Introduction
Osteoporosis is a systemic disease, which is
characterized by low bone mineral density (BMD) and deterioration
of skeletal microarchitecture, leading to increased risk of
fragility fractures (1). The
number of patients presenting clinically with osteoporosis is
increasingly common; a major hazard of osteoporosis is the
associated increased risk of fracture and the potential induction
of numerous other complications (2). It has been shown in epidemiological
investigations that osteoporosis has become a common disease in
clinics, and the importance of its associated risks has been
increasingly recognized by clinicians (2,3).
Osteoporosis is a type of metabolic disease, which
is characterized by low BMD, deterioration of skeletal
microarchitecture and increased risk of fragility fracture
(4). It is associated with a
diverse pathogenesis and complex molecular mechanisms, and has
become an epidemic affecting patient quality of life. The human
body lives in an oxygen-rich environment, and the metabolic process
of oxygen produces reactive oxygen species (ROS) continuously. The
body experiences oxidative stress when the balance between the
generation of ROS and the elimination of ROS is disrupted as a
result of aging and disease (5).
Increasingly, it has been found that ROS-induced oxidative stress
is important in osteoporosis; the excess of ROS regulates multiple
signaling pathways through the activation or inhibition of multiple
cytokines and enzyme activities, in addition to the upregulation or
downregulation of the expression of receptor ligands. This affects
the expression of endonuclear genes, accelerates the apoptosis of
osteogenesis-related cells, including bone mesenchymal stem cells
(BMSCs), osteoblasts and osteocytes, and increases the
proliferation and differentiation of osteoclasts. This results in a
reduced bone absorption rate relative to the bone formation rate,
and alters the dynamic balance between osteoclasts absorbing bone
tissue and osteoblasts forming bone tissue, resulting in
osteoporosis (6,7).
As an essential cell transcriptional factor during
the maturation process of adipocytes, the expression and activity
of peroxisome proliferators-activated receptor γ (PPAR-γ) may
determine the differentiation direction of mouse BMSCs into
osteoblasts or adipocytes (8). In
previous years, OS has attracted increased attention as a risk
factor for osteoporosis, and investigations on the role of the
PPAR-γ/WNT pathway in OS-mediated osteoporosis have been the most
extensive and thorough (9).
Consequently, the focus on anti-osteoporosis has gradually changed
from an estrogen-centered approach to an aging and oxidative
stress-centered approach. In our previous study, isopsoralen
inhibited the differentiation of mouse BMSCs into mature adipocytes
in a concentration-dependent manner; in addition, the
above-mentioned effects had marked effects on improving ovarian
hormone deficiency-induced osteoporosis in addition to bone
damage.
Traditional Chinese medicine has a history dating
back several thousand years and, in traditional medicine use in
China, the fruit of the Psoralea corylifolia has been used
extensively for improving performance of the kidney, spleen and
stomach. It is also termed Fructus psoraleae, Pogizhi and
Hufeizi (10), and has been
frequently used for treating fractures and osteoarthropathy, with
marked effects (11). Clinically,
P. corylifolia has also been used in treating dermatosis,
cardiovascular lesions, tumors and asthma. Isopsoralen (Fig. 1), an isomer with psoralen, is the
major effective component in the extract of P. corylifolia
(12). Numerous investigations
have revealed that psoralen is capable of promoting osteoblast
differentiation and maturation, and stimulating bone formation
(13). The present study aimed to
determine how isopsoralen regulates osteoporosis and to elucidate
the mechanism involved.
Materials and methods
Animals and experimental design
Six-week-old female Sprague-Dawley rats (weighing
140–160 g) were purchased from Inner Mongolia Medical University
(Inner Mongolia, China) and housed in polycarbonate cages in
temperature-controlled rooms (22±2°C) with relative humidity of
55±5% and a 12-h light/dark cycle. The experimental protocol was
approved by the Animal Care and Use Review Committee of The Inner
Mongolia People's Hospital. The rats were fed a standard laboratory
diet and were provided with water ad libitum for an
adaptation period of 7 days.
The rats were randomly distributed into four groups:
Sham-operated control (sham), ovariectomized rats without treatment
(osteoporosis), ovariectomized rats treated with isopsoralen 10
mg/kg/every 3 days (ISO-10), and ovariectomized rats treated with
isopsoralen 20 mg/kg every 3 days (ISO-20). All groups were treated
for 12 weeks. Following adaptation, the female ovariectomized rats
were anesthetized with 2% isoflurane, and ovaries were removed
bilaterally.
