Introduction
Alcohol abuse is characterized by an escalation from
low or moderate alcohol consumption to excessive consumption and
compulsive alcohol uptake, and is associated with numerous
environmental and genetic factors (1,2).
Harmful alcohol use is associated with substantial morbidity and
mortality, through disrupting the function of the brain, liver,
gastrointestinal tract and pancreas (3,4). It
has previously been indicated that alcohol consumption is
influenced by stressful stimuli that serve as risk factors,
including inescapable foot shock, restraint, forced swim and social
defeat in animals, and life stressors (such as job loss, divorce,
earthquakes or other traumatic events) in humans (5,6).
Among these stimuli, trauma is the most common, experienced at
least once by 50–70% of people in their lifetimes (7). Trauma not only destroys healthy body
tissue, but can also lead to the development of functional
disorders within the nerve-endocrine-immune system. Therefore,
traumatic stress was used as a stressor in our previous study,
which indicated that immunosuppression is accompanied by a decrease
in splenocyte proliferation and natural killer cell activity 1–3
days post-trauma; however, complete recovery was observed by day 7
(8). Furthermore, inflammation
induced by some factors, such as lipopolysaccharides, has been
deemed to result in a persistent increase in alcohol
self-administration (9,10). It has thus been speculated that a
causal relationship exists between traumatic stress and alcohol
consumption; and days 1, 3 and 7 post-trauma appear to be
associated with triggering alcohol problems.
Cocaine-and-amphetamine-regulated transcript (CART)
was originally described as an mRNA, which is increased in the rat
striatum following acute cocaine administration (11). Peptides produced by CART exhibit a
high to moderate distribution in the hypothalamus, in particular
the paraventricular nucleus (PVN), arcuate and supraoptic nuclei,
and in the anterior lobe of the pituitary and adrenal medulla
(12). It has been reported that
these peptides are involved in the regulation of biological
processes, including feeding, stress, reward, metabolism, anxiety
and depression (13,14). Kuhar and Yoho (15) extracted and identified CART 55–102
and CART 62–102 as two major CART fragments in rat tissues, by
western blotting. As a bioactive peptide fragment, CART 55–102 has
been reported to regulate behavioral and physiological responses to
stress (16). CART 55–102
signaling in the PVN may mediate the neuroendocrine response to
2,4,5-trimethyl-3-thiazoline-induced predator stress (17). CART was originally discovered
following the acute administration of cocaine and amphetamine into
rats (11); however, it is now
fully appreciated that CART 55–102 is involved in the actions of
other psychostimulants, including cocaine, morphine and alcohol
(18–20). As the PVN is an anatomical area
that is involved in stress and CART 55–102 signaling, the
modulation of CART 55–102 in the PVN in response to traumatic
stress and alcohol use is highly plausible.
It is well known that stress responses generally
involve the neuroendocrine system, in particular the
hypothalamus-pituitary-adrenal (HPA) axis (21,22).
These responses are activated by the limbic and ascending
brainstem, and the pontine pathways; characterized by a significant
increase in the release of neuropeptide and corticotropin releasing
factor from the PVN of the hypothalamus, and the secretion of
adrenocorticotropic hormone (ACTH) from the anterior pituitary and
glucocorticoids [corticotropin (CORT) in rats, cortisol in humans]
from the adrenal glands (21).
CART exists abundantly in all three levels of the HPA axis
(12), suggesting its role in
mediating the stress response. Circulating ACTH and CORT levels can
be influenced by intracerebroventricular CART injection in male
rats, indicating a marked effect of CART on the regulation of HPA
axis-associated activity (23).
HPA axis activation has previously been observed to be rapidly
evoked by traumatic stress (24);
however, it is unknown whether CART 55–102 can regulate the
activation induced by trauma.
The present study aimed to determine whether
alcohol-drinking behavior may be affected by traumatic stress, to
further explore and confirm the role of CART 55–102 within the PVN
with the administration of alcohol, and to examine the effects of
CART 55–102 within the PVN on the HPA axis during trauma.
Materials and methods
Animals
A total of 120 male Sprague-Dawley rats (weight,
~150 g; age, postnatal day 33; n=6/group) were purchased from the
Experimental Animal Center of Chinese Academy of Sciences
(Shanghai, China). The rats were maintained in a controlled
environment under a 12-h light-dark cycle (lights on at 7:00 a.m.),
at 23±1°C and 50% humidity, with ad libitum access to food
and water. Only male rates were used as female hormones may
influence behavioral alterations following surgery. All
experimental procedures were approved by the Animal Use and Care
Committee of Fudan University (Shanghai, China) and were in
accordance with the guidelines of the National Institutes of Health
(Institute of Laboratory Animal Resources) on animal care (25).
Drugs
Absolute ethanol (concentration ≥99.7% HeYing
Chemical Corporation, Shanghai, China) was diluted to 10% (v/v)
with filtered water for drinking. For intra-PVN injection, CART
55–102 (3337; Tocris Bioscience, Bristol, UK) was dissolved in
normal saline (NS) to produce the following concentrations: 0.025,
0.625 and 1.25 μg/0.5 μl. In the control group, 0.5 μl NS was
injected into the PVN.
Model
The rats were allowed to acclimate for a minimum of
5 days prior to experimentation. The traumatic stress paradigm was
performed as previously described (24,26).
In the control group, rats were administered 2% sodium
pentobarbital [intraperitoneal injection (i.p.), 62 mg/kg] for
anesthesia, but did not undergo trauma. Rats in the traumatic
stress group were initially anesthetized in the same manner as the
controls, after which a 5 cm incision was made along the abdominal
median line. Viscera were exposed for 1 min to verify the absence
of tissue damage, and the wounds were subsequently sutured.
Similarly, the rats were then incised 6 cm along the dorsal median
line and were sutured. Finally, all rats were returned to their
cages and kept warm. No post-operative infection occurred.
Alcohol drinking behavior test
Rats were provided with 24 h/day access to
two-bottle choice drinking (one bottle contained 10% alcohol, the
other contained filtered water) for 14 days from 12:00 p.m. on days
1, 3 and 7 post-trauma (T1, T3 and T7, respectively). Non-trauma
control rats were provided with two-bottle choice drinking from
12:00 p.m. on T0. The two-bottle choice drinking paradigm was
applied, according to the protocol outlined by Beckwith and
Czachowski (27). The positions of
the alcohol bottle and water bottle were alternated daily to avoid
place preference. Spillage and evaporation capacity was measured
via a water bottle placed in an empty cage. The volume in the
bottle prior to and following drinking activity, and the body
weight of the rats were measured daily. These measurements were
used to calculate the average alcohol intake over 14 days (g/kg/d).
