posttranslational modifications of NFkappaB: Another layer of
regulation for NF-kappaB signaling pathway. Cell Signal.
22:1282–1290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen LF, Mu Y and Greene WC: Acetylation
of RelA at discrete sites regulates distinct nuclear functions of
NF-kappaB. EMBO J. 21:6539–6548. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun T, Luo J, Jia M, Li H, Li K and Fu Z:
Small interfering RNA-mediated knockdown of NF-κBp65 attenuates
neuropathic pain following peripheral nerve injury in rats. Eur J
Pharmacol. 682:79–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramachandran S, Liaw JM, Jia J, Glasgow
SC, Liu W, Csontos K, Upadhya GA, Mohanakumar T and Chapman WC:
Ischemia-reperfusion injury in rat steatotic liver is dependent on
NFκB P65 activation. Transpl Immunol. 26:201–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andrade-Silva AR, Ramalho FS, Ramalho LN,
Saavedra-Lopes M, Jordão AA Jr, Vanucchi H, Piccinato CE and
Zucoloto S: Effect of NFkappaB inhibition by CAPE on skeletal
muscle ischemia-reperfusion injury. J Surg Res. 153:254–262. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suetsugu H, Iimuro Y, Uehara T, Nishio T,
Harada N, Yoshida M, Hatano E, Son G, Fujimoto J and Yamaoka Y:
Nuclear factor {kappa}B inactivation in the rat liver ameliorates
short term total warm ischaemia/reperfusion injury. Gut.
54:835–842. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang C, Li X and Liu Q: Sorbitol
dehydrogenase inhibitor protects the liver from
ischemia/reperfusion-induced injury via elevated glycolytic flux
and enhanced sirtuin 1 activity. Mol Med Rep. 11:283–288. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yan H, Jihong Y, Feng Z, Xiaomei X,
Xiaohan Z, Guangzhi W, Zhenhai M, Dongyan G, Xiaochi M, Qing F, et
al: Sirtuin 1-mediated inhibition of p66shc expression alleviates
liver ischemia/reperfusion injury. Crit Care Med. 42:e373–e381.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pantazi E, Zaouali MA, Bejaoui M, Serafin
A, Folch-Puy E, Petegnief V, De Vera N, Ben Abdennebi H, Rimola A
and Roselló-Catafau J: Silent information regulator 1 protects the
liver against ischemia-reperfusion injury: Implications in
steatotic liver ischemic preconditioning. Transpl Int. 27:493–503.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yeung F, Hoberg JE, Ramsey CS, Keller MD,
Jones DR, Frye RA and Mayo MW: Modulation of NF-kappaB-dependent
transcription and cell survival by the SIRT1 deacetylase. EMBO J.
23:2369–2380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Institute of laboratory animal resources
(US), . Committee on care, use of laboratory animals and national
institutes of health (US)Division of research resources: Guide for
the care and use of laboratory animals. 8th edition. National
academies press; Washington, DC: 2011
|
|
21
|
Pizarro MD, Rodriguez JV, Mamprin ME,
Fuller BJ, Mann BE, Motterlini R and Guibert EE: Protective effects
of a carbon monoxide-releasing molecule (CORM-3) during hepatic
cold preservation. Cryobiology. 58:248–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Balaban CL, Rodriguez JV and Guibert EE:
Delivery of the bioactive gas hydrogen sulfide during cold
preservation of rat liver: Effects on hepatic function in an ex
vivo model. Artif Organs. 35:508–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schlegel A, Kron P, Graf R, Dutkowski P
and Clavien PA: Warm vs. cold perfusion techniques to rescue rodent
liver grafts. J Hepatol. 61:1267–1275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamada N and Calne RY: A surgical
experience with five hundred thirty liver transplants in the rat.
Surgery. 93:64–69. 1983.PubMed/NCBI
|
|
25
|
Minor T, Manekeller S, Sioutis M and
Dombrowski F: Endoplasmic and vascular surface activation during
organ preservation: Refining upon the benefits of machine
perfusion. Am J Transplant. 6:1355–1366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Minor T and Manekeller S: Assessment of
hepatic integrity after ischemic preservation by isolated perfusion
in vitro: The role of albumin. Cryobiology. 54:188–195. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khandoga A, Biberthaler P, Enders G and
Krombach F: 5-Aminoisoquinolinone, a novel inhibitor of
poly(adenosine disphosphate-ribose) polymerase, reduces
microvascular liver injury but not mortality rate after hepatic
ischemia-reperfusion. Crit Care Med. 32:472–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lentsch AB, Kato A, Yoshidome H, McMasters
KM and Edwards MJ: Inflammatory mechanisms and therapeutic
strategies for warm hepatic ischemia/reperfusion injury.
Hepatology. 32:169–173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto T, Ono T, Ito T, Yamanoi A,
Maruyama I and Tanaka T: Hemoperfusion with a high-mobility group
box 1 adsorption column can prevent the occurrence of hepatic
ischemia-reperfusion injury in rats. Crit Care Med. 38:879–885.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peralta C, Jiménez-Castro MB and
Gracia-Sancho J: Hepatic ischemia and reperfusion injury: Effects
on the liver sinusoidal milieu. J Hepatol. 59:1094–1106. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia JJ, Zhang J, Li JH, Chen XD, Jiang L,
Zhou YF, He N, Xie HY, Zhou L and Zheng SS: Influence of perfusate
on liver viability during hypothermic machine perfusion. World J
Gastroenterol. 21:8848–8857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vekemans K, Liu Q, Brassil J, Komuta M,
Pirenne J and Monbaliu D: Influence of flow and addition of oxygen
during porcine liver hypothermic machine perfusion. Transplant
Proc. 39:pp. 2647–2651. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pamukcu B, Lip GY and Shantsila E: The
nuclear factor-kappa B pathway in atherosclerosis: A potential
therapeutic target for atherothrombotic vascular disease. Thromb
Res. 128:117–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin SJ, Ford E, Haigis M, Liszt G and
Guarente L: Calorie restriction extends yeast life span by lowering
the level of NADH. Genes Dev. 18:12–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vaziri H, Dessain SK, Ng Eaton E, Imai SI,
Frye RA, Pandita TK, Guarente L and Weinberg RA: hSIR2(SIRT1)
functions as an NAD-dependent p53 deacetylase. Cell. 107:149–159.
2001. View Article : Google Scholar : PubMed/NCBI
|