Calcium and urinary analysis
Blood was collected from the eye sockets of mice
under 2% isoflurane, and serum and urine were collected following
4,000 × g centrifugation for 10 min at 4°C. The serum and urinary
calcium levels were determined using a Technicon SMAC analyzer
(Technicon Instruments Corporation, Tarrytown, NY, USA). Serum
levels of leptin and parathyroid hormone (PTH) were determined
using Novartis Pharma Ag with a Luminex 200™ Multiplexing
instrument. A high-resolution desktop micro-CT system (SkyScan
1174v2; Bruker MicroCT, Kontich, Belgium) was used to analyze the
bone mineral density (BMD).
ELISA analysis
Blood was collected from the eye sockets under 2%
isoflurane and serum was collected following 4,000 × g
centrifugation for 10 min at 4°C. The levels of alkaline
phosphatase (ALP), the oxidative stress indicators, superoxide
dismutase (SOD), malondialdehyde (MDA), glutathione (GSH) and
glutathione peroxidase (GSH-PX), and the activities of caspase-3/9
were measured using ELISA assay kits (Elabscience Biotechnology
Co., Ltd., Wuhan, China) according to the manufacturers protocol,
respectively.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was prepared from cartilage tissue using
an RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA). cDNA was
synthesized using 1–2 µg of total RNA using reverse transcriptase
(Takara Bio, Inc., Otsu, Japan). The ABI 7500 sequencing detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used for qPCR analysis using the SYBR Premix
Ex Tag kit (Takara Bio, Inc.) with 200 ng cDNA (total reaction
volume: 10 µl; 1–2 µl cDNA, 2 µl primers, 2 µl SYBR and 4 µl
water). The primer sets used are listed in Table I. The detector was programmed with
the following PCR conditions: 40 cycles of 15 sec denaturation at
95°C, 40 sec amplification at 60°C and 30 sec at 72°C. The mRNA
levels were calculated using the 2−ΔΔCq analysis method
(14).
 | Table I.Primer sets used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sets used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer (5–3) |
|---|
| Col I | F:
ACCTAAGGGTACCGCTGGA |
|
| R:
TCCAGCTTCTCCATCTTTGC |
| OCN | F:
TCTCTCTGACCTCACAGATGCC |
|
| R:
TCCAGCTTCTCCATCTTTGC |
| OPG | F:
TGTTCCTACCAAGATTATACCAAAT |
|
| R:
CGCTCGATTTGCAGGTCTTT |
| GAPDH | F:
ACCACAGTCCATGCCATCAC |
|
| R:
TCCACCACCCTGTTGCTGTA |
Western blot analysis
Cartilage tissues were collected and lysed in lysis
buffer. The protein content of the supernatant was determined using
a BCA™ protein assay kit (Beyotime Institute of Biotechnology,
Haimen, China) following 10,000 × g centrifugation for 10 min at
4°C. Total protein (50 µg) was separated by 8–10% SDS-PAGE and
subsequently electrotransferred onto a PVDF membrane (EMD
Millipore, Bedford, MA, USA). The membrane was blocked with 5%
non-fat milk for 1 h at 37°C and incubated with the indicated
antibodies: Anti-Wnt (cat. no. sc-5630; 1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) anti-β-catenin (cat. no.
sc-7199; 1:500; Santa Cruz Biotechnology, Inc.), ant-PPAR-γ (cat.
no. sc-7196; 1:500; Santa Cruz Biotechnology, Inc.) and anti-GAPDH
(cat. no. sc-25778; 1:500; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. Following washing three times for 15 min with TBST, the
membrane was detected by incubation with anti-rabbit horseradish
peroxidase (HRP)-conjugated secondary antibodies (cat. no. sc-2004;
1:2,000; Santa Cruz Biotechnology, Inc.) for 1 h, and
immunodetection with enhanced chemiluminescence (Thermo Fisher
Scientific, Inc.) and quantified by Image Lab version 3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA).
The experimental groups were compared using one-way analysis of
variance and Duncans test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Isopsoralen regulates urine
calcium/creatinine (Ca/Cr) in osteoporosis
At 12 weeks post-isopsoralen treatment, the urine
level of Ca/Cr in the osteoporosis model group was higher, compared
with that in the sham group (Fig.
2). Treatment with 10 or 20 mg/kg isopsoralen effectively
inhibited the increased urine Ca/Cr in the rats with osteoporosis,
compared with the osteoporosis rat model group (Fig. 2).