Alcohol preference was calculated by dividing the volume of alcohol
consumed by the total volume of alcohol and water consumed. Food
was available ad libitum during measurements.
Blood ethanol concentration (BEC)
test
At the end of the 14th day of drinking behavior
analysis, rats were anesthetized with sodium pentobarbital (i.p.;
62 mg/kg) then immediately decapitated to collect blood through the
carotid artery, which was centrifuged (30 min, 931 × g at 4°C) to
obtain serum. The serum was frozen at −80°C until use to avoid
repeated freeze-thawing. BEC was measured using the
EnzyChrom™ Ethanol Assay kit (ECET-100; BioAssay
Systems, Hayward, CA, USA) according to the manufacturer's
protocol.
Radioimmunoassay (RIA)
At 10:00-12:00 a.m. on T0, T1, T3 and T7, rats were
first anesthetized with pentobarbital sodium (i.p.; 62 mg/kg), and
blood was collected through the carotid artery and then the rats
were sacrificed via decapitation. The serum was separated via
centrifugation (30 min, 931 × g, 4°C). Serum ACTH and CORT
concentrations were measured with the RIA method (assisted by the
Beijing Hua Ying Institute, Beijing, China) to evaluate HPA axis
activity. Serum ACTH and CORT levels at 30 and 60 min following
intra-PVN injection of saline or 1.25 μg/0.5 μl/side CART 55–102 on
T3 were also obtained.
Immunohistochemistry
Between 10:00-12:00 a.m. on T0, T1, T3 and T7, rats
were fully anaesthetized with pentobarbital sodium (i.p.; 62
mg/kg), and brains were removed following intracardial perfusion
with 0.1 M PBS and 4% paraformaldehyde, following with decapitation
for euthanasia of the rats, then fixed overnight at 4°C with 4%
paraformaldehyde and dehydrated with sucrose (20–30% gradient
sucrose) and embedded with an optimum cutting temperature compound
(cat. no. 4583; Sakura Finetek USA, Inc., Torrance, CA, USA) in
order to obtain 30-μm sections. Sections were used for
immunohistochemical and immunofluorescence staining of CART 55–102.
For immunohistochemical staining, sections were washed three times
with PBS, antigen retrieval was conducted using buffer P0083
(Beyotime Institute of Biotechnology, Shanghai, China), and the
sections were subsequently treated with 3% hydrogen peroxide for 1
h at 37°C. Following PBS washes and blocking with 5% donkey serum
(017-000-121; Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA) in 0.3% Triton X-100 for 1 h at room temperature,
the sections were incubated with a primary rabbit anti-rat antibody
against CART 55–102 (1:10,000; cat. no. H-003-62; Phoenix
Pharmaceuticals, Inc., Burlingame, CA, USA) overnight at 4°C. The
sections were washed three times with PBS and were then incubated
with goat anti-rabbit immunoglobulin G antibody (1:150; A0277;
Beyotime Institute of Biotechnology) for 1 h at 37°C, the following
day. Sections were washed and incubated with avidin-biotin complex
reagent for 1 h at 37°C (1:100; PK-6100; Vector Laboratories, Inc.,
Burlingame, CA, USA). Sections were subsequently incubated with DAB
(1:100; P0202; Beyotime Institute of Biotechnology). Finally,
sections were washed, dehydrated with gradient alcohol, cleared
with xylene and mounted with cover glass. Images of the sections
were then captured with a light microscope (Zeiss GmbH, Jena,
Germany).
Immunofluorescence
The sections were washed with PBS and incubated with
blocking agent for 1 h, prior to incubation overnight at 4°C with
the following primary antibodies: Rabbit anti-CART 55–102
polyclonal antibody (1:1,000; H-003-62; Phoenix Pharmaceuticals,
Inc.), mouse anti-neuronal marker (NeuN) monoclonal antibody
(1:1,000; MAB377; EMD Millipore, Billerica, MA, USA), mouse
anti-glial fibrillary acidic protein (GFAP) monoclonal antibody
(1:400; 3670; Cell Signaling Technology Inc., Danvers, MA, USA),
and goat anti-ionized calcium-binding adaptor 1 (IBA1) polyclonal
antibody (1:400; ab5076; Abcam, Cambridge, MA, USA). The sections
were rinsed three times for 10 min in PBS and incubated with
corresponding secondary antibodies (cat. nos. A-11008; conjugated
with Alexa Fluor 488 and A-21203; conjugated with Alexa Fluor 594;
1:1000; cat. no. A-11058; conjugated with Alexa Fluor 594; 1:2,000;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 2 h at room
temperature. Immunofluorescent images were captured under a
confocal scanning laser microscope (FluoView 10-ASW; Olympus
Corporation, Tokyo, Japan) following washes of the sections.
PVN injection of CART 55–102
When Sprague Dawley rats were ~200 g, they were
anesthetized with sodium pentobarbital (i.p.; 62 mg/kg), and
maintained on isoflurane then a guide cannula was bilaterally
implanted in the PVN of the hypothalamus (anterior/posterior, −1.5
mm; medial/lateral, ±0.4 mm; and dorsal/ventral, −8.0 mm) and
cannulas were anchored to the skull with screws and dental cement.
For analgesia, fentanyl (10 μg/kg) was intramuscularly injected
immediately after the surgery (28). The rats recovered for a period of 8
days; recovery of the circadian rhythm was ensured prior to
conducting traumatic stress, performed as described in the
Model subsection. Between 10:00-12:00 a.m. on T3, rats were
injected with 0.5 μl NS or 0.025, 0.625 and 1.25 μg/0.5 μl/side
CART 55–102 at a rate of 0.5 μl/min (0.5 μl/side NS was injected
into the PVN, and employed as the control group in the experiment).
Prior to the 14-day drinking behavior experiment, the injection
cannulae were maintained for an additional 1 min. Measurements of
alcohol consumption, preference and BEC were conducted as
aforementioned.
