Introduction
Despite efforts to identify and clinically apply
more effective anticancer agents, human colon cancer remains one of
the most frequent cancer types worldwide, as well as in China
(1). This is partly due to the
limitations associated with chemotherapy as a result of drug
resistance, and tumor recurrence and metastasis (2). Thus, considering the limitations
associated with chemotherapy, the identification and development of
novel therapeutic strategies are urgently required in order to
prolong the survival of patients with colon cancer.
Melatonin (5-methoxy-N-acetyltryptamine) is an
endogenous indole compound primarily secreted by the pineal gland
(3). Previous studies have
demonstrated that melatonin regulates biological activities,
including circadian rhythm regulation, seasonal changes, sleep,
reproduction and cardiovascular functions (3,4). In
addition, melatonin increases the anticancer effects of some
chemotherapeutics by regulating a number of signal transduction
pathways that are associated with cancer cell proliferation,
apoptosis and migration (5–7).
Previous studies have also revealed that melatonin inhibits ruffle
formation in RKO colon cancer cells, and modulates microtubule and
microfilament structure formation in epithelial cells, thereby
suppressing cell migration (8,9).
Ordoñez et al (10)
confirmed that inhibition of the nuclear factor-kB signaling
pathway contributed to the melatonin-induced suppression of HepG2
liver cancer cell migration and invasion.
Cell migration is critical for the invasion of
surrounding tissues and in turn, into blood or lymph; it is
therefore also important in the formation of metastases. Many of
these processes require cell motility, which is driven by cycles of
actin polymerization, cell adhesion and actomyosin contraction
(11). Tumor cells, particularly
those with high metastatic potential, often exhibit a loss of tight
junctions (TJ). TJs are complexes comprised of multiple proteins,
including occludin, claudins and zonula occludens-1 (ZO-1), which
regulate the paracellular flux or permeability between adjacent
cells (12). Downregulation of
ZO-1 and occludin proteins have been associated with the migration
and invasion of cancer cells (13,14).
In addition, previous findings have shown that cytoskeletal
contraction, regulation of tight junction barrier function and the
disruption of tight junction structure, are induced by the
phosphorylation of myosin light chains (MLC) (15). MLCs are believed to be involved in
the generation of the contractile force used for cell migration.
Zou et al (8) also
identified that melatonin inhibited the phosphorylation of MLC by
downregulating the MLC kinase (MLCK) and p38 mitogen-activated
protein kinase (MAPK) signaling pathway. However, Rho-associated
protein kinase (ROCK) can phosphorylate the myosin phosphatase
targeting subunit (MYPT), thereby inactivating MLC phosphatase,
which results in the inhibition of the dephosphorylation of MLC
(16). Therefore, inhibition of
MLC phosphorylation may be a result of ROCK downregulation. ROCKs
belong to the AGC family of serine-threonine kinases, and mainly
regulate the structure and movement of cells by acting on the
cytoskeleton. The MYPT, as the protein phosphatase-1-binding
component, is a critical component of the myosin phosphatase
complex (17). A previous study
revealed that ROCK controls cell polarity in neutrophils and
enhances actomyosin contractility (18). ROCK inhibition has also been
demonstrated to activate Rac in Swiss 3T3 cells and increase
membrane ruffling in HUVECs (19,20).
However, the inhibition of myosin phosphatase, and not ROCK
inhibition, increased MLC phosphorylation and inhibited cell
migration in fibroblasts (21).
Thus, ROCK activation may decrease the migration of RKO colon
cancer cells. In addition, inhibiting ROCK also suppressed the
phosphorylation of p38 MAPK following interleukin-1 stimulation
(22). The MAPK signaling pathway
regulates TJ paracellular transport by modulating the expression of
TJ proteins and thus, altering the molecular structure (16). These observations suggested that,
within the different signaling pathways, ROCK, ZO-1 and occludin
may control non-muscle cell motility. In addition, the MAPK
signaling pathways, which include extracellular signal-regulated
kinase (ERK), c-JUN N-terminal kinase (JNK) and p38 kinase, serve
pivotal roles in cell proliferation, migration and apoptosis in
mammals (23). The p38 signaling
pathway has been associated with the regulation of important
processes in colon cancer cells, including apoptosis, migration and
proliferation (24,25). A previous study has also indicated
that melatonin may possess anti-invasive/anti-metastatic actions
that involve the inhibition of the p38 MAPK signaling pathway in
breast cancer (26).
However, it is unknown whether melatonin can
suppress the migration of RKO cells via the phosphosphorylated
(p)-p38 signaling pathway by inhibiting ROCK and/or inducing the
expression of TJ proteins. Therefore, the aim of the present study
was to investigate the inhibitory effect of melatonin on the
migration of RKO cells. In addition, the expression of p-MYPT1,
ROCK, p-MLC, ZO-1, occludin and p-p38 in the signal transduction
pathway were assessed.
Materials and methods
Reagents
Melatonin was provided by the School of Pharmacy,
Anhui Medical University (Anhui, China), and was dissolved in DMSO
prior to addition to the complete cell culture medium.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
and DMSO were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). The ROCK inhibitor,
5-[[(2s)-hexahydro-2-methyl-1H-1,4-diazepin-1-yl]
sulfonyl]-4-methyl-isoquinoline, dihydrochloride (H-1152) was
purchased from Cayman Chemical Company (Ann Arbor, MI, USA), and
was dissolved in DMSO at the stock concentration of 10 mM. The
specific p38 MAPK inhibitor SB203580 and the protein kinase C (PKC)
activator phorbol 12-myristate 13-acetate (PMA) were purchased from
Cayman Chemical Company. PMA and SB203580 were dissolved in DMSO
with concentrations of 16 and 2 mM, respectively, and stored at
−20°C. Dulbecco's modified Eagle's medium (DMEM) was obtained from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Fetal
bovine serum (FBS) was obtained from the Zhejiang Tianhang
Biotechnology Co., Ltd. (Zhejiang, China). Primary antibodies (used
for western blotting and immunofluorescence) against ROCK1 (cat.
no. sc-17794), ROCK2 (cat. no. sc-1851), MLC (cat. no. sc-48414),
p-p38 (cat. no. sc-7675-R), p38 (cat. no. sc-7149), ZO-1 (cat. no.
sc-8147), occludin (cat. no. sc-8144), MYPT1 (cat. no. sc-514261),
p-MYPT1 (cat. no. sc-17432), β-actin (cat. no. sc-47778) and
Na+/K+-ATPase (cat. no. sc-21712) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
The antibody against p-MLC (cat. no. 3674) was purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The donkey anti-goat
immunoglobulin (Ig)G fluorescein isothiocyanate (FITC)-conjugated
secondary antibody and DAPI were obtained from Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd. (Beijing, China). All the
secondary antibodies (goat anti-mouse IgG antibody, cat. no.
AP124P; rabbit anti-goat IgG antibody, cat. no. AP106P; goat
anti-rabbit IgG antibody, cat. no. AP132P) were purchased from EMD
Millipore (Billerica, MA, USA). The enhanced chemiluminescence
(ECL) reagent was purchased from Pierce; Thermo Fisher Scientific,
Inc. The bicinchoninic acid kit was obtained from Beyotime
Institute of Biotechnology (Haimen, China).