Isopsoralen regulates BMD and
structure of the proximal tibial metaphysis (PTM) in
osteoporosis
There was a significant decrease in BMD and increase
in PTM scores in the osteoporosis model rats, compared with the
levels in the sham group (Fig. 3A and
B). Treatment with 10 or 20 mg/kg isopsoralen significantly
reversed the inhibited BMD and increased PTM scores in the rats
with osteoporosis, compared with those in the osteoporosis rat
model group (Fig. 3A and B).
Isopsoralen regulates leptin and
calcium in osteoporosis
Inhibition of leptin levels and increasing levels of
calcium were observed in the osteoporosis model, compared with
levels in the sham group (Fig. 4A and
B). However, treatment with 10 or 20 mg/kg isopsoralen
significantly increased the levels of leptin and reduced calcium in
the rats with osteoporosis, compared with the osteoporosis rat
model group (Fig. 4A and B).
Isopsoralen regulates levels of ALP,
collagen type I (Col I), osteocalcin (OCN) and osteoprotegerin
(OPN) in osteoporosis
The present study investigated the pro-osteogenic
effects of isopsoralen on ALP, Col I, OCN and OPN in the rats with
osteoporosis. As shown in Fig.
5A-D, the levels of ALP, Col I, OCN and OPN were stimulated in
the osteoporosis model rat, compared with the levels in the sham
group. In the later stage, the induced activities of ALP, Col I,
OCN and OPN were significantly inhibited by 10 and 20 mg/kg
isopsoralen in the rats with osteoporosis (Fig. 5A-D).
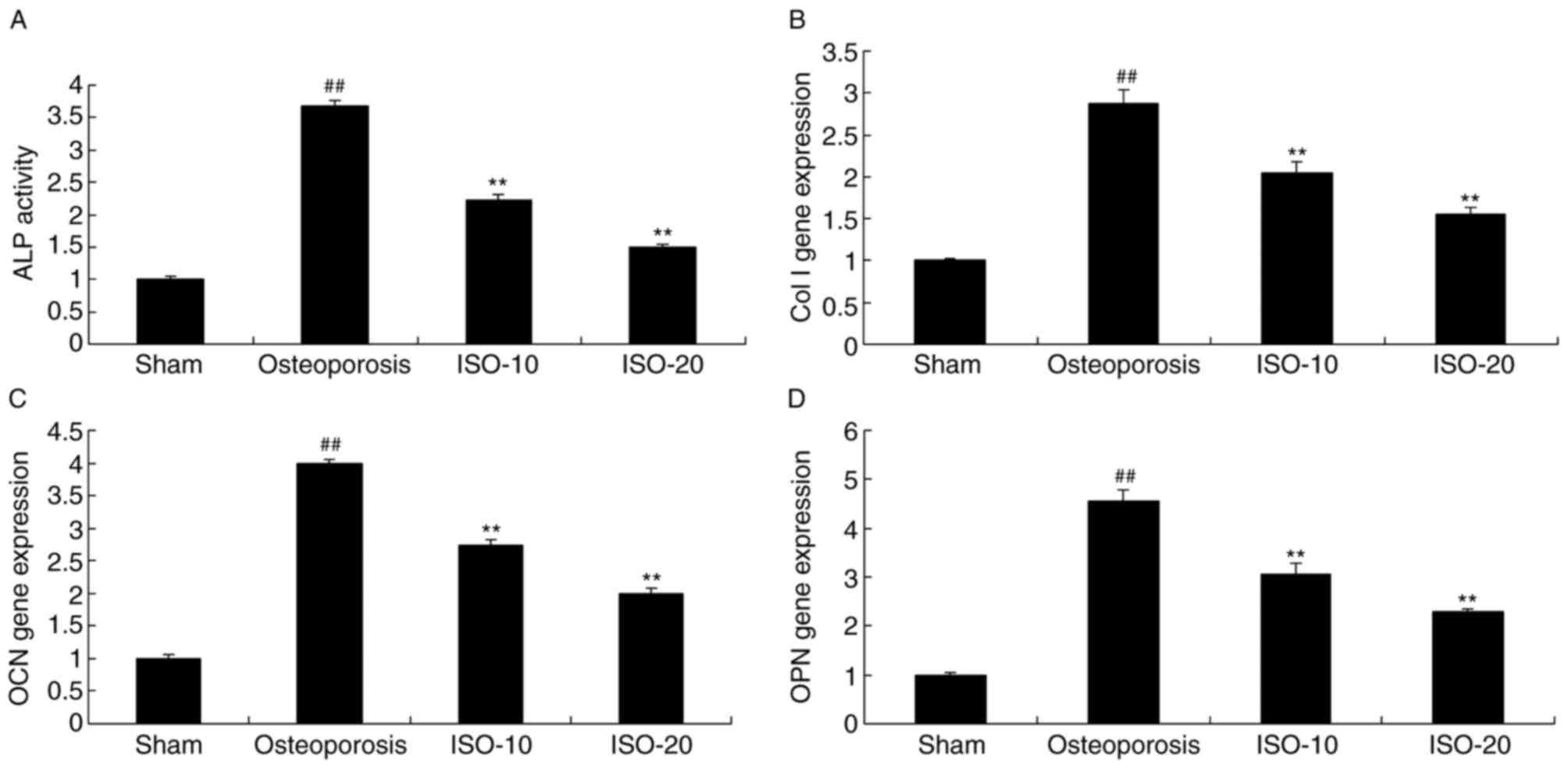 | Figure 5.Isopsoralen regulates ALP, Col I, OCN
and OPN in osteoporosis. Isopsoralen regulated (A) ALP, (B) Col I,
(C) OCN and (D) OPN in osteoporosis. ##P<0.01,
compared with the sham group; **P<0.01, compared with the
osteoporosis model group. ISO, isopsoralen; Sham, sham group;