Statistical analysis
Data are presented as the mean ± standard error of
the mean of 5 repeated experiments. Data obtained from measuring
alcohol drinking behavior, CART 55–102 expression levels, and ACTH
and CORT levels post-trauma of the groups were compared by one-way
analysis of variance followed by Tukey or Newman-Keuls post hoc
tests using Statistical Package for the Social Sciences (SPSS)
v19.0 software (IBM Corp., Armonk, NY, USA). To compare levels of
ACTH and CORT following CART 55–102 micro-injection, an unpaired
Student's t-test was conducted using SPSS. Pearson's Correlation
Analysis (2-tailed) between alcohol preference and ACTH and CORT
levels was also performed through SPSS. P<0.05 was considered to
indicate a statistically significant difference.
Results
Traumatic stress enhances subsequent
alcohol drinking behavior in rats from T3
To determine the effects of traumatic stress on
subsequent alcohol drinking behavior, rats exposed to trauma were
randomly assigned to the T1, T3 and T7 groups, which were given
access to 10% alcohol or water on T1, T3 and T7, respectively, for
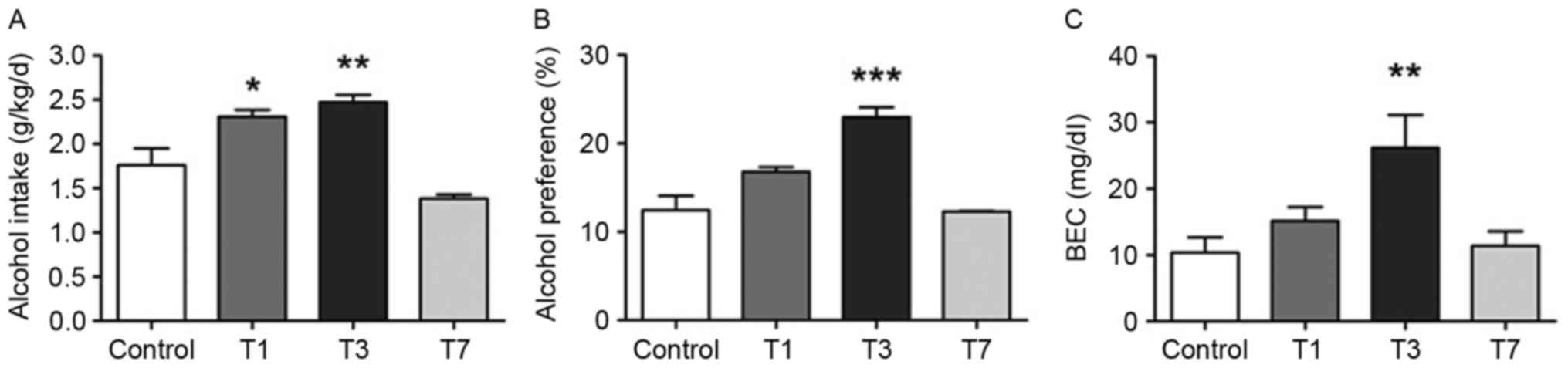
14 days. As presented in Fig. 1A,
alcohol intake in the T1 and T3 groups was significantly increased
compared with in the control group (P=0.016, P=0.001; Fig. 1A); alcohol preference in the T3
group also exhibited a similar increase (P<0.001; Fig. 1B). Additionally, the BEC of T3 rats