Cell lines
The human RKO colon cancer cell line was purchased
from the American Type Culture Collection (Manassas, VA, USA).
Cells were cultured in high glucose DMEM culture media supplemented
with 10% FBS, 40 U/ml penicillin and 100 U/ml streptomycin at 37°C
in a humidified atmosphere containing 5% CO2 throughout
the present study. Cells were grown to 70% confluency prior to the
start of the experiment.
Wound healing assay
Cells were seeded in 12-well plates at a density of
6×104 cells/well and incubated at 37°C until cells grew
to 100% confluency. Each well was then manually scratched with a
sterile 200 µl pipette tip to create the wound, and incubated at
37°C with melatonin (2.5 mM) or H-1152 (final concentration, 10
µM), alone or in combination. Images of the gap between the two
cell edges at the same position on the wound were captured at 0, 24
and 48 h using an inverted phase contrast microscope at a
magnification of ×100 (Leica DMI3000 B). The width of the wound was
measured using Quantity One software (version 4.6.2; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and the following
calculation was applied: Half of migration distance = (end distance
- start distance)/2.
Immunofluorescence assay
RKO cells were seeded in a 12-well
Costar® cell culture cluster dish at a density of
6×104 cells/well with sterile coverslips placed in the
wells prior to seeding and cultured at 37°C. Once the cells formed
a monolayer, they were treated with 2.5 mM melatonin, or 0.1% DMSO
as the control group, daily for 10 days; medium containing the
different treatments was replaced every day. Following treatment,
the cells were washed three times with 1X PBS and fixed with 4%
paraformaldehyde for 30 min at room temperature. Cells were then
washed again with PBS and blocked with blocking buffer (PBS
containing 1% bovine serum albumin; Sigma-Aldrich; Merck KGaA) for
2 h at room temperature. The coverslips with cells were incubated
with goat anti-human ZO-1 and occludin (both 1:50) primary
antibodies overnight at 4°C. Subsequently, the coverslips were
washed and incubated with a donkey anti-goat IgG FITC-conjugated
secondary antibody (1:100) for 2 h at room temperature away from
light. The cells were washed, incubated with DAPI for 5 min at room
temperature, washed again, mounted with aqueous-based anti-fade
mounting medium and then fixed with colorless nail polish on
microscope slides. Images of stained cells were captured by
fluorescence microscopy at a magnification of ×200 (Leica DMI4000
B). The immunofluorescence optical density was quantified from
three independent experiments using Image J software (version 1.3;
National Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to quantify the mRNA levels of
ROCK1/2, ZO-1 and occludin. Total RNA was extracted from RKO cells,
which were treated with 0.1% DMSO or melatonin (2 and 3 mM) for 48
h at 37°C, using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. A total of 4 µg of
total RNA was reverse transcribed using the PrimeScript™ RT reagent
kit with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's protocol. The thermocycling
conditions were as follows: at 42°C for 2 min, 37°C for 15 min and
85°C for 5 sec. The complementary DNAs were amplified using the
SYBR-Green qPCR Master mix (Takara Biotechnology Co., Ltd.) and a
Real-Time PCR system. All the primers were synthesized by the
Sangon Biotech Co., Ltd. (Shanghai, China) and the sequences are
listed in Table I. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C
for 30 sec. Relative quantification was performed using the
2−ΔΔCq method (27) and
the results were normalized to those of GAPDH.
 | Table I.Sequences of the primers used in
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of the primers used in
reverse transcription-quantitative polymerase chain reaction.
| Primer name | Primer sequences
(5′-3′) |
|---|
| GAPDH-F |
AGGTCGGAGTCAACGGATTTG |
| GAPDH-R |
CCTGGAAGATGGTGATGGGAT |
| ROCK1-F |
CTCTACCACTTTCCTGCCAA |
| ROCK1-R |
GTGGCACTTAACATGGCATC |
| ROCK2-F |
ACCAATGCTTTACTGCGAAC |
| ROCK2-R |
TCTCCAGCAGGCAGTTTTTA |
| ZO-1-F |
CTCTCAACAGGTGTATAGAAAGGATCC |
| ZO-1-R |
CTACGTATGGGAGTTGGGGTTC |
| Occludin-F |
AGAACTCTCCCGTTTGGATAAAGA |
| Occludin-R |
TTTGTAATCTGCAGATCCCTTCAC |
Western blot analysis
Following treatment, the RKO cells, which had be
treated with DMSO, melatonin, SB203580 and PMA (1 µM) for 48 h at
37°C, were collected and washed three times with PBS. Total protein
extracts were prepared with radioimmunoprecipitation buffer [hepes
25 mM, 1.5% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, NaCl
0.5 M, EDTA 5 mM, NaF 50 mM, sodium vanadate 0.1 mM,
phenylmethylsulfonyl fluoride 1 mM, and leupeptin 0.1 g/l, (pH
7.8)] and measured using a bicinchoninic protein assay kit
(Beyotime Institute of Biotechnology, Haimen, China). To further
verify ZO-1 and occludin expression, membrane proteins were also
extracted using the Membrane, Nuclear and Cytoplasmic Protein
Extraction kit (Sangon Biotech Co., Ltd.) according to the
manufacturer's instruction. Cell lysates were solubilized in SDS
sample buffer, separated by 8–15% SDS-PAGE with 60 µg protein
loaded/lane, and transferred to a PVDF membrane, which was blocked
with blocking buffer (5% non-fat dry milk) for 2 h at room
temperature. Membranes were then incubated with the primary
antibodies against MLC, p-MLC, p-p38, p38, ROCK1, ROCK2, MYPT1,
p-MYPT1, ZO-1, occludin, Na+/K+-ATPase and
β-actin (dilution of all primary antibodies 1:1,000) overnight at
4°C, followed by incubation with the corresponding horseradish
peroxidase-conjugated secondary antibody (1:3,000; EMD Millipore)
for 2 h at room temperature. The reactive bands were visualized
using ECL (Pierce; Thermo Fisher Scientific, Inc.) and a Kodak XAR
film. The images were scanned using a ScanPrisa 1240UT scanner
(Acer America Corporation, San Jose, CA, USA) and data were
quantified from three independent experiments using Quantity One
software (version 4.6.2; Bio-Rad Laboratories, Inc.). The results
obtained from total proteins were normalized to those of β-actin
and those generated from membrane proteins were normalized to those
of Na+/K+-ATPase.
