Introduction
Lung cancer continues to be the leading cause of
cancer-associated mortality in men and women worldwide (1). The average survival rate for a
patient diagnosed with lung cancer is <5 years. Among the lung
cancer subtypes, non-small cell lung cancer is the most common
(2,3). In an attempt to overcome this
problem, a growing number of therapeutic interventions have been
developed to target lung cancer, including targeted thermal
ablation, radiation therapy and biological therapy. In addition,
small molecule gene therapy is considered an effective therapy for
the treatment of lung cancer. In particular, using a combined-gene
therapeutic approach has been reported to increase curative
effects, and is often more effective than single gene therapy alone
(4,5).
p53 is a well-known tumor suppressor gene that can
activate genes responsible for regulating cell proliferation,
apoptosis, cell cycle control, senescence, transcriptional
regulation and DNA repair (6). In
addition, p53 is able to regulate the tumor microenvironment by
inhibiting the development of tumor vasculature. Approximately half
of all human tumors contain a mutation in p53, which is associated
with poor patient prognosis. Murine double minute 2 (MDM2) is an E3
ubiquitin ligase that can induce p53 inactivation by directly
binding to it and promoting its ubiquitination (7). Ultimately, MDM2-mediated
ubiquitination of p53 leads to its degradation by the 26S
proteasome. Consequently, increased expression levels of MDM2 can
inhibit p53 activity, thereby preventing its tumor suppressor
function. Therefore, the present study knocked down MDM2 expression
using small interfering (si)RNA to promote p53 transcriptional
activity, and determined its effects on a lung cancer model
(5,8).
One important characteristic of tumors is
uncontrolled cell growth. Under normal conditions, cell
proliferation is tightly regulated by the cell cycle. The
eukaryotic cell cycle is regulated by the cyclin-dependent kinase
(CDK) family (9), which contains
protein kinases that comprise catalytic (CDK) and regulatory
subunits (cyclin). Cyclin D-CDK4/6 and cyclin E-CDK2 are two types
of G1 cyclin-CDK complexes that are required for entry
into S phase of the cell cycle. Notably, increasing p53 levels have
been reported to result in an upregulation in the protein
expression levels of p21, which is an inhibitor of CDKs that can
prevent the development of G1 CDK-cyclin complexes
(10). In addition, p21 can
prevent DNA synthesis and inhibit proliferating cell nuclear
antigen; therefore, by increasing p53 levels, it is possible to
induce p21 to cause G1 phase cell cycle arrest.
Small molecule gene therapy has received increasing
amounts of attention; however, due to the complexity of tumors, a
single gene therapy strategy is unlikely to be successful (11). Conversely, a combined-gene
therapeutic approach has been reported to be more effective
(4,7). Therefore, the present study induced
co-expression of MDM2-specific siRNA and wild-type p53
(si-MDM2-p53) in H1299 lung cancer cells, and determined its
effects on cell cycle progression and cancer cell viability.
Materials and methods
Cell culture and transfection
H1299 lung cancer cells (Sangon Biotech Co., Ltd.,
Shanghai, China) were cultured in RPMI-1640 medium (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (Hyclone; GE Healthcare Life Sciences) and
penicillin-streptomycin (1,000 µg/ml) at 37°C and in 5%
CO2. When cells reached 70–90% confluence, they were
transfected with scrambled siRNA
[5′-GATCCGTATAAGTCAACTGTTGACttcaagagaGTCAACAGTTGACTTATACTTTTTTGGAAA-3′
(sense strand) and
5′-AGCTTTTCCAAAAAAGTATAAGTCAACTGTTGACtctcttgaaGTCAACAGTTGACTTATACG-3′
(antisense strand)] The lower case letters indicate non-homologus
sequences. The plasmid of si-MDM2, the plasmid of p53 and the
plasmid of si-MDM2-p53 (Jilin University, Jilin, China) were
prepared as previously described (4,5). The
plasmid si-MDM2 sequence: forward,
5′-CGTCGCGAGGGCTATGAACTAATGACCC-3′ and reverse,
5′-GCAGATCTTGCTTCGCGATGTACGGGCC-3′. The plamid p53 sequence:
forward, 5′-CCATCTACAAGCAGTCACAG-3′ and reverse,
5′-CAAATCTACAAGCAGTCACAG-3′. PGCsiRNA-MDM2 (si-MDM2), pcDNA3.1-p53
(p53), pcDNA3.1-p53/U6 siRNA-MDM2 and p53 (si-MDM2-p53) (Jilin
University, Jilin, China), these eukaryotic expression vectors were
used to transfect the plasmids into cells using a transfection
reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA). When
the cell density reached to 85–90%, 6 µg of every plasmid was used
to transfect into cells at 37°C. Following 48 h transfection, cells
were then collected to analyze cell activity, protein and gene
expression, and cell cycle progression.
