Introduction
The World Health Organization estimates that 240
million persons globally are chronically infected with hepatitis B
virus (HBV), a hepatotropic DNA virus that replicates via reverse
transcription (1). HBV may induce
acute and chronic infections. Chronically infected individuals are
at an increased risk of liver cirrhosis and hepatocellular
carcinoma (2). A critical clinical
symptom of chronic hepatitis B is severe liver damage (3). As HBV is noncytopathic to infected
hepatocytes, host-specific immune responses are predominantly
responsible for the liver damage (4). Cytotoxic T lymphocytes (CTLs) not
only clear HBV, but may also destroy infected hepatocytes (4). Furthermore, intrahepatic stellate
cells are activated by the HBV infection via the stimulation of a
variety of cytokines, which is followed by matrix deposition,
fibrosis and eventually liver cirrhosis (3). Two types of agents are currently
available for the treatment of hepatitis B: Nucleotide analogues
and immune system modulator interferons (5). Nucleotide analogues may suppress
viral replication but are frequently associated with an increase in
drug-resistant HBV mutants, which results in treatment failure and
progression to liver disease (6).
Immune system modulator interferons may also suppress viral
replication but the treatment is costly and side effects associated
with interferons limit their clinical use (7). Although these treatments are able to
suppress virus replication, they cannot relieve liver damage
effectively in the long term.
Mesenchymal stem cells (MSCs) are multipotent adult
stem cells present in bone marrow, adipose tissue and cord blood,
and have been identified as an attractive candidate for liver
repair (8). Various factors
contribute to this promise of MSCs, including the easy isolation
and expansion of these cells in culture, ability to evade the host
immune response recognition, immunomodulatory properties and
migratory behavior (9). MSCs have
also been demonstrated to form functional hepatocytes in
vitro (10) and possess the
ability to secrete soluble factors stimulating endogenous
parenchymal cells to support tissue recovery (11). Furthermore, MSC transplantation in
a liver fibrosis model has been indicated to reduce fibrosis and
restore depleted hepatic function (12).
A short hairpin (sh)RNA is an artificial RNA
molecule with a tight hairpin turn that may be used to silence
target gene expression via RNA interference (RNAi) (13). The pregenomic RNA, which is longer
than genome length, is an essential replication intermediate that
may be disabled using RNAi-based methods (14). There are four major overlapping
open reading frames of the compact viral genome: PreC/core,
polymerase, surface and X (15).
HBV transcripts also overlap with each other and have common 3′
sequences that are defined by the single transcription termination
signal (16). Single RNAi effector
molecules may therefore have cognates in more than one of the viral
RNAs (17) and multiple sites on
the viral genome have been successfully targeted by small
interfering (si)RNAs (14).
In the present study, MSCs and shRNAs were combined
to treat liver injury induced by HBV in a mouse model. Using in
vivo and in vitro assays, it was assessed whether
combined treatment may ameliorate liver injury and suppress virus
replication at the same time.
Materials and methods
Cell culture
Mouse immortal mesenchymal stem cells (miMSCs;
C3H10T1/2) acquired from China-Japan Union Hospital of Jilin
University (Changchun, China) were cultured in Human Umbilical Cord
Blood MSC Complete Medium (cat. no. HUXUB-90011; Cyagen
Biosciences, Inc., Guangzhou, China). A human hepatoblastoma cell
line (HepG2) and mouse hepatocellular carcinoma cell line (Hepa1-6)
were purchased from the Cell Bank of the Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China) and were cultured
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) (both from HyClone; GE Healthcare Life
Sciences, Logan, UT, USA). An HBV-transfected human hepatoblastoma
cell line (HepG2.2.15) was purchased from Jennio Biological
Technology (Guangzhou, China). HepG2.2.15 cells were cultured in
DMEM supplemented with 10% FBS. Cell cultures were performed at
37°C in a humidified atmosphere containing 5% CO2.
Plasmids
Lx-shRNA157, Lx-shRNA1694 were obtained by cloning
the 157i shRNA expression cassette and the 1694i shRNA expression
cassette into a pLVX-shRNA1 vector (AXYBIO, Changsha, China); while
Lx-shRNA157-1694 was obtained by cloning both 157i and 1694i shRNA
expression cassettes into one pLVX-shRNA1 vector. Lx-shRNA157
expresses 157i, which targets the HBV S gene; Lx-shRNA1694 can
express 1694i, which targets the HBV X gene; and Lx-shRNA157-1694
has the ability to express 157i and 1694i. Hygro-157-1694 contains
the same RNAi target as Lx-shRNA157-1694, was constructed by Suzhou
GenePharma Co., Ltd. (Suzhou, China) (http://www.genepharma.cn/index.asp). pZAC-1.2HBV, an
HBV genomic expression plasmid, was obtained from Professor Panyong
Mao (Beijing 302 Hospital, Beijing, China).
Evaluation of effect of transfection
with Lx-shRNA157, Lx-shRNA1694 and Lx-shRNA157-1694
HepG2.2.15, a reported HBV-producing cell line
(13) was used to evaluate the
effect of LxshRNA157, Lx-shRNA1694 and Lx-shRNA157-1694. Three
types of plasmids were transfected into HepG2.5.15 cells in
6-well-plates using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 3 µg/well. The
transfection was done according to the manufacturer's instructions
for Lipofectamine 2000. At 48 h post transfection, mRNA expression
of HBV S and HBV X genes were detected using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
levels of HBsAg/HBeAg in the cell culture medium were also
detected.
Construction of miMSCs stably
expressing shRNA
The shRNA expression plasmid Hygro-157-1694 (2
µg/well in 6-well tissue culture plates) was transfected into
miMSCs using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The transfection was performed according to the
manufacturer's instructions for Lipofectamine 2000. At 24 h
post-transfection, the cells were cultured with Human Umbilical
Cord Blood MSC Complete Medium (cat. no. HUXUB-90011; Cyagen
Biosciences, Inc.) containing hygromycin B (300 µg/ml; v900372;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 10% FBS for 14
days. The medium was removed and replaced with fresh Human
Umbilical Cord Blood MSC Complete Medium containing hygromycin B
(300 µg/ml) every 2 days. Following antibiotic selection, miMSCs
stably expressing shRNA (miMSC-shRNA) were plated in Human
Umbilical Cord Blood MSC Complete Medium containing hygromycin B
(50 µg/ml) at ~1 cell/well in 96-well tissue culture plates to
select monoclones. Following culture to 1×106
cells/clone, miMSC-shRNA monoclones were stored at −80°C until use.
All cell culture steps were performed at 37°C in a humidified
atmosphere containing 5% CO2.
Evaluation of interference function of
miMSC-shRNA monoclones
HBV-expressing plasmid (pZAC-1.2HBV, 3 µg) was
transfected into miMSCs or two miMSCs stably expressing shRNA
monoclones (miMSC-shRNA1, miMSC-shRNA2) in 6-well plates using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). At
48 h post-transfection, HBV S and X mRNA expression levels in
miMSC, miMSC-shRNA1 and miMSC-shRNA2 cells were tested using
RT-qPCR. HBV preS2 antigen levels in miMSCs, miMSC-shRNA1 and
miMSC-shRNA2 cells were tested by immunofluorescence.
Hepatogenic differentiation of miMSCs
in vitro
Hepatogenic differentiation was performed using
OriCell Human Mesenchymal Stem Cell Hepatogenic Differentiation
Medium (cat. no. HUXMX-90101; Cyagen Biosciences, Inc.), which
contains three types of media: Pre-treatment medium (containing 20
ng/ml epidermal growth factor and 10 ng/ml basic fibroblast growth
factor), hepatocyte differentiation medium [containing 20 ng/ml
hepatocyte growth factor (HGF), 10 ng/ml basic fibroblast growth
factor and 0.61 g/l nicotinamide] and hepatocyte maturation medium
(containing 20 ng/ml oncostatin M, 1 µmol/l dexamethasone and 50
mg/ml insulin-transferrin-selenium premix). Three steps, including
pretreatment, differentiation and maturation, were needed in this
experiment. In pretreatment step, miMSCs were plated in Human
Umbilical Cord Blood MSC Complete Medium with 10% FBS at a density
of 1.5×104 cells/cm2 in a 6-well tissue
culture plate that had been pre-coated with 0.1% gelatin solution.
After 24 h, the cells were serum-deprived for 2 days in the
pretreatment medium. Then, differentiation was induced by treating
miMSCs with hepatocyte differentiation media for 7 days. During the
7 days, the differentiation medium was refreshed every 2 days.