Statistical analysis
At least three independent experiments were
performed for each experiment and the data are expressed as mean ±
standard deviation. The difference between treatment and control
groups were analyzed by one-way analysis of variance followed by
Dunnett's or least significant difference post hoc tests using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). Quantitative analysis
of immunofluorescence was performed using two independent samples
t-tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
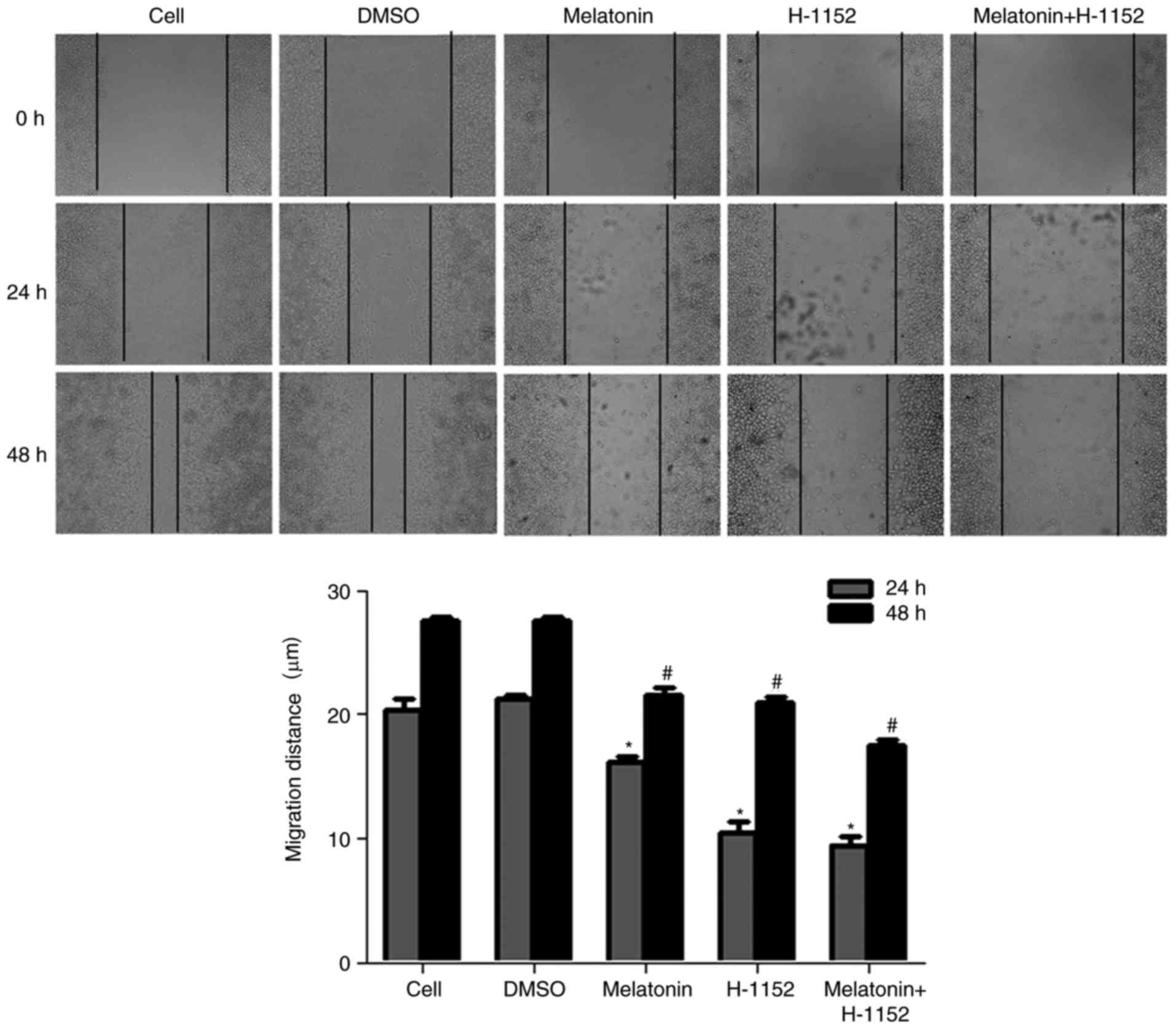
Effect of melatonin on RKO cell
migration
A previous study indicated that melatonin inhibited
the migration of RKO cells in a concentration-dependent manner and
may also inhibit the migration of RKO cells following treatment
with SB203580 and PMA in vitro via the p38 MAPK signaling
pathway (8). To further explore
the association between cell migration and ROCK, a wound-healing
assay was performed on RKO cells treated with H-1152, a ROCK
inhibitor. Following treatment with melatonin alone or in
combination with H-1152 for 24 and 48 h, the contrast half of
migration distance was significantly inhibited by all melatonin and
H-1152 treatments when compared with the control groups (Fig. 1 and Table II).
 | Table II.Effects of melatonin on migration in
RKO cells. |
Table II.
Effects of melatonin on migration in
RKO cells.
|
| Half migration
distance (mm) |
|---|
|
|
|
|---|
| Treatment
group | Dose | 24 h | 48 h |
|---|
| Cells in media | – | 20.379±0.855 | 27.551±0.273 |
| DMSO | – | 21.243±0.332 | 27.500±0.390 |
| Melatonin | 2.5 mM |
16.104±0.546a |
21.522±0.568a |
| H-1152 | 10 µM |
10.482±0.844a |
20.955±0.410a |
| Melatonin+ | 2.5 mM+ |
9.474±0.623a |
17.534±0.453a |
| H-1152 | 10 µM |
|
|
Melatonin decreases the expression of
ROCK2 and the phosphorylation of MYPT1, and increases the
expression of occludin in RKO cells
Following cell treatment with different
concentrations of melatonin (1, 2 and 3 mM) for 48 h, the results
revealed that the protein expression levels of ROCK2 and p-MYPT1
were significantly decreased in a concentration-dependent manner
when compared to the DMSO control treatment (Fig. 2A and B). In addition, the present
study also assessed the effect of melatonin on the mRNA expression
of ROCK and several TJ proteins in RKO cells by qPCR and western
blotting. ROCK1 expression was not altered at the mRNA and protein
levels, however, ROCK2 mRNA and protein was significantly
downregulated by melatonin when compared with DMSO treatment
(Fig. 2B and C). Melatonin also
increased the mRNA level of ZO-1 and occludin in a
concentration-dependent manner (Fig.
2D and Table III). In
addition, the protein expression levels of occludin were
significantly increased with high concentration of melatonin (3
mM), however, ZO-1 was only marginally increased with melatonin
treatment (Fig. 2E). To further
confirm this, membrane proteins were isolated using a Membrane,
Nuclear and Cytoplasmic Protein Extraction kit. The results
demonstrated that the membrane protein expression of ZO-1 and
occludin were significantly upregulated following melatonin
treatment when the results were normalized to those of
Na+/K+-ATPase instead of the β-actin level
(Fig. 2F).