MTT assay
A total of ~4.5×103 cells/well were
incubated in a 96-well plate, and were cultured under normal growth
conditions prior to transfection with si-MDM2, p53 or si-MDM2-p53
plasmids (Jilin University). Cell viability was measured by adding
20 µl MTT (Sangon Biotech Co., Ltd.) to each well 24, 48 and 72 h
post-transfection. Following 4 h, 150 µl dimethyl sulfoxide (Sangon
Biotech Co., Ltd.) was used to dissolve the crystals at room
temperature, and the absorbance was measured at 490 nm using a
microplate reader.
Flow cytometric analysis (FCM) to
determine cell cycle progression
Cells were collected for FCM by washing twice with
cold PBS (centrifuged at 1,000 × g for 5 min/wash, 4°C) and were
digested with 0.25% EDTA-free trypsin. Following two further
washes, 75% ethanol was added to the cells, and the cells were
cultured at 4°C overnight. Subsequently, the cells were centrifuged
at 4°C, 1,000 × g for 5 min, and the ethanol was discarded before
washing the cells two more times. Finally, ~8×104 cells
were resuspended in 200–500 µl PBS, stained with propidium iodide
(100 µg/ml), and maintained in the dark for 30 min at room
temperature. Cell cycle progression was analyzed using the
Epics-XL-MCL flow cytometer (Beckman Coulter, Inc., Brea, CA,
USA).
Western blot analysis
Cells were lysed with 100–150 µl
radioimmunoprecipitation assay buffer supplemented with
phenylmethylsulfonyl fluoride (Roche Diagnostics, Basel,
Switzerland), and were sonicated 3–5 times (0°C, 5 sec every time)
using ultrasound pyrolysis apparatus (Tomy Seiko Co., Ltd., Tokyo,
Japan) to shear the cells. Subsequently, the cells were centrifuged
for 20 min at 4°C, 12,000 × g, and the protein content in the
supernatant was measured using Bradford reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The lysates (20 µg protein)
were separated by 12% SDS-PAGE and were transferred onto
polyvinylidene fluoride membranes at 0°C for 50 min, then blocking
with 5 % nonfat milk at room temperature for 1 h (EMD Millipore,
Bedford, MA, USA). The protein expression levels of p53, p21,
cyclin D1 and MDM2 were detected by western blot analysis. Rabbit
polyclonal anti-p21 (1:2,000; cat. no. 10355-1-AP) and rabbit
polyclonal anti-p53 (1:1,000; cat. no. 10442-1-AP), were purchased
from ProteinTech Group, Inc. (Chicago, IL, USA). Mouse polyclonal
anti-cyclin D1 (1:200; cat. no. sc-450) and MDM2 (1:200; cat. no.
sc-81218), were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA); and rabbit polyclonal anti-pan-actin (1:1,000;
cat. no. 8456) was purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). All the primary antibodies were incubated at
room temperature for 4 h. Anti-rabbit (1:1,000; cat. no. SA00001-2)
and anti-mouse (1:1,000; cat. no. SA00001-1) were purchased from
ProteinTech Group, Inc. (Chicago, IL, USA), they were incubated at
room temperature for 1 h. Proteins were detected using an enhanced
chemiluminescence kit (cat. no. 120702-74; Advansta, Inc., Menio
Park, CA, USA). The images were captured using a Syngene Bio
Imaging System (Synoptics, Cambridge, UK).
Reverse transcription
semi-quantitative polymerase chain reaction [RT-(sq)PCR]
H1299 cells were harvested from a 6-well plate. The
cells were washed twice, following this 500 µl TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) was added to each well
and incubated at room temperature for 5 min. Subsequently,
trichloromethane was added, and following centrifugation at 4°C,
2,000 × g for 5 min, minisopropanol was added to the aqueous phase.
Finally, 75% ethanol was used to wash RNA prior to resuspending it
in RNase-free water. Subsequently, 5 µg total RNA (purified
following DNase I treatment) from each sample was converted to cDNA
using an RT kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA),
which was performed according to the manufacturer's protocol. The
primer sequences and reaction conditions are presented in Tables I and II. The PCR products were separated on a
2% agarose gel, and visualized by ethidium bromide staining then
images were captured using the image processing system (Tanon,
1600R; Tanon Science and Technology Co., Ltd., Shanghai, China).