Finally, cells were cultured with hepatocyte maturation medium for
total 14 days to induce maturation and the maturation medium was
refreshed every 2 days. Cells were placed at 37°C in a humidified
atmosphere containing 5% CO2 during incubation and
culture. Images of treatment, differentiation and maturation were
captured at the end of each step with a fluorescence microscope
(Olympus Corporation, Tokyo, Japan) at magnification, ×200.
Immunofluorescence
miMSCs were fixed with 4% paraformaldehyde for 10
min. Following permeabilization with 0.2% Triton X-100 for 8 min,
the cells were blocked with 5% FBS for 10 min and incubated
sequentially for 90 min with primary antibody (mouse anti-preS2
monoclonal immunoglobulin G, 1:200, cat. no. sc-516176; Santa Cruz
Biotechnology Inc., Dallas, TX, USA). Then, cells were incubated
with secondary antibody (Alexa Fluor 488-conjugated goat anti-mouse
immunoglobulin G, 1:200, B40941; Thermo Fisher Scientific, Inc.)
for 40 min. Between each step, cells were washed with PBS three
times (3 min/time) and subsequently nuclear staining was performed
using 4′,6-diamidino-2-phenylindole (1:10,000; Beyotime Institute
of Biotechnology, Shanghai, China) for 5 min. Cells were examined
with a fluorescence microscope (Olympus Corporation) at
magnification, ×200. All steps were carried out at room
temperature.
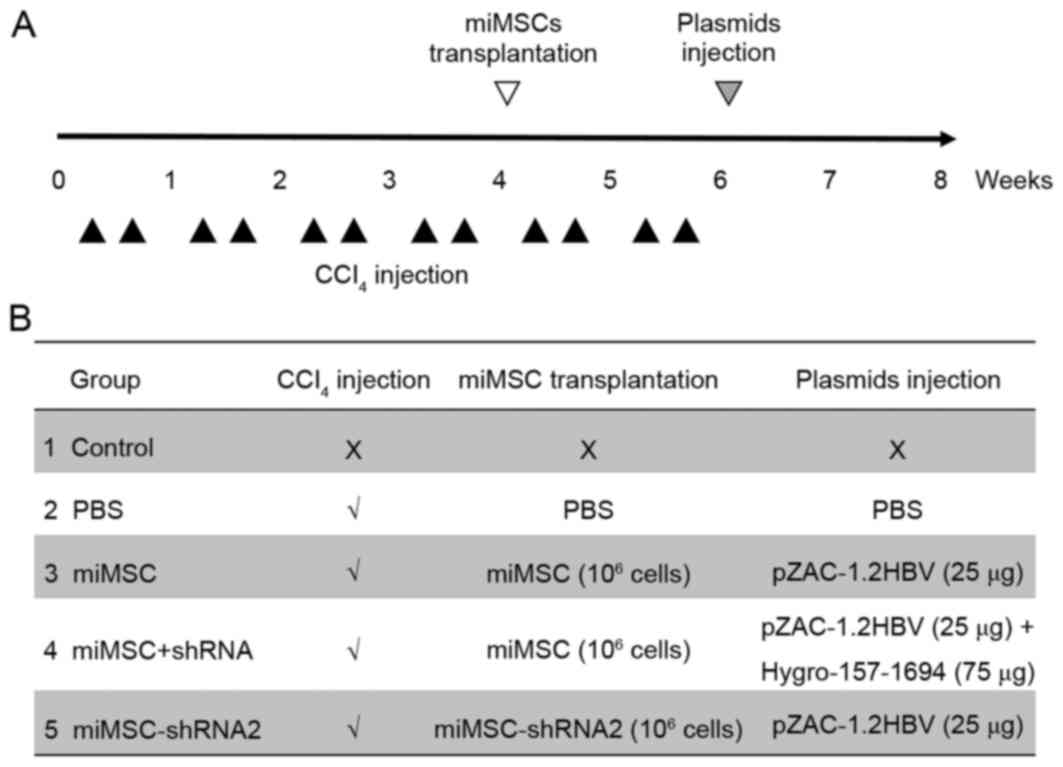
Animal model construction and
miMSC/shRNA treatment
The present study was approved by the Institutional
Animal Care and Use Committee of Jilin University (Changchun,
China) and followed the national guidelines for the treatment of
animals. A total of 25 female Balb/c mice (6–8 weeks old, 20±2 g
weight) were purchased from Liaoning Changsheng Biotechnology Co.,
Ltd. (Liaoning, China), allowed to acclimatize for 1 week and were
maintained under standard environmental conditions (at room
temperature with a 12-h light/dark cycle, humidity 50–60%) with
ad libitum access to water and rodent chow. Mice were
divided into five groups (5 mice/group). In the control group, no
procedure was administered. The other four groups were treated with
1 ml/kg CCl4 in olive oil (1:9) intraperitoneally twice
a week for 4 weeks. After 4 weeks, miMSC cells (1×106
cells/mouse) were injected into the spleen in the miMSC group and
the miMSC+shRNA, while miMSC-shRNA2 groups received miMSC-shRNA
cells (1×106 cells/mouse) in spleen. The PBS group
received an equal volume of PBS injections in the spleen. The
CCl4 toxin (1 ml/kg) was administered twice a week for 2
further weeks following miMSC transplantation (12 injections in
total) to induce a high selective pressure for transplanted cells
to engraft and differentiate. Subsequently, mice in the control,
PBS, miMSC and miMSC+shRNA groups were injected in the tail vein
with 2 ml PBS containing 25 µg HBV expression plasmid pZAC-1.2HBV
plus 75 µg shRNA expression plasmid, whereas the miMSC-shRNA2 group
only received 25 µg HBV expression plasmid Hygro-157-1694. Blood
serum was collected from all groups for 10 days following plasmid
injection to examine the levels of HBsAg/HBeAg. At the end of the
procedure, serum samples of animals were collected for ALT/AST
assays. Additionally, liver samples were taken for liver weight
(LW)/body weight (BW) ratio analysis, total RNA isolation or sirius
red staining. The whole animal experimental design was exhibited in
Fig. 1.
RNA isolation, RT-q
and-semi-quantitative PCR for gene expression analysis
RNA was isolated from HepG2.2.15 cells and miMSCs or
mouse liver tissues using TRIzol™ reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). cDNA synthesis was performed with
2 µg RNA sample using a Prime Script RT Reagent kit with gDNA
eraser (Takara Bio, Inc., Otsu, Japan). Then, qPCR was performed
with SYBR Green PCR Super Mix (Transgene, Inc., Cambridge, MA,
USA), 8 mM each primer and 100–500 ng/ml template cDNA using a
Bio-Rad CFX96 Real-Time PCR Detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). In brief, the qPCR went through 3 steps:
Initialization (98°C, 5 min); 30 cycles of denaturation (94°C, 30
sec), annealing (58°C, 30 sec), extension (72°C, 30 sec); final
elongation (72°C, 5 min). When the RT-PCR completed, temperature
was held on 4°C. The relative ratio and standard deviation between
the normal and treated samples was calculated using the
2−ΔΔCq method (18).
The expression of HBV S and HBV X genes was estimated using Bio-Rad
CFX Manager 3.0 software (Bio Rad Laboratories, Inc.). β-actin was
used as internal control. Semi-quantification was performed using
2X EasyTaq PCR SuperMix (Transgene, Inc.) to evaluate the gene
expression levels of tyrosine transaminase (TAT),
glucose-6-phosphatedehydrogenase (G-6-P), transthyretin (TTR),
human albumin (ALB), cytokeratin 18 (CK18), hepatocyte nuclear
factor-3β (HNF-3β) and α-fetoprotein (AFP). The PCR conditions
were: Initialization (98°C, 5 min); 25 cycles of denaturation
(94°C, 30 sec), annealing (58°C, 30 sec), extension (72°C, 30 sec);
final elongation (72°C, 5 min). When the qPCR cycle was completed
the temperature was held at 4°C. mRNA expression levels of genes
were estimated using 1.5% agarose gel electrophoresis. Ethidium
bromide was used for the visualization. β-actin was used as
internal control. All primer sequences are listed in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Sequence
(5′-3′) |
|---|
| β-actin | S:
TTCCTTCTTGGGTATGGAAT |
|
| A:
GAGCAATGATCTTGATCTTC |
| TAT | S:
ACCTTCAATCCCATCCGA |
|
| A:
TCCCGACTGGATAGGTAG |
| G-6-P | S:
CAGGACTGGTTCATCCTT |
|
| A:
GTTGCTGTAGTAGTCGGT |
| TTR | S:
TCTCTCAATTCTGGGGGTTG |
|
| A:
TTTCACAGCCAACGACTCTG |
| ALB | S:
TCAACTGTCAGAGCAGAGAAGC |
|
| A:
AGACTGCCTTGTGTGGAAGACT |
| CK18 | S:
TGGTACTCTCCTCAATCTGCTG |
|
| A:
CTCTGGATTGACTGTGGAAGTG |
| HNF-3β | S:
AGACTCCGGCGGGCACCGAG |
|
| A:
GTGGTTGAAGGCGTAATGGT |
| AFP | S:
GTGAAACAGACTTCCTGGTCCT |
|
| A:
GCCCTACAGACCATGAAACAAG |
| HBV S gene | S:
GCAGGAGGCGGATTTGC |
|
| A:
CAAGGTAGGAGCTGGAGCATTC |
| HBV X gene | S:
AGTCCAAGAGTCCTCTTATGTAAGACCTT |
|
| A:
CCGTCTGTGCCTTCTCATCTG |
Detection of HBV viral antigens
hepatitis B surface antigen (HBsAg) and hepatitis B e-antigen
(HBeAg) by ELISA
Levels of HBsAg and HBeAg in the cell culture medium
of HepG2.2.15 cells and sera from mice were examined using 75
µl/sample and commercial ELISA kits (cat. nos. KH-T-01 and KH-T-03;
Shanghai Kehua Bio-Engineering Co., Ltd., Shanghai, China)
according to the manufacturer's instructions. All experiments were
performed in triplicate and repeated at least two times
independently.