measured near the termination of the alcohol behavior test
exhibited a similar trend to intake and preference (P=0.003;
Fig. 1C). Therefore, initiation of
drinking from T3 may increase subsequent alcohol drinking behavior.
Conversely, drinking from T7 reversed the rising trend, suggesting
that T3 may be a key time point for evoking reinforced alcohol
self-administration during stress development.
CART 55–102 in the PVN exhibits an
inhibitory effect on subsequent drinking behavior
To evaluate the effects of CART 55–102 in the PVN on
alcohol drinking behavior, immunohistochemistry and
immunofluorescence were performed on samples from T0, T1, T3 and
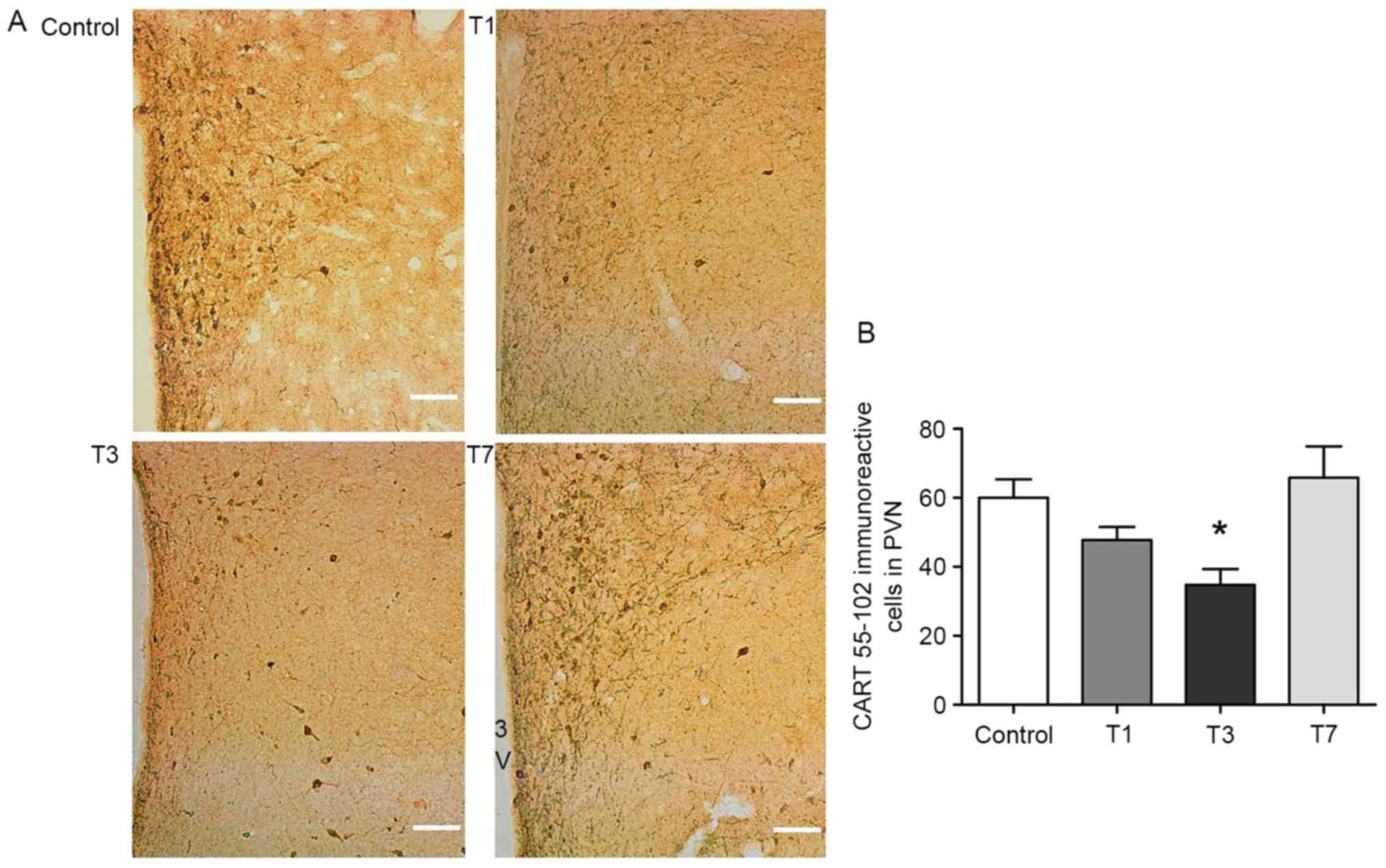
T7. Immunohistochemistry demonstrated that CART 55–102 in the PVN
was markedly decreased in the T1 and T3 groups compared with in the
control group, particularly on T3 (P=0.015; Fig. 2A and B); this appeared to correlate
with the concomitant increase in alcohol consumption and preference
from T3. The data of the present study suggested that CART 55–102
in the PVN may suppress subsequent and long periods of drinking
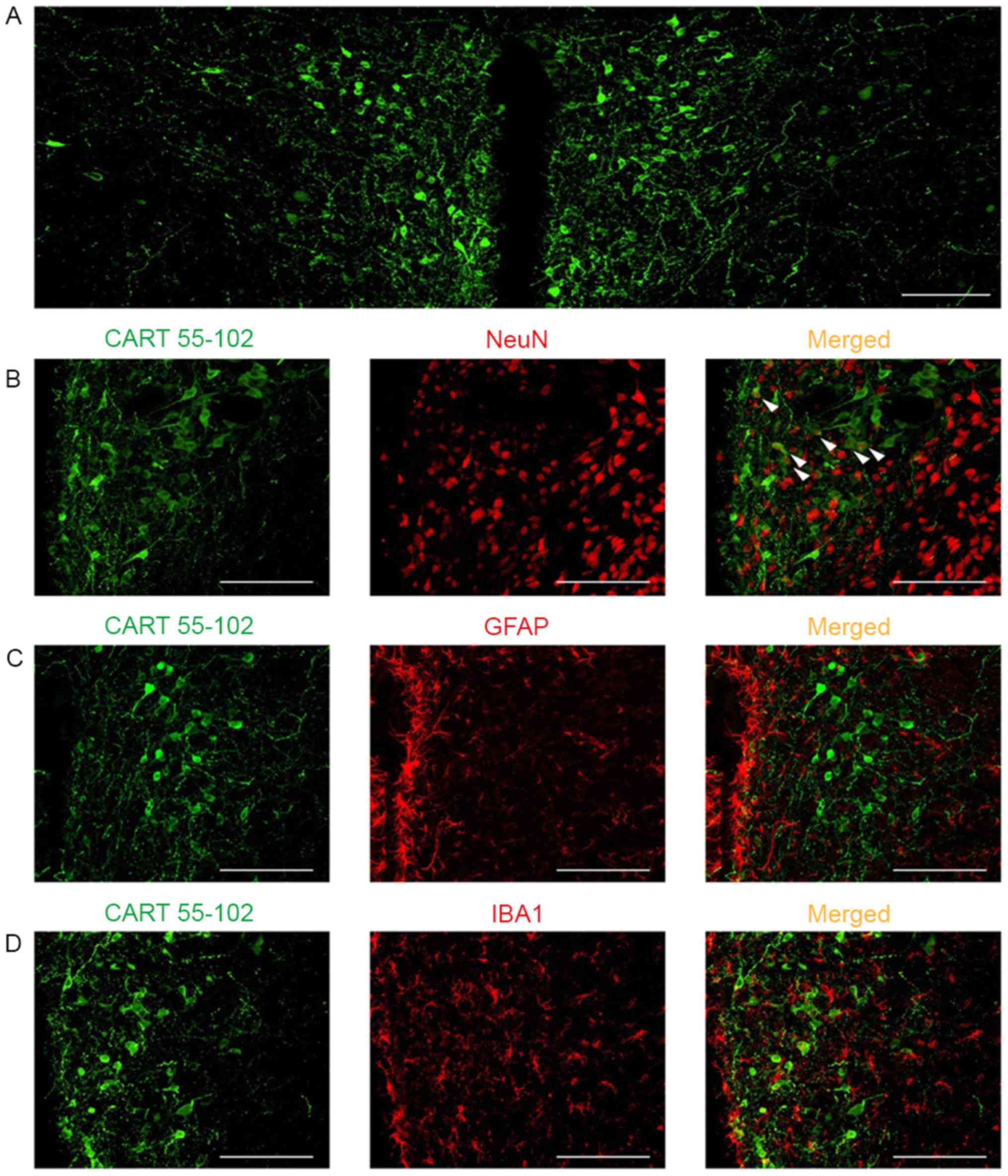
behavior. Confocal immunofluorescence labeling indicated that CART
55–102 was abundantly expressed in the PVN (Fig. 3A). In the PVN, the majority of CART
55–102 immunoreactive cells were co-localized with NeuN (Fig. 3B), but not with GFAP, an astrocytic
marker (Fig. 3C) or IBA1, a
microglial marker (Fig. 3D).