Statistical analysis was then carried out.
 | Table I.Primer sequences for reverse
transcription polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription polymerase chain reaction.
| Gene | Forward primer
sequence | Reverse primer
sequence |
|---|
| GAPDH |
5′-AGAAGGCTGGGGCTCATTTG-3′ |
5′-AGGGGCCATCCACAGTCTTC-3′ |
| p53 |
5′-CCATCTACAAGCAGTCACAG-3′ |
5′-CAAATCTACAAGCAGTCACAG-3′ |
| p21 |
5′-GTGGCTCTGATTGGCTTTCTG-3′ |
5′-CTGAAAACAGGCAGCCCAAGG-3′ |
| Cyclin D1 |
5′-TGGATGCTGGAGGTCTGCGAG-3′ |
5′-GGCTTCGATCTGCTCCTGGC-3′ |
| CDK4 |
5′-CGTGAGGTGGCTTTACTGAG-3′ |
5′-CTTGATCGTTTCGGCTGG-3′ |
| MDM2 |
5′-CGTCGCGAGGGCTATGAACTAATGACCC-3′ |
5′-GCAGATCTTGCTTCGCGATGTACGGGCC-3′ |
 | Table II.Polymerase chain reaction
thermocycling conditions. |
Table II.
Polymerase chain reaction
thermocycling conditions.
| Gene | Denaturation | Annealing | Extension | Cycle no. |
|---|
| GAPDH | 94°C, 30 sec | 55°C, 30 sec | 72°C, 30 sec | 28 |
| p53 | 94°C, 30 sec | 56°C, 30 sec | 72°C, 30 sec | 30 |
| p21 | 94°C, 30 sec | 55°C, 30 sec | 72°C, 30 sec | 30 |
| Cyclin D1 | 94°C, 30 sec | 55°C, 30 sec | 72°C, 30 sec | 28 |
| CDK4 | 94°C, 30 sec | 55°C, 30 sec | 72°C, 30 sec | 28 |
| MDM2 | 94°C, 30 sec | 56°C, 30 sec | 72°C, 30 sec | 28 |
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used to statistically
analyze the data using single factor analysis of variance and least
significant difference to test the multiple comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of si-MDM2, p53 or si-MDM2-p53
plasmids on the proliferation of H1299 cells
To determine whether simultaneously inhibiting MDM2
and overexpressing wild-type p53 was able to inhibit the
proliferation of lung cancer cells, H1299 cells were transfected
with si-MDM2, p53 or si-MDM2-p53 plasmids and cell proliferation
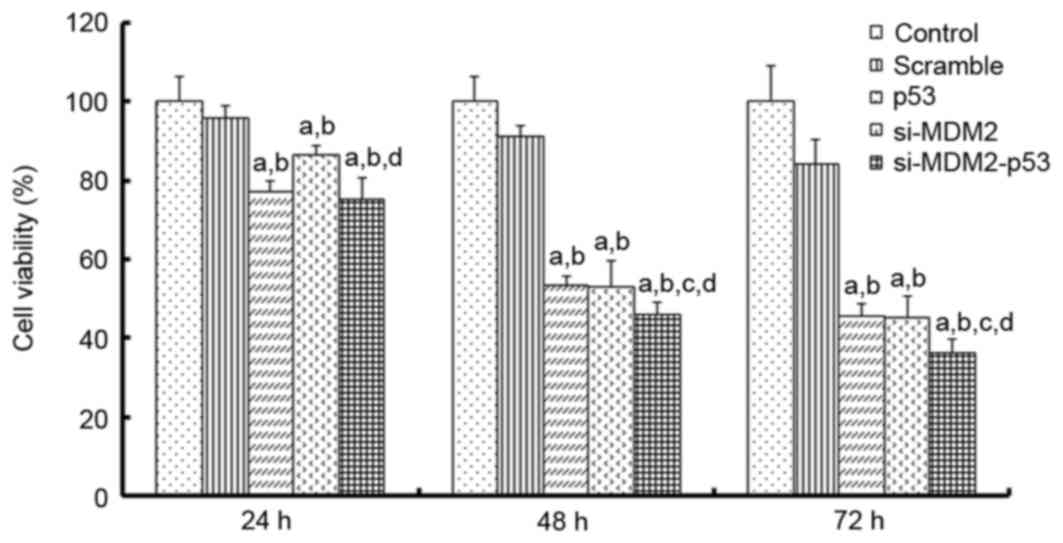
was analyzed at 24, 48 and 72 h using an MTT assay. The results
indicated that si-MDM2, p53 and si-MDM2-p53 plasmids inhibited cell
proliferation (Fig. 1). Notably,
after 48 h the si-MDM2-p53 plasmid was able to inhibit cell
proliferation by ~50%, which was more than p53 or si-MDM2 alone
(P<0.05). Taken together, these results indicated that the
si-MDM2-p53 plasmid may significantly inhibit the proliferation of
H1299 cells.