Biochemical tests
At days 0, 3, 7, 10, 13, 17 and 21 post
cell-treatment with hepatocyte differentiation medium, levels of
urea in the cell culture medium of miMSCs were tested using the
Urea Enzymatic Assay kit (Bioo Scientific, Austin, TX, USA)
according to the manufacturer's instructions. Blood samples (~0.5
ml) were taken from all experimental groups prior to sacrifice.
Serum was isolated and levels of alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) were estimated using ALT kit (cat.
no. C001-a) and AST kit (cat. no. C002-a) (all from Changchun Huili
Biotech Co., Ltd., Changchun, China), respectively. HepG2 cells,
which was originally misidentified as a hepatocellular carcinoma
cell line but was later indicated to be derived from a
hepatoblastoma (16), were used as
positive controls (hepatocyte/hepatocyte-like cells) in urea
production and glycogen storage assays.
Sirius red stain
Liver tissues were taken immediately, fixed in 4%
paraformaldehyde (diluted in PBS) at 4°C overnight and then
dehydrated with 20% gradient and 30% gradient sucrose-PBS
solutions. Liver tissues were frozen in isopentane and kept at
−80°C overnight. Liver sections (30-µm thick) were obtained using a
sliding microtome for following sirius red staining. Murine liver
sections were picro-sirius red stained (Direct red 80; Sangon
Biotech Co., Ltd., Shanghai, China) for 1 h at room temperature.
Following staining, the sections were sealed with neutral gum. The
red region in liver sections indicated areas that contained a high
collagen level. Images were captured using a fluorescent microscope
(Olympus Corporation) under natural light. Magnification, ×200.
Statistical analysis
Statistical calculations were performed using
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). An
unpaired t-test or one-way analysis of variance followed by the
Newman-Keuls test were performed to analyze the data. The results
are expressed as the mean ± standard error of the mean. P<0.05
was considered to indicate a statistically significant
difference.
Results
Construction and interference function
of Lx-shRNA157, Lx-shRNA1694 and Lx-shRNA157-1694
The use of an adeno-associated virus (AAV) vector to
simultaneously deliver two shRNAs targeting different HBV-related
genes (157i targeting the HBV S gene and 1694i targeting the HBV X
gene) has been demonstrated to result in greater antiviral effects
than vectors that delivered a single shRNA in vitro and
in vivo (19). In the
present study, one or two shRNA expression cassettes were subcloned
into a pLVX-shRNA1 vector (Fig. 2A and
B). In vitro anti-HBV activities of all the constructs
were investigated with the aim of determining efficient vectors for
anti-HBV gene therapy.
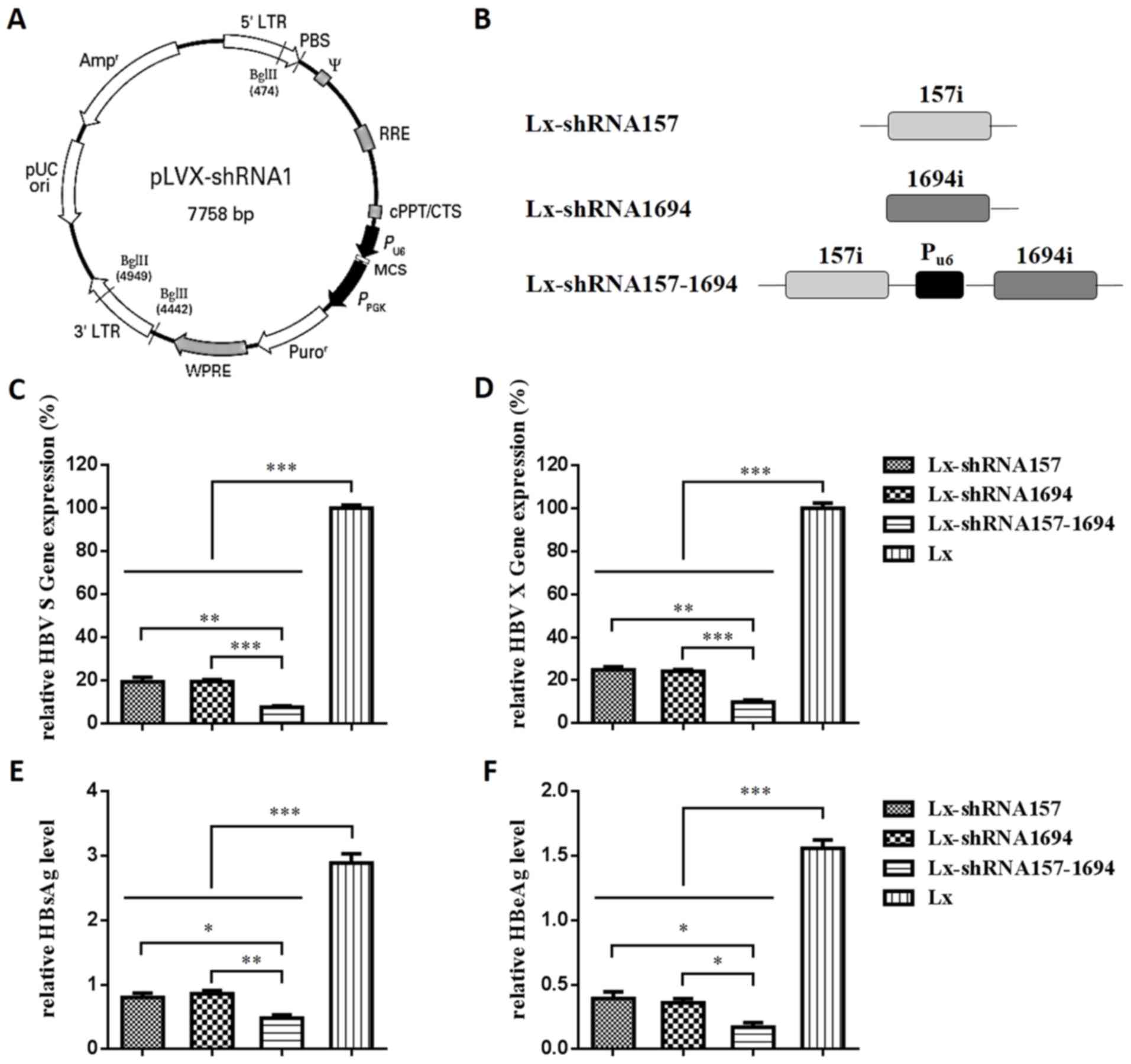 | Figure 2.Construction and interference
function of Lx-shRNA157, Lx-shRNA1694 and Lx-shRNA157-1694. (A)
pLVX-shRNA1 vector map. (B) Schematic diagram of shRNA expression
cassettes. RT-qPCR was used to assess the interference function of
the shRNA expression plasmids against the (C) ‘HBV S gene’ and (D)
‘HBV X gene’. ELISA was used to assess the interference function of
shRNA expression plasmids against (E) HBsAg and (F) HBeAg in
HepG2.2.15 cells. Lx, Lx-shRNA157 or Lx-shRNA1694, Lx-shRNA157-1694
(3 µg) were transfected into HepG2.2.15 cells in 6-well plates. At
48 h post-transfection, HBV S and X gene expression levels in
HepG2.2.15 cells were tested using RT-qPCR. All values are
expressed as the mean + standard error of the mean (n=3).