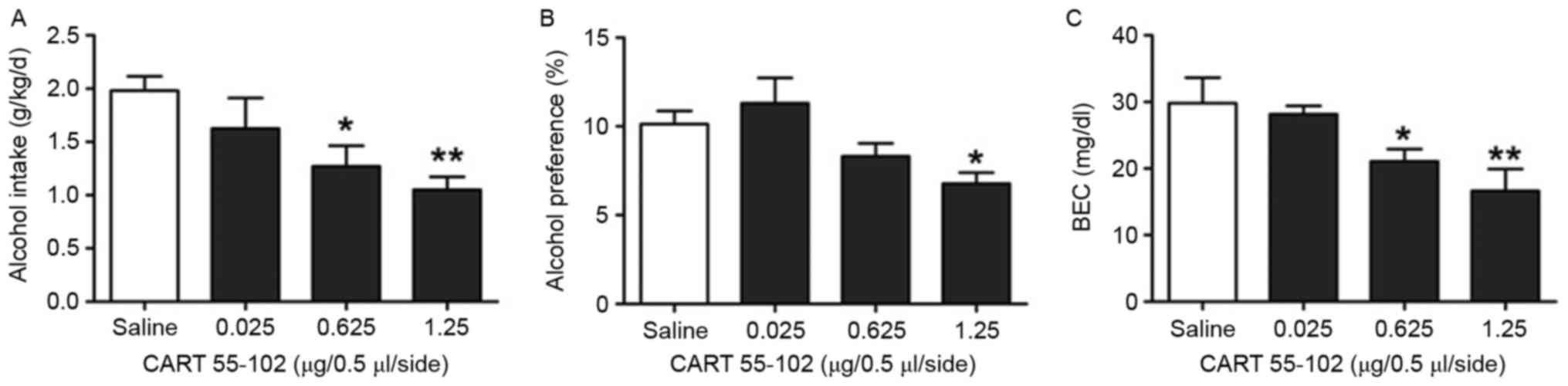
Subsequently, recombinant CART 55–102 or saline was
bilaterally injected into the PVN on T3, followed by the 14-day
drinking behavior test. Repeated analysis of one-way ANOVA
indicated that alcohol intake, preference and BEC exhibited a
dose-dependent decrease in rats treated with 0.025, 0.625 and 1.25
μg/0.5 μl/side CART 55–102 (Fig.
4). The intake, preference and BEC in the group treated with
1.25 μg/0.5 μl/side CART 55–102 exhibited a marked decrease
compared with the saline group (P=0.002, P=0.013, P=0.002; Fig. 4). These findings suggested that
CART 55–102 in the PVN may exert a negative role in the regulation
of alcohol drinking behavior post-trauma.
HPA axis hyperactivity may be reversed
by CART 55–102 administration into the PVN
Traumatic stress has been reported to serve a key
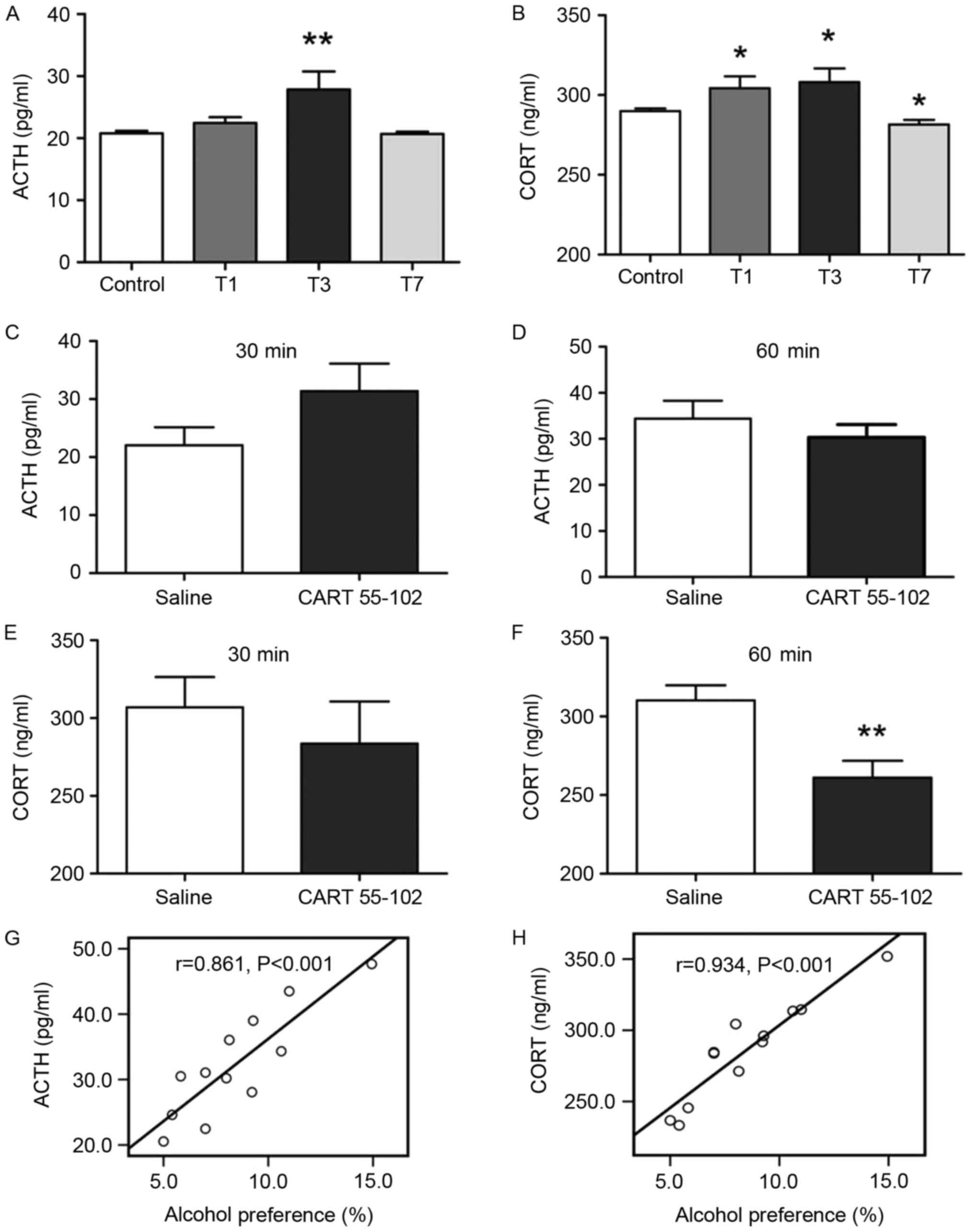
role in the endocrine system, particularly the HPA axis (29). The results of a RIA indicated that
the HPA axis exhibited hyperactivity in the T3 group as measured by
serum ACTH and CORT levels (P=0.003, P=0.02; Fig. 5A and B); however, CORT
concentration on T1 and T7 also revealed a partial increase. The
correlation between CART 55–102 and the HPA axis has been noted
previously (23,30). To explore the effects of CART
55–102 administration on HPA axis activity, the ACTH and CORT
concentrations on T3 at 30 and 60 min following intra-PVN CART
55–102 injection (1.25 μg) were examined, since 1.25 μg CART 55–102
had the most significant effect on drinking behavior in rats. A
significant alteration in ACTH and CORT levels was not observed at
30 min between the CART 55-102- and saline-treated groups (Fig. 5C and D). However, at 60 min, CORT
levels in CART 55-102-treated rats were significantly decreased
compared with in the saline group (P=0.0068; Fig. 5F), indicating that CART 55–102 may
reverse the excessive CORT levels on T3. In addition, the
correlation analysis between alcohol preference and ACTH and CORT
levels at 60 min following CART 55–102 microinjection revealed a
positive correlation (P<0.001, P<0.001; Fig. 5G and 5H). These data suggested that
the HPA axis may be involved in the regulation of alcohol drinking
behavior.