Osteoporosis, osteoporosis model; ISO-10, 10 mg/kg ISO; ISO-20, 20
mg/kg ISO; ALP, alkaline phosphatase; Col I, collagen type I; OCN,
osteocalcin; OPN, osteoprotegerin. |
Isopsoralen regulates oxidative stress
in osteoporosis
To detect the anti-oxidative effects of isopsoralen
on oxidative stress in the osteoporosis rat model, the activities
of SOD, MDA, GSH and GSH-PX were examined. In the osteoporosis
model rats, it was found that the activities of SOD, GSH and GSH-PX
were decreased, and the activity of MDA was increased, compared
with activities in the sham group (Fig. 6A-D). Treatment with 10 or 20 mg/kg
isopsoralen significantly decreased the activity of MDA, and
increased the activities of SOD, GSH and GSH-PX in the rats with
osteoporosis (Fig. 6A-D).
 | Figure 6.Isopsoralen regulates oxidative stress
in osteoporosis. Isopsoralen regulated (A) SOD, (B) MDA, (C) GSH
and (D) GSH-PX in osteoporosis. ##P<0.01, compared
with the sham group; **P<0.01, compared with the osteoporosis
model group. ISO, isopsoralen; Sham, sham group; Osteoporosis,
osteoporosis model; ISO-10, 10 mg/kg ISO; ISO-20, 20 mg/kg ISO;
SOD, superoxide dismutase; MDA, malondialdehyde, GSH, glutathione,
GSH-PX, glutathione peroxidase. |
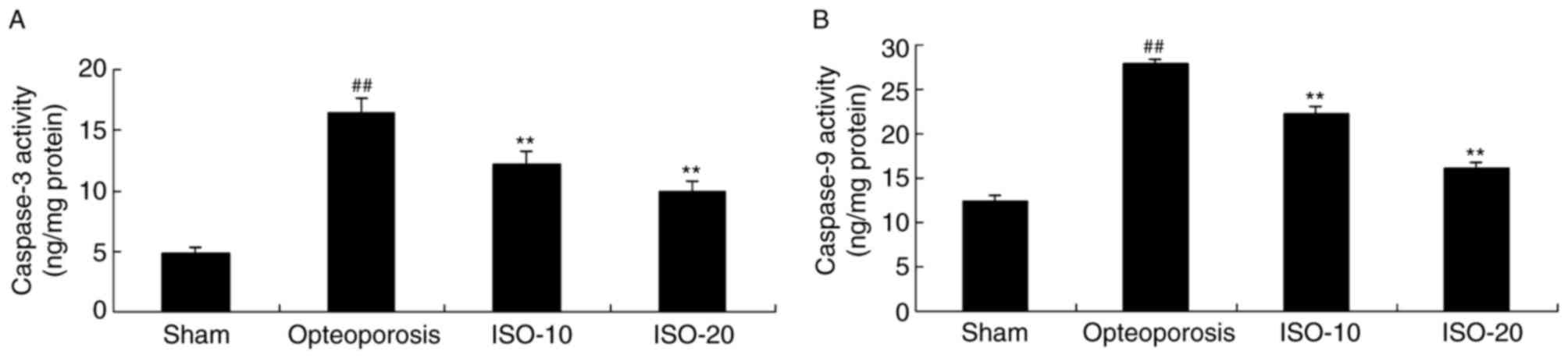
Isopsoralen regulates caspase-3/9
activity in osteoporosis
In order to examine the effect of isopsoralen on the
apoptosis in bone of rats with osteoporosis, the present study
examined caspase-3/9 activity in the osteoporosis rat model. As
shown in Fig. 7A and B,
significant increases in caspase-3/9 activity were observed in the
osteoporosis rat model, compared with the sham group. Treatment
with 10 or 20 mg/kg isopsoralen significantly weakened caspase-3/9
activity in the rats with osteoporosis (Fig. 7A and B).