*P<0.05, **P<0.01, ***P<0.001. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; shRNA, short
hairpin RNA; HBV, hepatitis B virus; HBsAg, hepatitis B surface
antigen; HBeAg, hepatitis B e-antigen. |
HepG2.2.15 cells were reported to derive from a
hepatoblastoma (20), which was
previously misidentified as a human hepatocellular carcinoma cell
line. In the present study, HepG2.2.15 cells were used to test the
anti-HBV activity of shRNA as HepG2.2.15 is an HBV-producing cell
line. To examine anti-HBV activity, shRNA expression plasmids were
transfected into HepG2.2.15 cells. From the results of RT-qPCR, HBV
S mRNA expression levels in HepG2.2.15 cells transfected with
Lx-shRNA157, Lx-shRNA1694 and Lx-shRNA157-1694 were 19.2, 19.3 and
7.4%, respectively (Fig. 2C). HBV
X mRNA expression levels in HepG2.2.15 cells transfected with
Lx-shRNA157, Lx-shRNA1694 and Lx-shRNA157-1694 were 24.8, 24.1 and
9.8%, respectively (Fig. 2D).
Based on the ELISA results (Fig. 1E
and F), the interference capacities of Lx-shRNA157,
Lx-shRNA1694 and Lx-shRNA157-1694 on expression of HBV surface
antigens HBsAg and HBsAg were consistent with the RT-qPCR results.
Furthermore, Lx-shRNA157-1694 demonstrated significantly greater
antiviral effects compared with Lx-shRNA157 or Lx-shRNA1694 at mRNA
(both P<0.005) and protein (P<0.05 and P<0.005,
respectively) expression levels.
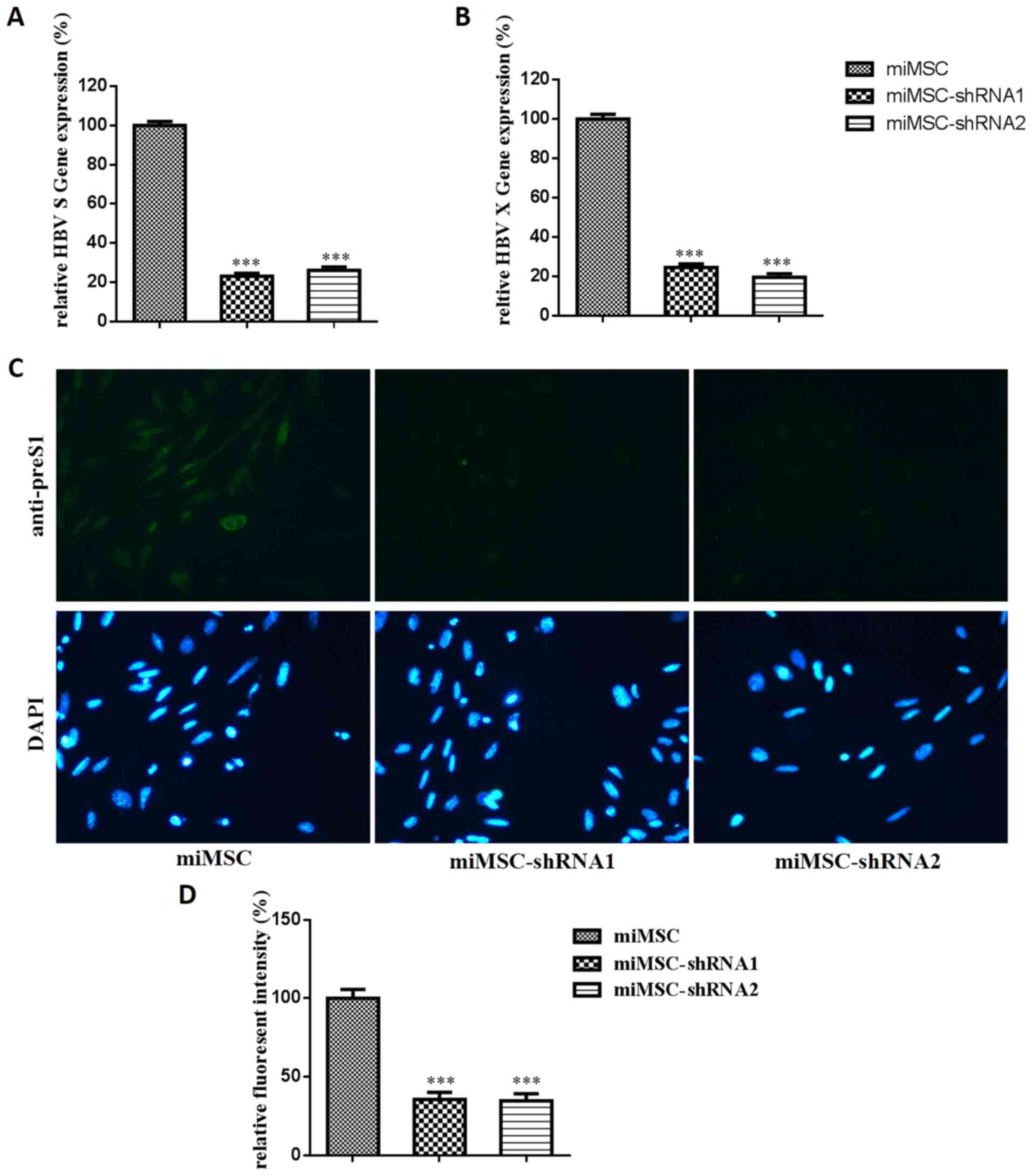
Interference function of miMSCs stably
expressing shRNA
Hygro-157-1694, which contains the same RNAi target
as Lx-shRNA157-1694, was used to select miMSCs that were stably
expressing shRNA. Two monoclones of miMSCs that stably expressed
shRNA, miMSC-shRNA1 and miMSC-shRNA2, were successfully selected.
pZAC-1.2HBV is an HBV genomic expression plasmid. To examine
anti-HBV activity, pZAC-1.2HBV was transfected into miMSCs,
miMSC-shRNA1 or miMSC-shRNA2. HBV S mRNA expression levels in
miMSC-shRNA1 and miMSC-shRNA2 were 23.2 and 26.1%, respectively,
whereas HBV X mRNA expression levels in miMSC-shRNA1 and
miMSC-shRNA2 were 24.6 and 19.7%, respectively (Fig. 3A and B). HBV preS2 antigen protein
expression levels in miMSC-shRNA1 and miMSC-shRNA2 were determined
as 35.6 and 34.7%, respectively (Fig.
3C and D). These results indicated that miMSCs stably
expressing shRNA monoclones were successfully selected and both
monoclones indicated similar antiviral effects at mRNA and protein
expression levels.
Hepatocyte differentiation of miMSCs,
miMSC-shRNA1 and miMSC-shRNA2 in vitro
To investigate whether stable-expression of shRNAs
influenced the hepatogenic differentiation capability of miMSCs,
hepatogenic differentiation of miMSCs, miMSC-shRNA1 and
miMSC-shRNA2 was induced in vitro. The use of specific
culture conditions has been reported to cause MSCs to undergo a
phenotypic change, express genes which are typically expressed in
hepatocytes and fulfill specific metabolic functions similar to
hepatocytes (21). In the present
study, phenotypic changes were recorded during hepatogenic
differentiation. Prior to differentiation, cells exhibited a
fibroblast-like morphology and did not markedly change during
pretreatment. Following ~12 days of differentiation, cells
developed a broadened flattened shape. During maturation, a
polygonal cell shape was developed. Results indicated that all
three cell types progressed through the same phenotypic changes as
previously reported (Fig. 4A). The
mRNA expression patterns of seven hepatocyte-specific markers and
β-actin during the hepatogenic differentiation were investigated
using semi-quantitative PCR. TAT was expressed in the three cell
types between days 0–21 throughout the whole hepatogenic
differentiation process, whereas G-6-P and TTR expression levels
were undetectable. ALB, CK18, HNF-3β and AFP mRNA expression levels
in the three cell types increased gradually during hepatogenic
differentiation; however, AFP mRNA expression levels declined on
day 21 (Fig. 4B), which is likely
due to the fact that AFP is a marker of the early stage of the
hepatogenic differentiation (22).
Compared with Hepa1-6, ALB, CK18 and AFP mRNA expression levels in
cells on day 21 were similar. However, TAT and HNF-3β mRNA
expression levels in cells on day 21 were lower compared with
Hepa1-6 expression levels in all cell types. These results
suggested that although miMSCs, miMSC-shRNA1 and miMSC-shRNA2 were
able to differentiate into hepatocyte-like cells, there were
differences between hepatocyte-like cells and hepatocytes in the
mRNA expression levels of specific hepatocyte-specific markers.