In summary, administration of CART 55–102 into the
PVN may suppress reinforced alcohol drinking behavior induced by
traumatic stress, potentially through the disturbance of HPA axis
hyperactivity.
Discussion
The physiological and behavioral response of the
body to counteract any factors that perturb and then reinstate
homeostasis is known as the stress response. Stress has been
reported to have a determinant effect on producing behavioral
sequelae (31–33). In humans, traumatic stress is a
common stressor, and can include surgical trauma, brain injury and
skin damage. The Defense Survey of Health Related Behaviors among
Active Duty Military Personnel (HRB Survey) indicated that military
trauma can alcohol abuse in soldiers (34). Koob and Le Moal (35) also suggested drug abuse as a
pathology induced by direct ties to the stress response via an
allostatic mechanism. The present study explored the time-course
effect of traumatic stress on subsequent alcohol drinking behavior
in Sprague Dawley rats. Notably, traumatic stress markedly
increased subsequent alcohol consumption and preference,
particularly following drinking from T3. During traumatic stress,
the organism undergoes a constantly changing recovery process, from
a progressively pathological to a normal state. Wan et al
(36) reported that
hepatolobectomy in rats can trigger astroglial and microglial
excessive activation in the brain on day 3, whereas the neuroglia
may fully return to a nonreactive state on day 7 post-surgery.
Therefore, T3 may be considered a turning point that means turning
to the recovery mode. Additionally, other studies support the
findings of the present study, since they have demonstrated that
stress is an important factor correlated with changes to drinking
behavior (32,37). Notably, the present study indicated
the important role of traumatic stress in the regulation of
voluntary alcohol consumption, and provided a broader line of
thought for the treatment of patients that abuse alcohol, although
several differences between drinking patterns in humans and
research animals exist.
CART is synthesized in neuroendocrine cells of the
parvocellular and the magnocellular hypothalamus; CART 55–102 is
abundant in the nuclear area of the PVN. The present study
suggested that CART 55–102 immunoreactivity within the PVN was
prominently reduced on T3, with the majority co-localized within
neurons. In addition, the administration of CART 55–102 into the
PVN prevented reinforced drinking behavior on T3 in a
dose-dependent manner. These results suggested that CART 55–102
within the PVN serves a protective role for the organism by
suppressing alcohol consumption. In other studies, it has also been
observed that CART 55–102 can negatively modulate drug-seeking
behaviors. King et al (38)
demonstrated the role of CART in mediating the rewarding or
motivational properties of ethanol; intracerebroventricular
administration of CART 55–102 prevented the context-induced
reinstatement of alcohol-seeking behaviors in rats. Intra-accumbens
nucleus shell injection of CART 55–102 also attenuated the
reinstatement of alcohol-seeking behaviors in a dose-dependent
manner; no effect was reported with the CART 1–27 fragment
(20). The effects of CART 55–102
on psychostimulants are complex, and brain region- and
dose-dependent. Administration of active CART 55–102 into the
ventral tegmental area was reported to promote a conditioned place
preference, similar to that induced by cocaine or amphetamine
(39). In addition,
context-induced reinstatement of alcohol-seeking behaviors was
reduced with active CART 55–102 administration into the
intra-nucleus accumbens (20). The
suppressive and reinforcing roles of CART 55–102 in drug abuse are
associated with its location and interactions. However, the role of
CART 55–102 in supporting and inhibiting the effects of drugs is
complex and is not only observed in rodent behavioral studies.
The present study focused on the functional role of
the hypothalamic PVN in alcohol consumption. As the primary site of
the HPA axis, the PVN harbors the cholinergic basal forebrain
neurons that serve a crucial role in regulating the ACTH-CORT
system in response to stress (40). Dandekar et al (41) revealed a significant increase in
CART-immunoreactive cells and fibers following 24 h of alcohol
withdrawal and a marked loss of CART-immunoreactivity from cells in
the PVN at 48 h. Chen et al (42) supported the suggestion that several
peptides in the hypothalamic PVN can increase alcohol consumption
by promoting distinct aspects of the drinking response. These
studies have provided the evidence that suggest the profound role
of PVN in alcohol drinking behavior. The results of the present
study indicated that CART 55–102 in the PVN may mediate the
association between traumatic stress and subsequent alcohol
consumption. Therefore, CART 55–102 may be a biological factor in
determining vulnerability to drug abuse as well as a potential
therapeutic target to post-trauma alcohol abuse.
The modulatory role served by CART 55–102 has been
noted to be involved in the stress response (17,43).
Expression of CART 55–102 in the PVN was modulated with traumatic
stress; the lowest level of expression was reported on T3. HPA axis
activity was markedly upregulated on T3, with excessive circulating
ACTH and CORT levels. However, the elevated levels of CORT were
reversed via CART 55–102 microinjection into the PVN at 60 min; the
ACTH levels exhibited an insignificant decrease at 60 min. These
data suggested that CART 55–102 may serve a role in the control of
adrenal function during trauma. An association between CART 55–102
and the HPA axis has been identified; however, it has also been
suggested that CART 55–102 administration into the PVN may
stimulate HPA axis activity with an associated increase in plasma
ACTH (23,44). This discrepancy may be explained by
the use of different animal models. Sprague Dawley rats exposed to
trauma were used in the present study compared with healthy Wistar
rats used by Stanley et al (23). The neurobiological mechanisms
differ significantly in different animal models. The HPA axis may
be involved in the regulation of alcohol drinking behavior as
suggested by the positive correlation between alcohol preference
and ACTH and CORT levels; CART 55–102 may serve an inhibitory role
in alcohol drinking behavior via the HPA axis in traumatic
rats.
In the present study, alcohol intake and preference
following treatment with CART 55–102 were measured over 14 days.