Effects of si-MDM2, p53 or si-MDM2-53
plasmids on cell cycle arrest
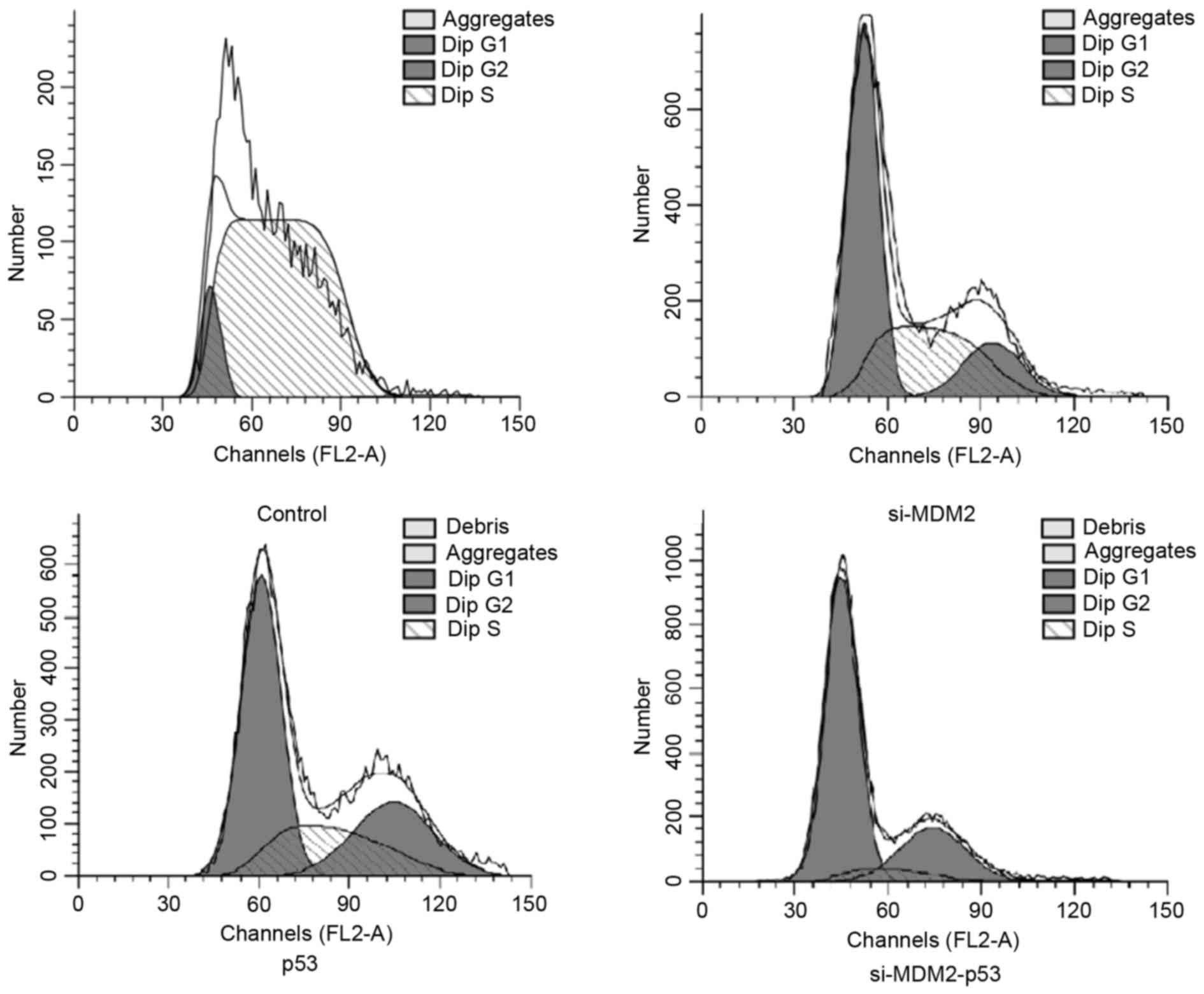
In order to determine whether manipulating the
MDM2/p53 pathway could affect the cell cycle, FCM was used to
analyze cell cycle progression in H1299 cells. The results
demonstrated that the percentage of cells in G1 phase
was increased when cells were transfected with si-MDM2, p53 or
si-MDM2-53 plasmids (Fig. 2).