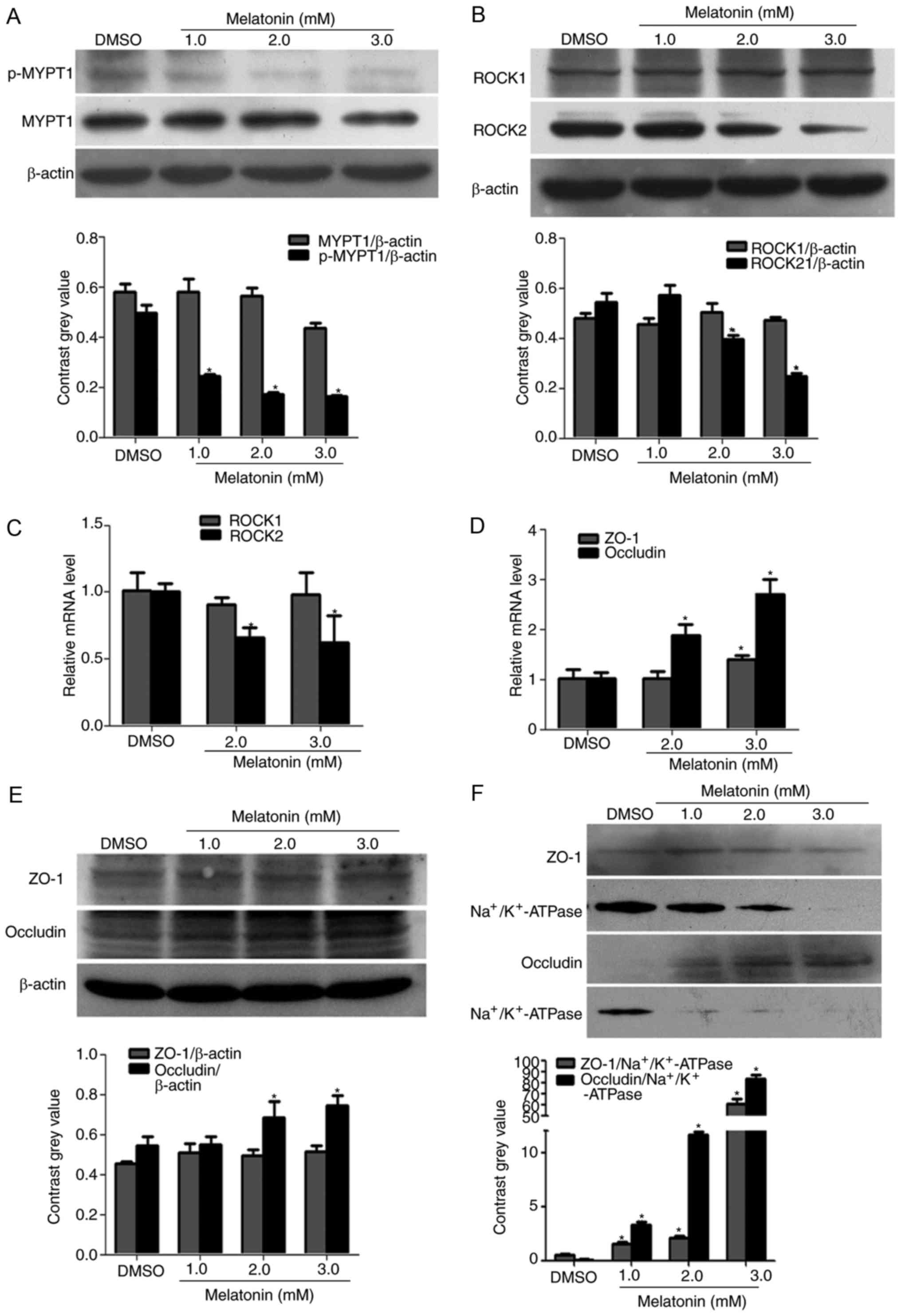 | Figure 2.Effect of melatonin on ROCK
expression and the phosphorylation of MYPT1. Western blotting was
performed to determine the protein expression of (A) MYPT1 and
p-MYPT1, and (B) ROCK1/2 in RKO cells. Treatment for 48 h with
different concentrations (1, 2 and 3 mM) of melatonin markedly
decreased the protein expression of ROCK2 and p-MYPT1 in RKO cells.
Reverse transcription-quantitative polymerase chain reaction was
performed to evaluate the relative mRNA levels of (C) ROCK1/2 and
(D) ZO-1 and occludin. The mRNA expression of ROCK2 was decreased
with melatonin, which reflected the results of western blotting. In
addition, the mRNA expression of ZO-1 and occludin were increased
with melatonin treatment. (E) In the total proteins, melatonin only
significantly increased the protein expression of occludin;
treatment had no effect on ZO-1 expression. (F) However, in the
membrane proteins the expression of ZO-1 and occludin was
significantly increased when compared with the control group.
*P<0.05 vs. the DMSO group. ROCK, Rho-associated protein kinase;
p-, phospho-; MYPT1, myosin phosphatase targeting subunit 1; ZO-1,
zona occludens-1. |
 | Table III.Effects of melatonin on the mRNA
expression levels in RKO cells. |
Table III.
Effects of melatonin on the mRNA
expression levels in RKO cells.
|
|
| Relative mRNA
expression |
|---|
|
|
|
|
|---|
| Treatment
group | Dose (mM) | ROCK1 | ROCK2 | ZO-1 | Occludin |
|---|
| DMSO | – | 1.007±0.142 | 1.001±0.060 | 1.010±0.177 | 1.007±0.136 |
| Melatonin | 2.0 | 0.908±0.052 |
0.659±0.076a | 1.012±0.147 |
1.877±0.214a |
|
| 3.0 | 0.982±0.162 |
0.623±0.202a |
1.391±0.089a |
2.703±0.280a |
As shown in Fig. 3,
following treatment with melatonin and H-1152, alone or in
combination, for 48 h, the expression of p-MYPT1, ROCK2 and p-MLC
were significantly decreased, however, the expression of MYPT1,
ROCK1 and MLC were not altered (Fig.
3A-C). Our previous study (8)
suggested that the phosphorylation of p38 was inhibited by
melatonin in a concentration-dependent manner. The present study
further determined p-p38 expression and revealed that when compared
with the control group, p38 phosphorylation was downregulated in
cells treated with H-1152 for 4 h (Fig. 3D).