The results revealed that a single injection of CART 55–102 on T3
prolonged the 14-day inhibitory effect on drinking behavior.
Hyperactivity observed in the HPA axis on T3 was followed by
long-term reinforced drinking behavior; however, CART 55–102
reversed the increased serum levels CORT and may regulate long-term
alcohol self-administration. The effects of CORT on alcohol
consumption were not determined in the present study; however, the
results detected a positive correlation between the two factors.
Previous studies have revealed that the interaction between CORT
and the dopaminergic system in the nucleus accumbens may facilitate
the reinforcing effects of alcohol and other drugs (45–47).
Therefore, CART 55–102 may serve an inhibitory role in drinking
behavior through the disruption of excessive CORT serum levels;
however, more evidence is required from future investigations.
In conclusion, the results of the present study
demonstrated that traumatic stress significantly increased
subsequent alcohol intake and preference from T3. In addition, the
majority of CART 55–102 in the PVN was localized to neurons. The
results suggested that CART 55–102 may serve an inhibitory effect
on alcohol consumption in a dose-dependent manner, which may be due
to reduced hyperactivity of the HPA axis. The results suggested
that CART 55–102 may negatively modulate the behavioral effect of
alcohol abuse and may serve as a therapeutic target to reduce
alcohol addiction in the clinical setting.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81471370
and 81573712). The authors would like to thank Dr. Qiu-Qin Han
(School of Basic Medical Sciences, Fudan University, Shanghai,
China) for her help in revising the manuscript.
References
|
1
|
Koob GF, Ahmed SH, Boutrel B, Chen SA,
Kenny PJ, Markou A, O'Dell LE, Parsons LH and Sanna PP:
Neurobiological mechanisms in the transition from drug use to drug
dependence. Neurosci Biobehav Rev. 27:739–749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koob G and Kreek MJ: Stress, dysregulation
of drug reward pathways, and the transition to drug dependence. Am
J Psychiat. 164:1149–1159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Federico A, Cotticelli G, Festi D,
Schiumerini R, Addolorato G, Ferrulli A, Merli M, Lucidi C, Milani
S, Panella C, et al: The effects of alcohol on gastrointestinal
tract, liver and pancreas: Evidence-based suggestions for clinical
management. Eur Rev Med Pharmacol Sci. 19:1922–1940.
2015.PubMed/NCBI
|
|
4
|
Sun C, Shen L, Li X, Liu C and Zhou Y:
Risk of pneumonia in central nervous system injury with alcohol
intake: A meta-analysis. Int J Clin Exp Med. 8:15738–15744.
2015.PubMed/NCBI
|
|
5
|
Becker HC, Lopez MF and Doremus-Fitzwater
TL: Effects of stress on alcohol drinking: A review of animal
studies. Psychopharmacology (Berl). 218:131–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anthenelli R and Grandison L: Effects of
stress on alcohol consumption. Alcohol Res Current Rev. 34:381–382.
2012.
|
|
7
|
Ursano RJ, Zhang L, Li H, Johnson L,
Carlton J, Fullerton CS and Benedek DM: PTSD and traumatic stress.
Brain Res. 1293:2–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao S, Wang J, Jiang J, Cao X, Wu G and
Zhao H: Characterization of Fyn signaling on the age-dependent
immuno-modulation on traumatic rats. Brain Res. 1255:162–169. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blednov YA, Benavidez JM, Geil C, Perra S,
Morikawa H and Harris RA: Activation of inflammatory signaling by
lipopolysaccharide produces a prolonged increase of voluntary
alcohol intake in mice. Brain Behav Immun. 25 Suppl 1:S92–S105.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blednov YA, Ponomarev I, Geil C, Bergeson
S, Koob GF and Harris RA: Neuroimmune regulation of alcohol
consumption: Behavioral validation of genes obtained from genomic
studies. Addict Biol. 17:108–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Douglass J, Mckinzie AA and Couceyro P:
PCR differential display identifies a rat brain mRNA that is
transcriptionally regulated by cocaine and amphetamine. J Neurosci.
15:2471–2481. 1995.PubMed/NCBI
|
|
12
|
Koylu EO, Couceyro PR, Lambert PD, Ling
NC, DeSouza EB and Kuhar MJ: Immunohistochemical localization of
novel CART peptides in rat hypothalamus, pituitary and adrenal
gland. J Neuroendocrinol. 9:823–833. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kristensen P, Judge ME, Thim L, Ribel U,
Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang
N, et al: Hypothalamic CART is a new anorectic peptide regulated by
leptin. Nature. 393:72–76. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rogge G, Jones D, Hubert GW, Lin Y and
Kuhar MJ: CART peptides: Regulators of body weight, reward and
other functions. Nat Rev Neurosci. 9:747–758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuhar MJ and Yoho LL: CART peptide
analysis by Western blotting. Synapse. 33:163–171. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koylu EO, Balkan B, Kuhar MJ and Pogun S:
Cocaine and amphetamine regulated transcript (CART) and the stress
response. Peptides. 27:1956–1969. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma A, Rale A, Utturwar K, Ghose A and
Subhedar N: Identification of the CART neuropeptide circuitry
processing TMT-induced predator stress. Psychoneuroendocrino.
50:194–208. 2014. View Article : Google Scholar
|
|
18
|
Bakhtazad A, Vousooghi N, Garmabi B and
Zarrindast MR: Evaluation of CART peptide level in rat plasma and
CSF: Possible role as a biomarker in opioid addiction. Peptides.
84:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jaworski JN, Vicentic A, Hunter RG, Kimmel
HL and Kuhar MJ: CART peptides are modulators of mesolimbic
dopamine and psychostimulants. Life Sci. 73:741–747. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Millan EZ and McNally GP: Cocaine- and
amphetamine-regulated transcript in the nucleus accumbens shell
attenuates context-induced reinstatement of alcohol seeking. Behav
Neurosci. 126:690–698. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herman JP, Figueiredo H, Mueller NK,
Ulrich-Lai Y, Ostrander MM, Choi DC and Cullinan WE: Central
mechanisms of stress integration: Hierarchical circuitry
controlling hypothalamo-pituitary-adrenocortical responsiveness.