Furthermore, FCM indicated that co-expression of si-MDM2 and the
p53 overexpression plasmid resulted in a significant increase in
the number of cells in G1 phase cell cycle arrest,
compared with the cell transfected with si-MDM2 or p53 plasmids
alone (P<0.05). The percentages of cells in G1 phase
from each group are presented in Table III.
 | Table III.Percentage of cells in each phase of
the cell cycle. |
Table III.
Percentage of cells in each phase of
the cell cycle.
|
| Phase |
|---|
|
|
|
|---|
| Group | G1 | S | G2 |
|---|
| Control |
10.14±1.2 |
89.86±2.0 |
0.0±0.0 |
| si-MDM2 |
51.30±4.2a |
33.74±1.3a |
14.95±3.4a |
| p53 |
51.05±2.3a |
23.71±2.6a |
25.25±2.8a |
| si-MDM2-p53 |
68.42±2.5a–c |
7.21±3.1a–c |
24.37±4.3a–c |
Effects of si-MDM2, p53 or si-MDM2-53
plasmids on the expression of cell cycle-associated genes and
proteins in H1299 cells
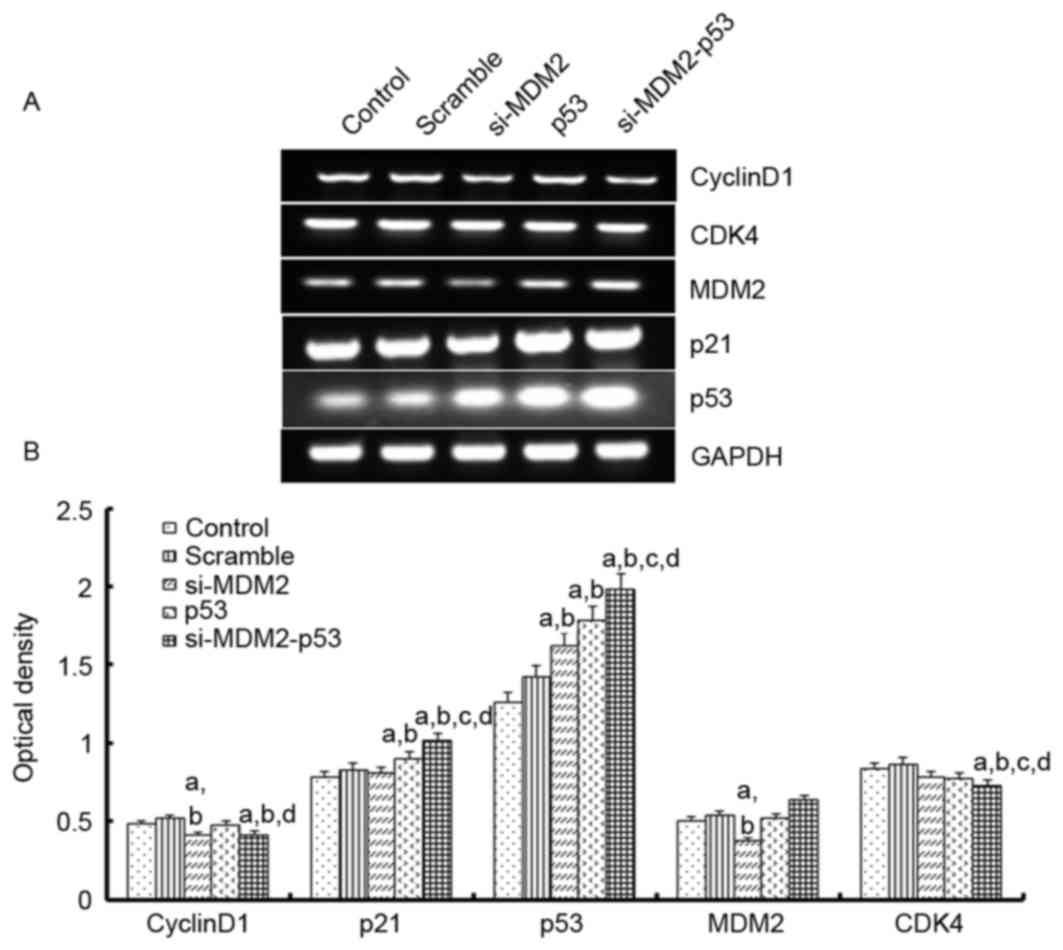
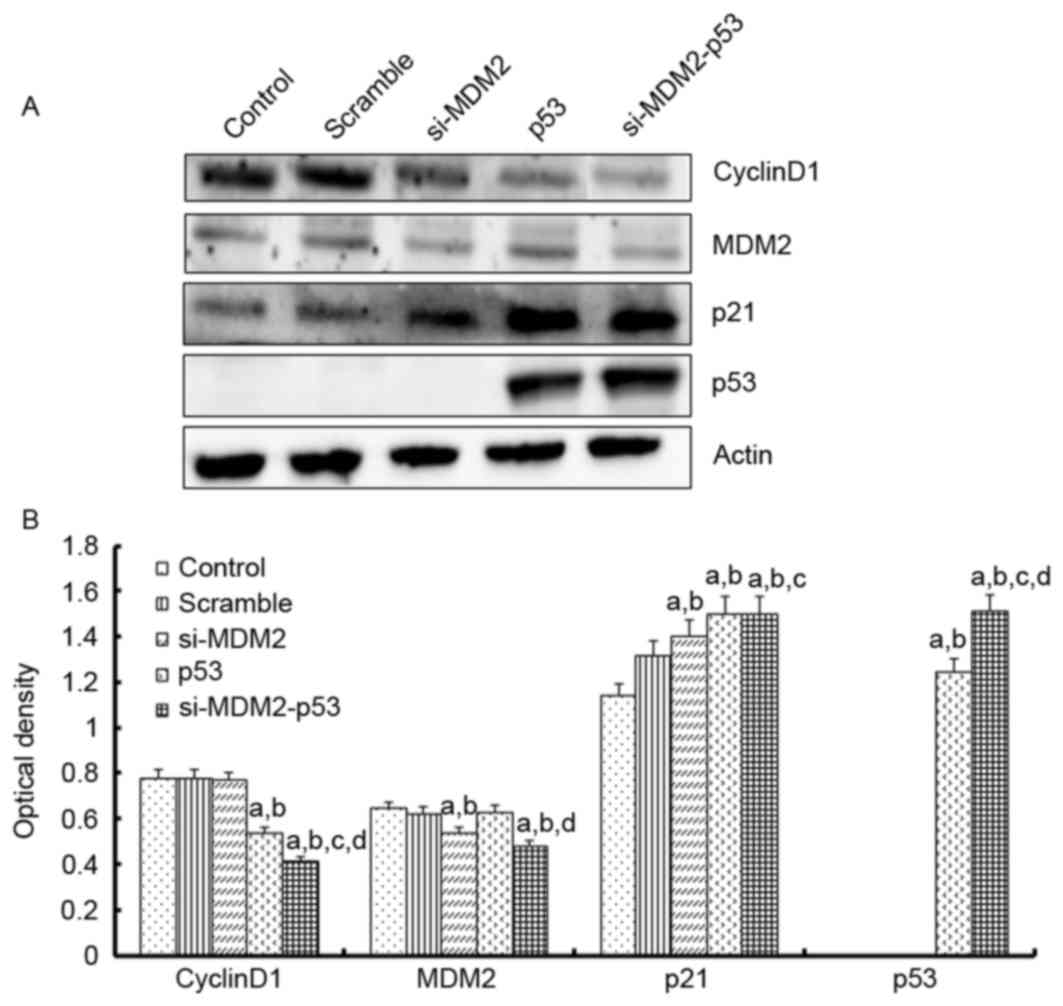
To investigate the mechanism underlying the effects
of the si-MDM2, p53 and si-MDM2-53 plasmids on G1 phase
cell cycle arrest and cell proliferation, the mRNA expression
levels of p21, cyclin D1, CDK4 and MDM2 were determined by
RT-(sq)PCR, and the protein expression levels of p53, p21, cyclin
D1 and MDM2 were determined by western blot analysis, respectively
(Figs. 3 and 4). The results indicated that the protein
and mRNA expression levels of p53 and p21 were significantly
increased when the H1299 cells were transfected with si-MDM2, p53
or si-MDM2-53 plasmids, compared with the control or scramble
groups (P<0.05). In addition, there was a significant decrease
in the protein and mRNA expression levels of cyclin D1 and MDM2,
and a significant decrease in the mRNA expression levels of cyclin
D1, MDM2 and CDK4 (P<0.05).
Discussion
Effective therapeutic strategies for the treatment
of lung cancer remain a major obstacle, particularly because
patients often present at terminal stages. Furthermore, the low
diagnostic accuracy of clinical staging delays the optimum time for
effective treatment (12,13). Small molecule gene therapies for
lung cancer are considered a potentially effective therapeutic
approach; however, care must be taken to identify the appropriate
combination of gene therapies for the effective treatment of lung
cancer.