Furthermore, the similar expression levels of ALB mRNA in all three
cell types on day 13, 17 and 21 indicated that they had the similar
synthetic function as hepatocytes.
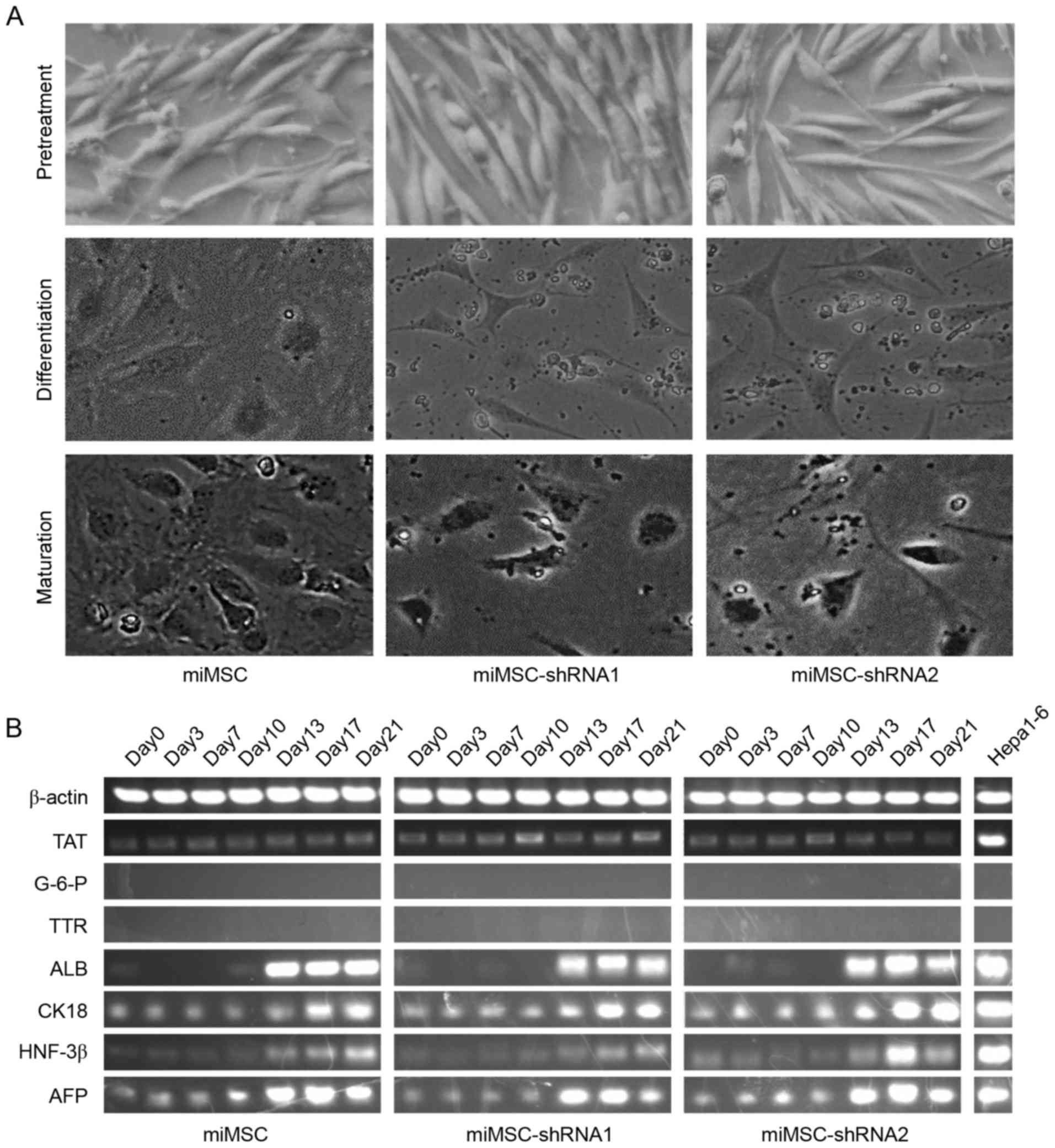 | Figure 4.Analysis of hepatocyte-like cell
characteristics of miMSCs, miMSC-shRNA1 and miMSC-shRNA2 during
hepatogenic differentiation in vitro. (A) Morphology of
differentiated hepatocytes. Images at ×200 magnification were taken
at the end of treatment, differentiation, maturation phase,
respectively. (B) Semi-quantitative polymerase chain reaction
analyses of temporal mRNA expression patterns of
hepatocyte-specific markers. miMSC, mouse immortal mesenchymal stem
cell. shRNA, short hairpin RNA; TAT, tyrosine transaminase; G-6-P,
glucose-6-phosphatedehydrogenase; TTR, transthyretin; ALB, human
albumin; CK18, cytokeratin 18; HNF-3β, hepatocyte nuclear factor
3β; AFP, α-fetoprotein. |
Urea production and glycogen storage levels of
miMSCs, miMSC-shRNA1 and miMSC-shRNA2 were also examined and
indicated that these three cell types had similar urea production
and glycogen storing ability (data not shown).
These results indicated that miMSCs, miMSC-shRNA1
and miMSC-shRNA2 exhibited the same ability to differentiate into
hepatocyte-like cells, and our alteration had no influence on the
hepatogenic differentiation capability of miMSCs.
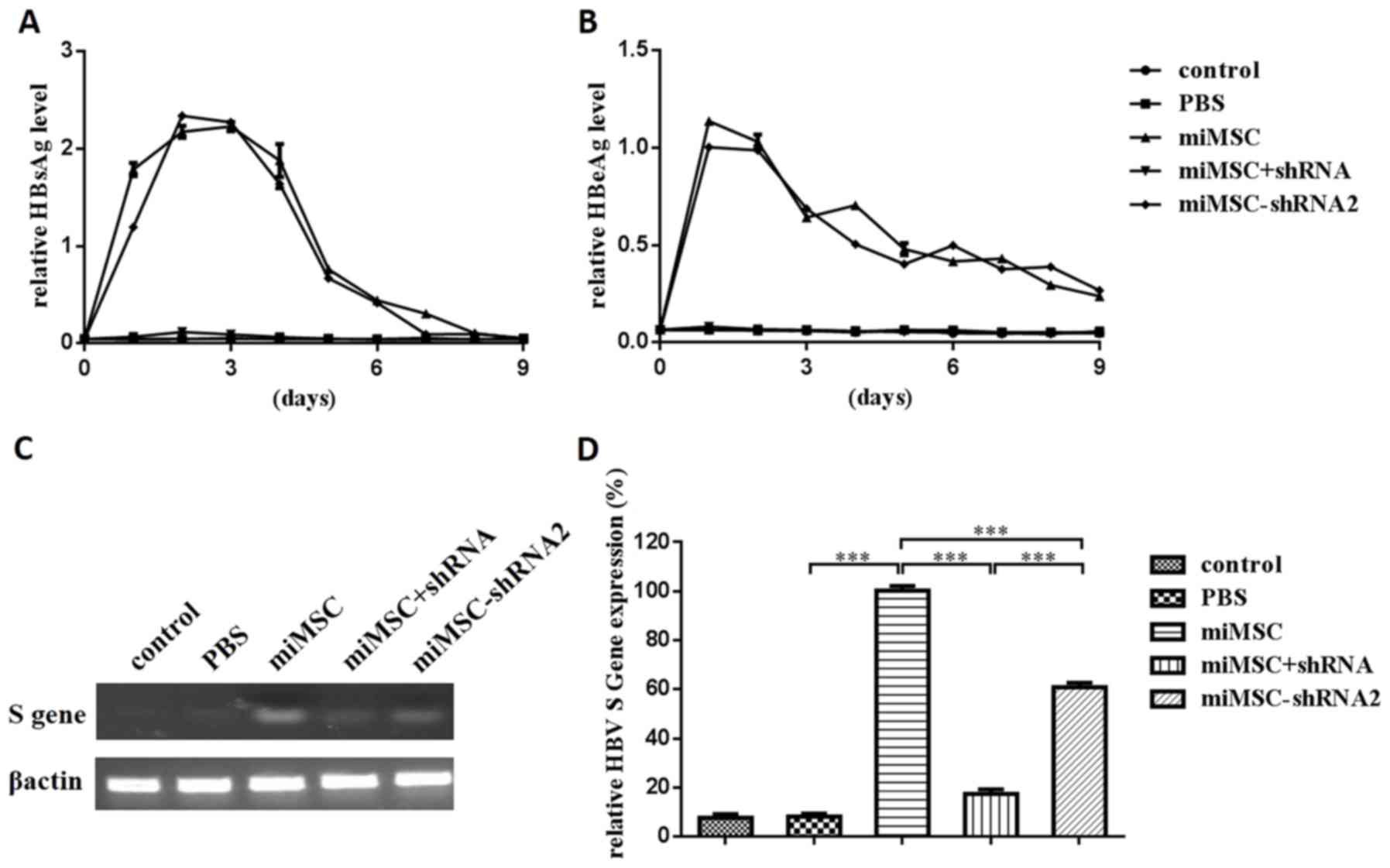
Therapeutic effects of miMSCs combined
with shRNA on HBV expression
To evaluate the therapeutic effects of miMSCs
combined with shRNA on HBV expression, an in vivo mouse
model was established. HBsAg and HBeAg levels in murine sera were
subsequently analyzed using ELISA (Fig. 5A and B). Results demonstrated that
HBsAg and HBeAg levels in the sera of mice in the control and PBS
groups were almost undetectable, whereas those in the miMSC group
reached a maximum level on day 2 or 1 following injection of the
HBV expression plasmid injection and decreased gradually from day 3
or 2, respectively. These findings suggested that the HBV
expression plasmid was expressed successfully following injection
into mice. The expression trends of HBsAg and HBeAg levels in the
control, PBS and miMSC+shRNA groups were similar, which indicated
that administration of shRNA expression plasmid through the tail
vein successfully decreased the expression of HBsAg and HBeAg in
sera. The expression trends of HBsAg and HBeAg levels in the miMSC
and miMSC-shRNA2 groups demonstrated no significant differences,
and this indicated that the transplantation of miMSCs stably
expressing shRNA had nearly no influence on the expression of HBsAg
and HBeAg in murine sera.