Front Neuroendocrinol. 24:151–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Linthorst ACE and Reul JM: Stress and the
brain: Solving the puzzle using microdialysis. Pharmacol Biochem
Behav. 90:163–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stanley SA, Small CJ, Murphy KG, Rayes E,
Abbott CR, Seal LJ, Morgan DG, Sunter D, Dakin CL, Kim MS, et al:
Actions of cocaine- and amphetamine-regulated transcript (CART)
peptide on regulation of appetite and hypothalamo-pituitary axes in
vitro and in vivo in male rats. Brain Res. 893:186–194. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mou Huilin XZXL: NRSF and CCR5 established
neuron-glia communication during acute and chronic stresses. Drug
Metabol Toxicol. 5:2016.
|
|
25
|
Janet C and Garber RWBJ: Guide for the
care and use of lab animals. Washington, DC: National Academy
Press; 2010
|
|
26
|
Zhao H, Huang HW, Wu GC and Cao XD: Effect
of orphanin FQ on interleukin-1beta mRNA transcripts in the rat
CNS. Neuroscience. 114:1019–1031. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beckwith SW and Czachowski CL:
Alcohol-preferring P rats exhibit elevated motor impulsivity
concomitant with operant responding and self-administration of
alcohol. Alcohol Clin Exp Res. 40:1100–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng XM, Mi WL, Xia F, Mao-Ying QL, Jiang
JW, Xiao S, Wang ZF, Wang YQ and Wu GC: Involvement of spinal
orexin A in the electroacupuncture analgesia in a rat model of
post-laparotomy pain. BMC Complement Altern Med. 12:2252012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dimopoulou I, Tzanela M, Vassiliadi D,
Mavrou I, Kopterides P, Orfanos S, Kotanidou A, Kontogiannopoulou
S, Vasdekis S, et al: Pituitary-adrenal responses following major
abdominal surgery. Hormones (Athens). 7:237–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Smith SM, Vaughan JM, Donaldson CJ, Rivier
J, Li C, Chen A and Vale WW: Cocaine- and amphetamine-regulated
transcript activates the hypothalamic-pituitary-adrenal axis
through a corticotropin-releasing factor receptor-dependent
mechanism. Endocrinology. 145:5202–5209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marco EM, Ballesta JA, Irala C, Hernández
MD, Serrano ME, Mela V, López-Gallardo M and Viveros MP:
Sex-dependent influence of chronic mild stress (CMS) on voluntary
alcohol consumption; study of neurobiological consequences.
Pharmacol Biochem Behav. 152:68–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weil ZM, Karelina K, Gaier KR, Corrigan TE
and Corrigan JD: Juvenile traumatic brain injury increases alcohol
consumption and reward in female mice. J Neurotraum. 33:895–903.
2016. View Article : Google Scholar
|
|
33
|
Ostroumov A, Thomas AM, Kimmey BA, Karsch
JS, Doyon WM and Dani JA: Stress increases ethanol
self-administration via a shift toward excitatory gaba signaling in
the ventral tegmental area. Neuron. 92:493–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adams RS, Larson MJ, Corrigan JD, Ritter
GA and Williams TV: Traumatic brain injury among U.S. active duty
military personnel and negative drinking-related consequences.
Subst Use Misuse. 48:821–836. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koob GF and Le Moal M: Drug addiction,
dysregulation of reward, and allostasis. Neuropsychopharmacol.
24:97–129. 2001. View Article : Google Scholar
|
|
36
|
Wan Y, Xu J, Meng F, Bao Y, Ge Y, Lobo N,
Vizcaychipi MP, Zhang D, Gentleman SM, Maze M and Ma D: Cognitive
decline following major surgery is associated with gliosis,
β-amyloid accumulation, and τ phosphorylation in old mice. Crit
Care Med. 38:2190–2198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sperling RE, Gomes SM, Sypek EI, Carey AN
and McLaughlin JP: Endogenous kappa-opioid mediation of
stress-induced potentiation of ethanol-conditioned place preference
and self-administration. Psychopharmacology (Berl). 210:199–209.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
King BJ, Furlong TM and McNally GP:
Cocaine and amphetamine related transcript (CART) inhibits context
induced reinstatement of reward seeking. Behav Neurosci.
124:423–427. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kimmel HL, Gong W, Vechia SD, Hunter RG
and Kuhar MJ: Intra-ventral tegmental area injection of rat cocaine
and amphetamine-regulated transcript peptide 55–102 induces
locomotor activity and promotes conditioned place preference. J
Pharmacol Exp Ther. 294:784–792. 2000.PubMed/NCBI
|
|
40
|
Cook CJ: Stress induces CRF release in the
paraventricular nucleus, and both CRF and GABA release in the
amygdala. Physiol Behav. 82:751–762. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dandekar MP, Singru PS, Kokare DM and
Subhedar NK: Transient up-regulation of cocaine- and
amphetamine-regulated transcript peptide (CART) immunoreactivity
following ethanol withdrawal in rat hypothalamus. Brain Res.
1240:119–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen YW, Barson JR, Chen A, Hoebel BG and
Leibowitz SF: Hypothalamic peptides controlling alcohol intake:
Differential effects on microstructure of drinking bouts. Alcohol.
48:657–664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Balkan B, Gozen O, Yararbas G, Koylu EO,
Akinturk S, Kuhar MJ and Pogun S: CART expression in limbic regions
of rat brain following forced swim stress: Sex differences.
Neuropeptides. 40:185–193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stanley SA, Murphy KG, Bewick GA, Kong WM,
Opacka-Juffry J, Gardiner JV, Ghatei M, Small CJ and Bloom SR:
Regulation of rat pituitary cocaine- and amphetamine-regulated
transcript (CART) by CRH and glucocorticoids. Am J Physiol
Endocrinol Metab. 287:E583–E590. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Galesi FL, Ayanwuyi LO, Mijares MG,
Cippitelli A, Cannella N, Ciccocioppo R and Ubaldi M: Role of
Hypothalamic-Pituitary-Adrenal axis and corticotropin-releasing
factor stress system on cue-induced relapse to alcohol seeking. Eur
J Pharmacol. 788:84–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fahlke C, Hård E, Eriksson CJ, Engel JA
and Hansen S: Consequence of long-term exposure to corticosterone
or dexamethasone on ethanol consumption in the adrenalectomized
rat, and the effect of type I and type II corticosteroid receptor
antagonists. Psychopharmacology (Berl). 117:216–224. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Deroche V, Marinelli M, Le Moal M and
Piazza PV: Glucocorticoids and behavioral effects of
psychostimulants. II: cocaine intravenous self-administration and
reinstatement depend on glucocorticoid levels. J Pharmacol Exp
Ther. 281:1401–1407. 1997.PubMed/NCBI
|