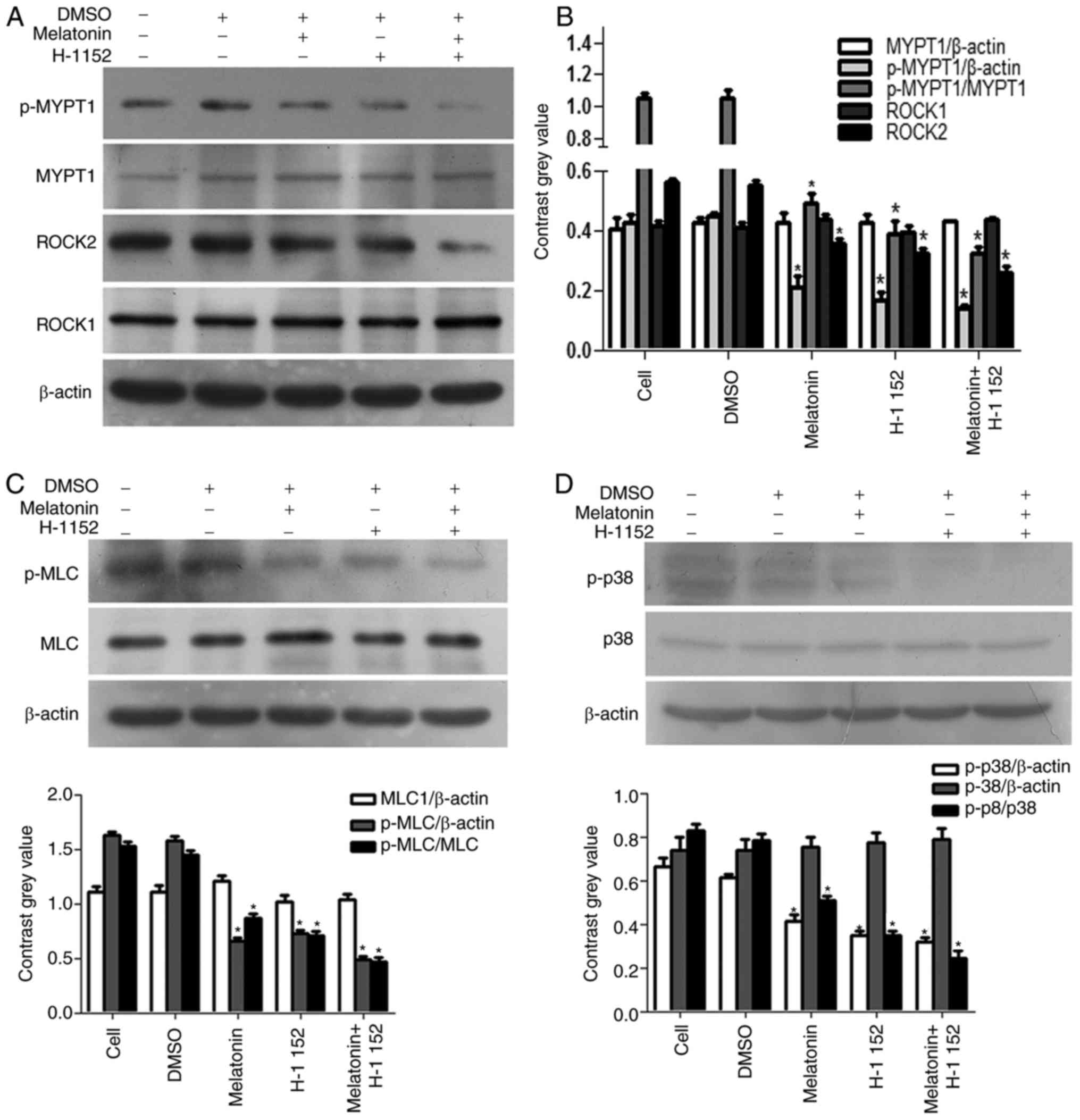 | Figure 3.Effect of melatonin and H-1152 on
ROCK expression, and the phosphorylation of MYPT1 and p38. H-1152
(10 µM) was used to treat RKO cells, alone and in combination with
melatonin, and the expression of (A) p-MYPT1 and ROCK2 were
significantly decreased. (B) Quantification of the protein
expression levels of p-MYPT1 and ROCK2. (C) p-MLC was significantly
decreased. (D) In addition, the phosphorylation of p38 was also
decreased in RKO cells following H-1152 treatment, alone or in
combination with melatonin (2.5 mM) for 4 h, thereby exhibiting the
same trend as ROCK2 expression. *P<0.05 vs. the DMSO group.
H-1152, 5-[[(2s)-hexahydro-2-methyl-1H-1,4-diazepin-1-yl]
sulfonyl]-4-methyl-isoquinoline, dihydrochloride; ROCK,
Rho-associated protein kinase; MYPT1, myosin phosphatase targeting
subunit 1; p-, phospho-; MLC, myosin light chains. |
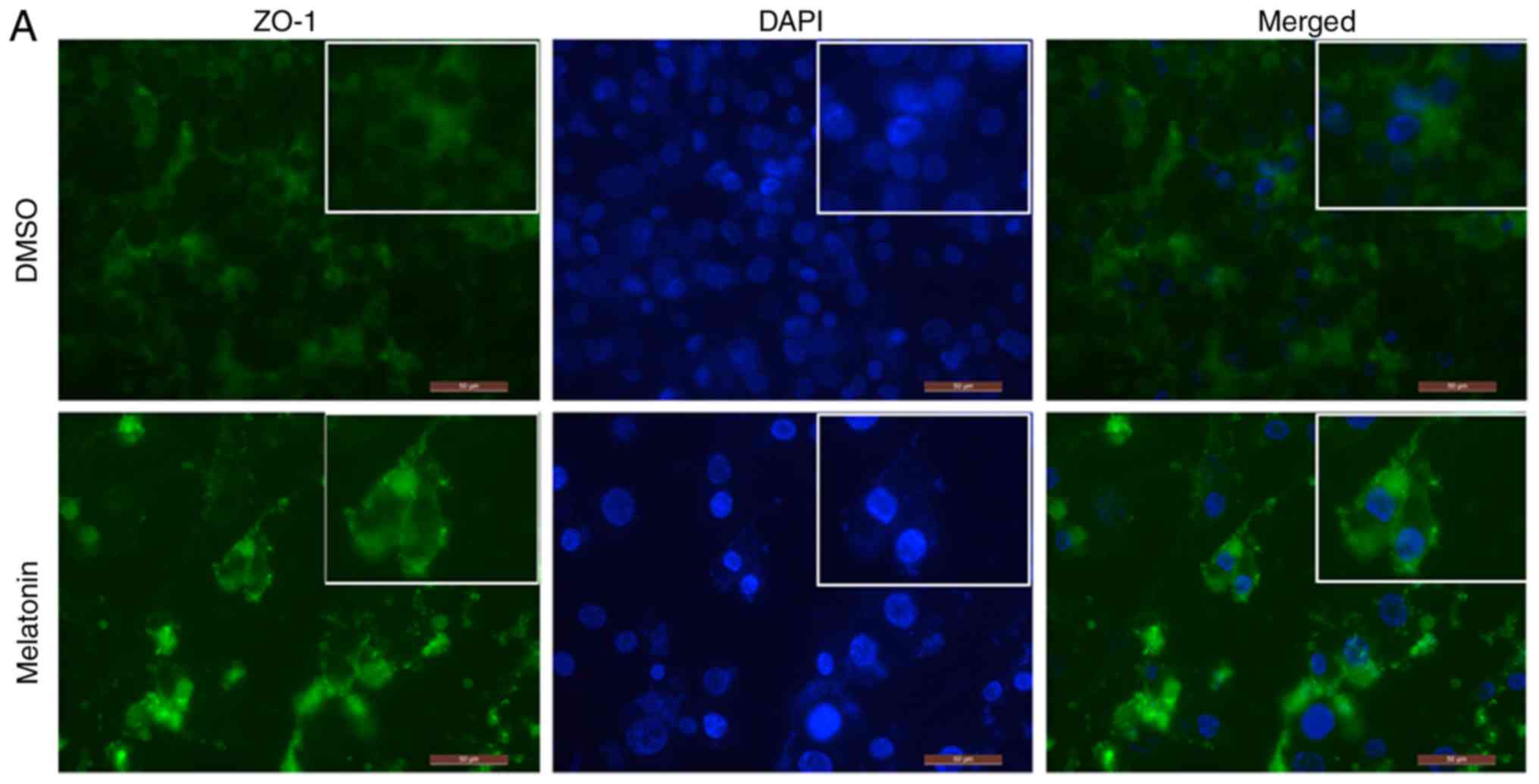
Melatonin increases the expression of
ZO-1 and occludin in RKO cell TJs
In order to detect the expression and localization
of ZO-1 and occludin proteins, an immunofluorescence assay was
performed. Following RKO cell treatment with melatonin, the
fluorescence intensity of ZO-1 and occludin was increased,
particularly in regions with TJs, when compared with the control
group (Fig. 4A and B).