Isopsoralen regulates the protein
expression of WNT in osteoporosis
To confirm the osteogenic effects of isopsoralen on
osteoporosis, the protein expression of WNT was measured using
western blot analysis. There was a significant decrease in the
protein expression of WNT in the osteoporosis model rat, compared
with that in the sham group (Fig. 8A
and B). Treatment with 10 or 20 mg/kg isopsoralen significantly
induced the protein expression of WNT in the rats with osteoporosis
(Fig. 8A and B).
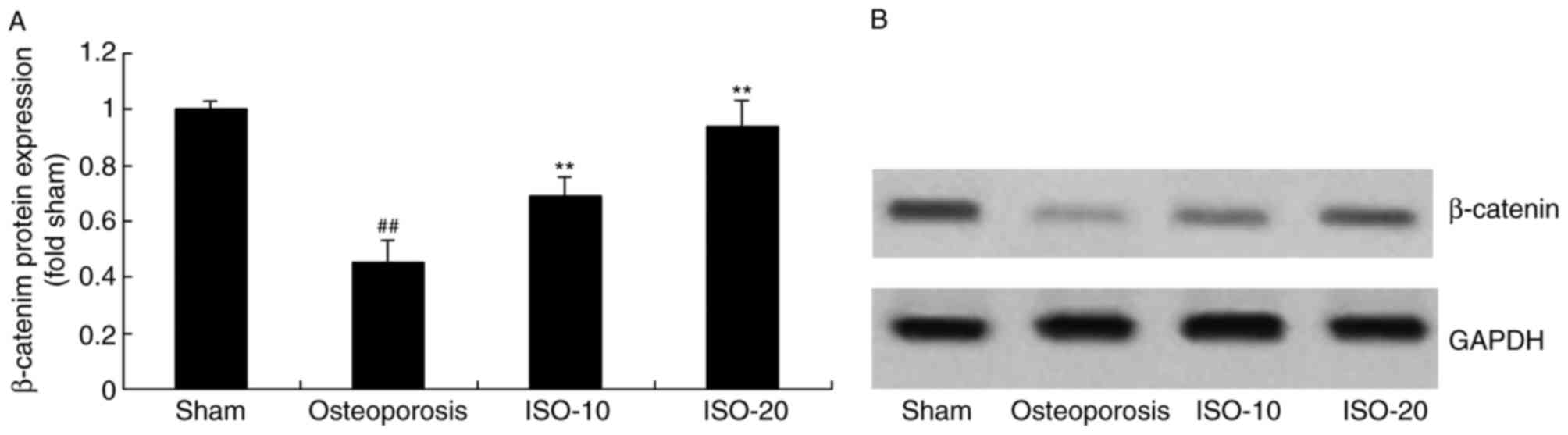
Isopsoralen regulates the protein
expression of β-catenin in osteoporosis
The present study also examined whether the protein
expression of β-catenin is involved in the osteogenic effects of
isopsoralen on osteoporosis. It was found that the protein
expression of β-catenin was significantly inhibited in the
osteoporosis rat model, compared with that in the sham group
(Fig. 9A and B). However, the
inhibition of the protein expression of β-catenin was significantly
reduced in the rats with osteoporosis by 10 or 20 mg/kg isopsoralen
(Fig. 9A and B).
Isopsoralen regulates the protein
expression of PPAR-γ in osteoporosis
Finally, the present study investigated whether
PPAR-γ affects the osteogenic effects of isopsoralen on
osteoporosis. As shown in Fig. 10A
and B, the protein expression of PPAR-γ was significantly
induced in the osteoporosis model rats, compared with that in the
sham group. Treatment with 10 or 20 mg/kg isopsoralen significantly
suppressed the protein expression of PPAR-γ in the rats with
osteoporosis (Fig. 10A and
B).