The administration of HBV expression plasmid through
the tail vein induced HBsAg and HBeAg expression throughout the
bodies of mice. To evaluate HBV expression in the liver, HBV S mRNA
levels in murine liver tissues were tested using semi-quantitative
PCR (Fig. 5C) or qPCR (Fig. 5D). The results demonstrated that
HBV S mRNA expression levels in the miMSC+shRNA and miMSC-shRNA2
groups were significantly decreased compared with the miMSC group
(P<0.001). Compared with the miMSC group, HBV S mRNA expression
levels in the control, PBS, miMSC+shRNA and miMSC-shRNA2 groups
were 7.7, 8.3, 17.5 and 60.9%, respectively.
These findings demonstrated that administration of
shRNA-expressing plasmid through the tail vein successfully
decreased the expression of HBsAg and HBeAg in murine sera and
liver tissue compared with the miMSC group. Notably, miMSC-shRNA
transplantation decreased the HBV S mRNA expression levels in
murine liver; however, expression of HBV antigens in the sera was
not suppressed.
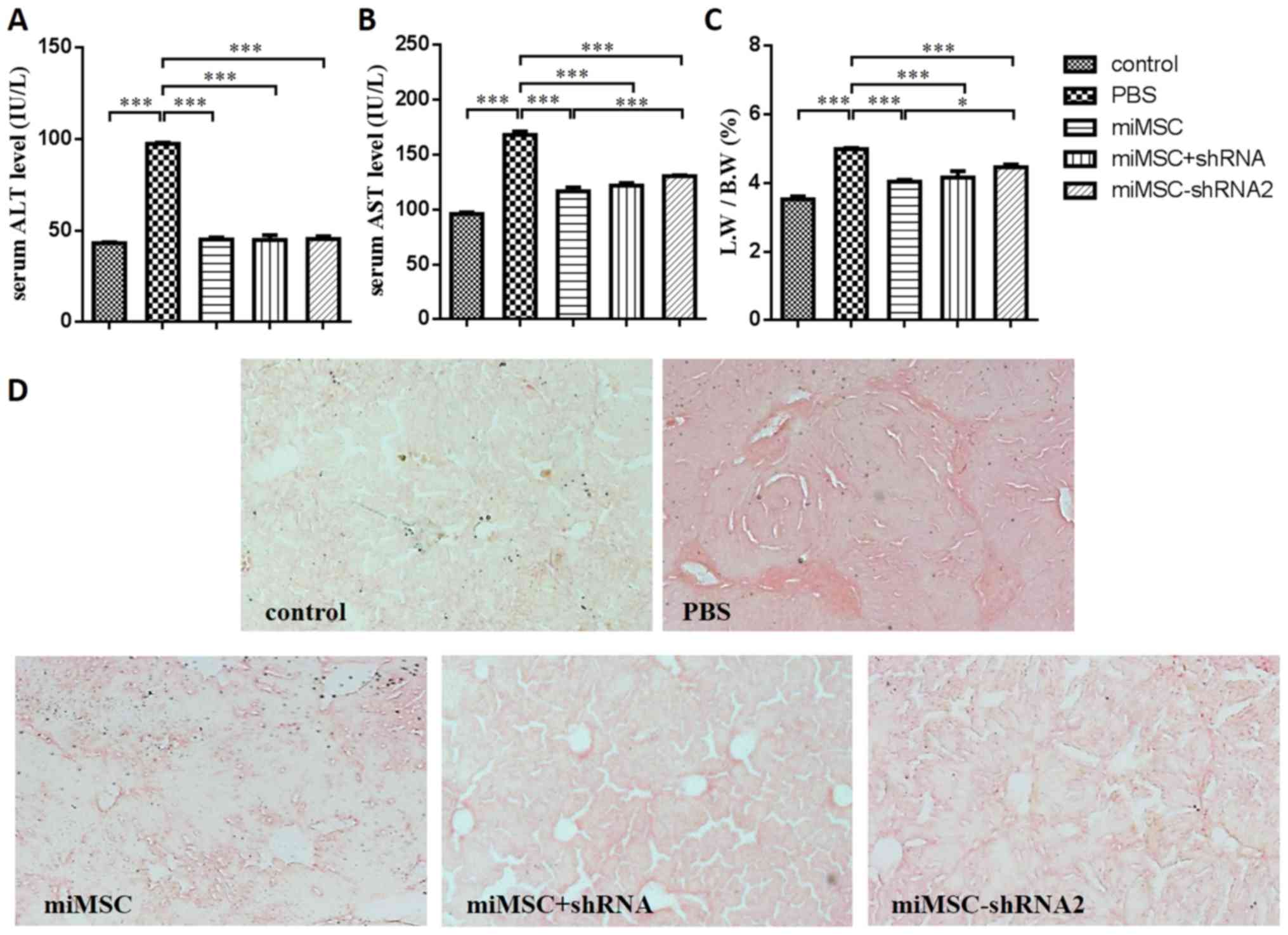
Therapeutic effects of miMSCs combined
with shRNA on liver injury
The release of ALT and AST enzymes, which are
markers for liver injury (23),
was measured using a commercial biochemistry analyzer (Fig. 6A and B). Results demonstrated that,
following miMSC transplantation (miMSC, miMSC+shRNA and
miMSC-shRNA2 groups), ALT and AST levels in the serum decreased
significantly compared with the PBS group (P<0.001). However,
the AST levels in the serum of mice in the miMSC-shRNA2 group were
significantly greater compared with that in the miMSC group
(P<0.001). These findings revealed that our alteration of miMSCs
had influenced the therapeutic effects of miMSCs on liver injury to
some degree. The LW/BW ratio is also a marker for liver injury and
is increased when the liver is damaged (24). The LW/BW ratio of the PBS group was
significantly increased compared with the control group, indicating
that the liver injury was induced by CCl4 (P<0.001).
In addition, results indicated that, following miMSC
transplantation (miMSC, miMSC+shRNA and miMSC-shRNA2 groups), the
LW/BW ratio decreased significantly compared with the PBS group
(P<0.001; Fig. 6C).
Furthermore, the LW/BW of the miMSC-shRNA2 group was significantly
increased compared with the miMSC group (P<0.05), which was
consistent with results of the AST levels in serum.
As collagen deposition is a marker for liver
fibrosis (25), liver fibrosis in
murine liver sections was assessed using sirius red staining in the
present study (Fig. 6D). Liver
sections of miMSC, miMSC+shRNA and miMSC-shRNA2 groups indicated
marked reductions in fibrosis compared with that in the PBS group.
Additionally, no notable fibrosis was observed in the liver
sections of the control group. These results suggested that a
marked reduction of liver injury had occurred in the miMSC+shRNA
and miMSC-shRNA2 groups. However, the therapeutic effects of
miMSC-shRNA2 transplantation against liver damage were weaker
compared with miMSC transplantation and treatment with miMSC
combined with shRNA, which was indicated by analyses of AST levels
and LW/BW ratio.