Quantitative analysis of immunofluorescence revealed that the
expression of ZO-1 and occludin was increased in the
melatonin-treated group, which was consistent with the western
blotting results (Fig. 4C and
D).
Melatonin downregulates the expression
of ROCK via the p38/MAPK signaling pathway in RKO cells
Following the aforementioned experiments, it was
speculated whether the expression and activity of ROCK may be
relevant in p38 MAPK signaling. Therefore, the effect of PMA, a PKC
activator, and SB203580, a p38 inhibitor, on the expression of
p-MYPT1 was determined (Fig. 5).
Western blot analysis demonstrated that melatonin inhibited the
expression of the p-MYPT1. In addition, treatment with melatonin
and SB combined further reduced the expression levels of p-MYPT1.
However, although the expression of p-MYPT1 was significantly
decreased when cells were treated in combination with PMA compared
with PMA treatment alone, this decrease was not as marked as that
observed with melatonin and SB treatments.
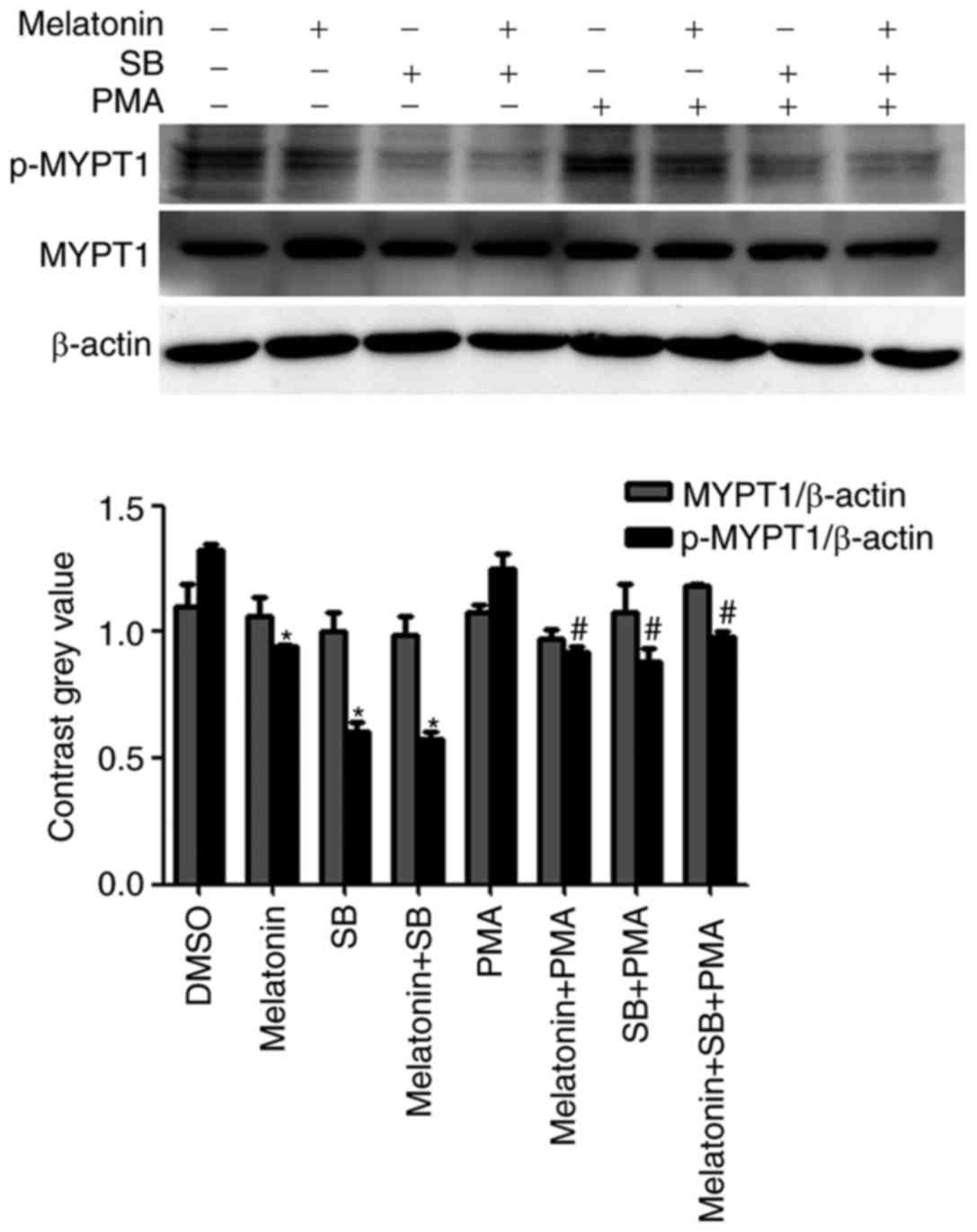 | Figure 5.Effect of melatonin, SB203580 and PMA
on the expression of p-MYPT1. Cells were treated with DMSO, or with
melatonin (2.5 mM), SB203580 (10 µM) and PMA (1 µM), alone or in
combination, for 48 h. The expression of p-MYPT1 decreased when
cells were exposed to SB203580. Combined treatment of melatonin
and/or SB203580 with PMA inhibited the phosphorylation of MYPT1
however, the decrease in p-MYPT1 expression observed was not as
marked. Thus, PMA partially prevented the melatonin or
SB203580-induced decrease in p-MYPT1 expression. *P<0.05, vs.
the DMSO group; #P<0.05, vs. the PMA only group. PMA,
Phorbol 12-myristate 13-acetate; p-, phospho-; MYPT1, myosin
phosphatase targeting subunit 1; SB, SB203580. |
Discussion
The hallmarks of cancer include sustaining
proliferative signaling, evading growth suppressors, resisting cell
death, enabling replicative immortality, inducing angiogenesis, and
activating cell invasion and metastasis (28). Recently, several studies have
indicated that melatonin can modulate microtubule and microfilament
structure formation, and suppress the invasive and metastatic
potential of invasive and metastatic potential of breast (6), colon (8), liver (10) and lung (13) cancer cells via different signaling
pathways. However, the underlying mechanism of melatonin on RKO
cells migration inhibition is poorly understood. In the present
study, melatonin suppressed the migration of human colon cancer RKO
cells, potentially via the P-38/MAPK signaling pathway, and also
decreased the expression of ROCK and TJ associated proteins.