Discussion
Numerous studies have been performed on psoralen,
the results of which demonstrate that psoralen has excellent
protective effect on the steady state of bone health (15). Osteoporosis is a systemic disease,
which is characterized by low BMD and the deterioration of skeletal
microarchitecture, leading to increased risk of fragility fractures
(16). Osteoporosis can be divided
into the two broad categories of primary and secondary
osteoporosis; the former can be further divided into
post-menopausal osteoporosis (type I), senile osteoporosis (type
II) and idiopathic osteoporosis; and the latter refers to any
disease affecting the bone physiology or drugs leading to
osteoporosis, for example, osteoporosis induced by long-term
administration of a high-dose glucocorticoid (2,17).
Generally, post-menopausal osteoporosis occurs within 5–10 years of
menopause in women, whereas senile osteoporosis occurs in women
>70 years old, and idiopathic osteoporosis primarily occurs in
adolescents, with an unclear pathogenesis (18). The present study is the first, to
the best of our knowledge, to demonstrate that isopsoralen treated
or prevented osteoporosis, leading to decreased urine Ca/Cr,
decreased BMD and PTM, and increased leptin and decreased calcium
in rats with osteoporosis. Ming et al (19) also reported that isopsoralen
prevented anti-osteoporotic and BMSC differentiation in rats.
OCN and Runt-related transcription factor 2 (RUNX2)
are key regulatory factors in osteoblast differentiation, which is
important during bone formation. They are also used in
investigations to measure the osteogenic and adipogenic abilities
of cells. In the present study, it was found that isopsoralen
significantly inhibited the activities of ALP, Col I, OCN and OPN
in rats with osteoporosis.
Bone cells are a type of long-life cell, which is
more susceptible to oxidative stress than osteoblasts and
osteoclasts (20). Several
experiments on rodents and humans have indicated that under
oxidative stress induced by aging of the body, the number of bone
cells gradually decreases, with weakened viability (21). The extracellular matrix in tissue
is comprised of organic and inorganic components; the organic
components are predominantly composed of a variety of collagens,
whereas the inorganic components are hydroxyapatite crystals
(5). The abnormally increased
levels of ROS, in addition to the matrix metalloproteinase and
cysteine protease within osteoclasts, may damage the sulfhydryl
group and amino group of the protein, leading to protein
denaturation and crosslinking, and damage to collagen and
fibronectin (22). In the present
study, isopsoralen significantly decreased the activity of MDA, and
increased the activities of SOD, GSH and GSH-PX in the rats with
osteoporosis. Feng et al (23) reported that isopsoralen also
effectively inhibited H2O2-induced oxidative
damage in HLE-B3 cells.
In bone metabolic pathways, the signal transduction
of multiple pathways are involved in cell differentiation, among
which the WNT signaling pathway has been a consistent focus of
investigations. WNT can control the fate of several types of cells,
is involved in the processes of cell proliferation,
differentiation, migration, polarization and apoptosis, and
sustains cellular dynamic balance (24). The WNT pathway is the most
extensively investigated of the WNT signaling pathways. When the
WNT signaling pathway is activated, the extracellular WNT protein
binds with the cell surface specific receptor, transmembrane
receptor frizzled protein, and the co-receptor, low-density
lipoprotein receptor associated protein 5/6 (LRP5/6) to form the
WNT-Fzd-LRP5/6 complex. This complex activates the disheveled
protein (Dvl) in T cells, and the activated Dvl sends signals to
the cytoplasm to promote the binding of Dvl with the Fzd receptor,
leading to the binding of LRP5/6 with the axis protein complex and
phosphorylation. In this manner, the process of the downstream
casein kinase-adenomatous polyposis protein-glycogen synthase
kinase 3P-axis protein complex in the degradation and acidification
of catenin in the cytoplasm is inhibited. As a result, β-catenin
accumulates in the cell, enters the cell nucleus, and forms a
complex with T cytokines and lymphoid enhancement factor; this
specifically binds with the whip gene transcription promoter and
jointly regulates the transcription process, leading to WNT signal
activation (25–27). The classical WNT signaling pathway
inhibits osteoclast formation through driving the differentiation
of BMSCs into osteoblasts, resulting in the promotion of bone
formation and affecting bone remodeling (9). The present study revealed that
isopsoralen significantly weakened caspase-3/9 activity and induced
the protein expression of WNT/β-catenin in rats with
osteoporosis.