Discussion
HBV is responsible for ~350 million chronic
infections worldwide and >1 million annual fatalities (26). Liver failure is a serious clinical
syndrome characterized by massive necrosis of hepatocytes due to a
variety of acute or chronic infections of HBV (27). Therefore, for patients with
hepatitis B, suppression of HBV replication is not sufficient and
the damaged liver function must be restored. Liver transplantation
is considered the standard treatment for patients with liver
failure. However, lack of tissue sources, high cost and associated
complications have restricted the use of liver transplantation
clinically (28). MSC
transplantation therapy has become a novel and potential treatment
for liver injury recently (29).
Previous studies have demonstrated that the infusion of bone
marrow-derived (BM)-MSCs may ameliorate liver fibrosis (30,31)
and reverse fulminant hepatic failure in experimental mice
(32). Furthermore, infusions of
autologous BM-MSCs or umbilical cord MSCs have significantly
improved liver function in patients with liver cirrhosis (33) and liver failure (34).
Due to the lack of a proofreading function of its
polymerase, HBV continuously undergoes rapid mutagenesis that
creates multiple HBV drug-resistant variants during viral
replication (35). This phenomenon
affects chemotherapy outcomes in the majority of patients with
chronic HBV (36). Subsequently,
the requirement for alternative therapeutic approaches is urgent.
Previous studies have suggested that two shRNAs that targeted the
HBV S (157i) and X (1694i) genes may be employed to avoid the
phenomenon of resistance of HBV mutants to a single siRNA (19). And the dual shRNA expression vector
(AAV-157i-1694i) demonstrated greater antiviral effects in
vitro and in vivo compared with those vectors only
expressing a single shRNA (19).
In the present study, Lx-shRNA157-1694 (shRNA
expression plasmid) containing two shRNA expression cassettes was
constructed to suppress HBV replication and employed with miMSC
transplantation as treatment for liver injury. miMSCs were combined
with shRNA in two different ways to evaluate the therapeutic
effects on liver injury induced by HBV infection: By injecting
miMSCs and the shRNA expression plasmid separately, or only
injecting miMSCs stably expressing shRNA into the mouse model. The
former therapeutic regimen successfully suppressed HBV expression
in murine sera and liver tissue, whereas the latter suppressed HBV
expression in liver tissue alone. Both therapeutic regimens
significantly relieved liver injury. Yan et al (37) previously identified sodium
taurocholate cotransporting polypeptide (NTCP) as a functional
receptor for HBV. Consistent with HBV liver tropism, NTCP is
primarily expressed in hepatocytes and localized to the sinusoidal
plasma membrane. Therefore, in patients with chronic hepatitis B,
HBV infection and replication occurs predominantly in liver tissue
(38). Transplantation of miMSCs
stably expressing shRNA that are able to suppress HBV expression
only in liver tissue would be sufficient for clinical treatment. To
the best of the authors' knowledge, the present study is the first
to combine miMSCs with shRNA to treat liver injury and suppress HBV
replication at the same time in a mouse model. Therefore, the
present study provides a novel therapeutic strategy for the
treatment of liver injury induced by HBV infection.
The present results revealed that the therapeutic
effects of miMSC-shRNA2 transplantation on suppressing HBV
expression in the liver and on relieving liver damage were weaker
compared with shRNA expression plasmid injection or miMSC
transplantation. This result indicated that our alteration may have
influenced the migration and proliferation ability of miMSCs, such
that only a small proportion of miMSC-shRNA2 had migrated to the
liver and resulted in a low shRNA level in liver tissue. Incubation
with recombinant HGF has been reported to increase the homing
ability of MSCs towards injured liver tissue (9). Further study is required to
investigate whether incubation with HGF may improve the therapeutic
effects of miMSC-shRNA2 transplantation. Previous research has also
indicated that double-stranded AAV vector serotype 8 (a
hepatotropic AAV vector) carrying shRNA effectively reduced HBV
replication and gene expression in murine liver tissue (39). miMSCs combined with an AAV8 vector
carrying shRNA to treat liver injury induced by HBV infection will
be used in our further study. This therapeutic regimen may improve
the suppression of HBV expression in the liver and liver
damage.
As HBV has a narrow host range, which does not
include mice, a combined hydrodynamic injection mouse model
(40) with a
CCl4-induced liver injury mouse model (41) was constructed to simulate HBV
replication and liver injury. Hydrodynamic injection of plasmids
containing the HBV genome into mice has been reported to not cause
liver damage (42). Therefore,
liver injury in the present mouse model was caused by
CCl4 injection. However, in patients with chronic
hepatitis B, liver damage is caused by CTLs during the elimination
of HBV-infected hepatocytes. Therefore, further investigation into
the therapeutic effects of miMSC combined with shRNA in a more
suitable animal model that can simulate liver injury caused by HBV
infection is required.
In conclusion, the present study evaluated the
therapeutic effects of miMSC combined with shRNAs in treating liver
injury induced by HBV in a mouse model. The in vitro and
in vivo results support the therapeutic strategy using a
combination of miMSC and shRNAs and this has potential as a future
therapy for the treatment of liver injury induced by HBV
injection.
Acknowledgements
The authors would like to thank the China-Japan
Union Hospital of Jilin University (Changchun, China) for providing
miMSCs. The present study was supported by the National Natural
Science Foundation of China (grant nos. 81472816 and 81673002) and
the Science and Technology Development Plan of Jilin Province
(grant nos. 20160101240JC and 20160519018JH). The authors also
would like to thank Miss Phuong Thi Sarkis (sarkis-science-editing.com) for editorial support in
the preparation of this study.
Glossary
Abbreviations
Abbreviations:
|
HBV
|
hepatitis B virus
|
|
MSCs
|
mesenchymal stem cells
|
|
shRNA
|
short hairpin RNA
|
|
miMSCs
|
mouse immortal mesenchymal stem
cells
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
References
|
1
|
Lin GG, Zhang K and Li JM: Application of
CRISPR/Cas9 Technology to HBV. Int J Mol Sci. 16:26077–26086. 2015.
View Article : Google Scholar :
|
|
2
|
Hassan MM, Li D, El-Deeb AS, Wolff RA,
Bondy ML, Davila M and Abbruzzese JL: Association between hepatitis
B virus and pancreatic cancer. J Clin Oncol. 26:4557–4562. 2008.
View Article : Google Scholar :
|
|
3
|
Suhail M, Abdel-Hafiz H, Ali A, Fatima K,
Damanhouri GA, Azhar E, Chaudhary AG and Qadri I: Potential
mechanisms of hepatitis B virus induced liver injury. World J
Gastroentero. 20:12462–12472. 2014. View Article : Google Scholar
|
|
4
|
Guidotti LG and Chisari FV: Immunobiology
and pathogenesis of viral hepatitis. Annu Rev Pathol. 1:23–61.
2006. View Article : Google Scholar
|
|
5
|
Stein LL and Loomba R: Drug targets in
hepatitis B virus infection. Infect Disord Drug Targets. 9:105–116.
2009. View Article : Google Scholar
|
|
6
|
Kayaaslan B and Guner R: Adverse effects
of oral antiviral therapy in chronic hepatitis B. World J Hepatol.
9:227–241. 2017. View Article : Google Scholar :
|
|
7
|
Yu HB, Liu EQ, Lu SM and Zhao SH:
Treatment with peginterferon versus interferon in Chinese patients
with hepatitis B. Biomed Pharmacother. 64:559–564. 2010. View Article : Google Scholar
|
|
8
|
Nasir GA, Mohsin S, Khan M, Shams S, Ali
G, Khan SN and Riazuddin S: Mesenchymal stem cells and
Interleukin-6 attenuate liver fibrosis in mice. J Transl Med.
11:782013. View Article : Google Scholar :
|
|
9
|
Liu J, Pan G, Liang T and Huang P:
HGF/c-Met signaling mediated mesenchymal stem cell-induced liver
recovery in intestinal ischemia reperfusion model. Int J Med Sci.
11:626–633. 2014. View Article : Google Scholar :
|
|
10
|
Le Blanc K and Ringdén O: Immunobiology of
human mesenchymal stem cells and future use in hematopoietic stem
cell transplantation. Biol Blood Marrow Transplant. 11:321–334.
2005. View Article : Google Scholar
|
|
11
|
Parekkadan B, van Poll D, Megeed Z,
Kobayashi N, Tilles AW, Berthiaume F and Yarmush ML:
Immunomodulation of activated hepatic stellate cells by mesenchymal
stem cells. Biochem Biophys Res Commun. 363:247–252. 2007.
View Article : Google Scholar :
|
|
12
|
Seo KW, Sohn SY, Bhang DH, Nam MJ, Lee HW
and Youn HY: Therapeutic effects of hepatocyte growth
factor-overexpressing human umbilical cord blood-derived
mesenchymal stem cells on liver fibrosis in rats. Cell Biol Int.