The two ROCK isoforms, ROCK1 and ROCK2, which share
65% homology in their amino acid sequences and 92% homology in
their kinase domains, are highly homologous. Although the two
isoforms are ubiquitously expressed, the expression of ROCK1 and
ROCK2 are more prominent in different tissues including the liver
and lung, and the brain and heart, respectively (29,30).
In the present study, the data demonstrated that the protein
expression of ROCK2 in RKO cells treated with various
concentrations of melatonin was downregulated. In addition, the
mRNA levels of ROCK2 were suppressed with increasing concentrations
of melatonin, however, the expression of ROCK1 was not altered.
These results indicate that melatonin may reduce ROCK2 expression
at the transcriptional and protein level. In the author's previous
study (8), the phosphorylation of
MYPT1 and MLC was inhibited by melatonin in a
concentration-dependent manner. Therefore, ROCK2 may serve a role
in the dephosphorylation of MLC, as opposed to ROCK1, in RKO cells.
There is an increasing body of evidence that has indicated that
ROCK may be active and augment tumor aggressive properties such as
metastasis and invasion in gastric cancer cells (31,32).
Thus, a reduction in ROCK activity may inhibit the migration and
invasion of cancer cells. Despite their extensive homology and
substrate promiscuity, the two ROCK isoforms have distinct
functions. Notably, ROCK2 depletion has been reported to enhance
microfilament bundle assembly into stress fibers and the formation
of focal adhesion, while ROCK1-depleted cells exhibit the opposite
phenotype (33).
Our previous findings suggested that the migration
of RKO cells was inhibited in a concentration-dependent manner
following treatment with melatonin (8). To confirm the association between
ROCK and RKO cell migration, the present study treated cells with
H-1152 to inhibit ROCK function, which demonstrated that RKO cell
migration was markedly inhibited when compared with the control
group. H-1152, a specific inhibitor of ROCK, also decreased ROCK
protein expression; ROCK2 protein expression and the
phosphorylation of MYPT1 were inhibited following treatment with
melatonin and H-1152, alone or in combination. In addition, the
phosphorylation level of MLC was also decreased. These results
indicate that melatonin may exert inhibitory effects on RKO cell
migration by attenuating the expression of ROCK2 and the
phosphorylation of MLC. Previous studies have reported that MLC
phosphorylation is essential to initiate actin-myosin interactions.
Phosphorylation of MLC activates myosin ATPase activity, which
couples with actin-myosin filaments to the plasma membrane, thereby
increasing the generation of actin-myosin contractile force and
cell contractility (8,11). Pretreatment with the specific ROCK
inhibitor, Y27632, has also been revealed to abrogate MLC
phosphorylation and suppress membrane contraction (34).
In the present study, the membrane proteins ZO-1 and
occludin were observed to be increased in RKO cells treated with
melatonin. Immunofluorescence assay analyses also revealed that
melatonin induced the localized expression of ZO-1 and occludin at
cell TJs. In addition, some studies have also suggested that
melatonin may inhibit tumor invasion by increasing the expression
of TJ-associated proteins ZO-1 and occludin (13,14).
These results suggest that translocation of ZO-1 and occludin may
contribute to the anti-migration effect of melatonin.
Our previous study demonstrated that the
phosphorylation of p38 was decreased by melatonin treatment in a
concentration-dependent manner (8). In addition, p38 MAPK has been
reported to be a key signaling molecule in the regulation of cancer
invasion and metastasis (35,36).
In the present study, western blotting suggested that the
phosphorylation of p38 was suppressed by H-1152, thus exhibiting a
similar trend to that of ROCK2 expression. Therefore, ROCK2 may
contribute to the migration of RKO cells by inhibiting p38 MAPK.
The authors' previous study verified that SB203580, an inhibitor of
the p38 MAPK signaling pathway, further enhanced the
melatonin-induced inhibition of RKO migration, while PMA, a PKC
activator, weakened this inhibitory effect (8), the inhibitory effect of SB203580 was
demonstrated to be similar to H-1152-mediated migration. The
present study confirmed that the expression of p-MYPT1 was more
markedly reduced following the combined treatment with melatonin
and SB203580 when compared with melatonin treatment alone, while
the combined treatment with PMA attenuated this inhibitory effect.
In addition, SB203580 markedly decreased the expression of p-MYPT1
same as p-p38 in the PMA-stimulated group in the authors' previous
study. Due to its inhibitory effect on p38 phosphorylation,
previous studies have suggested that melatonin is a promising
anti-invasion factor that may also be effective in cancer therapies
targeting breast cancer (37,38);
this is consistent with the results of the present study.
Collectively, these results indicate that the anti-migration effect
of melatonin may be associated with the downregulation of the p38
MAPK signaling pathway and ROCK expression.
In conclusion, melatonin inhibited the migration of
RKO cells by decreasing ROCK expression, and increasing the
expression of ZO-1 and occludin on the cell membrane, which may be
associated, at least in part, with the p38 MAPK signaling pathway.
These results provide evidence for the potential clinical
application of melatonin in the treatment of metastatic colorectal
cancers. However, tumor cell migration and the formation of tumor
metastases are extremely complex processes, thus further studies
are required to elucidate the comprehensive molecular mechanisms
underlying melatonin-induced inhibition of tumor cell
migration.
Acknowledgements
The present study was funded by the Science
Foundation for the Excellent Youth Scholars of Universities of
Anhui Province (grant no. 2013SQRL101ZD), the Anhui Natural Science
Foundation (grant no. 1508085QH167) and the National Natural
Science Foundation of China (grant no. 81272399).
References
|
1
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vellinga TT, Borovski T, de Boer VC,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: SIRT1/PGC1α dependent increase in
oxidative phosphorylation supports chemotherapy resistance of colon
cancer. Clin Cancer Res. 21:2870–2879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akbulut KG, Aktas SH and Akbulut H: The
role of melatonin, sirtuin2 and FoXO1 transcription factor in the
aging process of colon in male rats. Biogerontol. 16:99–108. 2015.