The results of the present study demonstrated that
p53 serves an important role in regulating the oncogenic behavior
of H1299 cells. Under normal conditions, p53 is suppressed by MDM2,
which results in the maintenance of low p53 nuclear levels
(6,14–16).
Notably, MDM2 inhibits p53-mediated apoptosis, DNA repair and cell
cycle arrest; however, in response to stress, including oxidative
or DNA replication stress, hypoxia or oncogenic activation, the
negative loop between p53 and MDM2 is broken, and p53 expression is
stabilized by post-translational modifications (17,18).
Specifically, MDM2 releases p53, thereby allowing p53 to
translocate to the nucleus, bind its target genes and activate
downstream genes that combat the source of stress. In the present
study, it was indicated that si-MDM2-p53 was able to significantly
inhibit the proliferation of H1299 cells, and that si-MDM2 and p53
co-expression could increase the expression of p53 (19,20).
Regulation of the cell cycle is a complex procedure
that is regulated by complex mechanisms, and is controlled by
critical regulatory genes, including CDKs and their regulatory
inhibitors (21,22). It has previously been reported that
p21 is associated with cell cycle regulation. For example, p21 is
an inhibitor of CDKs that inhibits the expression of G1
CDK-cyclin complexes. Notably, p21 is a downstream target gene of
p53 (23). Upon being subjected to
stress, p53 protein levels are increased, which results in a
corresponding increase in p21 protein levels. In addition, CDK2,
CDK4 and CDK6 are downregulated by p21 (24,25).
In the present study, FCM was used to detect alterations in the
cell cycle progression of H1299 cells in response to si-MDMD2, p53
overexpression or both. A significant increase in G1
phase cell cycle arrest was detected following transfection with
the si-MDM2-53 plasmid, compared with si-MDM2 or p53 alone.
In conclusion, the present study used a si-MDM2 and
p53 co-expression plasmid to disrupt the negative feedback loop
between MDM2 and p53, and to increase the expression levels of p53
(26,27). The results indicated that in H1299
lung cancer cells transfected with the si-MDM2-53 plasmid, p53 and
p21 expression levels were increased, whereas CDK4 and cyclin D1
were downregulated, at the protein and mRNA levels. These results
suggested that downregulation of MDM2 and upregulation of p53 may
induce inhibition of H1299 cell proliferation and cell cycle
arrest. Taken together, the use of a si-MDM2-p53 co-expression
plasmid may offer a novel gene therapy that targets lung
cancer.
Acknowledgements
The present study was supported by the Research Fund
for the National Natural Science Foundation of China (grant no.
81572927), the National Natural Science Foundation of China (grant
no. 81501982) and the Scientific and Technological Research
Planning Project of Education in Jilin Province (grant no.
20150414025GH).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Zhang G, Li X, Li B and Zhang X:
The effect of ribosomal protein S15a in lung adenocarcinoma. PeerJ.
4:e17922016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riley T, Sontag E, Chen P and Levine A:
Transcriptional control of human p53-regulated genes. Nat Rev Mol
Cell Biol. 9:402–412. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gu J, Wang B, Liu Y, Zhong L, Tang Y, Guo
H, Jiang T, Wang L, Li Y and Cai L: Murine double minute 2 siRNA
and wild-type p53 gene therapy interact positively with zinc on
prostate tumours in vitro and in vivo. Eur J Cancer. 50:1184–1194.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji K, Wang B, Shao YT, Zhang L, Liu YN,
Shao C, Li XJ, Li X, Hu JD, Zhao XJ, et al: Synergistic suppression
of prostatic cancer cells by coexpression of both murine double
minute 2 small interfering RNA and wild-type p53 gene in vitro and
in vivo. J Pharmacol Exp Ther. 338:173–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ayroldi E, Petrillo MG, Bastianelli A,
Marchetti MC, Ronchetti S, Nocentini G, Ricciotti L, Cannarile L
and Riccardi C: L-GILZ binds p53 and MDM2 and suppresses tumor
growth through p53 activation in human cancer cells. Cell Death
Differ. 22:118–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu J, Tang Y, Liu Y, Guo H, Wang Y, Cai L,
Li Y and Wang B: Murine double minute 2 siRNA and wild-type p53
gene therapy enhances sensitivity of the SKOV3/DDP ovarian cancer
cell line to cisplatin chemotherapy in vitro and in vivo. Cancer
Lett. 343:200–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo H, Li Y, Gu J, Wang Y, Liu L, Zhang P
and Liu Y: Effect of vascular endothelial growth factor siRNA and
wild-type p53 co-expressing plasmid in MDA-MB-231 cells. Mol Med
Rep. 13:461–468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elmetwali T, Salman A and Palmer DH:
NORE1A induction by membrane-bound CD40L (mCD40L) contributes to
CD40L-induced cell death and G1 growth arrest in p21-mediated
mechanism. Cell Death Dis. 7:e21462016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng QH, Ma LW, Zhu WG, Zhang ZY and Tong
TJ: p21Waf1/Cip1 plays a critical role in modulating senescence
through changes of DNA methylation. J Cell Biochem. 98:1230–1248.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hensing T, Chawla A, Batra R and Salgia R:
A personalized treatment for lung cancer: molecular pathways,
targeted therapies, and genomic characterization. Adv Exp Med Biol.