As described above, OCN and RUNX2 are key regulatory
factors in osteoblast differentiation, which are important during
bone formation, and examined in studies for measuring the
osteogenic and adipogenic abilities of cells (28). As osteoblasts and adipocytes are
derived from BMSCs, the results of the present study suggested that
the mechanism of action of isopsoralen against osteoporosis may be
to exert its estrogen-like effect, and regulate the balance between
RUNX2 and PPAR-γ, thus inhibiting the differentiation of BMSCs into
adipocytes (8). In the present
study, isopsoralen significantly suppressed the protein expression
of PPAR-γ in the rats with osteoporosis.
In conclusion, the findings of the present study
showed that isopsoralen effectively inhibited urine Ca/Cr, restored
the inhibition of BMD and increase of PTM, and increased leptin and
decreased calcium in rats with osteoporosis. This may occur through
the regulation of PPAR-γ/WNT to inhibit oxidative stress in
osteoporosis. Therefore, isopsoralen is a promising candidate for
development as a therapeutic agent against osteoporosis in
postmenopausal women.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (grant no. 81560368).
References
|
1
|
Tu MY, Chen HL, Tung YT, Kao CC, Hu FC and
Chen CM: Short-Term Effects of Kefir-Fermented milk consumption on
bone mineral density and bone metabolism in a randomized clinical
trial of osteoporotic patients. PLoS One. 10:e01442312015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung HY, Koo J, Kwon SK, Kang MI, Moon
SH, Park JY, Shin CS, Yoon BK, Yoon HK, Chang JS, et al: Early
changes in 25-hydroxyvitamin D levels and bone markers after
monthly risedronate with cholecalciferol in Korean patients with
osteoporosis. Clin Interv Aging. 8:597–603. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takakuwa M and Iwamoto J: Elcatonin in
combination with risedronate is more effective than risedronate
alone for relieving back pain in postmenopausal women with
osteoporosis. Biol Pharm Bull. 35:1159–1165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu FB, Wang JY, Zhang YL, Quan RF, Yue
ZS, Zeng LR, Zheng WJ, Hou Q, Yan SG and Hu YG: Curculigoside
regulates proliferation, differentiation, and pro-inflammatory
cytokines levels in dexamethasone-induced rat calvarial
osteoblasts. Int J Clin Exp Med. 8:12337–12346. 2015.PubMed/NCBI
|
|
5
|
Li J, He C, Tong W, Zou Y, Li D, Zhang C
and Xu W: Tanshinone IIA blocks dexamethasone-induced apoptosis in
osteoblasts through inhibiting Nox4-derived ROS production. Int J
Clin Exp Pathol. 8:13695–13706. 2015.PubMed/NCBI
|
|
6
|
Liao L, Su X, Yang X, Hu C, Li B, Lv Y,
Shuai Y, Jing H, Deng Z and Jin Y: TNF-α Inhibits FoxO1 by
Upregulating miR-705 to aggravate oxidative damage in bone
Marrow-Derived mesenchymal stem cells during osteoporosis. Stem
Cells. 34:1054–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin H, Gao X, Chen G, Sun J, Chu J, Jing
K, Li P, Zeng R and Wei B: Indole-3-carbinol as inhibitors of
glucocorticoid-induced apoptosis in osteoblastic cells through
blocking ROS-mediated Nrf2 pathway. Biochem Biophys Res Commun.
460:422–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ge C, Cawthorn WP, Li Y, Zhao G,
Macdougald OA and Franceschi RT: Reciprocal control of osteogenic
and adipogenic differentiation by ERK/MAP kinase phosphorylation of
Runx2 and PPARgamma transcription factors. J Cell Physiol.
231:587–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu C, Wang J, Zhu T, Shen Y, Tang X, Fang
L and Xu Y: Cross-Talking Between PPAR and WNT signaling and its
regulation in mesenchymal stem cell differentiation. Curr Stem Cell
Res Ther. 11:247–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu D, Ji L, Zheng L, Cao J, Peng Y and
Zheng J: Mechanism-based inactivation of cytochrome P450 2B6 by
isopsoralen. Xenobiotica. 46:335–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu H, Zhang L, Liu D, Tang P and Song F:
Isolation and purification of psoralen and isopsoralen and their
efficacy and safety in the treatment of osteosarcoma in nude rats.