View Article : Google Scholar
|
|
4
|
Xu C, Wu A, Zhu H, Fang H, Xu L, Ye J and
Shen J: Melatonin is involved in the apoptosis and necrosis of
pancreatic cancer cell line SW-1990 via modulating of Bcl-2/Bax
balance. Biomed Pharmacother. 67:133–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Guo W, Chen W, Yu W, Tian Y, Fu L,
Shi D, Tong B, Xiao X, Huang W and Deng W: Melatonin potentiates
the antiproliferative and pro-apoptotic effects of ursolic acid in
colon cancer cells by modulating multiple signaling pathways. J
Pineal Res. 54:406–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mao L, Yuan L, Slakey LM, Jones FE, Burow
ME and Hill SM: Inhibition of breast cancer cell invasion by
melatonin is mediated through regulation of the p38
mitogen-activated protein kinase signaling pathway. Breast Cancer
Res. 12:R1072010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi C, Zhang Y, Yu Z, Xiao Y, Wang J, Qiu
H, Yu W, Tang R, Yuan Y, Guo W and Deng W: Melatonin enhances the
antitumor effect of fisetin by inhibiting COX-2/iNOS and NF-kB/p300
signaling pathways. PLoS One. 9:999432014. View Article : Google Scholar
|
|
8
|
Zou DB, Wei X, Hu RL, Yang XP, Zuo L,
Zhang SM, Zhu HQ, Zhou Q, Gui SY and Wang Y: Melatonin inhibits the
migration of colon cancer RKO cells by down-regulating myosin light
chain kinase expression through cross-talk with p38 MAPK. Asian Pac
J Cancer Prev. 16:5835–5842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benítez-King G, Soto-Vega E and
Ramírez-Rodriguez G: Melatonin modulates microfilament phenotypes
in epithelial cells: Implications for adhesion and inhibition of
cancer cell migration. Histol Histopathol. 24:789–799.
2009.PubMed/NCBI
|
|
10
|
Ordoñez R, Carbajo-Pescador S,
Prieto-Dominguez N, García-Palomo A, González-Gallego J and Mauriz
JL: Inhibition of matrix metalloproteinase-9 and nuclear factor
kappa B contribute to melatonin prevention of motility and
invasiveness in HepG2 liver cancer cells. J Pineal Res. 56:20–30.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Runkle EA and Mu D: Tight junction
proteins: From barrier to tumorigenesis. Cancer Lett. 337:41–48.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Q, Gui S, Zhou Q and Wang Y:
Melatonin inhibits the migration of human lung adenocarcinoma A549
cell lines involving JNK/MAPK pathway. PLoS One. 9:1011322014.
View Article : Google Scholar
|
|
14
|
Hoover KB, Liao SY and Bryant PJ: Loss of
the tight junction MAGUK ZO-1 in breast cancer: Relationship to
glandular differentiation and loss of heterozygosity. Am J Pathol.
153:1767–1773. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vicente-Manzanares M, Ma X, Adelstein RS
and Horwitz AR: Non-muscle myosin II takes centre stage in cell
adhesion and migration. Nat Rev Mol Cell Biol. 10:778–790. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
González-Marisca L, Tapia R and Chamorro
D: Crosstalk of tight junction components with signaling pathways.
Biochim Biophys Acta. 1778:729–756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito M, Nakano T, Erdodi F and Hartshorne
DJ: Myosin phosphatase: Structure, regulation and function. Mol
Cell Biochem. 259:197–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pellegrin S and Mellor H: Actin stress
fibres. J Cell Sci. 120:3491–3499. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsuji T, Ishizaki T, Okanoto M, Higashida
C, Kimura K, Furuyashiki T, Arakawa Y, Birge RB, Nakamoto T, Hirai
H and Narumiya S: ROCK and mDia1 antagonize in Rho-dependent Rac
activation in Swiss 3T3 fibroblasts. J Cell Biol. 157:819–830.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wojciak-Stothard B and Ridley AJ: Shear
stress-induced endothelial cell polarization is mediated by Rho and
Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 161:429–439. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Totsukawa G, Wu Y, Sasaki Y, Hartshorne
DJ, Yamakita Y, Yamashiro S and Matsumura F: Distinct roles of MLCK
and ROCK in the regulation of membrane protrusions and focal
adhesion dynamics during cell migration of fibroblasts. J Cell
Biol. 164:427–439. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Banerjee S and McGee DW: ROCK activity
affects IL-1-induced signaling possibly through MKK4 and p38 MAPK
in Caco-2 cells. In Vitro Cell Dev Biol Anim. 52:878–884. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang L and Karin M: Mammalian MAP kinase
signaling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cumaoglu A, Dayan S, Agkaya AO, Ozkul Z
and Ozpozan NK: Synthesis and pro-apoptotic effects of new
sulfonamide derivatives via activating p38/ERK phosphorylation in
cancer cells. J Enzyme Inhib Med Chem. 30:413–419. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grossi V, Peserico A, Tezil T and Simone
C: p38α MAPK pathway: A key factor in colorectal cancer therapy and
chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hill SM, Belancio VP, Dauchy RT, Xiang S,
Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, et al:
Melatonin: An inhibitor of breast cancer. Endocr Relat Cancer.
22:R183–R204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa O, Fujisawa K, Ishizaki T, Saito
Y, Nakao K and Narumiya S: ROCK-I and ROCK-II, two isoforms of
Rho-associated coiled-coil forming protein serine/threonine kinase
in mice. FEBS Lett. 392:189–193. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hahmann C and Schroeter T: Rho-kinase
inhibitors as therapeutics: From pan inhibition to isoform
selectivit. Cell Mol Life Sci. 67:171–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsuoka T, Yashiro M, Kato Y, Shinto O,
Kashiwagi S and Hirakawa K: RhoA/ROCK signaling mediates plasticity
of scirrhous gastric carcinoma motility. Clin Exp Metastasis.
28:627–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu N, Bi F, Pan Y, Sun L, Xue Y, Shi Y,
Yao X, Zheng Y and Fan D: Reversal of the malignant phenotype of
gastric cancer cells by inhibition of RhoA expression and activity.
Clin Cancer Res. 10:6239–6247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zucchini C, Martinelli M, De Sanctis P,
Rodia MT, Mattei G, Ugolini G, Montroni I, Ghignone F and Solmi R:
Possible gender-related modulation by the ROCK1 gene in colorectal
cancer susceptibility. Pathobiology. 82:252–258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hsu HH, Kuo WW, Day CH, Shibu MA, Li SY,
Chang SH, Shih HN, Chen RJ, Viswanadha VP, Kuo YH and Huang CY:
Taiwanin E inhibits cell migration in human LoVo colon cancer cells
by suppressing MMP-2/9 expression via p38 MAPK pathway. Environ
Toxicol. 32:2021–2031. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shin I, Kim S, Song H, Kim HR and Moon A:
H-Ras-specific activation of Rac-MKK3/6-p38 pathway: Its critical
role in invasion and migration of breast epithelial cells. J Biol
Chem. 280:14675–14683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mao L, Yuan L, Slakey LM, Jones FE, Burow
ME and Hill SM: Inhibition of breast cancer cell invasion by
melatonin is mediated through regulation of the p38
mitogen-activated protein kinase signaling pathway. Breast Cancer
Res. 2:R1072010. View Article : Google Scholar
|
|
37
|
Hill SM, Belancio VP, Dauchy RT, Xiang S,
Brimer S, Mao L, Hauch A, Lundberg PW, Summers W, Yuan L, et al:
Melatonin: An inhibitor of breast cancer. Endocr Relat Cancer.
22:R183–R204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lai JM, Hsieh CL and Chang ZF: Caspase
activation during phorbol ester-induced apoptosis requires ROCK
dependent myosin-mediated contraction. J Cell Sci. 116:3491–3501.
2003. View Article : Google Scholar : PubMed/NCBI
|