799:85–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang HQ, Jin JJ and Wang J: Arctigenin
enhances chemosensitivity to cisplatin in human nonsmall lung
cancer H460 cells through downregulation of survivin expression. J
Biochem Mol Toxicol. 28:39–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Wang X, Chen T and Qu J: p38
inhibitor SB203580 sensitizes the resveratrol-induced apoptosis in
human lung adenocarcinoma (A549) cells. J Biochem Mol Toxicol.
26:251–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vassilev LT, Vu BT, Graves B, Carvajal D,
Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et
al: In vivo activation of the p53 pathway by small-molecule
antagonists of MDM2. Science. 303:844–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shinohara T and Uesugi M: In-vivo
activation of the p53 pathway by small-molecule antagonists of
MDM2. Tanpakushitsu Kakusan Koso. 52(13 Suppl): S1816–S1817.
2007.(In Japanese).
|
|
16
|
Marine JC and Lozano G: Mdm2-mediated
ubiquitylation: P53 and beyond. Cell Death Differ. 17:93–102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Zhang C, Wang XL, Ly P, Belyi V,
Xu-Monette ZY, Young KH, Hu W and Feng Z: E3 ubiquitin ligase
TRIM32 negatively regulates tumor suppressor p53 to promote
tumorigenesis. Cell Death Differ. 21:1792–1804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harris SL and Levine AJ: The p53 pathway:
Positive and negative feedback loops. Oncogene. 24:2899–2908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pajalunga D, Mazzola A, Salzano AM, Biferi
MG, De Luca G and Crescenzi M: Critical requirement for cell cycle
inhibitors in sustaining nonproliferative states. J Cell Biol.
176:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Odkhuu E, Mendjargal A, Koide N, Naiki Y,
Komatsu T and Yokochi T: Lipopolysaccharide downregulates the
expression of p53 through activation of MDM2 and enhances
activation of nuclear factor-kappa B. Immunobiology. 220:136–141.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valesky EM, Hrgovic I, Doll M, Wang XF,
Pinter A, Kleemann J, Kaufmann R, Kippenberger S and Meissner M:
Dimethylfumarate effectively inhibits lymphangiogenesis via p21
induction and G1 cell cycle arrest. Exp Dermatol. 25:200–205. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SY, Kim JE, Lee KW and Lee HJ:
Lactococcus lactis ssp. lactis inhibits the proliferation of SNU-1
human stomach cancer cells through induction of G0/G1 cell cycle
arrest and apoptosis via p53 and p21 expression. Ann N Y Acad Sci.
1171:270–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chu K, Gao G, Yang X, Ren S, Li Y, Wu H,
Huang Y and Zhou C: miR-512-5p induces apoptosis and inhibits
glycolysis by targeting p21 in non-small cell lung cancer cells.
Int J Oncol. 48:577–586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baharuddin P, Satar N, Fakiruddin KS,
Zakaria N, Lim MN, Yusoff NM, Zakaria Z and Yahaya BH: Curcumin
improves the efficacy of cisplatin by targeting cancer stem-like
cells through p21 and cyclin D1-mediated tumour cell inhibition in
non-small cell lung cancer cell lines. Oncol Rep. 35:13–25. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Devany E, Zhang X, Park JY, Tian B and
Kleiman FE: Positive and negative feedback loops in the p53 and
mRNA 3′ processing pathways. Proc Natl Acad Sci USA. 110:pp.
3351–3356. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang Y, Cui Y, Li Z, Jiao Z, Zhang Y, He
Y, Chen G, Zhou Q, Wang W, Zhou X, et al: Radiation-induced
miR-208a increases the proliferation and radioresistance by
targeting p21 in human lung cancer cells. J Exp Clin Cancer Res.
35:72016. View Article : Google Scholar : PubMed/NCBI
|