Afr Health Sci. 14:641–647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuang XM, Zhong YH, Xiao WB, Li H and Lu
C: Identification and characterization of psoralen and isopsoralen
as potent CYP1A2 reversible and time-dependent inhibitors in human
and rat preclinical studies. Drug Metab Dispos. 41:1914–1922. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Hong C, Zhou C, Xu D and Qu HB:
Screening antitumor compounds psoralen and isopsoralen from
Psoralea corylifolia L. Seeds. Evid Based Complement
Alternat Med. 2011:3630522011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiao G, Pan B, Zhou Z, Zhou L, Li Z and
Zhang Z: MicroRNA-21 regulates cell proliferation and apoptosis in
H2O2-stimulated rat spinal cord neurons. Mol
Med Rep. 12:7011–7016. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gatti D, Viapiana O, Idolazzi L, Fracassi
E, Ionescu C, Dartizio C, Troplini S, Kunnathully V, Adami S and
Rossini M: Distinct effect of zoledronate and clodronate on
circulating levels of DKK1 and sclerostin in women with
postmenopausal osteoporosis. Bone. 67:189–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leder BZ, Tsai JN, Uihlein AV,
Burnett-Bowie SA, Zhu Y, Foley K, Lee H and Neer RM: Two years of
Denosumab and teriparatide administration in postmenopausal women
with osteoporosis (The DATA Extension Study): A randomized
controlled trial. J Clin Endocrinol Metab. 99:1694–1700. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
LeBlanc A, Wang AT, Wyatt K, Branda ME,
Shah ND, Van Houten H, Pencille L, Wermers R and Montori VM:
Encounter decision aid vs. Clinical decision support or usual care
to support Patient-Centered treatment decisions in osteoporosis:
The osteoporosis choice randomized trial II. PLoS One.
10:e01280632015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krantz E, Trimpou P and Landin-Wilhelmsen
K: Effect of growth hormone treatment on fractures and quality of
life in postmenopausal osteoporosis: A 10-year follow-up study. J
Clin Endocrinol Metab. 100:3251–3259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ming L, Ge B, Chen K, Ma H and Zhai Y:
Effects of isopsoralen on bone marrow stromal stem cells
differentiate and proliferate in vitro. Zhongguo Zhong Yao Za Zhi.
36:2124–2128. 2011.(In Chinese). PubMed/NCBI
|
|
20
|
Schröder K: NADPH oxidases in bone
homeostasis and osteoporosis. Cell Mol Life Sci. 72:25–38. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SY, Lee KS, Yi SH, Kook SH and Lee JC:
Acteoside suppresses RANKL-mediated osteoclastogenesis by
inhibiting c-Fos induction and NF-κB pathway and attenuating ROS
production. PLoS One. 8:e808732013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Almeida M, Ambrogini E, Han L, Manolagas
SC and Jilka RL: Increased lipid oxidation causes oxidative stress,
increased peroxisome proliferator-activated receptor-gamma
expression, and diminished pro-osteogenic Wnt signaling in the
skeleton. J Biol Chem. 284:27438–27448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng CY, Huang XR, Qi MX, Tang SW, Hu YH,
Chen S and Ke FJ: Mitochondrial proteomic analysis of isopsoralen
protection against oxidative damage in human lens epithelial cells.
Chin J Integr Med. 18:529–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen JR, Lazarenko OP, Wu X, Tong Y,
Blackburn ML, Shankar K, Badger TM and Ronis MJ: Obesity reduces
bone density associated with activation of PPARγ and suppression of
Wnt/β-catenin in rapidly growing male rats. PLoS One. 5:e137042010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen G, Wang C, Wang J, Yin S, Gao H,
Xiang LU, Liu H, Xiong Y, Wang P, Zhu X, et al: Antiosteoporotic
effect of icariin in ovariectomized rats is mediated via the
Wnt/β-catenin pathway. Exp Ther Med. 12:279–287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sang C, Zhang Y, Chen F, Huang P, Qi J,
Wang P, Zhou Q, Kang H, Cao X and Guo L: Tumor necrosis factor
alpha suppresses osteogenic differentiation of MSCs by inhibiting
semaphorin 3B via Wnt/β-catenin signaling in estrogen-deficiency
induced osteoporosis. Bone. 84:78–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lv J, Sun X, Ma J, Ma X, Xing G, Wang Y,
Sun L, Wang J, Li F and Li Y: Involvement of
periostin-sclerostin-Wnt/β-catenin signaling pathway in the
prevention of neurectomy-induced bone loss by naringin. Biochem
Biophys Res Commun. 468:587–593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar : PubMed/NCBI
|