38:106–116. 2014. View Article : Google Scholar
|
|
13
|
Paddison PJ, Caudy AA, Bernstein E, Hannon
GJ and Conklin DS: Short hairpin RNAs (shRNAs) induce
sequence-specific silencing in mammalian cells. Gene Dev.
16:948–958. 2002. View Article : Google Scholar :
|
|
14
|
Sun DX, Rösler C, Kidd-Ljunggren K and
Nassal M: Quantitative assessment of the antiviral potencies of 21
shRNA vectors targeting conserved, including structured, hepatitis
B virus sites. J Hepatol. 52:817–826. 2010. View Article : Google Scholar
|
|
15
|
Beck J and Nassal M: Hepatitis B virus
replication. World J Gastroentero. 13:48–64. 2007. View Article : Google Scholar
|
|
16
|
Wooddell CI, Yuen MF, Chan HL, Gish RG,
Locarnini SA, Chavez D, Ferrari C, Given BD, Hamilton J, Kanner SB,
et al: RNAi-based treatment of chronically infected patients and
chimpanzees reveals that integrated hepatitis B virus DNA is a
source of HBsAg. Sci Transl Med. 9:eaan02412017. View Article : Google Scholar
|
|
17
|
Marimani M, Hean J, Bloom K, Ely A and
Arbuthnot P: Recent advances in developing nucleic acid-based HBV
therapy. Future Microbiol. 8:1489–1504. 2013. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Li Z, He ML, Yao H, Dong QM, Chen YC, Chan
CY, Zheng BJ, Yuen KY, Peng Y, Sun Q, et al: Inhibition of HBV
replication and gene expression in vitro and in vivo with a single
AAV vector delivering two shRNA molecules. BMB Rep. 42:59–64. 2009.
View Article : Google Scholar
|
|
20
|
Zhao R, Wang TZ, Kong D, Zhang L, Meng HX,
Jiang Y, Wu YQ, Yu ZX and Jin XM: HHepatoma cell line HepG2.2.15
demonstrates distinct biological features compared with parental
HepG2. World J Gastroentero. 17:1152–1159. 2011. View Article : Google Scholar
|
|
21
|
Meier RP, Müller YD, Morel P,
Gonelle-Gispert C and Bühler LH: Transplantation of mesenchymal
stem cells for the treatment of liver diseases, is there enough
evidence? Stem Cell Res. 11:1348–1364. 2013. View Article : Google Scholar
|
|
22
|
Sarvandi SS, Joghataei MT, Parivar K,
Khosravi M, Sarveazad A and Sanadgol N: In vitro differentiation of
rat mesenchymal stem cells to hepatocyte lineage. Iran J Basic Med
Sci. 18:89–97. 2015.
|
|
23
|
Qu M, Yuan X, Liu D, Ma Y, Zhu J, Cui J,
Yu M, Li C and Guo D: Bone marrow-derived mesenchymal stem cells
attenuate immune-mediated liver injury and compromise virus control
during acute hepatitis B virus infection in mice. Stem Cells Dev.
26:818–827. 2017. View Article : Google Scholar
|
|
24
|
Saat TC, van den Engel S, Bijman-Lachger
W, Korevaar SS, Hoogduijn MJ, IJzermans JN and de Bruin RW: Fate
and effect of intravenously infused mesenchymal stem cells in a
mouse model of hepatic ischemia reperfusion injury and resection.
Stem Cells Int. 2016:57614872016. View Article : Google Scholar :
|
|
25
|
Xie SR, An JY, Zheng LB, Huo XX, Guo J,
Shih D and Zhang XL: Effects and mechanism of adenovirus-mediated
phosphatase and tension homologue deleted on chromosome ten gene on
collagen deposition in rat liver fibrosis. World J Gastroentero.
23:5904–5912. 2017. View Article : Google Scholar
|
|
26
|
Oh IS and Park SH: Immune-mediated liver
injury in hepatitis B virus infection. Immune Netw. 15:191–198.
2015. View Article : Google Scholar :
|
|
27
|
Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z,
Zhang A, Shi J, Chen L, Lv S, et al: Human mesenchymal stem cell
transfusion is safe and improves liver function in acute-on-chronic
liver failure patients. Stem Cell Transl Med. 1:725–731. 2012.
View Article : Google Scholar
|
|
28
|
Pamecha V, Kumar S and Bharathy KG: Liver
transplantation in acute on chronic liver failure: Challenges and
an algorithm for patient selection and management. Hepatol Int.
9:534–542. 2015. View Article : Google Scholar
|
|
29
|
Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY,
Chen XY, Liu QL, Peng L, Li JG, Mei YY, et al: Allogeneic bone
marrow-derived mesenchymal stromal cells for hepatitis B
virus-related acute-on-chronic liver failure: A randomized
controlled trial. Hepatology. 66:209–219. 2017. View Article : Google Scholar
|
|
30
|
Rabani V, Shahsavani M, Gharavi M, Piryaei
A, Azhdari Z and Baharvand H: Mesenchymal stem cell infusion
therapy in a carbon tetrachloride-induced liver fibrosis model
affects matrix metalloproteinase expression. Cell Biol Int.
34:601–605. 2010. View Article : Google Scholar
|
|
31
|
Chang YJ, Liu JW, Lin PC, Sun LY, Peng CW,
Luo GH, Chen TM, Lee RP, Lin SZ, Harn HJ and Chiou TW: Mesenchymal
stem cells facilitate recovery from chemically induced liver damage
and decrease liver fibrosis. Life Sci. 85:517–525. 2009. View Article : Google Scholar
|
|
32
|
van Poll D, Parekkadan B, Cho CH,
Berthiaume F, Nahmias Y, Tilles AW and Yarmush ML: Mesenchymal stem
cell-derived molecules directly modulate hepatocellular death and
regeneration in vitro and in vivo. Hepatology. 47:1634–1643. 2008.
View Article : Google Scholar
|
|
33
|
Terai S, Ishikawa T, Omori K, Aoyama K,
Marumoto Y, Urata Y, Yokoyama Y, Uchida K, Yamasaki T, Fujii Y, et
al: Improved liver function in patients with liver cirrhosis after
autologous bone marrow cell infusion therapy. Stem Cells.
24:2292–2298. 2006. View Article : Google Scholar
|
|
34
|
Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie
C, Zheng YB and Gao ZL: Autologous bone marrow mesenchymal stem
cell transplantation in liver failure patients caused by hepatitis
B: Short-term and long-term outcomes. Hepatology. 54:820–828. 2011.
View Article : Google Scholar
|
|
35
|
Buti M, Rodriguez-Frias F, Jardi R and
Esteban R: Hepatitis B virus genome variability and disease
progression: The impact of pre-core mutants and HBV genotypes. J
Clin Virol. 34 Suppl 1:S79–S82. 2005. View Article : Google Scholar
|
|
36
|
Tillmann HL: Antiviral therapy and
resistance with hepatitis B virus infection. World J Gastroentero.
13:125–140. 2007. View Article : Google Scholar
|
|
37
|
Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z,
Huang Y, Qi Y, Peng B, Wang H, et al: Sodium taurocholate
cotransporting polypeptide is a functional receptor for human
hepatitis B and D virus. Elife. 1:e000492012. View Article : Google Scholar :
|
|
38
|
Schulze A, Schieck A, Ni Y, Mier W and
Urban S: Fine mapping of pre-S sequence requirements for hepatitis
B virus large envelope protein-mediated receptor interaction. J
Virol. 84:1989–2000. 2010. View Article : Google Scholar
|
|
39
|
Chen CC, Sun CP, Ma HI, Fang CC, Wu PY,
Xiao X and Tao MH: Comparative study of anti-hepatitis B virus RNA
interference by double-stranded adeno-associated virus serotypes 7,
8, and 9. Mol Ther. 17:352–359. 2009. View Article : Google Scholar
|
|
40
|
Yang PL, Althage A, Chung J and Chisari
FV: Hydrodynamic injection of viral DNA: A mouse model of acute
hepatitis B virus infection. P Natl Acad Sci USA. 99:13825–13830.
2002. View Article : Google Scholar
|
|
41
|
Higashiyama R, Inagaki Y, Hong YY, Kushida
M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G
and Okazaki I: Bone marrow-derived cells express matrix
metalloproteinases and contribute to regression of liver fibrosis
in mice. Hepatology. 45:213–222. 2007. View Article : Google Scholar
|
|
42
|
Huang LR, Wu HL, Chen PJ and Chen DS: An
immunocompetent mouse model for the tolerance of human chronic
hepatitis B virus infection. P Natl Acad Sci USA. 103:17862–17867.
2006. View Article : Google Scholar
|